Abstract
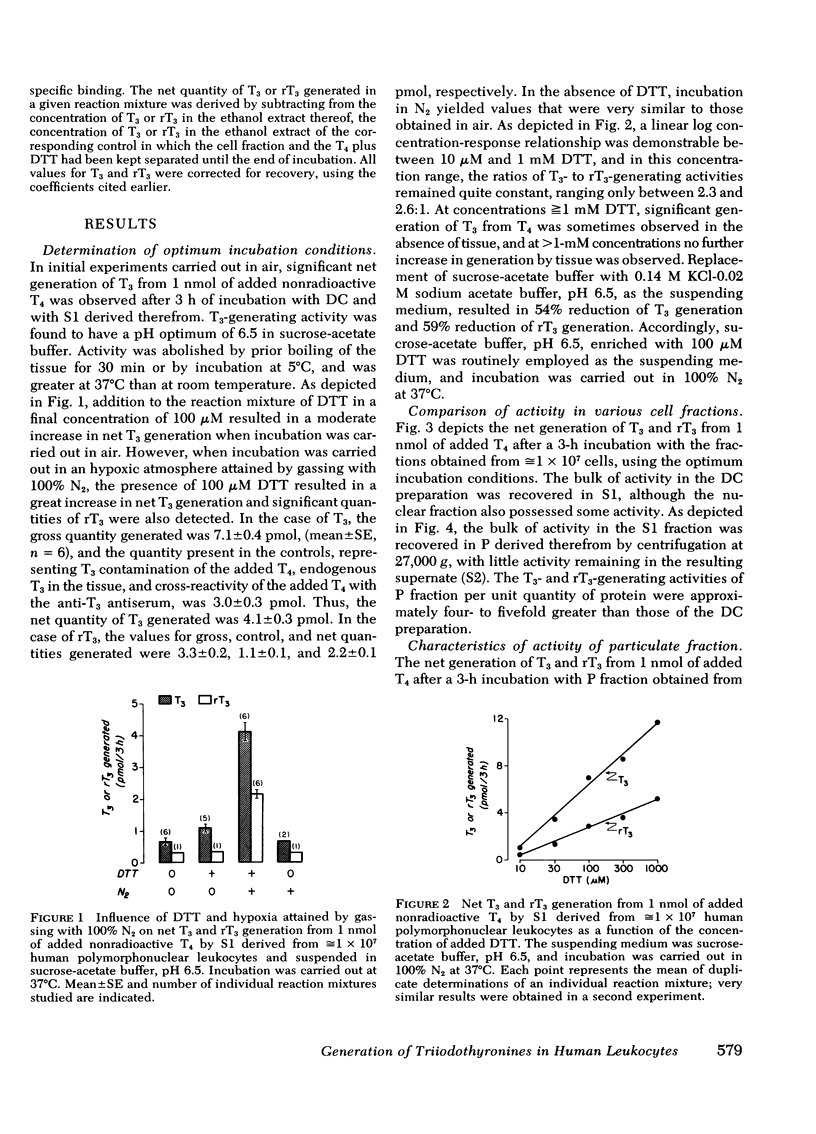
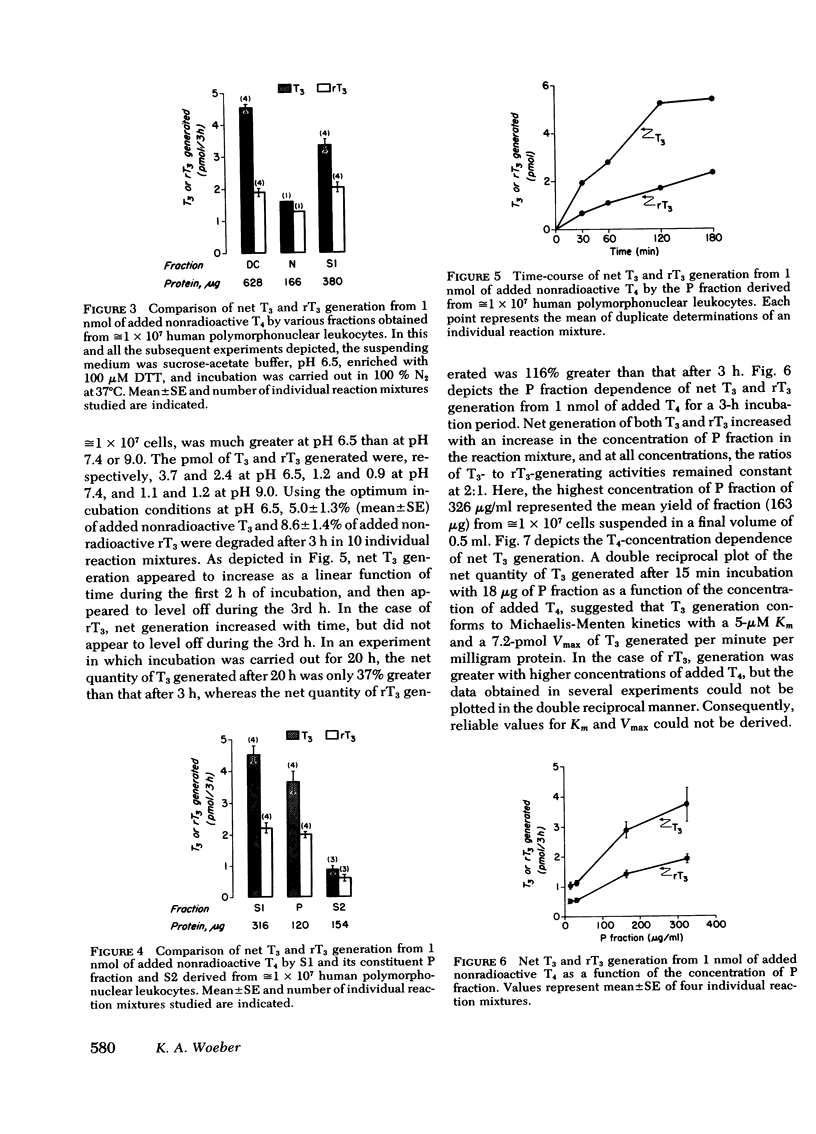
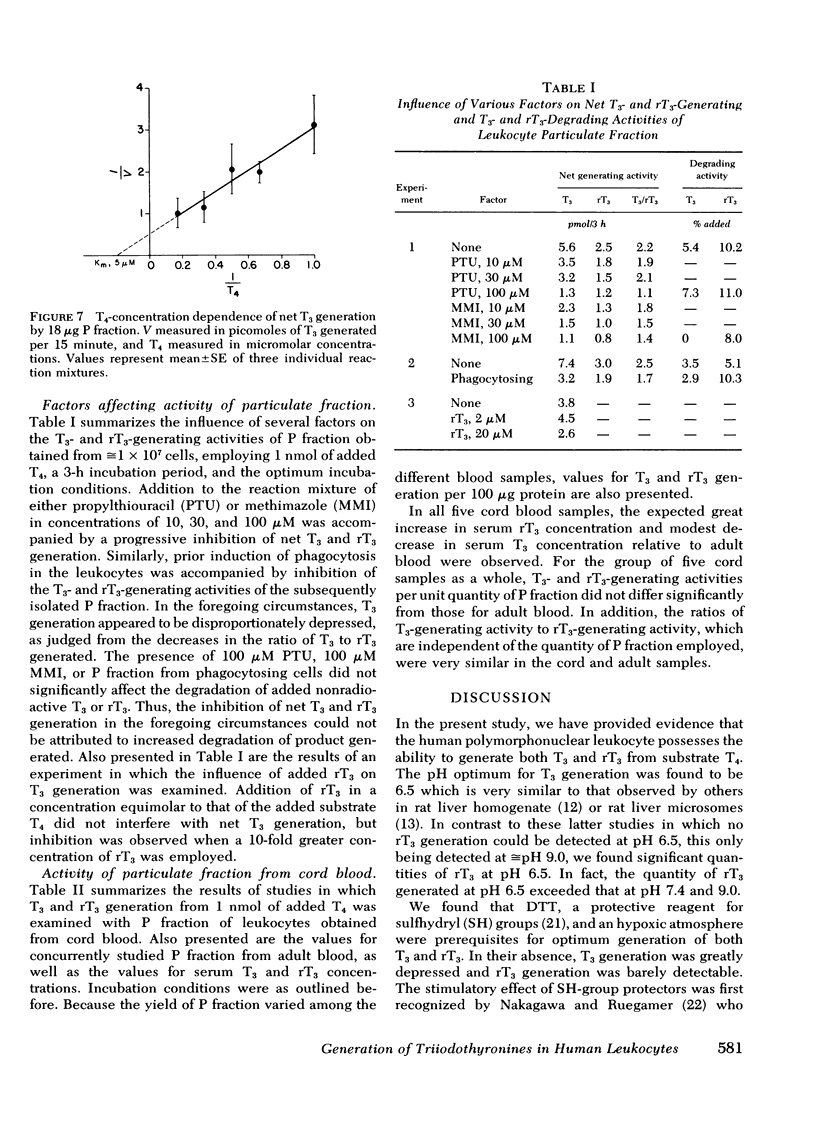
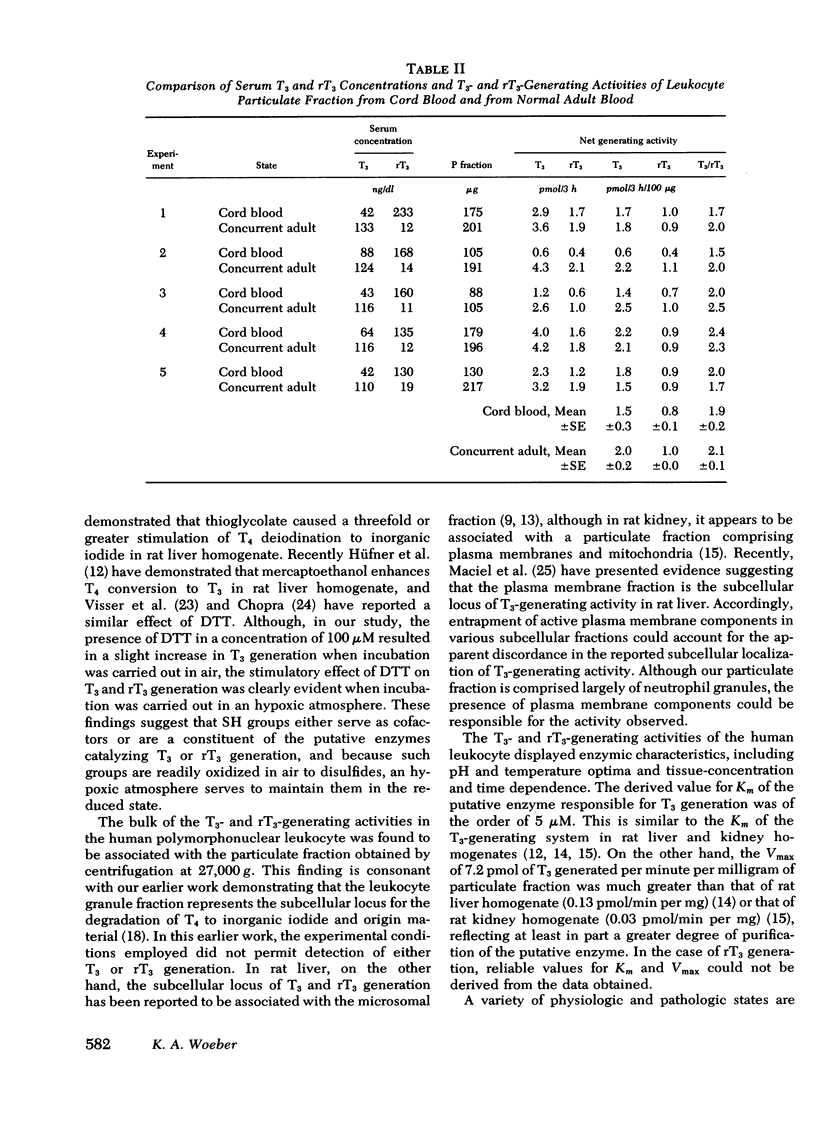
Extrathyroidal monodeiodination of l-thyroxine (T4) is the principal source of l-triiodothyronine (T3) and l-reverse-triiodothyronine (rT3) production. To define some of the cellular factors involved, we examined T3 and rT3 generation from added nonradioactive T4 in human polymorphonuclear leukocytes, using radioimmunoassays to quantify the T3 and rT3 generated. Under optimum incubation conditions which included a pH of 6.5 in sucrose-acetate buffer, the presence of dithiothreitol as a sulfhydryl-group protector, and incubation in an hypoxic atmosphere, significant net generation of T3 and rT3 was observed. Of the several subcellular fractions studied, the particulate fraction obtained by centrifugation at 27,000 g was found to possess the highest T3- and rT3-generating activities per unit quantity of protein. With respect to T3 generation from substrate T4, the Km was 5 μM and the Vmax was 7.2 pmol/min per mg protein. Propylthiouracil, methimazole, and prior induction of phagocytosis inhibited both T3 and rT3 generation, but T3 generation was inhibited to a greater extent. rT3, in a concentration equimolar to that of substrate T4, did not alter T3 generation, but inhibited T3 generation when the molar ratio of rT3 to T4 approached 10:1. Under the incubation conditions employed, particulate fractions of leukocytes obtained from five cord blood samples displayed an essentially normal relationship between T3- and rT3-generating activities, despite the distinctly divergent serum T3 and rT3 concentrations in these samples. From our findings, we draw the following conclusions: (a) the human polymorphonuclear leukocyte possesses the ability to generate T3 and rT3 from substrate T4; (b) the T3- and rT3-generating activities are associated principally with the 27,000 g particulate fraction and display enzymic characteristics with a sulfhydryl-group requirement; (c) T3-generating activity appears to be more susceptible to inhibitory influences than rT3-generating activity; and (d) in cord blood leukocytes, the putative enzymes catalyzing T3 and rT3 generation appear to be functionally intact under the experimental conditions employed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M., Curnutte J. T., McMurrich B. J. The particulate superoxide-forming system from human neutrophils. Properties of the system and further evidence supporting its participation in the respiratory burst. J Clin Invest. 1976 Oct;58(4):989–996. doi: 10.1172/JCI108553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braverman L. E., Ingbar S. H., Sterling K. Conversion of thyroxine (T4) to triiodothyronine (T3) in athyreotic human subjects. J Clin Invest. 1970 May;49(5):855–864. doi: 10.1172/JCI106304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braverman L. E., Vagenakis A., Downs P., Foster A. E., Sterling K., Ingbar S. H. Effects of replacement doses of sodium L-thyroxine on the peripheral metabolism of thyroxine and triiodothyronine in man. J Clin Invest. 1973 May;52(5):1010–1017. doi: 10.1172/JCI107265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLELAND W. W. DITHIOTHREITOL, A NEW PROTECTIVE REAGENT FOR SH GROUPS. Biochemistry. 1964 Apr;3:480–482. doi: 10.1021/bi00892a002. [DOI] [PubMed] [Google Scholar]
- Cavalieri R. R. Impaired peripheral conversion of thyroxine to triiodothyronine,. Annu Rev Med. 1977;28:57–65. doi: 10.1146/annurev.me.28.020177.000421. [DOI] [PubMed] [Google Scholar]
- Chiraseveenuprapund P., Buergi U., Goswami A., Rosenberg I. N. Conversion of L-thyroxine to triiodothyronine in rat kidney homogenate. Endocrinology. 1978 Feb;102(2):612–622. doi: 10.1210/endo-102-2-612. [DOI] [PubMed] [Google Scholar]
- Chopra I. J. A study of extrathyroidal conversion of thyroxine (T4) to 3,3',5-triiodothyronine (T3) in vitro. Endocrinology. 1977 Aug;101(2):453–463. doi: 10.1210/endo-101-2-453. [DOI] [PubMed] [Google Scholar]
- Chopra I. J. An assessment of daily production and significance of thyroidal secretion of 3, 3', 5'-triiodothyronine (reverse T3) in man. J Clin Invest. 1976 Jul;58(1):32–40. doi: 10.1172/JCI108456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra I. J. Sulfhydryl groups and the monodeiodination of thyroxine to triiodothyronine. Science. 1978 Feb 24;199(4331):904–906. doi: 10.1126/science.622575. [DOI] [PubMed] [Google Scholar]
- Conversion of thyroxine into tri-iodothyronine by rat liver homogenate. Biochem J. 1975 Sep;150(3):489–493. doi: 10.1042/bj1500489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin L., Castle J., McMahon F., Martin P., Hammond M., Cavalieri R. R. Extrathyroidal conversion of thyroxine to 3,3',5'-triiodothyronine (reverse-T3) and to 3,5,3'-triiodothyronine (T3) in humans. J Clin Endocrinol Metab. 1977 Apr;44(4):733–742. doi: 10.1210/jcem-44-4-733. [DOI] [PubMed] [Google Scholar]
- Hesch R. D., Brunner G., Söling H. D. Conversion of thyroxine (T4) and triiodothyronine (T3) and the subcellular localisation of the converting enzyme. Clin Chim Acta. 1975 Mar 10;59(2):209–213. doi: 10.1016/0009-8981(75)90031-5. [DOI] [PubMed] [Google Scholar]
- Höffken B., Ködding R., Hesch R. D. Conversion of T4 to T3 and rT3 and their cytoplasmic binding: pH dependency. Clin Chim Acta. 1977 Jul 15;78(2):261–266. doi: 10.1016/0009-8981(77)90314-x. [DOI] [PubMed] [Google Scholar]
- Hüfner M., Grussendorf M., Ntokalou M. Properties of the thyroxine (T4) monodeiodinating system in rat liver homogenate. Clin Chim Acta. 1977 Jul 15;78(2):251–259. doi: 10.1016/0009-8981(77)90313-8. [DOI] [PubMed] [Google Scholar]
- Kaplan M. M., Schimmel M., Utiger R. D. Changes in serum 3,3',5'-triiodothyronine (reverse T3) concentrations with altered thyroid hormone secretion and metabolism. J Clin Endocrinol Metab. 1977 Sep;45(3):447–456. doi: 10.1210/jcem-45-3-447. [DOI] [PubMed] [Google Scholar]
- Kaplan M. M., Utiger R. D. Iodothyronine metabolism in rat liver homogenates. J Clin Invest. 1978 Feb;61(2):459–471. doi: 10.1172/JCI108957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J., Green W. L. Degradation of thyroid hormones by phagocytosing human leukocytes. J Clin Invest. 1973 Jan;52(1):60–72. doi: 10.1172/JCI107174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nakagawa S., Ruegamer W. R. Properties of a rat tissue iodothyronine deiodinase and its natural inhibitor. Biochemistry. 1967 May;6(5):1249–1261. doi: 10.1021/bi00857a005. [DOI] [PubMed] [Google Scholar]
- Nicod P., Burger A., Strauch G., Vagenakis A. G., Braverman L. E. The failure of physiologic doses of reverse T3 to effect thyroid-pituitary function in man. J Clin Endocrinol Metab. 1976 Aug;43(2):478–481. doi: 10.1210/jcem-43-2-478. [DOI] [PubMed] [Google Scholar]
- Pittman C. S., Chambers J. B., Jr, Read V. H. The extrathyroidal conversion rate of thyroxine to triiodothyronine in normal man. J Clin Invest. 1971 Jun;50(6):1187–1196. doi: 10.1172/JCI106596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refetoff S., Matalon R., Bigazzi M. Metabolism of L-thyroxine (T4) and L-triiodothyronine (T3) by human fibroblasts in tissue culture: evidence for cellular binding proteins and conversion of T4 to T3. Endocrinology. 1972 Oct;91(4):934–947. doi: 10.1210/endo-91-4-934. [DOI] [PubMed] [Google Scholar]
- Sterling K., Brenner M. A., Saldanha V. F. Conversion of thyroxine to triiodothyronine by cultured human cells. Science. 1973 Mar 9;179(4077):1000–1001. doi: 10.1126/science.179.4077.1000. [DOI] [PubMed] [Google Scholar]
- Surks M. I., Schadlow A. R., Stock J. M., Oppenheimer J. H. Determination of iodothyronine absorption and conversion of L-thyroxine (T 4 ) to L-triiodothyronine (T 3 ) using turnover rate techniques. J Clin Invest. 1973 Apr;52(4):805–811. doi: 10.1172/JCI107244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser T. J., Does-Tobé I., Docter R., Hennemann G. Subcellular localization of a rat liver enzyme converting thyroxine into tri-iodothyronine and possible involvement of essential thiol groups. Biochem J. 1976 Aug 1;157(2):479–482. doi: 10.1042/bj1570479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woeber K. A. A granule-associated L-thyroxine deiodinating system in the human leukocyte. Endocrinology. 1976 Mar;98(3):802–806. doi: 10.1210/endo-98-3-802. [DOI] [PubMed] [Google Scholar]
- Woeber K. A., Ingbar S. H. Metabolism of L-thyroxine by phagocytosing human leukocytes. J Clin Invest. 1973 Aug;52(8):1796–1803. doi: 10.1172/JCI107361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woeber K. A. Observations concerning the binding of L-triiodothyronine in the human polymorphonuclear leukocyte. J Clin Endocrinol Metab. 1977 Jan;44(1):62–68. doi: 10.1210/jcem-44-1-62. [DOI] [PubMed] [Google Scholar]



