Abstract
Although most monoclonal antibodies developed for cancer therapy are of the IgG class, antibodies of the IgE class have certain properties that make them attractive as cancer therapeutics. These properties include the superior affinity for the Fc epsilon receptors (FcεRs), the low serum level of IgE that minimizes competition of endogenous IgE for FcεR occupancy, and the ability to induce a broad and vigorous immune response through the interaction with multiple cells including mast cells, basophils, monocytes, macrophages, dendritic cells, and eosinophils. Tumor-targeted IgE antibodies are expected to harness the allergic response against tumors and activate a secondary, T-cell-mediated immune response. Importantly, the IgE antibody can be used for passive immunotherapy and as an adjuvant of cancer vaccines. However, there are important limitations in the use of animal models including the fact that human IgE does not interact with rodent FcεRs and that there is a different cellular distribution of FcεRs in humans and rodents. Despite these limitations, different murine models have been used with success to evaluate the in vivo anti-cancer activity of several IgE antibodies. These models include wild-type immunocompetent animals bearing syngeneic tumors, xenograft models using immunocompromised mice bearing human tumors and reconstituted with human effector cells, and human FcεRIα transgenic mice bearing syngeneic tumors. In addition, non-human primates such as cynomolgus monkeys can be potentially used for toxicological and pharmacokinetic studies. This article describes the advantages and disadvantages of these models and their use in evaluating the in vivo properties of IgE antibodies for cancer therapy.
Keywords: IgE, Animal models, Cancer, AllergoOncology, Immunotherapy
Introduction
The use of antibodies as cancer therapeutics has rapidly grown since the 1990s. Several antibodies containing human constant regions of the IgG class are now commonly used in the clinic for cancer therapy [1]. Examples of these antibodies are trastuzumab (Herceptin®, humanized IgG1κ), which is specific for HER2/neu and used for the treatment of HER2/neu-positive breast cancer, and rituximab (Rituxan®, chimeric IgG1κ), which is specific for CD20 and used for the treatment of non-Hodgkin’s lymphoma and other B-cell malignancies. The success of these and other IgG antibodies has been clearly shown by the prolongation of patient survival.
Although in general therapeutic anti-cancer antibodies are of the IgG class, antibodies of the IgE class have certain properties that support the study of these molecules as potential therapeutics for the immunotherapy of cancer. Research devoted to the relationship between cancer and IgE falls within the field of “AllergoOncology,” which aims to reveal the function of IgE-mediated immune responses against cancer cells in order to elucidate the biology of IgE and to develop novel IgE-based treatment options against malignant diseases [2, 3]. IgE has been suggested to provide protection against parasitic infections [4]; nevertheless, this function is controversial [5, 6]. While the physiological function of IgE remains unclear, IgE is known to mediate allergic reactions through its interaction with FcεRI on the surface of effector cells. The unique IgE-mediated allergic (Type I hypersensitivity/anaphylactic) response is due to the presence of mast cells and basophils that are sensitized by IgE bound to FcεRI on their surface [4]. Allergens bind IgE and cross-link the receptors leading to rapid degranulation of these effector cells, resulting in the release of multiple factors, including histamine, enzymes, and lipid mediators [4]. The release of all these factors leads to tissue damage and an acute inflammatory response. Thus, it is possible that if IgE antibodies specific for a tumor antigen were present in the tumor microenvironment, such a hypersensitivity reaction would lead to tumor cell death and phagocytosis of dead cells by antigen-presenting cells (APC), such as dendritic cells (DC) and macrophages. Cognate T cells could then be activated by these APC leading to an adaptive T-cell immune response against the tumor. This immune response has the potential to be specific for not only the targeted tumor antigen, but other tumor antigens due to epitope spreading, a phenomenon in which an immune response is induced to epitopes or antigens other than the initially targeted epitope or antigen [7]. Importantly, IgE can also mediate antigen presentation via the interaction with FcεRs expressed on APC [8–12].
Several studies such as those describing an inverse association between allergies and/or IgE antibody levels and several types of malignancies suggest a natural anti-cancer effect of IgE [2, 13]. Whether or not this is the case, there are several properties of the IgE antibody that make it an attractive option for cancer therapy. One of these properties is the low endogenous serum level of this class of antibody. IgE constitutes roughly 0.02% of all circulating antibodies, which has been attributed to a short serum half-life as well as a low synthesis rate [14, 15]. The IgG class is the most abundant and constitutes around 85% of all antibodies in the serum [14]. Thus, the competition for FcR occupancy on the surface of effector cells is expected to be lower for therapeutic IgE compared to IgG. In fact, a limitation of IgG therapeutics is the fact that high doses of the antibody are required to overcome the competition for binding to FcγR by endogenous IgG, which interferes with the ability of the therapeutic antibody to mediate effector functions such as antibody-dependent cell-mediated cytotoxicity (ADCC) [16], a critical mechanism of anti-tumor protection. Another property unique to IgE that makes it a meaningful cancer therapeutic option is its exceptionally high affinity for the FcεRs. IgE mediates immune activation via its interaction with two FcεRs: the high-affinity receptor, FcεRI, and the so-called low-affinity receptor, FcεRII (also known as CD23). IgE binds FcεRI with very high affinity (K a = 1010 M−1), which is two orders of magnitude higher than the affinity of IgG for its high-affinity FcγR (FcγR1/CD64) [2, 3, 8, 17–19]. Membrane CD23 is trimeric and has three lectin domains that are connected to the membrane via a triple α-helical coiled-coil stalk [4]. The affinity of human IgE for a single lectin domain is K a = 106–107 M−1 [4, 20–22]. However, the avidity of human IgE for CD23 on the cell surface (trimeric form) is K a = 108–109 M−1, which is roughly equivalent to the affinity of IgG for CD64 [4, 18, 20–22].
Murine and human CD23 are both type II receptors that show similar structure; however, their pattern of expression differs. In humans, CD23 is expressed on eosinophils, monocytes, lymphocytes, follicular DC, Langerhans cells, and platelets [21, 23]. In the mouse, CD23 is expressed mostly on B cells and a subset of T cells [21, 23]. Substantial differences also exist between rodent and human FcεRI. Murine FcεRI is expressed solely on mast cells and basophils [8], while the rat FcεRI is also expressed on eosinophils and macrophages [24]. The human receptor, on the other hand, is expressed on mast cells, basophils, eosinophils, monocytes, Langerhans cells, and DC [8]. However, it is important to note that the expression level is much higher on human mast cells and basophils compared to the other human cell types [8]. Additionally, the structure of the murine FcεRI is different compared to the rat and human receptors. Murine FcεRI is always a tetramer that consists of an α chain (responsible for IgE binding), a β chain, and two disulfide-linked γ chains (αβγ2) [8]. Functional human and rat FcεRI can exist either as an αβγ2 tetramer or as an αγ2 trimer [8, 24]. Therefore, the β chain is not required in humans or rats for cell surface expression. Furthermore, the α chain of the human receptor is sufficient to bind human IgE [25].
Human IgG interacts with rodent FcγRs, and thus, rodent models, such as mice, can readily be used to evaluate antibody effector functions and Fc-mediated immune activation elicited by these antibodies and, ultimately, their anti-cancer efficacy and toxicity. In contrast, human IgE does not bind murine FcεRs [8, 26]. Interestingly, murine and rat IgE bind the human FcεRI although the affinity for the human receptor is lower [8, 27, 28]. Due to these inter-species limitations, the evaluation of human IgE in rodent models is more difficult compared to human IgG. In the present article, we discuss the different options of animal models that are available to study IgE-mediated anti-cancer activity. A summary of the mouse models that have been used to study the anti-tumor effect of IgE antibodies in vivo is presented in Table 1.
Table 1.
Examples of mouse models used to evaluate the anti-tumor efficacy of IgE molecules
| IgE species | IgE specificity | Targeted cancer cells | Mouse model | Refs |
|---|---|---|---|---|
| Murine | gp36 of MMTV | H2712 murine mammary carcinoma (s.c. and i.p.) | C3H/HeJ | [29] |
| Rat/human chimeric | Murine Ly-2 | E3 murine thymoma (s.c.) | C57BL/6 | [30] |
| Murine | DNP | MC38 murine colon carcinoma cells expressing human CEA (s.c.)a | C57BL/6 | [35] |
| Murine | DNP | TS/A-LACK murine mammary carcinoma cells coated with DNP (s.c.) | BALB/c | [36] |
| Murine and murine/human chimeric | Colorectal cancer antigen | Human COLO 205 (s.c.) | SCID | [39] |
| Rat/human chimeric | Murine Ly-2 | E3 murine thymoma (i.p.) | NOD-SCID | [41] |
| Mouse/human chimeric | FBP | IGROV-1 human ovarian carcinoma cells (s.c.) | C.B-17 scid/scid | [43] |
| HUA patient-derived ovarian carcinoma (i.p.) | nu/nu | [44–46] | ||
| Mouse/human chimeric | NIP | TS/A-LACK murine mammary carcinoma cells coated with NIP (s.c.) | Human FcεRIα transgenic BALB/c | [36] |
| Human | HER2/neu | D2F2/E2 murine mammary carcinoma cells expressing human HER2/neu (i.p.) | Human FcεRIα transgenic BALB/c | [57] |
s.c. Subcutaneous, i.p. intraperitoneal, MMTV murine mammary tumor virus, DNP dinitrophenol, FBP folate binding protein, NIP 4-hydroxy-5-iodo-3-nitrophenylacetyl
aTumor targeting occurred via a biotinylated anti-CEA IgG followed by streptavidin and then a biotinylated IgE
Wild-type immunocompetent mice
Animal models with a fully functioning immune system (immunocompetent) bearing tumor cell lines from the same genetic background (syngeneic) are commonly used to evaluate antibody-targeted therapy. Rodents are mostly used for this purpose, with mice being the predominant species of choice. Due to the lack of species reactivity between human IgE and murine FcεRs, these models cannot be used to evaluate the immune activation promoted by human IgE but are useful for the evaluation of the direct cytotoxic effects of human IgE that do not involve antibody Fc effector functions. Additionally, these models are appropriate to examine the efficacy and potential toxicity of murine IgE. Since murine IgE is not immunogenic in these models, numerous administrations of the therapeutic IgE are possible without the potential neutralization of the therapy by a murine antibody immune response. Although the use of these models can result in meaningful studies on murine IgE, the interpretation of these data and extrapolation to human IgE must be carried out with caution. Another limitation of these models is that the pattern of expression of murine FcεRI is different from that of humans, being limited to basophils and mast cells, while in humans, FcεRI is also expressed on other cell types, including APC and eosinophils. Therefore, the type of immune activation may not be the same in humans, and thus, the downstream effects may also be different. Moreover, human tumors cannot be used in this model due to their immunogenicity in mice, which prevents the establishment of such xenograft tumors (cancer cells from an individual of one species transplanted into an organism of another species).
The first study carried out with a tumor-targeted IgE was conducted by Nagy et al. in the early 1990s [29]. A murine IgE specific for the major envelope glycoprotein (gp36) of the mouse mammary tumor virus (MMTV) was produced and the anti-cancer efficacy evaluated in C3H/HeJ mice bearing syngeneic H2712 (MMTV positive) cells. Mice (n = 6) received 105 cells subcutaneously (s.c.) simultaneously with mouse ascites containing 12.5 μg of the anti-gp36 IgE or an equivalent amount of normal mouse serum. Additionally, every 4 days for 8 weeks, the animals were treated intraperitoneally (i.p.) with 100 μL of ascites containing 25 μg of the targeted IgE or 100 μL of normal mouse serum. In this experiment, animals receiving normal mouse serum succumbed to disease by day 44. Four of the 6 animals treated with the murine IgE were alive at that time. After the cessation of treatment, 1 mouse treated with the IgE started developing a tumor on day 58 and died on day 72. The remaining 3 animals survived past 175-days post-tumor challenge. In a similar experiment where only the number of tumor cells was changed (increased to 106), control animals died from excess tumor burden within 32 days. Only 2 mice treated with the IgE died within this time frame, while the remaining 4 died by day 161. This study also evaluated the efficacy of the IgE against tumors growing in the peritoneal cavity. H2712 cells (105) were injected i.p. Treatment was similar to the previously described experiments. However, the dosing was carried out only for 6 weeks. In this experiment, all control animals died within 34 days, whereas 2 of the 6 in the IgE treatment group survived until day 139 [29]. These data show that murine IgE antibodies are able to protect mice from a lethal dose of cancer cells, whether the tumors were growing s.c. or i.p. The mechanism of anti-cancer activity was not described in this study. However, it is possible that immune activation and the induction of a local hypersensitivity response in the tumor microenvironment were responsible for the effect.
A syngeneic animal model has also been used to examine the anti-cancer effects of a rat/human chimeric IgE specific for murine Ly-2 [30]. Ly-2 is a cell surface marker for murine CD8 cells that can also be expressed on tumors of T-cell origin [31, 32]. Cytotoxic T lymphocytes (CTL) play a key role in anti-tumor immunity, however, in certain cases tumors do not express cell surface proteins that allow CTL activation, which has been considered to be a reason for poor responses to CTL-mediated therapies [33]. In order to direct CTL to target tumors in a non–major histocompatibility (non-MHC)–dependent manner, a strategy where the anti-Ly-2 IgE and a chimeric receptor expressed on CTL has been utilized [30]. This chimeric receptor, which consisted of the human FcεRIα chain (the IgE binding chain), the human CD3-ζ signaling molecule, and the transmembrane human FcγRIIa domain, was stably expressed in the murine CTLL-R8 T-cell line resulting in “3H2” effector cells [30]. An FcγR was not used due to the complications caused by high IgG serum level, relative low affinities, and the potential for complement activation. 3H2 effector cells were incubated simultaneously with 106 murine E3 thymoma cells (that express Ly-2 [34]) syngeneic to C57BL/6 at an effector to target cell ratio of 10:1 in the presence or absence of the anti-Ly-2 IgE for 4 h. This combination was then injected s.c. into C57BL/6 mice. Control animals received E3 thymoma cells alone or E3 cells plus 3H2 cells (without the IgE). The thymoma cells grew rapidly in animals receiving only E3 cells and caused death in all 5 mice within 5–7 days. The addition of the 3H2 effector cells significantly prolonged survival. However, 4 out of 5 five of these animals developed tumors and succumbed to the disease. The greatest effect was observed in the presence of the anti-Ly-2 IgE, where survival was prolonged at an even higher level than that using 3H2 cells alone, with only 1 of 5 animals developing a tumor. A second experiment using an effector to target ratio of 3:1 showed that all 5 control animals died within 15 days. Three of 5 animals given E3 and 3H2 effector cells combined with a non-specific IgE died within this same time frame. However, in the presence of the tumor-specific IgE, all mice survived tumor free. These data suggest that an anti-tumor IgE can be used to redirect engineered CTL to lyse tumor cells in an adoptive transfer therapy setting. Importantly, different anti-tumor IgE with human constant regions can be used in this strategy.
Syngeneic mouse models have also been used to evaluate the ability of murine IgE to act as an adjuvant for cancer immunotherapy [35]. The authors postulated that IgE on the surface of tumor cells could affect tumor immunogenicity by the activation of innate immunity within the tumor microenvironment and that these changes might affect tumor growth. A biotin-avidin bridge strategy was used to target IgE to the tumor. C57BL/6 mice were injected s.c. with 3 × 105 syngeneic MC38-CEA-2 tumor cells. Two days later, mice were injected with a biotinylated tumor-specific murine IgG (40 μg anti-CEA-2 antibody) to target the tumor. One day later, mice were given 50 μg avidin i.p followed by 50 μg streptavidin 4 h later. Finally on day 4, mice were given 30 μg/mL biotinylated anti-dinitrophenyl (DNP) murine IgE (or biotinylated IgG control antibody) i.p. IgE treatment significantly reduced tumor growth and prolonged survival compared to IgG treatment. Two of 10 mice receiving the IgE treatment completely rejected the tumor. Additionally, these 2 mice also resisted tumor cell growth for up to 60 days after rechallenge with MC38 parental cells, showing the induction of a memory response beyond that of the antigen that was initially targeted. The Fc region of the IgE was shown to play a critical role since heat inactivation of the biotinylated IgE prior to injection on day 4 abrogated the anti-cancer effects of the treatment. Depletion of eosinophils, CD8, or CD4 also abrogated the anti-tumor effects, demonstrating the requirement for these three cell types in the IgE-mediated growth inhibitory effects. Additionally, these studies were confirmed in a more immunogenic tumor model using the syngeneic murine lymphoma RMA-Thy1.1 cell line under similar conditions [35].
To further explore the properties of murine IgE as an adjuvant and to determine whether IgE could be used for the purpose of preventative immunization, cellular vaccines were developed using irradiated tumor cells coated with IgE [35]. Again, the biotin-avidin bridging technique described in the previous paragraph was used. C57BL/6 mice were vaccinated twice (2 weeks apart) by s.c. administration of IgE-coated MC38-CEA-2-irradiated tumor cells in the left flank. Three different doses of tumor cells were used ranging from 1–30 × 104. Two weeks after the second immunization, the animals were challenged with 3 × 105 live MC38-CEA-2 cells. Cells coated with murine IgG molecules only showed significant delay in tumor growth at the highest dose of vaccination. However, all doses of cells coated with IgE showed significant anti-cancer properties. These studies were confirmed using a similar vaccination schedule and RMA tumor cells [35]. For these studies, animals were vaccinated only once with 1–50 × 103 irradiated tumor cells. Two weeks after administration, mice were challenged with 5 × 104 live RMA cells. Taken together, these studies suggest that IgE-coated tumor cells can affect tumor immunogeneicity and drive tumor-specific T-cell-mediated responses both in therapeutic and preventative vaccination strategies.
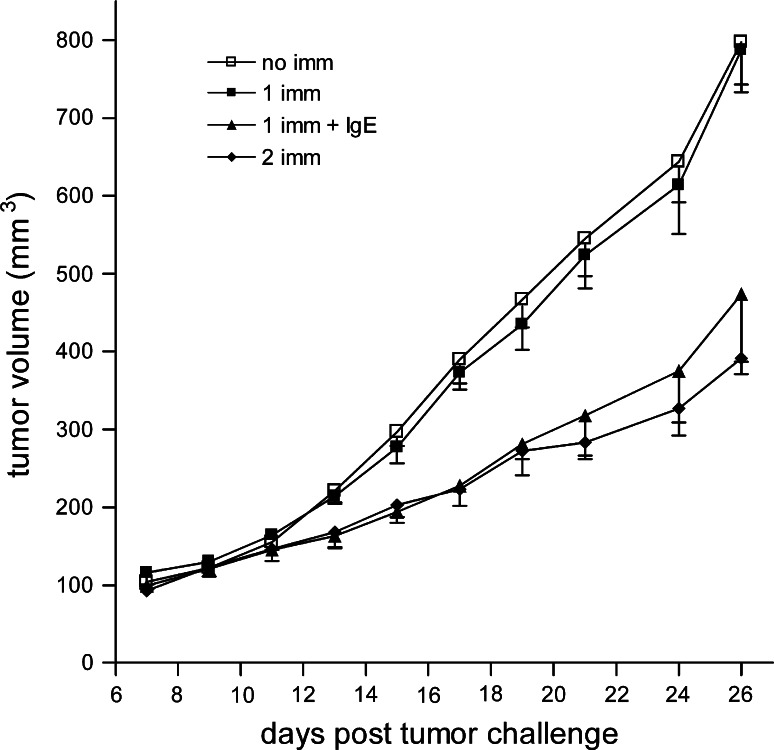
The adjuvant effect of IgE-coated tumor cells was confirmed in BALB/c mice using a slightly different strategy [36]. TS/A-LACK murine mammary adenocarcinoma cells were infected with modified vaccinia virus Ankara (MVA) in order to avoid the need for irradiation of tumor cells prior to vaccination since MVA kills infected cells within a few days. Additionally, MVA was used to exploit the high immunogenicity of the vaccinia virus. TS/A-LACK-infected cells were then conjugated with the hapten DNP. These haptenized cells were coated with murine anti-DNP IgE and used to vaccinate BALB/c mice. Mice were vaccinated s.c. and then challenged s.c. with 105 live TS/A-LACK tumor cells 15 days after vaccination. A strong anti-cancer effect was observed in these animals (Fig. 1, P < 0.0001 compared with a single immunization in the absence of IgE). Vaccination with haptenized tumor cells (not bearing IgE) did not induce an anti-tumor response. However, when mice were vaccinated twice with haptenized tumor cells, an anti-tumor effect was observed. This effect was similar to that of animals vaccinated once with IgE-coated tumor cells (Fig. 1). The same experiments were carried out in either FcεRI or CD23 knockout mice [36]. The anti-cancer effects of vaccination with IgE-coated tumor cells were not observed in FcεRI knockout animals, but remained evident in the CD23 knockout animals. These data show that the FcεRI is a key mediator of the anti-cancer effects of the vaccination, suggesting the possibility that the IgE adjuvanticity could result from an inflammatory response similar to that observed in an allergic reaction followed by antigen presentation by well-known APC such as DC. However, it is tempting to speculate that basophils and/or mast cells may also act as APC under these conditions since both cell types have been shown in murine models to act as APC in the presence of murine IgE complexed with the antigen [37, 38]. All together, these data demonstrate that the presence of the IgE in the vaccination protocol resulted in stronger immune activation and thus provides further evidence that IgE can act as an adjuvant for cancer therapy.
Fig. 1.
Anti-tumor protection induced by IgE-loaded MVA-infected TS/A-LACK cells. BALB/c mice (n = 5 for all groups) were vaccinated s.c. with 105 IgE-free (2 imm) MVA-infected TS/A-LACK cells at days -30 and -15, or were not vaccinated (no imm). Two other groups of mice received only one s.c. vaccination with 105 IgE-loaded (1 imm + IgE) or IgE-free (1 imm) MVA-infected TS/A-LACK cells at day-15. At day 0, all vaccinated mice were challenged with s.c. administration of 2 × 105 living TS/A-LACK cells. Results are mean ± the standard error of the mean and were obtained from one of three experiments yielding similar results. Reprinted from Figure 3 of Nigro et al. [36] with permission. Copyright 2009. The American Association of Immunologists, Inc
Xenograft models using immunocompromised mice
In contrast to the syngeneic murine tumor models, xenograft models allow the use of human cancer cells in order to evaluate the anti-cancer efficacy of a potential new-targeted drug. Thus, the tumor cells themselves more effectively reflect the human disease, which is often the ultimate target for cancer therapy. This type of model is frequently the only option when studying the efficacy of monoclonal antibodies since they are often species specific and do not show cross-reactivity between humans and mice. This approach requires the use of immunosupressed animals such as severe combined immunodeficiency (SCID) and nude mice that have impaired cellular and humoral immune responses. Therefore, these models cannot be used to evaluate the adaptive immune response elicited by immunotherapies, including therapuetic antibodies. Moreover, xenograft models cannot be used to examine the anti-cancer activity triggered by human IgE effector functions since it does not interact with murine FcεRs. However, these models can be used to study the direct cytotoxic effects on cancer cells mediated by a human IgE. Since the animals are immunosupressed, the human IgE will not be immunogenic allowing multiple administrations of the therapeutic IgE. Additionally, these models can be used to assess the anti-cancer effects of a murine IgE specific for a human tumor antigen.
The anti-cancer effects of a tumor-specific IgE have been reported using this type of animal model [39]. The murine IgE (30.6) is specific for an antigen expressed on the surface of human colorectal carcinoma cells, such as the COLO 205 cell line [39]. SCID mice were injected s.c. with 5 × 106 COLO 205 cells. Systemic treatment with 1 μg of either the 30.6 murine IgE or the 30.6 mouse/human chimeric IgE was given via intravenous (i.v.) injection 5 days after tumor challenge. The chimeric IgE had no effect on tumor growth. However, the murine IgE induced a rapid, but transient (48 h in duration) inhibition of tumor growth. Increasing the dosing schedule to three treatments on days 5, 7, and 9 after tumor challenge did not increase the inhibitory activity [39]. Anti-cancer activity was also observed in animals bearing s.c. HT29 human colon carcinoma cells treated with 1 μg of the 30.6 murine IgE [39]. Additionally, no effect was observed against E3 thymoma cells that lack expression of the antigen targeted by 30.6. Furthermore, no anti-tumor effect was observed with a non-specific murine IgE even when used at 100 μg. These data show that the anti-cancer effects of murine anti-30.6 IgE are antigen specific and require the murine IgE constant regions. A mouse/human chimeric IgG1 containing the 30.6 variable region had also previously shown anti-cancer activity in this tumor model [40]. The murine 30.6 IgE effect was observed at antibody concentrations that were 250-fold lower than those previously reported for the effects seen using 30.6 IgG1, consistent with the superior anti-cancer activity of the IgE. However, these two antibodies were not tested simultaneously.
The strategy using chimeric receptors expressed on murine CTLL-R8 T cells for adoptive transfer described above was also used in immunosupressed animals utilizing human T cells and human tumor cells. A chimeric receptor similar to that described above (consisting of human FcεRIα, the human CD3-ζ signaling molecule, and the transmembrane human FcγRII hinge) was produced by adding the cytoplasmic domain of the human costimulatory molecule CD28 in order to potentiate the activation signal of the CTL [41]. Primary human T lymphocytes expressing this chimeric receptor (named T-CL9) were used for adoptive transfer into non-obese diabetic-severe combined immunodeficiency (NOD-SCID) mice. For this study, these animals were irradiated (2.5 Gy) and challenged i.p. the following day with 104 E3 (Ly-2 positive) thymoma cells. Anti-Ly-2 IgE-loaded T-CL9 or control T cells not expressing the chimeric receptor were injected i.p. 4 times at 6, 24, 48, and 96 h following tumor challenge. Adoptive transfer of the IgE-coated TCL-9 cells significantly prolonged survival. However, complete tumor rejection and long-term survival were not observed, which were explained by the fact that the TCL-9 did not persist in the animal and could not be detected in the blood, spleen, or peritoneal cavity after 1-week post-administration. This effect was shown to be due to the presence of the chimeric receptor since primary T cells not expressing the receptor showed no anti-tumor protection. Furthermore, tumor targeting by the anti-Ly-2 IgE was also necessary since TCL-9 cells transferred with a non-specific IgE also did not show a protective effect. These data show that primary human T cells expressing the chimeric IgE receptor can suppress tumor growth in the presence of a tumor-targeted IgE. This strategy of adoptive transfer is an alternative to the use of a chimeric receptor containing scFv [42], which requires the production of a new chimeric receptor for each targeted tumor antigen. The use of the FcεRI-containing chimeric receptor can eliminate this problem through the combinatorial use with multiple IgE antibodies targeting different tumor antigens.
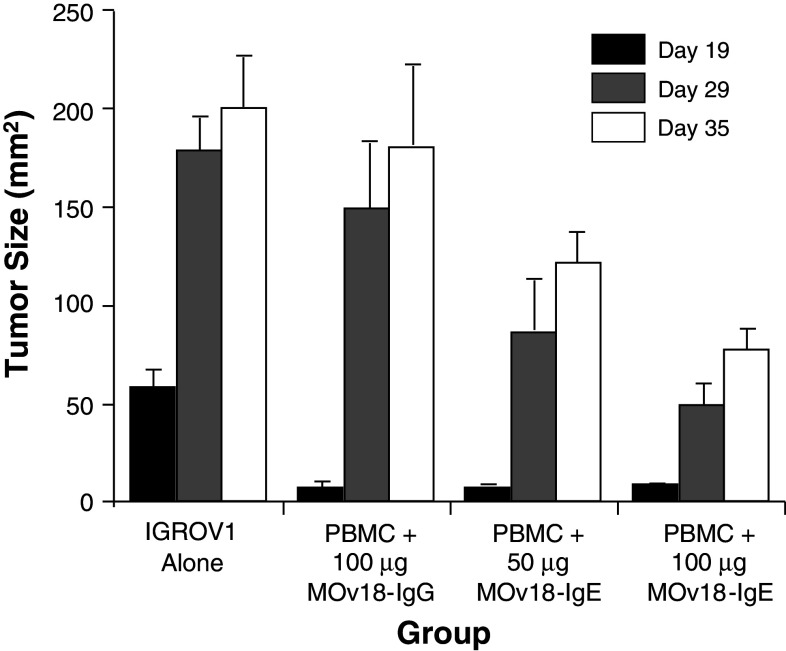
Another option available for the use of immunocompromised animal models is the reconstitution of the animal with natural human effector cells. For studies with human IgE, this would mean using human FcεR-expressing effector cells. This strategy has been utilized with success to evaluate the anti-cancer efficacy of a mouse/human chimeric IgE specific for the ovarian cancer-associated antigen, folate binding protein (FBP) [43]. This IgE was named MOv18 IgE, and its anti-cancer activity was evaluated in an immunocompromised mouse xenograft model of ovarian carcinoma. C.B-17 scid/scid (SCID) animals were challenged s.c. with 2.5 × 106 IGROV1 human ovarian carcinoma cells followed by systemic injection (i.v.) of 50–100 μg of the MOv18 IgE or MOv18 IgG (also a mouse/human chimeric antibody containing the same variable region) simultaneously with human peripheral blood mononuclear cells (PBMC). Both the MOv18 IgG or the IgE, injected together with human PBMC, inhibited tumor growth up to 19 days [43]. However, by day 35, the effect of the IgG was no longer apparent, while the IgE continued to show protection (Fig. 2). Compared to the control treated animals, those treated with PBMC plus the 100 μg MOv18 IgE showed a 72% inhibitory rate on day 29 (P < 0.001) and a 62% inhibitory rate on day 35 (P < 0.005). Thus, the inhibitory effect of the IgE was greater and of longer duration than that of the IgG. The anti-cancer effect of the MOv18 IgE was only observed in the presence of human PBMC suggesting that the mechanism of tumor protection was mediated by the ability of the antibody to recruit human FcεR-expressing cells. A non-specific IgE was also tested in this model, and no inhibitory effect was observed, indicating that tumor targeting via binding of the MOv18 IgE to FBP on the surface of targeted cells was required.
Fig. 2.
MOv18-IgE inhibits the growth of IGROV1 human ovarian adenocarcinoma cells in vivo in the presence of human PBMC as effectors. A matched variable region set of mouse/human chimeric IgE and IgG1 antibodies specific for FBP was tested for their ability to inhibit the s.c. growth of cells in a SCID mouse xenograft model of human ovarian cancer. Mice were treated i.p. with anti-asialo GM1 to eliminate host natural killer cells. One day later, mice were challenged s.c. with 2 × 106 IGROV1 cells followed by i.v. injection of 2.5 × 106 human PBMC combined with either 100 μg MOv18-IgG1, 100 μg MOv18-IgE or 50 μg MOv18-IgE. The mean tumor size ± the standard error of the mean measured on days 19, 29, and 35 is shown (n = 4 for all groups). Adapted and printed from Figure 4A Gould et al. [43] with permission. Copyright Wiley–VCH Verlag GmbH & Co. KGaA
The anti-cancer activity of the MOv18 IgE was confirmed using a nude mouse model of ovarian carcinoma [44]. Patient-derived HUA human ovarian carcinoma xenografts were implanted in nu/nu female mice by i.p. injection. The following day mice were treated i.p. with 4 × 106 human PBMC alone or combined with 100 μg MOv18 IgE or MOv18 IgG. Again the effect was significantly stronger in the presence of the MOv18 IgE compared to the IgG. Additionally, in this model, it was also shown that treatment with the MOv18 IgE resulted in infiltration of human monocytes into the tumor [44]. It was subsequently shown that human monocytes mediate the effect of the MOv18 IgE in this tumor model [45]. In addition, use of the human monocyte (histiocytic lymphoma) cell line U-937 showed that these cells can also serve as effectors and mediate anti-tumor protection by the MOv18 IgE [46]. This effect is increased when the U-937 cell line is prestimulated with IL-4 to increase the expression of FcεRII (CD23). Interestingly, in vitro studies suggested that both FcεRI and CD23 contribute to the anti-tumor activity of the MOv18 IgE through ADCC and antibody-dependent cell-mediated phagocytosis (ADCP), respectively [46].
It is important to note that a limitation of this type of animal model is that it does not have a self-replenishing supply of human effector cells. The human PBMC were injected once and are expected to be removed from the circulation rapidly due to a short life span in the mouse [47]. Additionally, certain human cytokines secreted by human effector cells may not interact with the murine immune system [48]. Moreover, the types of effector cells that express FcεRs in the PBMC preparations is restricted to certain cell types such as monocytes [49]. Thus, the full spectrum of human effector cells (including basophils and mast cells) is not active in these mice. Therefore, the anti-tumor effect of antibodies tested in this model, such as the MOv18 IgE, may be even greater in models without the drawbacks noted above and where the source of FcεR-expressing effector cells is natural and constant.
Human FcεRIα transgenic mouse model
In order to have a mouse model in which human IgE is capable of interacting with FcεRI-expressing cells, a transgenic mouse with the murine FcεRIα knocked out and its human equivalent knocked in (since IgE binding occurs through the FcεRIα subunit [25]) has been described in both a BALB/c and C57BL/6 background [50–52]. Surprisingly, in these animals expressing the “humanized” FcεRI, the cellular distribution of human FcεRI surface expression mimics that of humans. FcεRI is expressed on mast cells, basophils, monocytes, eosinophils, Langerhans cells, and DC. Thus, this model provides a more accurate and constant repertoire of effector cells for the study of human IgE. This model was initially intended to be used to study anaphylaxis [50–53] and the efficacy of molecules that inhibit anaphylaxis [54–56]. However, it can also be used to evaluate both the anti-cancer effects mediated by FcεRI and the potential toxicity of IgE containing human constant regions. Whether or not the entire toxicity profile may be studied in this model depends on the species cross-reactivity of the antibody, and whether or not it will interact with the murine homolog of the antigen. Additionally, these animals are only transgenic for human FcεRI, but not FcεRII (CD23). Therefore, the role of CD23 in the observed anti-cancer effects cannot be measured, and thus, the overall anti-cancer activity may be underestimated. Moreover, these transgenic animals are not transgenic for human IgE. This makes multiple, long-term administrations of human IgE problematic since these mice may potentially mount a murine anti-human IgE humoral response. Furthermore, human cell lines cannot be used in these transgenic animals since tumors would not be expected to establish in these immunocompetent animals.
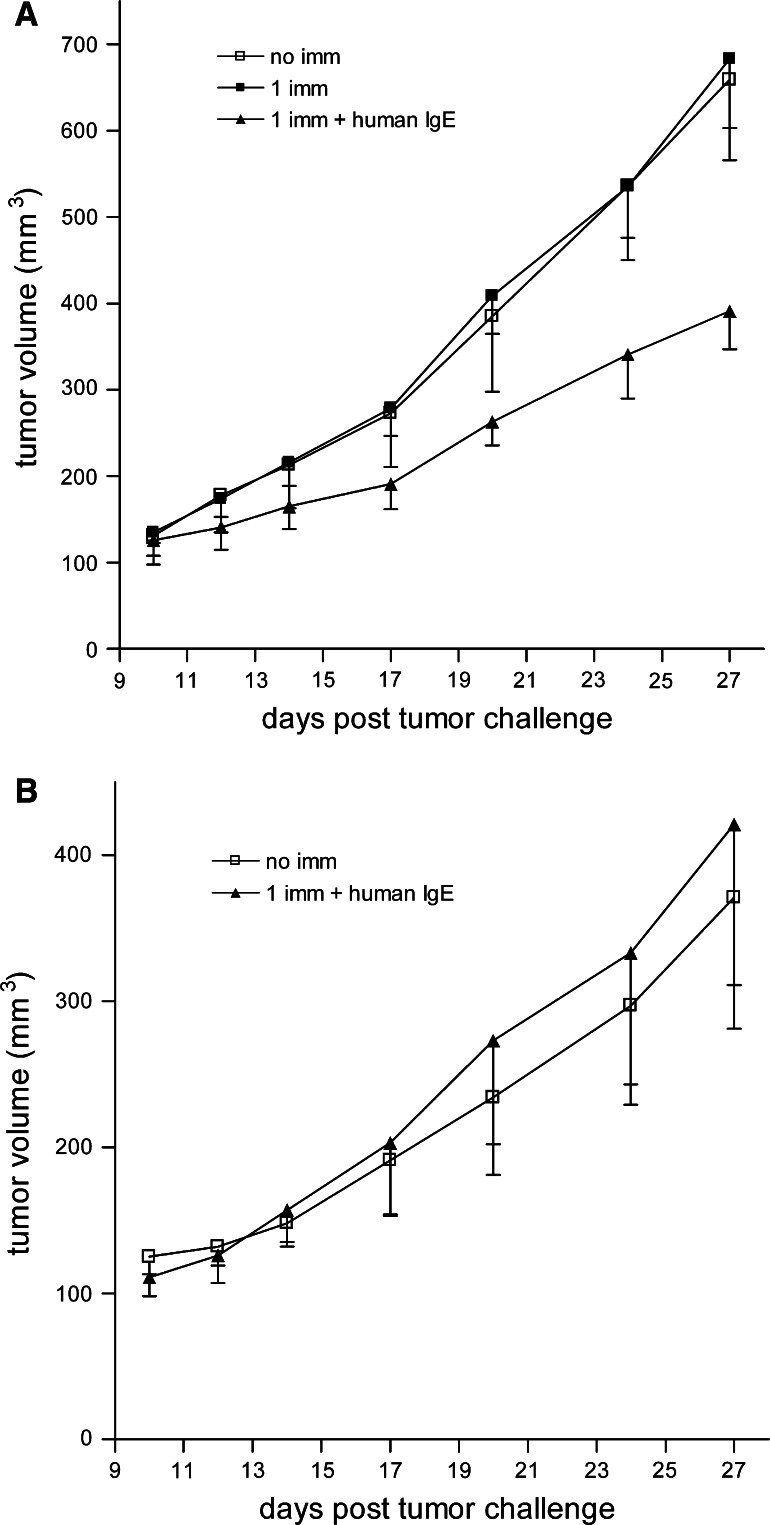
As described above in the section covering the wild-type immunocompetent mouse models, murine IgE can serve as an adjuvant for cancer therapy. This has also been shown for IgE containing human constant regions in the human FcεRIα transgenic mouse model [36]. MVA-infected TS/A-LACK cells (syngenic for BALB/c) were haptenized with NIP-OSu (4-hyroxy-5-iodo-3-nitrophenylacetyl-hydroxysuccinimidyl ester). Immunization consisted of s.c. injection of cells loaded with 20 μg of the mouse/human chimeric anti-NIP IgE (105 per mouse). Two weeks after immunization, animals were challenged s.c. with TS/A-LACK cells. Cells loaded with the anti-NIP IgE significantly blocked tumor growth compared to animals immunized with cells alone (Fig. 3a, P < 0.0001 compared with a single immunization in the absence of IgE). Additionally, immunization of wild-type BALB/c mice did not yield an inhibitory effect (Fig. 3b). These results are expected since chimeric IgE does not interact with the murine FcεRs and demonstrate that FcεRI-expressing cells are involved in mediating the anti-tumor effect, since the animals are only transgenic for human FcεRIα. These studies provide support for the possible use of IgE as an adjuvant for cancer vaccines in humans.
Fig. 3.
Anti-tumor IgE adjuvant effect in human FcεRIα transgenic mice. a Mice (n = 5 for all groups) were vaccinated s.c. with 105 chimeric IgE-loaded (1 imm + human IgE) or chimeric IgE-free (1 imm) MVA-infected TS/A-LACK cells at day -15. At day 0, all vaccinated mice were challenged with s.c. administration of 2 × 105 living TS/A-LACK cells. Non-immunized mice challenged with live TS/A-LACK cells were used as controls (no imm). b As a control of the experiment in a, BABL/c wildtype mice (n = 5 for all groups) were vaccinated s.c. with 105 chimeric IgE-loaded (1 imm + human IgE) MVA-infected TS/A-LACK cells at day-15. At day 0, all vaccinated mice were challenged with s.c. administration of 2 × 105 living TS/A-LACK cells. Non-immunized mice challenged with 2 × 105 living TS/A-LACK cells were used as controls (no imm). Results are mean ± the standard error of the mean and were obtained from one of three experiments yielding similar results. Reprinted from Figure 5 of Nigro et al. [36] with permission. Copyright 2009. The American Association of Immunologists, Inc
This model has been recently used to characterize a fully human anti-HER2/neu IgE containing the variable regions of the scFv C6MH3-B1 [57], which does not compete with trastuzumab (Herceptin®) for binding to HER2/neu [58]. This model was first used to evaluate the ability of the IgE to induce a local cutaneous anaphylaxis reaction in the skin of the animals. Transgenic animals were sensitized with 1 μg of the anti-HER2/neu IgE by intradermal injection. Four hours later, the animals were systemically given an anti-human κ antibody to artificially cross-link the FcεRI on sensitized effector cells. This cross-linking antibody was given in the presence of Evan’s Blue, a dye that aids in the visualization of the local hypersensitivity reaction. A reaction was observed in these animals, while no reaction was observed in animals sensitized with the human anti-HER2/neu IgE and systemically given 2 μg of the soluble form of the extracellular domain of the antigen (ECDHER2) [57]. The fact that no reaction occurred in the presence of the soluble antigen is consistent with a monoepitopic interaction with the antigen that is unable to induce cross-linking of the FcεRI. Given that the blood volume of a mouse is 1–2 mL, this dose is equivalent to 1–2 μg/mL. In cancer patients, a serum concentration of ECDHER2 above 15 ng/mL is considered to be elevated, with highly elevated levels being above 100 ng/mL [59, 60]. Thus, even at the high concentration of ECDHER2 used in these in vivo studies, soluble ECDHER2 did not induce a local hypersensitivity reaction. Additionally, in preliminary studies, systemic injection of complexes of ECDHER2 and anti-HER2/neu IgE (with fourfold molar excess of ECDHER2) was well tolerated in human FcεRIα transgenic mice. Similar results were observed with systemic injection of 100 μg of the anti-HER2/neu IgE followed 4 h later by systemic injection of 38 μg of ECDHER2. These data suggest that the soluble form of the antigen is unable to cross-link the FcεR and thus, this anti-HER2/neu IgE may be well tolerated in humans.
The anti-cancer efficacy of the anti-HER2/neu IgE was also evaluated in these animals [57]. Transgenic animals were challenged i.p. with 2 × 105 D2F2/E2 murine mammary carcinoma cells expressing human HER2/neu. These cells have been used previously for the evaluation of anti-tumor efficacy of HER2/neu-targeted therapies [61–63] and overcome the need to use a human tumor since they express the human antigen on their surface. Animals received two treatments of 100 μg of the anti-HER2/neu IgE (n = 18) or buffer alone (n = 17) on days 2 and 4 after tumor challenge. The IgE significantly prolonged the survival of tumor-bearing animals (P < 0.001) [57]. The median survival of control animals was 28 days, which increased to 39 days in the IgE-treated animals. Systemic treatment of these animals was well tolerated. These data show that a tumor-specific IgE can mediate anti-cancer effects in vivo, and thus, additional studies on this IgE are warranted to further evaluate both its efficacy and safety for human use.
Macaca fascicularis (cynomolgus monkey) model
A study conducted in the early 1980s examined the regulatory process involved in mediator synthesis and release during anaphylaxis in sensitized lungs of the cynomolgus monkey Macaca fascicularis [64]. For this study, sections of lung tissue from these monkeys were sensitized with human serum containing high titers of IgE. The lungs were then incubated with an anti-human IgE and assayed for the release of mediators of anaphylaxis, including histamine. The lungs sensitized with human serum showed increased release of these mediators, presumably due to a successful interaction between human IgE and the monkey FcεRI, suggesting the usefulness of this animal for safety studies of IgE containing human constant regions. If the targeted human protein is highly homologous to the cynomolgous monkey protein, this model is even more meaningful. This is the case with HER2/neu, which shows high sequence homology (about 99%) between the two species and thus these monkeys have been used in studies on HER2/neu-targeted IgG antibodies [65, 66]. A preliminary study on the safety and initial pharmacokinetics of the fully human anti-HER2/neu IgE has been conducted in this animal model [57]. For this study, one male monkey was dosed with a low dose (0.0024 mg/kg) and one male monkey was dosed with a higher dose (0.08 mg/kg). The doses for this study were based on previous studies in humans using two different immune-stimulating therapeutic antibodies: oregovomab (a CA-125-specific murine monoclonal antibody) and EMD 273063 (a humanized anti-GD2 monoclonal antibody linked to interleukin-2) [67–69]. The anti-HER2/neu IgE was given to both monkeys via i.v. infusion over 20 min. Circulating human anti-HER2/neu IgE was observed in the serum on the two monkeys 30 min after administration [57]. One week later, no human IgE was detected in the serum of either animal. Both animals were monitored for 1 week after dosing. No adverse events or changes in behavior were observed in either animal. It is important to note that HER2/neu is expressed in many tissues and a basal level of ECDHER2 is found in healthy individuals [70, 71] and still no adverse events were observed. These data represent encouraging yet preliminary findings that the anti-HER2/neu IgE can be safely infused into these animals and suggests that the antibody rapidly redistributes throughout the body, presumably interacting with cells expressing the FcεRs or HER2/neu. It is also important to note that the serum half-life of human IgE is 2 days. Additional studies are needed to further evaluate the toxicity, pharmacokinetics, and biodistribution of this IgE when infused at a wide range of doses. The cynomolgus monkey can be a highly meaningful animal model for the evaluation of toxicity of human IgE-based therapeutics. However, the limitations of this model are the high cost of using these animals and the lack of tumor models to assess IgE-mediated anti-cancer activity.
Closing remarks
IgE has exceptional potential for use as a novel platform for monoclonal antibody-based cancer therapeutics, due to several unique properties of this antibody class. These include the exceptionally high affinity for the FcεRs, the low serum levels of endogenous IgE that minimizes competition with therapeutic IgE for FcεR occupancy, and the ability to enhance a broad range of innate and adaptive immune response effector mechanisms mediated by various cells, including eosinophils and APC as well as mast cells and basophils. Results from the various animal models for the assessment of the anti-cancer properties of IgE-based therapeutics discussed in this article make it clear that IgE is functionally effective in mediating anti-cancer immune responses, which in some cases has been shown to be more effective than IgG-based approaches. Although these different animal models vary widely in terms of the immune competence of the animals used, the cellular origin of the malignancies studied, and the species of the IgE monoclonal antibodies, FcεRs, and immune cells involved in these model systems, the common denominator is the effectiveness of IgE-based therapies against cancer. In fact, given the diversity of these experimental systems, the consistent finding that IgE-based approaches are effective decreases the possibility that this outcome is an artifact of a particular experimental approach. However, despite the advantages of the models described in this article, it is important to note that all of these models have limitations. Further studies are needed to determine the safety and efficacy of cancer-targeted IgE.
Future work in human and non-human primate studies, as well as in murine model systems, needs to further address several issues: (1) Are IgE-based anti-cancer therapies safe? (2) What are the immune and inflammatory anti-tumor mechanisms that are marshaled by IgE-based monoclonals? (3) How do these immune/inflammatory mechanisms mediate their anti-cancer cell effects? (4) What is the pharmacodynamics of IgE-based therapeutic agents? Finally, the responsiveness of different kinds of malignancies to IgE-based experimental treatment needs to be better defined. It is an exciting period for AllergoOncology. Answers to these questions, and the full evolution of IgE-based therapies for cancer, are awaited eagerly.
Acknowledgments
Our work has been supported in part by the NIH/NCI grants K01CA138559, R41CA137881, R01CA1368413, K01CA138559, R01CA57152, R01CA121195, the Susan G. Komen Breast Cancer Foundation Basic, Clinical and Translational Research Grant BCTR0706771, and the NIH Fogarty AITRP-AIDS Malignancies Program D43TW000013-S1.
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
This paper is part of the Symposium in Writing: AllergoOncology: The Role of Th2 responses in cancer.
References
- 1.Helguera G, Daniels TR, Rodriguez JA, Penichet ML (2010) Monoclonal antibodies, human engineered. In: Flickinger M (ed) Encyclopedia of industrial biotechnology: bioprocess, bioseparation, and cell technology. Wiley, New York
- 2.Jensen-Jarolim E, Achatz G, Turner MC, Karagiannis S, Legrand F, Capron M, Penichet ML, Rodriguez JA, Siccardi AG, Vangelista L, Riemer AB, Gould H. AllergoOncology: the role of IgE-mediated allergy in cancer. Allergy. 2008;63:1255–1266. doi: 10.1111/j.1398-9995.2008.01768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Penichet ML, Jensen-Jarolim E. Cancer and IgE: introducing the concept of AllergoOncology. New York: Springer; 2010. [Google Scholar]
- 4.Gould HJ, Sutton BJ. IgE in allergy and asthma today. Nat Rev Immunol. 2008;8:205–217. doi: 10.1038/nri2273. [DOI] [PubMed] [Google Scholar]
- 5.Cooper PJ, Ayre G, Martin C, Rizzo JA, Ponte EV, Cruz AA. Geohelminth infections: a review of the role of IgE and assessment of potential risks of anti-IgE treatment. Allergy. 2008;63:409–417. doi: 10.1111/j.1398-9995.2007.01601.x. [DOI] [PubMed] [Google Scholar]
- 6.Watanabe N, Bruschi F, Korenaga M. IgE: a question of protective immunity in Trichinella spiralis infection. Trends Parasitol. 2005;21:175–178. doi: 10.1016/j.pt.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 7.Hardwick N, Chain B. Epitope spreading contributes to effective immunotherapy in metastatic melanoma patients. Immunotherapy. 2011;3:731–733. doi: 10.2217/imt.11.62. [DOI] [PubMed] [Google Scholar]
- 8.Kinet JP. The high-affinity IgE receptor (Fc epsilon RI): from physiology to pathology. Annu Rev Immunol. 1999;17:931–972. doi: 10.1146/annurev.immunol.17.1.931. [DOI] [PubMed] [Google Scholar]
- 9.Bieber T. Fc epsilon RI on human epidermal Langerhans cells: an old receptor with new structure and functions. Int Arch Allergy Immunol. 1997;113:30–34. doi: 10.1159/000237500. [DOI] [PubMed] [Google Scholar]
- 10.Bieber T. Fc epsilon RI-expressing antigen-presenting cells: new players in the atopic game. Immunol Today. 1997;18:311–313. doi: 10.1016/S0167-5699(97)01046-3. [DOI] [PubMed] [Google Scholar]
- 11.Maurer D, Ebner C, Reininger B, Fiebiger E, Kraft D, Kinet JP, Stingl G. The high affinity IgE receptor (Fc epsilon RI) mediates IgE-dependent allergen presentation. J Immunol. 1995;154:6285–6290. [PubMed] [Google Scholar]
- 12.Maurer D, Fiebiger S, Ebner C, Reininger B, Fischer GF, Wichlas S, Jouvin MH, Schmitt-Egenolf M, Kraft D, Kinet JP, Stingl G. Peripheral blood dendritic cells express Fc epsilon RI as a complex composed of Fc epsilon RI alpha- and Fc epsilon RI gamma-chains and can use this receptor for IgE-mediated allergen presentation. J Immunol. 1996;157:607–616. [PubMed] [Google Scholar]
- 13.Turner MC (2010) Epidemiological evidence: IgE, atopy, and solid tumors. In: Penichet ML, Jensen-Jarolim E (eds) Cancer and IgE: introducing the concept of AllergoOncology. Springer, New York, pp 47–78
- 14.Manz RA, Hauser AE, Hiepe F, Radbruch A. Maintenance of serum antibody levels. Annu Rev Immunol. 2005;23:367–386. doi: 10.1146/annurev.immunol.23.021704.115723. [DOI] [PubMed] [Google Scholar]
- 15.Achatz G, Achatz-Straussberger G, Feichtner S, Koenigsberger S, Lenz S, Peckl-Schmid D, Zaborsky N, Lamers M (2010) The biology of IgE: molecular mechanism restraining potentially dangerous high serum IgE titres in vivo. In: Penichet ML, Jensen-Jarolim E (eds) Cancer and IgE: introducing the concept of AllergoOncology. Springer, New York, pp 13–36
- 16.Preithner S, Elm S, Lippold S, Locher M, Wolf A, da Silva AJ, Baeuerle PA, Prang NS. High concentrations of therapeutic IgG1 antibodies are needed to compensate for inhibition of antibody-dependent cellular cytotoxicity by excess endogenous immunoglobulin G. Mol Immunol. 2006;43:1183–1193. doi: 10.1016/j.molimm.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 17.Daniels TR, Rodriguez JA, Ortiz-Sanchez O, Helguera G, Penichet ML (2010) The IgE antibody and its use in cancer immunotherapy. In: Penichet ML, Jensen-Jarolim E (eds) Cancer and IgE: introducing the concept of allergooncology. Springer, New York, pp 159–184
- 18.Ravetch JV, Kinet JP. Fc receptors. Annu Rev Immunol. 1991;9:457–492. doi: 10.1146/annurev.iy.09.040191.002325. [DOI] [PubMed] [Google Scholar]
- 19.Gould HJ, Sutton BJ, Beavil AJ, Beavil RL, McCloskey N, Coker HA, Fear D, Smurthwaite L. The biology of IgE and the basis of allergic disease. Annu Rev Immunol. 2003;21:579–628. doi: 10.1146/annurev.immunol.21.120601.141103. [DOI] [PubMed] [Google Scholar]
- 20.Hibbert RG, Teriete P, Grundy GJ, Beavil RL, Reljic R, Holers VM, Hannan JP, Sutton BJ, Gould HJ, McDonnell JM. The structure of human CD23 and its interactions with IgE and CD21. J Exp Med. 2005;202:751–760. doi: 10.1084/jem.20050811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conrad DH. Fc epsilon RII/CD23: the low affinity receptor for IgE. Annu Rev Immunol. 1990;8:623–645. doi: 10.1146/annurev.iy.08.040190.003203. [DOI] [PubMed] [Google Scholar]
- 22.McCloskey N, Hunt J, Beavil RL, Jutton MR, Grundy GJ, Girardi E, Fabiane SM, Fear DJ, Conrad DH, Sutton BJ, Gould HJ. Soluble CD23 monomers inhibit and oligomers stimulate IgE synthesis in human B cells. J Biol Chem. 2007;282:24083–24091. doi: 10.1074/jbc.M703195200. [DOI] [PubMed] [Google Scholar]
- 23.Delespesse G, Sarfati M, Wu CY, Fournier S, Letellier M. The low-affinity receptor for IgE. Immunol Rev. 1992;125:77–97. doi: 10.1111/j.1600-065X.1992.tb00626.x. [DOI] [PubMed] [Google Scholar]
- 24.Dombrowicz D, Quatannens B, Papin JP, Capron A, Capron M. Expression of a functional Fc epsilon RI on rat eosinophils and macrophages. J Immunol. 2000;165:1266–1271. doi: 10.4049/jimmunol.165.3.1266. [DOI] [PubMed] [Google Scholar]
- 25.Hakimi J, Seals C, Kondas JA, Pettine L, Danho W, Kochan J. The alpha subunit of the human IgE receptor (FceRI) is sufficient for high affinity IgE binding. J Biol Chem. 1990;265:22079–22081. [PubMed] [Google Scholar]
- 26.Bettler B, Hofstetter H, Rao M, Yokoyama WM, Kilchherr F, Conrad DH. Molecular structure and expression of the murine lymphocyte low-affinity receptor for IgE (Fc epsilon RII) Proc Natl Acad Sci USA. 1989;86:7566–7570. doi: 10.1073/pnas.86.19.7566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang B, Rieger A, Kilgus O, Ochiai K, Maurer D, Fodinger D, Kinet JP, Stingl G. Epidermal Langerhans cells from normal human skin bind monomeric IgE via Fc epsilon RI. J Exp Med. 1992;175:1353–1365. doi: 10.1084/jem.175.5.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conrad DH, Wingard JR, Ishizaka T. The interaction of human and rodent IgE with the human basophil IgE receptor. J Immunol. 1983;130:327–333. [PubMed] [Google Scholar]
- 29.Nagy E, Berczi I, Sehon AH. Growth inhibition of murine mammary carcinoma by monoclonal IgE antibodies specific for the mammary tumor virus. Cancer Immunol Immunother. 1991;34:63–69. doi: 10.1007/BF01741326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kershaw MH, Darcy PK, Trapani JA, Smyth MJ. The use of chimeric human Fc(epsilon) receptor I to redirect cytotoxic T lymphocytes to tumors. J Leukoc Biol. 1996;60:721–728. doi: 10.1002/jlb.60.6.721. [DOI] [PubMed] [Google Scholar]
- 31.Panaccio M, Gillespie MT, Walker ID, Kirszbaum L, Sharpe JA, Tobias GH, McKenzie IF, Deacon NJ. Molecular characterization of the murine cytotoxic T-cell membrane glycoprotein Ly-3 (CD8) Proc Natl Acad Sci USA. 1987;84:6874–6878. doi: 10.1073/pnas.84.19.6874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mathieson BJ, Campbell PS, Potter M, Asofsky R. Expression of Ly 1, Ly 2, Thy 1, and TL differentiation antigens on mouse T-cell tumors. J Exp Med. 1978;147:1267–1279. doi: 10.1084/jem.147.4.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Topalian SL, Kasid A, Rosenberg SA. Immunoselection of a human melanoma resistant to specific lysis by autologous tumor-infiltrating lymphocytes. Possible mechanisms for immunotherapeutic failures. J Immunol. 1990;144:4487–4495. [PubMed] [Google Scholar]
- 34.Hogarth PM, Henning MM, McKenzie IF. Alloantigenic phenotype of radiation-induced thymomas in the mouse. J Natl Cancer Inst. 1982;69:619–626. [PubMed] [Google Scholar]
- 35.Reali E, Greiner JW, Corti A, Gould HJ, Bottazzoli F, Paganelli G, Schlom J, Siccardi AG. IgEs targeted on tumor cells: therapeutic activity and potential in the design of tumor vaccines. Cancer Res. 2001;61:5517–5522. [PubMed] [Google Scholar]
- 36.Nigro EA, Brini AT, Soprana E, Ambrosi A, Dombrowicz D, Siccardi AG, Vangelista L. Antitumor IgE adjuvanticity: key role of Fc epsilon RI. J Immunol. 2009;183:4530–4536. doi: 10.4049/jimmunol.0900842. [DOI] [PubMed] [Google Scholar]
- 37.Gong J, Yang NS, Croft M, Weng IC, Sun L, Liu FT, Chen SS. The antigen presentation function of bone marrow-derived mast cells is spatiotemporally restricted to a subset expressing high levels of cell surface FcepsilonRI and MHC II. BMC Immunol. 2010;11:34. doi: 10.1186/1471-2172-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoshimoto T, Yasuda K, Tanaka H, Nakahira M, Imai Y, Fujimori Y, Nakanishi K. Basophils contribute to T(H)2-IgE responses in vivo via IL-4 production and presentation of peptide-MHC class II complexes to CD4 + T cells. Nat Immunol. 2009;10:706–712. doi: 10.1038/ni.1737. [DOI] [PubMed] [Google Scholar]
- 39.Kershaw MH, Darcy PK, Trapani JA, MacGregor D, Smyth MJ. Tumor-specific IgE-mediated inhibition of human colorectal carcinoma xenograft growth. Oncol Res. 1998;10:133–142. [PubMed] [Google Scholar]
- 40.Mount PF, Sutton VR, Li W, Burgess J, Mc KIF, Pietersz GA, Trapani JA. Chimeric (mouse/human) anti-colon cancer antibody c30.6 inhibits the growth of human colorectal cancer xenografts in scid/scid mice. Cancer Res. 1994;54:6160–6166. [PubMed] [Google Scholar]
- 41.Teng MW, Kershaw MH, Jackson JT, Smyth MJ, Darcy PK. Adoptive transfer of chimeric FcepsilonRI gene-modified human T cells for cancer immunotherapy. Hum Gene Ther. 2006;17:1134–1143. doi: 10.1089/hum.2006.17.1134. [DOI] [PubMed] [Google Scholar]
- 42.Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci USA. 1993;90:720–724. doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gould HJ, Mackay GA, Karagiannis SN, O’Toole CM, Marsh PJ, Daniel BE, Coney LR, Zurawski VR, Jr, Joseph M, Capron M, Gilbert M, Murphy GF, Korngold R. Comparison of IgE and IgG antibody-dependent cytotoxicity in vitro and in a SCID mouse xenograft model of ovarian carcinoma. Eur J Immunol. 1999;29:3527–3537. doi: 10.1002/(SICI)1521-4141(199911)29:11<3527::AID-IMMU3527>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 44.Karagiannis SN, Wang Q, East N, Burke F, Riffard S, Bracher MG, Thompson RG, Durham SR, Schwartz LB, Balkwill FR, Gould HJ. Activity of human monocytes in IgE antibody-dependent surveillance and killing of ovarian tumor cells. Eur J Immunol. 2003;33:1030–1040. doi: 10.1002/eji.200323185. [DOI] [PubMed] [Google Scholar]
- 45.Karagiannis SN, Bracher MG, Hunt J, McCloskey N, Beavil RL, Beavil AJ, Fear DJ, Thompson RG, East N, Burke F, Moore RJ, Dombrowicz DD, Balkwill FR, Gould HJ. IgE-antibody-dependent immunotherapy of solid tumors: cytotoxic and phagocytic mechanisms of eradication of ovarian cancer cells. J Immunol. 2007;179:2832–2843. doi: 10.4049/jimmunol.179.5.2832. [DOI] [PubMed] [Google Scholar]
- 46.Karagiannis SN, Bracher MG, Beavil RL, Beavil AJ, Hunt J, McCloskey N, Thompson RG, East N, Burke F, Sutton BJ, Dombrowicz D, Balkwill FR, Gould HJ. Role of IgE receptors in IgE antibody-dependent cytotoxicity and phagocytosis of ovarian tumor cells by human monocytic cells. Cancer Immunol Immunother. 2008;57:247–263. doi: 10.1007/s00262-007-0371-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jackson DJ, Kumpel BM. Optimisation of human anti-tetanus toxoid antibody responses and location of human cells in SCID mice transplanted with human peripheral blood leucocytes. Hum Antibodies. 1997;8:181–188. [PubMed] [Google Scholar]
- 48.Chen Q, Khoury M, Chen J. Expression of human cytokines dramatically improves reconstitution of specific human-blood lineage cells in humanized mice. Proc Natl Acad Sci USA. 2009;106:21783–21788. doi: 10.1073/pnas.0912274106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fuss IJ, Kanof ME, Smith PD, Zola H. Isolation of whole mononuclear cells from peripheral blood and cord blood. Curr Protoc Immunol Chap. 2009;7:Unit7. doi: 10.1002/0471142735.im0701s85. [DOI] [PubMed] [Google Scholar]
- 50.Dombrowicz D, Brini AT, Flamand V, Hicks E, Snouwaert JN, Kinet JP, Koller BH. Anaphylaxis mediated through a humanized high affinity IgE receptor. J Immunol. 1996;157:1645–1651. [PubMed] [Google Scholar]
- 51.Dombrowicz D, Lin S, Flamand V, Brini AT, Koller BH, Kinet JP. Allergy-associated FcRbeta is a molecular amplifier of IgE- and IgG-mediated in vivo responses. Immunity. 1998;8:517–529. doi: 10.1016/S1074-7613(00)80556-7. [DOI] [PubMed] [Google Scholar]
- 52.Fung-Leung WP, De Sousa-Hitzler J, Ishaque A, Zhou L, Pang J, Ngo K, Panakos JA, Chourmouzis E, Liu FT, Lau CY. Transgenic mice expressing the human high-affinity immunoglobulin (Ig) E receptor alpha chain respond to human IgE in mast cell degranulation and in allergic reactions. J Exp Med. 1996;183:49–56. doi: 10.1084/jem.183.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dombrowicz D, Flamand V, Brigman KK, Koller BH, Kinet JP. Abolition of anaphylaxis by targeted disruption of the high affinity immunoglobulin E receptor alpha chain gene. Cell. 1993;75:969–976. doi: 10.1016/0092-8674(93)90540-7. [DOI] [PubMed] [Google Scholar]
- 54.Allen LC, Kepley CL, Saxon A, Zhang K. Modifications to an Fcgamma-Fcvarepsilon fusion protein alter its effectiveness in the inhibition of FcvarepsilonRI-mediated functions. J Allergy Clin Immunol. 2007;120:462–468. doi: 10.1016/j.jaci.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 55.Zhang K, Kepley CL, Terada T, Zhu D, Perez H, Saxon A. Inhibition of allergen-specific IgE reactivity by a human Ig Fcgamma-Fcepsilon bifunctional fusion protein. J Allergy Clin Immunol. 2004;114:321–327. doi: 10.1016/j.jaci.2004.03.058. [DOI] [PubMed] [Google Scholar]
- 56.Zhu D, Kepley CL, Zhang M, Zhang K, Saxon A. A novel human immunoglobulin Fc gamma Fc epsilon bifunctional fusion protein inhibits Fc epsilon RI-mediated degranulation. Nat Med. 2002;8:518–521. doi: 10.1038/nm0502-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Daniels TR, Leuchter RK, Quintero R, Helguera G, Rodríguez JA, Martínez-Maza O, Schultes BC, Nicodemus CF, Penichet ML (2011) Targeting HER2/neu with a fully human IgE to harness the allergic reaction against cancer cells. Cancer Immunol Immunother [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 58.Tang Y, Lou J, Alpaugh RK, Robinson MK, Marks JD, Weiner LM. Regulation of antibody-dependent cellular cytotoxicity by IgG intrinsic and apparent affinity for target antigen. J Immunol. 2007;179:2815–2823. doi: 10.4049/jimmunol.179.5.2815. [DOI] [PubMed] [Google Scholar]
- 59.Lennon S, Barton C, Banken L, Gianni L, Marty M, Baselga J, Leyland-Jones B. Utility of serum HER2 extracellular domain assessment in clinical decision making: pooled analysis of four trials of trastuzumab in metastatic breast cancer. J Clin Oncol. 2009;27:1685–1693. doi: 10.1200/JCO.2008.16.8351. [DOI] [PubMed] [Google Scholar]
- 60.Hoopmann M, Sachse K, Valter MM, Becker M, Neumann R, Ortmann M, Gohring UJ, Thomas A, Mallmann P, Schondorf T. Serological and immunohistochemical HER-2/neu statuses do not correlate and lack prognostic value for ovarian cancer patients. Eur J Cancer Care (Engl) 2010;19:809–815. doi: 10.1111/j.1365-2354.2009.01112.x. [DOI] [PubMed] [Google Scholar]
- 61.Triulzi C, Vertuani S, Curcio C, Antognoli A, Seibt J, Akusjarvi G, Wei WZ, Cavallo F, Kiessling R. Antibody-dependent natural killer cell-mediated cytotoxicity engendered by a kinase-inactive human HER2 adenovirus-based vaccination mediates resistance to breast tumors. Cancer Res. 2010;70:7431–7441. doi: 10.1158/0008-5472.CAN-10-0493. [DOI] [PubMed] [Google Scholar]
- 62.Helguera G, Rodriguez JA, Daniels TR, Penichet ML. Long-term immunity elicited by antibody-cytokine fusion proteins protects against sequential challenge with murine mammary and colon malignancies. Cancer Immunol Immunother. 2007;56:1507–1512. doi: 10.1007/s00262-007-0297-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Helguera G, Rodriguez JA, Penichet ML. Cytokines fused to antibodies and their combinations as therapeutic agents against different peritoneal HER2/neu expressing tumors. Mol Cancer Ther. 2006;5:1029–1040. doi: 10.1158/1535-7163.MCT-05-0488. [DOI] [PubMed] [Google Scholar]
- 64.Weichman BM, Hostelley LS, Bostick SP, Muccitelli RM, Krell RD, Gleason JG. Regulation of the synthesis and release of slow-reacting substance of anaphylaxis from sensitized monkey lung. J Pharmacol Exp Ther. 1982;221:295–302. [PubMed] [Google Scholar]
- 65.Adams CW, Allison DE, Flagella K, Presta L, Clarke J, Dybdal N, McKeever K, Sliwkowski MX. Humanization of a recombinant monoclonal antibody to produce a therapeutic HER dimerization inhibitor, pertuzumab. Cancer Immunol Immunother. 2006;55:717–727. doi: 10.1007/s00262-005-0058-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Braen AP, Perron J, Tellier P, Catala AR, Kolaitis G, Geng W. A 4-week intrathecal toxicity and pharmacokinetic study with trastuzumab in cynomolgus monkeys. Int J Toxicol. 2010;29:259–267. doi: 10.1177/1091581810361527. [DOI] [PubMed] [Google Scholar]
- 67.Berek J, Taylor P, McGuire W, Smith LM, Schultes B, Nicodemus CF. Oregovomab maintenance monoimmunotherapy does not improve outcomes in advanced ovarian cancer. J Clin Oncol. 2009;27:418–425. doi: 10.1200/JCO.2008.17.8400. [DOI] [PubMed] [Google Scholar]
- 68.Braly P, Nicodemus CF, Chu C, Collins Y, Edwards R, Gordon A, McGuire W, Schoonmaker C, Whiteside T, Smith LM, Method M. The Immune adjuvant properties of front-line carboplatin-paclitaxel: a randomized phase 2 study of alternative schedules of intravenous oregovomab chemoimmunotherapy in advanced ovarian cancer. J Immunother. 2009;32:54–65. doi: 10.1097/CJI.0b013e31818b3dad. [DOI] [PubMed] [Google Scholar]
- 69.King DM, Albertini MR, Schalch H, Hank JA, Gan J, Surfus J, Mahvi D, Schiller JH, Warner T, Kim K, Eickhoff J, Kendra K, Reisfeld R, Gillies SD, Sondel P. Phase I clinical trial of the immunocytokine EMD 273063 in melanoma patients. J Clin Oncol. 2004;22:4463–4473. doi: 10.1200/JCO.2004.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 71.Lafky JM, Wilken JA, Baron AT, Maihle NJ. Clinical implications of the ErbB/epidermal growth factor (EGF) receptor family and its ligands in ovarian cancer. Biochim Biophys Acta. 2008;1785:232–265. doi: 10.1016/j.bbcan.2008.01.001. [DOI] [PubMed] [Google Scholar]