Abstract
Critical advances in the early diagnosis of HIV now allow for treatment opportunities during acute infection. It remains unclear whether treatment of acute HIV infection with antiretroviral therapy improves long-term clinical outcomes for the individual and current guidelines are not definitive in recommending therapy at this stage of infection. However, treatment of acute HIV infection may have short-term benefit on viral set point when compared to delayed therapy as well as reducing the risk of transmission to others. Herein we review the immunological and clinical literature to discuss whether we should treat acute HIV infection, both from the perspective of the individual HIV-infected patient and from the public health perspective. As transmission of drug-resistant HIV variants are of concern, we also review recent clinical trial data to provide recommendations for which specific antiretroviral treatment regimens should be considered for the treatment of acute HIV infection.
Keywords: HIV, Antiretroviral therapy, Clinical trials, Observational studies, Viral load set point, Viral reservoir, Drug-resistant HIV transmission
Introduction
Acute HIV infection is the earliest stage of infection which occurs 1–2 weeks after transmission but before seroconversion. Acute infection lasts for approximately 2–4 weeks, during which the plasma p24 antigen and HIV RNA levels are detectable but the anti-HIV antibodies are not yet detectable [1••]. During this period the virus disseminates widely and viremia reaches very high levels. With the associated cytokines released by innate immune cells in response to the viremia [2], acutely HIV-infected patients often experience a viral syndrome. While peripheral blood CD4+ T-cell counts may remain stable or dip minimally, there is a profound and rapid loss of immune cells in gut-associated lymphoid tissue that is in part irreversible [3, 4].
The question of whether or not to treat acute HIV infection with antiretroviral therapy (ART) remains investigational. There is a paucity of randomized clinical trial data to guide recommendations. The most recent treatment guidelines from the Department of Health and Human Services (DHHS) 2011 [5] and International Antiviral Society-USA (IAS-USA) 2010 [6] conclude that there is insufficient data to routinely recommend treatment of acute HIV infection, but that treatment should be considered optional. In this review we will therefore summarize pertinent data to inform this critical decision: both from the perspective of potential individual benefit and also in terms of public health considerations.
Acute HIV Clinical Presentation and Diagnostics
Acute HIV infection is difficult to diagnose because the symptoms are transient and protean. However, making the correct diagnosis is critical because 1) treatment during acute HIV infection may provide benefit and 2) acutely HIV-infected patients are at increased risk of transmitting. During acute and early HIV infection the risk of transmission appears to be much higher than during chronic infection [7]. In the rhesus macaque model of SIV infection, plasma is up to 750 times more infectious, per-virion, in the acutely infected animals as compared to the chronically infected animals [8]. It has been hypothesized that this increased infectiousness is due to high viral loads, often in excess of one million RNA molecules per mL and homogeneity of highly infectious transmitted/founder viral variants at the time of acute infection [9, 10].
The diagnosis of acute HIV infection requires astute clinical acumen and correct use of specific diagnostic tests. It has been estimated that 40–90% of acutely HIV infected patients are symptomatic within days to weeks of initial exposure [11]. However, the most common symptoms are nonspecific and could be confused with symptoms of infectious mononucleosis, influenza, malaria, and rickettsial diseases, including fever, fatigue, rash, headache, lymphadenopathy, pharyngitis, myalgia, arthralgia, nausea, vomiting, and diarrhea. Additionally, meningoencephalitis and oral or genital mucocutaneous ulcers have been reported [12–15]. Symptoms have been reported to last up to 10 weeks, but most commonly they last less than 14 days [12]. Severe and prolonged symptoms portend rapid disease progression [16, 17]. Testing for acute HIV should be performed in anyone with these viral symptoms, particularly those with sexual contact with a person who is known to be HIV-infected or who is at high risk for having HIV infection (ie, men who have sex with men, sex workers, or persons who have recently had sex with anyone from highly endemic areas like sub-Saharan Africa) or those presenting with a sexually transmitted infection. Additionally, anyone who is found to have acute HIV infection should also be screened for other sexually transmitted infections.
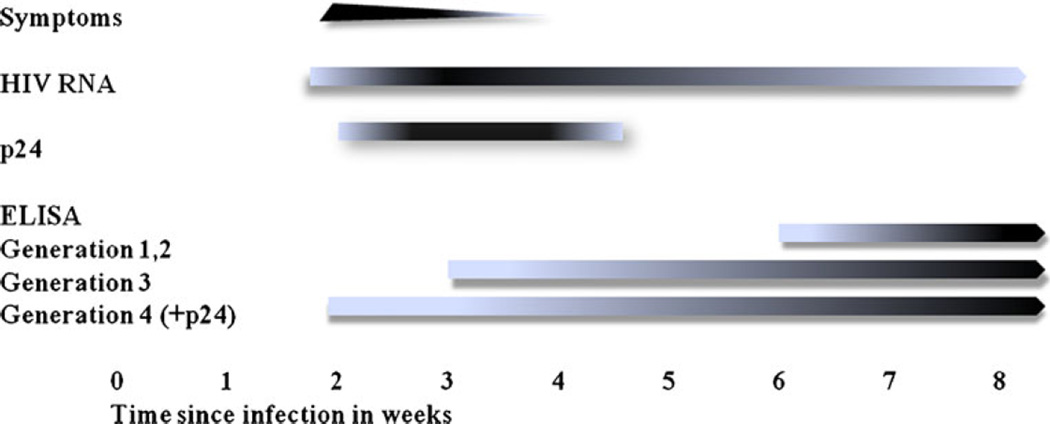
Whether HIV is transmitted through the mucosal, percutaneous, or intravenous route, the virus is not immediately detectable in plasma. This “eclipse” phase lasts from 7 to 21 days [18, 19]. Subsequently, virus can be detected in the plasma, either using nucleic acid amplification when HIV RNA is detectable at 1–5 copies per mL [20] or using clinically available HIV RNA viral loads when HIV RNA is detectable at ≥ 50 copies per mL [21]. Notably, false positives have been reported when HIV RNA < 10,000 [22, 23]; therefore, repeat testing of HIV RNA within 24 h is advisable, as the dynamics of HIV replication during acute infection are very rapid with doubling times of 10 h [24]. Gag p24 antigen appears next by about 14–21 days and lastly antibody responses become detectable after 21 days in plasma [25]. The enzyme-linked immunosorbent assay (ELISA) is the most common immunoassay used for the detection of HIV-1 and HIV-2 antibody. It has evolved from the first-generation viral lysate-based immunoglobulin G (IgG) tests, to the second-generation tests incorporating recombinant and/or synthetic peptide antigens, to the third-generation tests which detect IgG and IgM (antigen sandwich techniques), and to the third-generation-plus assays which also detect HIV-1 group O [26]. Antibody is detected in most individuals within 6–12 weeks after infection with the earlier generation assays, but antibody levels can be detected within 3–4 weeks after infection with third-generation antigen sandwich assays. The newest fourth-generation ELISAs incorporate p24 antigen testing, so that the window period for diagnosis can be shortened to about 2–3 weeks from the time of initial infection (Fig. 1) [27–30]. Because the highest viral loads occur between 2 weeks and 2 months [24], the diagnosis of acute HIV infection during this time provides a critical opportunity for the health care provider to offer both education and treatment.
Fig. 1.
Timeline of acute HIV presentation and diagnostics
Rationale for the Treatment of Acute HIV Infection: For the Individual
The principal rationales to treat acute HIV with ART, when considering the individual patient, are 1) to treat highly symptomatic patients as they are more likely to progress rapidly, 2) to preserve CD4 T + cell counts and reduce the viral set point, 3) to limit the size of viral reservoirs, 4) to preserve HIV-specific immunity, and 5) time to CD4 T + cell count ≤ 500 is short, why wait?
1. Treat Symptomatic Acute HIV Infection
Acutely HIV-infected patients who are symptomatic tend to progress more rapidly than those without symptoms. Antiviral therapy in acute infection rapidly reduces viral loads and alleviates symptoms as well [31–33]. The most recent IAS-USA guidelines recommend considering ART in the setting of symptomatic acute HIV [6].
2. Preserve CD4 T-Cell Counts and Reduce Viral Set Point (Table 1)
Table 1.
Summary of clinical trials of treatment of acute HIV with ART
| Study | Time to ART from diagnosis |
Duration of ART |
Outcome, as compared to untreated |
|---|---|---|---|
| Kinloch-De Loes (1995) [34] Placebo-controlled RCT | 25 days | 24 weeks | Less opportunistic infections during treatment |
| AZT monotherapy | |||
| Niu (1995) [35] Placebo-controlled RCT AZT monotherapy | 18 days | 24 weeks | Improved CD4+ T cell counts at 1 year after treatment interruption (TI) but no difference in viral load (VL) or clinical events |
| Hogan, SETPOINT (2011) [36••] RCT | Within 6 months | 36 weeks | Delayed time to CD4+ T cell counts ≤ 350 cells/mm3 after TI |
| 3-drug ART | |||
| Streeck (2006) [39] Observational prospective | 25 days | 24 weeks | Improved HIV-specific CD8+ T cell responses, no improved VL for 6 months after TI |
| 3-drug ART | |||
| Hecht (2006) [40] Observational prospective | 14 days | 12 weeks | Decreased VL, improved CD4+ T cell count for 72 weeks after TI |
| 3-drug ART | |||
| Fidler (2007) [41] Retrospective | “During primary infection” | 12 weeks | Slower CD4+ T-cell decline after TI |
| 3-drug ART | |||
| Von Wyl (2011) [42] Observational prospective | 16 weeks | 18 months | Decreased VL for 1 y but not 3 y after TI |
| 3-drug ART | |||
| Grijsen (2011) [37] Randomized 3-arm | “During primary infection” | 24 or 60 weeks | Decreased VL and decreased time to start ART 36 wk after TI |
| 3-drug ART | |||
| Fidler, SPARTAC (2011) [41] RCT | Within 6 months | 12 or 48 weeks | Delayed time to CD4 T-cell count < 350 cells/mm3 after TI |
| 3-drug ART | |||
| Hoen, QUEST (2007) [43] Observational prospective | “During primary infection” | 48 weeks | During treatment, improved CD4+ T-cell counts, decreased markers of immune activation (CD38 + CD8+ T cells), and decreased proviral DNA |
| 4-drug ART |
A. Randomized Controlled Clinical Trials of ART During Acute HIV
There have been a paucity of clinical trials addressing the question of whether acute HIV should be treated with ART. To date, there have only been two published placebo-controlled randomized clinical trials (RCTs). The first, reported in 1995, showed that there were significantly fewer opportunistic infections in acutely HIV-infected patients randomized to receive zidovudine (ZDV) monotherapy for 24 weeks as compared to placebo [34]. In 1998, another RCTcomparing ZDV monotherapy for 24 weeks to placebo showed improved CD4+ T cell counts at 1 year but no difference in viral load or clinical events, suggesting that there was early development of ZDV resistance when monotherapy was used in acute infection [35].
The results of the randomized controlled SETPOINT study were recently published in which early versus delayed ART in early (< 6 months) HIV infection was studied to determine whether viral load set point could be altered after treatment interruption [36••]. Early HIV-infected patients were randomized to receive 36 weeks of three-drug ART or to defer therapy until they met predefined criteria. Predefined criteria included having a CD4+ T-cell count ≤ 350 cells/mm3 at two consecutive determinations ≥ 4 weeks apart, having a CD4+ T-cell count ≤ 200 cells/mm3 or 14%, HIV RNA level ≥ 750,000 copies/mL plasma 4 weeks into the study or ≥ 200,000 copies/mL at 12 weeks into the study, or CDC category B or C diagnosis. This study was halted prematurely by the Data Safety Monitoring Board due to futility. The deferred treatment arm experienced more rapid disease progression than expected. Notably, by week 72, 50% of subjects in the deferred therapy arm met immunologic or virologic criteria, 20% within the first 36 weeks. Because of this higher than anticipated rate of disease progression, the virologic set point differences between the two groups could not be statistically evaluated. Guidelines have changed since this study began, with a recommendation to start ART during chronic infection once CD4+ T-cell counts drop ≤ 500 cells/mm3. It would be important to know what number of subjects in the deferred arm reached a CD4+ T-cell count ≤ 500 cells/mm3 within 36 weeks.
Recently, the preliminary findings of a Dutch open-label randomized three-arm study were reported, which compared 173 acutely HIV-infected subjects who were either untreated, treated with ART for 24 weeks or 60 weeks followed by a treatment interruption. The primary endpoint of needing to start/re-start ART if CD4+ T-cell counts fell below 350 cells/mm3 or if subjects had an AIDS-defining condition was reached at 0.7 years, 3.1 years, and 2.1 years respectively. Additionally, viral load set points (log10 c/mL) were reported at 36 weeks after randomization or treatment interruption as 4.8 in the untreated arm, and 3.9 and 4.2 in the 24-week and 60-week treated arms. Authors concluded that early and temporary ART lowered the viral set point and delayed treatment initiation during chronic HIV infection [37].
Results of a randomized controlled trial, SPARTAC, were reported at IAS 2011 [38]. Subjects with acute HIV infection were randomized to receive three-drug ART within 6 months of infection for 48 weeks (ART-48), 12 weeks (ART-12), or no therapy (standard of care, SOC). The primary endpoint was time to CD4+ T-cell counts < 350 cells/mm3 or long-term ART initiation after treatment interruption. Fifty percent of ART-48 participants reached the primary endpoint compared to 61% in each of ART-12 and SOC groups. ART-48 conferred a significant delay in time to reach primary end point compared with SOC, a median of 65 weeks longer, while ART-12 had no effect compared with SOC. ART-48 conferred a reduction in HIV RNA of 36 weeks after interrupting therapy compared with SOC. There were no significant differences between groups in progression rates to AIDS, death, or the incidence of serious adverse events. These investigators concluded that 48 weeks of ART in acute/early HIV infection delayed disease progression.
B. Observational Studies Evaluating Treatment Interruption After ART in Acute HIV
There have been a number of observational studies that have evaluated whether there is benefit to short-term ART initiated during acute HIV infection followed by treatment interruption. One 2006 study prospectively assessed whether initiation of three-drug ART during acute infection and given for 24 weeks would improve adaptive immune responses after treatment interruption. They compared 12 treated subjects to 6 untreated acutely HIV-infected controls. Treatment resulted in suppression of viremia, an increase in the CD4+ T-cell count, enhanced differentiation of HIV-specific CD8+ T cells from effector memory to effector cells at week 24 of treatment, and significantly higher virus-specific interferon-γ+ CD8+ T-cell responses after viral rebound (at week 48). However, no differences in viremia or in CD4+ T-cell counts were found 6 months after ART was stopped [39]. However, in a 2006 study, initiation of ART within 2 weeks of HIV infection and treatment for at least 12 weeks was associated with sustained viral load and CD4 cell count benefits for up to 72 weeks after termination of therapy [40]. In 2007, another study found that in patients who received 3 months of antiretroviral therapy during acute infection, the subsequent CD4+ T-cell count decline over 3 years was slower than in patients who did not receive acute therapy [41]. Similarly, in subjects treated during acute infection in the Swiss cohort study, treatment was interrupted after 18 months of ART and it appeared that viral loads was were lower in the first year but not 3 years after treatment interruption as compared to untreated control subjects, suggesting that treatment of acute HIV effects are likely transiently beneficial [42].
C. QUEST Study: Largest Prospective Observational Study
In 2007, the largest prospective trial of ART during acute infection, QUEST, showed that acutely HIV-infected patients treated with four-drug ART for 1 year experienced a significant improvement in CD4+ T-cell counts, decreased markers of immune activation (CD38 + CD8+ T cells), and decreased proviral DNA levels as compared to untreated acutely HIV infected controls [43, 44]. Notably, there was a high incidence of clinical depression (11%), of treatment non-adherence (36%), and of grade 3 or 4 liver function test elevations (12%–19%) in the treated patients.
D. Lack of Complete Mucosal Immune Reconstitution Despite ART in Acute and Early HIV
Although blood CD4+ T-cell counts are preserved when acute HIV is treated with ART, investigators evaluated whether gut-associated lymphoid tissue (GALT) similarly reconstitutes. They performed colonoscopies on acutely HIV-infected subjects who initiated ART during acute infection. Colon biopsies were performed 1–7 years following acute infection. As compared to uninfected control patients, blood CD4 T-cell counts and markers of immune activation normalized but gut lymphocyte populations were persistently 50%–60% depleted at 1–7 years on fully suppressive ART [4].
3. Decreased Viral Reservoir, Future Opportunities for Functional Cure
Cells latently infected with integrated HIV DNA serve as a viral reservoir that persists during ART and contributes to viral rebound after treatment discontinuation. Treating with ART during acute/early HIV infection may reduce the size of the latent reservoir. As new therapeutic vaccines and immunotherapies are developed, it is possible that a smaller viral reservoir may increase chances for successful HIV eradication, particularly as early proof of concept clinical trials of compounds such as histone deacetylase (HDAC) inhibitors are being performed.
At CROI 2011, Jain et al. presented data showing that subjects with acute/early HIV infection (< 6 months) who started ART early (< 6 months after estimated date of HIV infection) versus later (≥ 2 years) and who maintained ≥ 2 years of subsequent virologic suppression had lower levels of activated CD4+ and CD8+ T cells as compared to the group treated later, but still higher than uninfected control subjects [45]. Deferred therapy was also associated with a 4.8-fold higher level of proviral DNA and also higher cell-associated RNA levels. The size of the viral reservoir during ART was associated with the percentage of activated (CD38, HLADR, CCR5, PD1) CD4 and CD8+ T cells. Also at CROI 2011, Ananworanich et al. presented data of 20 Thai subjects with acute HIV infection who were treated within 3 weeks of infection with a four-drug regimen [46]. After 6 months of ART their colon and blood evidenced undetectable levels of HIV RNA (< 50 copies/mL). Colon and blood T-cell subsets were comparable to uninfected controls.
At the Fifth International Workshop of HIV Persistence during Therapy 2011 meeting, Buzon et al. presented data from a cohort of subjects who were treated with ART during acute HIV infection and followed for 10 years while fully suppressed on ART [47]. CD4+ T cells were isolated from these subjects and compared to that from treatment-naïve elite controllers (EC: undetectable viral load in the absence of ART) and subjects who initiated ART in the chronic phase of infection. In comparison to chronic treated patients, levels of integrated HIV DNA and total HIV DNA were significantly lower in EC and acutely treated, while no differences were seen between EC and acute treated, contributing to increasing evidence that prolonged ART initiated during acute HIV may result in reduced levels of HIV residing in established latent reservoirs.
4. Treatment During Acute Infection Preserves HIV-Specific Immunity
There is evidence that treatment during acute infection may preserve HIV-specific adaptive immune responses. Oxenius et al. evaluated HIV-specific CD4+ and CD8+ T cells responses in HIV-infected subjects who received ART within the first 6 months of infection as compared to those who deferred therapy [48]. They found that delayed initiation of ART is associated with a progressive loss of HIV-specific CD8+ T cells and absent HIV-specific CD4+ T cell responses, whereas even transient ART given early during acute infection preserved HIV-specific adaptive immune responses. Similarly, Rosenberg et al. showed that subjects treated early during infection maintained HIV-specific CD8+ and CD4+ T-cell responses, which correlated with a much decreased viral load set point after treatment interruption [49]. These studies have encouraged researchers to test immunomodulatory agents and to develop therapeutic vaccines to enhance immunologic control of HIV [32, 50–56].
5. Why Wait to Treat? Time to CD4 ≤ 500. After Seroconversion
The most recent HIV treatment guidelines recommend earlier therapy—advising initiation with ART when CD4+ T cell counts fall to levels at or below 500 cells/mm3, so as to reduce the development of co-morbidities such as cardiovascular, kidney, and liver disease, and non-AIDS malignancies [57, 58]. New data asks the intriguing question of how long it takes for patients to reach a CD4+ T cell count ≤ 500 after acute HIV infection. Using Concerted Action on Seroconversion to AIDS and Death in Europe (CASCADE) data, where 25 cohorts of subjects with well-estimated dates of HIV seroconversion are followed prospectively, mixed models were fitted to estimate time from seroconversion to CD4+ T cell counts below 500 cells/mm3. A total of 18,495 subjects were evaluated with the median time to CD4+ T cell counts to below 500 cells/mm3 being only 1.19 years, to below 350 cells/mm3 was 4.19 years, and to below 200 cells/mm3 was 7.93 years [59]. Notably, this CASCADE cohort was also investigated to understand whether time to start ART after seroconversion affected AIDS or death outcomes. They found that CD4+ T-cell counts less than 500 but not 500–799 cells/mm3 were associated with slower disease progression and increased mortality [60••]. Similarly, investigators compared the PRIMO Beijing MSM cohort to the MSM component of the CASCADE cohort. They showed that median CD4+ T-cell counts at diagnosis in the PRIMO cohort were 504/uL and in the CASCADE cohort were 554/ mm3. By 2 years following acute HIV, median CD4 T cell counts were 194 cells/mm3 lower in the PRIMO cohort and 149 cells/mm3 lower in the CASCADE cohort [61]. These data suggest that the time to development of CD4+ T cell counts to levels below 500 cells/mm3 may be more accelerated than presumed, and that the additional time on therapy represents a small percentage of the total time on ART for most patients.
Rationale Against Treatment of Acute HIV Infection: For the Individual
Although there are potential benefits associated with initiation of ART during acute HIV, there are also limitations to this approach. Concerns about long-term toxicity and the development of ART resistance have served as a rationale for the deferral of HIV therapy during acute HIV. Although newer ART regimens are generally better tolerated, more convenient, and more potent than older regimens, there are fewer longer-term safety data for the newer drugs. Earlier initiation of ART may extend exposure to ART by several years. The D:A:D study found an increased incidence of cardiovascular disease associated with cumulative exposure to some drugs within the nucleoside reverse transcriptase inhibitor (NRTI) and protease inhibitor (PI) classes [62, 63]. In the SMART study, continuous exposure to ART was associated with significantly greater loss of bone density compared with interruption or deferral of therapy [57].
Initiating ART during acute HIV may lead to an earlier onset of drug resistance selection in non-adherent patients. Consequently, patients may have limited options for future drug regimens and, importantly, may be more likely to transmit drug-resistant virus to others. Although studies support decreased development of drug resistance when ART is started at higher CD4+ T cell counts [64], patient “readiness” to start ART during acute HIV infection is of utmost importance. Patients must fully understand that absolute treatment adherence is necessary to prevent drug resistance before they initiate lifelong ART.
Although ART is costly, several modeling studies actually support the cost effectiveness of HIV therapy initiated soon after diagnosis [65–67]. It has been reported that the annual cost of care is 2.5 times higher for patients with CD4+ T-cell counts below 50 cells/mm3 compared with patients with CD4+ T-cell counts above 350 cells/mm3 [68]. However, no cost comparisons have been reported between those starting ART with a CD4+ T cell counts between 350 and 500 cells/mm3 as compared to CD4+ T cell counts above 500 cells/mm3.
Rationale for the Treatment of Acute HIV Infection: Public Health Implications
New data strongly support an evolving paradigm of “treatment as prevention,” such that treating HIV-infected individuals will decrease transmission to others. This principle strongly applies to the acutely HIV infected patient. The efficiency of HIV transmission is proportional to the amount of blood and genital viral load in the individual and acutely HIV infected patients have tremendously high viral loads in their blood and genital secretions [69–71].
Although it is biologically probable that acute HIV contributes strongly to sexual transmission, the proportion of transmission events attributable to acute HIV infection are challenging to study. Increased rates of transmission in early and late HIV were observed in the Rakai cohort in Uganda, with (8.2/1000) events of transmission per coital act during the first 2.5 months of infection, (0.7/1000) events during chronic infection, and (2.8/1000) in the last 6–24 months of life [7]. During acute HIV the hazard ratio was calculated to be 26 times higher when compared to chronic HIV infection [72].
In Quebec, related transmission clusters were investigated by evaluating phylogenies reconstructed from sampled viral gene sequences of persons who had seroconverted in the most recent 6 months [73]. Approximately half of the persons who seroconverted in the previous 6 months co-segregated into 75 transmission clusters, while the remaining individuals had unique sequences, suggesting that early HIV infection was responsible for approximately half of HIV transmission events. In related studies, about 30–35% of patients with acute HIV infection cosegregated into phylogenetically related clusters [74, 75].
Using mathematical modeling, sexual transmission events during the epidemic phase of HIV may be more influenced by acutely infected individuals than during the endemic phase [76–82]. All together, however, it is likely that acutely HIV-infected persons contribute disproportionately to sexual transmission. As “test and treat” strategies are being evaluated [83], it will be important to understand whether treating acutely HIV-infected persons will aid in curbing transmission.
Revisions to Clinical Practice Guidelines in 2010–2011
The HIV treatment guidelines from the Department of Health and Human Services (DHHS) [5] and International Antiviral Society-USA (IAS-USA) [6] have recently been revised in 2011 and 2010, respectively. Regarding acute/ primary HIV infection, both the older and the revised guidelines recommend starting ART if acute HIV is symptomatic (rated A1a; strongly recommended). For asymptomatic individuals, the revised guidelines recommend treating during acute HIV if CD4+ T cell count ≤ 500 whereas the earlier guidelines recommended treating when CD4 T + cell count ≤ 350. This change reflects changes in the guidelines to begin treating chronic HIV infection when CD4+ Tcell count ≤ 500. Both old and new guidelines recommend treatment of all HIV-infected pregnant women (A1) because acute or recent HIV infection is associated with a high risk of mother-to-child transmission of HIV [84]. Both older and revised guidelines conclude that there is insufficient data to recommend treatment of acute HIV infection at this time, but that treatment of acute HIV infection should be considered optional and that any potential benefits must be weighed against the risks of potential drug toxicities, development of drug resistance, and need for continuous therapy with strict adherence.
Choosing the Best ART Regimen for Treatment of Acute HIV Infection
Transmitted Drug Resistance
Transmitted drug resistance has been reported in 6%–16% of newly acquired HIV infections [85, 86]. For example, in a European multi-cohort study, 10,056 subjects were evaluated for drug resistance mutations prior to starting their first ART regimen. A total of 9.5% had evidence of transmitted drug resistance, with 15% experiencing treatment failure at 1 year, with the hazard ratio for the risk of failing their initial treatment regimen being twofold higher for those with a NNRTI-containing regimen [87]. Notably, clinically relevant transmitted PI resistance is quite uncommon [88–90]. Therefore, if ART is begun before genotype testing is available, a non-NNRTI, ie, a PI-based regimen should be chosen. Though an integrase inhibitor-based regimen could be considered as empiric therapy in the setting of acute infection there is little data regarding the durable antiviral efficacy of this regimen in patients with plasma HIV-1 RNA levels in excess of 106 copies/mL [91].
Conclusions
Acute HIV infection represents a unique treatment opportunity in the course of infection both for the individual and from a public health perspective. Given current treatment guideline recommendations regarding earlier therapy as well as improvements in safety and tolerability of current preferred first-line regimens and the promise of future immunologic interventions, it would appear that the benefits of early therapy are likely to outweigh the risks (Table 2). That said, if possible, when the diagnosis is made providers should consider enrolling patients with acute HIV infection in a clinical trial. From the evidence that we have presented in this review, it is paramount that we more clearly determine the role of ART in this setting. Information regarding such trials can be obtained at www.clinicaltrials.gov or from local HIV treatment experts.
Table 2.
Rationale for and against starting ART during acute HIV
| Rationale for ART in acute HIV |
|
| Disadvantages of ART in acute HIV |
|
Footnotes
Disclosure No potential conflicts of interest relevant to this article were reported.
Contributor Information
Meagan O’Brien, Division of Infectious Diseases, Cancer Institute, New York University School of Medicine, New York, NY 10016, USA.
Martin Markowitz, Aaron Diamond AIDS Research Center, an affiliate of the Rockefeller University, New York, NY 10016, USA, mmarkowitz@adarc.org.
References
Papers of particular interest, published recently, have been highlighted as:
•• Of major importance
- 1. Cohen MS, Shaw GM, McMichael AJ, Haynes BF. Acute HIV-1 Infection. N Engl J Med. 2011;364(20):1943–1954. doi: 10.1056/NEJMra1011874.This recent review gives an excellent up-to-date summary of the basic science of HIV transmission events along with important vaccine considerations
- 2.Stacey AR, Norris PJ, Qin L, Haygreen EA, Taylor E, Heitman J, et al. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J Virol. 2009;83(8):3719–3733. doi: 10.1128/JVI.01844-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tincati C, Biasin M, Bandera A, Violin M, Marchetti G, Piacentini L, et al. Early initiation of highly active antiretroviral therapy fails to reverse immunovirological abnormalities in gut-associated lym-phoid tissue induced by acute HIV infection. Antivir Ther. 2009;14(3):321–330. [PubMed] [Google Scholar]
- 4.Mehandru S, Poles MA, Tenner-Racz K, Jean-Pierre P, Manuelli V, Lopez P, et al. Lack of mucosal immune reconstitution during prolonged treatment of acute and early HIV-1 infection. PLoS Med. 2006;3(12):e484. doi: 10.1371/journal.pmed.0030484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Panel on Antiretroviral Guidelines for Adults and Adolescents: Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 2011 Jan 10; http://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL.pdf.
- 6.Thompson MA, Aberg JA, Cahn P, Montaner JS, Rizzardini G, Telenti A, et al. Antiretroviral treatment of adult HIV infection: 2010 recommendations of the International AIDS Society-USA panel. JAMA. 2010;304(3):321–333. doi: 10.1001/jama.2010.1004. [DOI] [PubMed] [Google Scholar]
- 7.Wawer MJ, Gray RH, Sewankambo NK, Serwadda D, Li X, Laeyendecker O, et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis. 2005;191(9):1403–1409. doi: 10.1086/429411. [DOI] [PubMed] [Google Scholar]
- 8.Ma ZM, Stone M, Piatak M, Jr, Schweighardt B, Haigwood NL, Montefiori D, et al. High specific infectivity of plasma virus from the pre-ramp-up and ramp-up stages of acute simian immunodeficiency virus infection. J Virol. 2009;83(7):3288–3297. doi: 10.1128/JVI.02423-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bollinger RC, Brookmeyer RS, Mehendale SM, Paranjape RS, Shepherd ME, Gadkari DA, et al. Risk factors and clinical presentation of acute primary HIV infection in India. JAMA. 1997;278(23):2085–2089. [PubMed] [Google Scholar]
- 10.Rosenberg ES, Billingsley JM, Caliendo AM, Boswell SL, Sax PE, Kalams SA, et al. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278(5342):1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 11.Kahn JO, Walker BD. Acute human immunodeficiency virus type 1 infection. N Engl J Med. 1998;339(1):33–39. doi: 10.1056/NEJM199807023390107. [DOI] [PubMed] [Google Scholar]
- 12.Schacker T, Collier AC, Hughes J, Shea T, Corey L. Clinical and epidemiologic features of primary HIV infection. Ann Intern Med. 1996;125(4):257–264. doi: 10.7326/0003-4819-125-4-199608150-00001. [DOI] [PubMed] [Google Scholar]
- 13.Ho DD, Rota TR, Schooley RT, Kaplan JC, Allan JD, Groopman JE, et al. Isolation of HTLV-III from cerebrospinal fluid and neural tissues of patients with neurologic syndromes related to the acquired immunodeficiency syndrome. N Engl J Med. 1985;313(24):1493–1497. doi: 10.1056/NEJM198512123132401. [DOI] [PubMed] [Google Scholar]
- 14.Niu MT, Stein DS, Schnittman SM. Primary human immunodeficiency virus type 1 infection: review of pathogenesis and early treatment intervention in humans and animal retrovirus infections. J Infect Dis. 1993;168(6):1490–1501. doi: 10.1093/infdis/168.6.1490. [DOI] [PubMed] [Google Scholar]
- 15.Quinn TC. Acute primary HIV infection. JAMA. 1997;278(1):58–62. [PubMed] [Google Scholar]
- 16.Dorrucci M, Rezza G, Vlahov D, Pezzotti P, Sinicco A, Nicolosi A, et al. Clinical characteristics and prognostic value of acute retroviral syndrome among injecting drug users. Italian Seroconversion Study. AIDS. 1995;9(6):597–604. doi: 10.1097/00002030-199506000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Henrard DR, Phillips JF, Muenz LR, Blattner WA, Wiesner D, Eyster ME, et al. Natural history of HIV-1 cell-free viremia. JAMA. 1995;274(7):554–558. [PubMed] [Google Scholar]
- 18.Keele BF, Giorgi EE, Salazar-Gonzalez JF, Decker JM, Pham KT, Salazar MG, et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci U S A. 2008;105(21):7552–7557. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee HY, Giorgi EE, Keele BF, Gaschen B, Athreya GS, Salazar-Gonzalez JF, et al. Modeling sequence evolution in acute HIV-1 infection. J Theor Biol. 2009;261(2):341–360. doi: 10.1016/j.jtbi.2009.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palmer S, Wiegand AP, Maldarelli F, Bazmi H, Mican JM, Polis M, et al. New real-time reverse transcriptase-initiated PCR assay with single-copy sensitivity for human immunodeficiency virus type 1 RNA in plasma. J Clin Microbiol. 2003;41(10):4531–4536. doi: 10.1128/JCM.41.10.4531-4536.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Damond F, Avettand-Fenoel V, Collin G, Roquebert B, Plantier JC, Ganon A, et al. Evaluation of an upgraded version of the Roche Cobas AmpliPrep/Cobas TaqMan HIV-1 test for HIV-1 load quantification. J Clin Microbiol. 2010;48(4):1413–1416. doi: 10.1128/JCM.01409-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daar ES, Little S, Pitt J, Santangelo J, Ho P, Harawa N, et al. Diagnosis of primary HIV-1 infection. Los Angeles County Primary HIV Infection Recruitment Network. Ann Intern Med. 2001;134(1):25–29. doi: 10.7326/0003-4819-134-1-200101020-00010. [DOI] [PubMed] [Google Scholar]
- 23.Rich JD, Merriman NA, Mylonakis E, Greenough TC, Flanigan TP, Mady BJ, et al. Misdiagnosis of HIV infection by HIV-1 plasma viral load testing: a case series. Ann Intern Med. 1999;130(1):37–39. doi: 10.7326/0003-4819-130-1-199901050-00007. [DOI] [PubMed] [Google Scholar]
- 24.Little SJ, McLean AR, Spina CA, Richman DD, Havlir DV. Viral dynamics of acute HIV-1 infection. J Exp Med. 1999;190(6):841–850. doi: 10.1084/jem.190.6.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fiebig EW, Wright DJ, Rawal BD, Garrett PE, Schumacher RT, Peddada L, et al. Dynamics of HIV viremia and antibody serocon-version in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS. 2003;17(13):1871–1879. doi: 10.1097/00002030-200309050-00005. [DOI] [PubMed] [Google Scholar]
- 26.Saville RD, Constantine NT, Cleghorn FR, Jack N, Bartholomew C, Edwards J, et al. Fourth-generation enzyme-linked immunosorbent assay for the simultaneous detection of human immunodeficiency virus antigen and antibody. J Clin Microbiol. 2001;39(7):2518–2524. doi: 10.1128/JCM.39.7.2518-2524.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sickinger E, Jonas G, Yem AW, Goller A, Stieler M, Brennan C, et al. Performance evaluation of the new fully automated human immunodeficiency virus antigen-antibody combination assay designed for blood screening. Transfusion. 2008;48(4):584–593. doi: 10.1111/j.1537-2995.2007.01583.x. [DOI] [PubMed] [Google Scholar]
- 28.Branson BM. State of the art for diagnosis of HIV infection. Clin Infect Dis. 2007;45(Suppl 4):S221–S225. doi: 10.1086/522541. [DOI] [PubMed] [Google Scholar]
- 29.Eshleman SH, Khaki L, Laeyendecker O, Piwowar-Manning E, Johnson-Lewis L, Husnik M, et al. Detection of individuals with acute HIV-1 infection using the ARCHITECT HIV Ag/Ab Combo assay. J Acquir Immune Defic Syndr. 2009;52(1):121–124. doi: 10.1097/QAI.0b013e3181ab61e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daar ES, Pilcher CD, Hecht FM. Clinical presentation and diagnosis of primary HIV-1 infection. Curr Opin HIV AIDS. 2008;3(1):10–15. doi: 10.1097/COH.0b013e3282f2e295. [DOI] [PubMed] [Google Scholar]
- 31.Rieder P, Joos B, von Wyl V, Kuster H, Grube C, Leemann C, et al. HIV-1 transmission after cessation of early antiretroviral therapy among men having sex with men. AIDS. 2010;24(8):1177–1183. doi: 10.1097/QAD.0b013e328338e4de. [DOI] [PubMed] [Google Scholar]
- 32.Markowitz M, Vaida F, Hare CB, Boden D, Mohri H, Hecht FM, et al. The virologic and immunologic effects of cyclosporine as an adjunct to antiretroviral therapy in patients treated during acute and early HIV-1 infection. J Infect Dis. 2010;201(9):1298–1302. doi: 10.1086/651664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Attia S, Egger M, Muller M, Zwahlen M, Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS. 2009;23(11):1397–1404. doi: 10.1097/QAD.0b013e32832b7dca. [DOI] [PubMed] [Google Scholar]
- 34.Kinloch-De Loes S, Hirschel BJ, Hoen B, Cooper DA, Tindall B, Carr A, et al. A controlled trial of zidovudine in primary human immunodeficiency virus infection. N Engl J Med. 1995;333(7):408–413. doi: 10.1056/NEJM199508173330702. [DOI] [PubMed] [Google Scholar]
- 35.Niu MT, Bethel J, Holodniy M, Standiford HC, Schnittman SM. Zidovudine treatment in patients with primary (acute) human immunodeficiency virus type 1 infection: a randomized, doubleblind, placebo-controlled trial. DATRI 002 Study Group. Division of AIDS Treatment Research Initiative. J Infect Dis. 1998;178(1):80–91. doi: 10.1086/515612. [DOI] [PubMed] [Google Scholar]
- 36. Hogan CM, Degruttola V, Sun X, Fiscus SA, Del Rio C, Hare CB, et al. The Setpoint Study (ACTG A5217): Effect of Immediate Versus Deferred Antiretroviral Therapy on Virologic Set Point in Recently HIV-1-Infected Individuals. J Infect Dis. 2011;205(1):87–96. doi: 10.1093/infdis/jir699.This important randomized controlled study compared early versus delayed ART in early (< 6 months) HIV infection. Although this study was powered to determine whether viral load setpoint could be altered after treatment interruption, the study was stopped early (and the viral load setpoint outcome could not be evaluated) because subjects who were not treated within 6 months of acute HIV diagnosis experienced more rapid disease progression.
- 37.Grijsen M, Steingrover R, Wit F, de Wolf F, Lange J, Verbon A, Brinkman K, van der Ende M, Schuitemaker H, Prins J. An RCT Comparing No Treatment with 24 or 60 Weeks of Temporary ART during Primary HIV Infection. Conference on Retroviruses and Opportunistic Infections(CROI).2011. [Google Scholar]
- 38.Fidler S. Spartac Trial Investigators. The effect of short-course antiretroviral therapy in primary HIV infection: final results from an international randomised controlled trial; SPARTAC. 6th International AIDS Society conference on HIV pathogenesis, treatment and prevention. 2011 [Google Scholar]
- 39.Streeck H, Jessen H, Alter G, Teigen N, Waring MT, Jessen A, et al. Immunological and virological impact of highly active antire-troviral therapy initiated during acute HIV-1 infection. J Infect Dis. 2006;194(6):734–739. doi: 10.1086/503811. [DOI] [PubMed] [Google Scholar]
- 40.Hecht FM, Wang L, Collier A, Little S, Markowitz M, Margolick J, et al. A multicenter observational study of the potential benefits of initiating combination antiretroviral therapy during acute HIV infection. J Infect Dis. 2006;194(6):725–733. doi: 10.1086/506616. [DOI] [PubMed] [Google Scholar]
- 41.Fidler S, Fox J, Touloumi G, Pantazis N, Porter K, Babiker A, et al. Slower CD4 cell decline following cessation of a 3 month course of HAART in primary HIV infection: findings from an observational cohort. AIDS. 2007;21(10):1283–1291. doi: 10.1097/QAD.0b013e3280b07b5b. [DOI] [PubMed] [Google Scholar]
- 42.von Wyl V, Gianella S, Fischer M, Niederoest B, Kuster H, Battegay M, et al. Early antiretroviral therapy during primary HIV-1 infection results in a transient reduction of the viral setpoint upon treatment interruption. PLoS One. 2011;6(11):e27463. doi: 10.1371/journal.pone.0027463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoen B, Cooper DA, Lampe FC, Perrin L, Clumeck N, Phillips AN, et al. Predictors of virological outcome and safety in primary HIV type 1-infected patients initiating quadruple antiretroviral therapy: QUEST GW PROB3005. Clin Infect Dis. 2007;45(3):381–390. doi: 10.1086/519428. [DOI] [PubMed] [Google Scholar]
- 44.Tilling R, Kinloch S, Goh LE, Cooper D, Perrin L, Lampe F, et al. Parallel decline of CD8+/CD38++ T cells and viraemia in response to quadruple highly active antiretroviral therapy in primary HIV infection. AIDS. 2002;16(4):589–596. doi: 10.1097/00002030-200203080-00010. [DOI] [PubMed] [Google Scholar]
- 45.Jain V, Hartogensis W, Bacchetti P, Hunt P, Epling L, Sinclair E, Lee T-H, Busch M, Hecht F, Deeks S. ART Initiation during Acute/Early HIV Infection Compared to Later ART Initiation Is Associated with Improved Immunologic and Virologic Parameters during Suppressive ART, Paper # 517; Conference on Retroviruses and Opportunistic Infections (CROI).2011. [Google Scholar]
- 46.Ananworanich J, Schuetz A, Sereti I, Rerknimitr R, deSouza M, Dewar R, Chomont N, Phanuphak N, Phanuphak P, Kim J RV254/SEARCH 010 Study Group. Mega-HAART Suppresses HIV Viremia, Reduces Viral Reservoir, and Restores Immunity in Peripheral Blood and Sigmoid Colon of Acute HIV-infected Subjects, Paper # 516; Conference on Retroviruses and Opportunistic Infections (CROI).2011. [Google Scholar]
- 47.Buzon MJSK, Stone AB, Pereyra P, Rosenberg E, Yu XG, Lichterfeld M. Reduced HIV-1 reservoir size after 10 years of sup-pressive antiretroviral therapy in patients initiating treatment during primary infection, Abstract 33. The Fifth International Workshop on HIV Persistence During Therapy. 2011 [Google Scholar]
- 48.Oxenius A, Price DA, Easterbrook PJ, O'Callaghan CA, Kelleher AD, Whelan JA, et al. Early highly active antiretroviral therapy for acute HIV-1 infection preserves immune function of CD8+ and CD4+ T lymphocytes. Proc Natl Acad Sci U S A. 2000;97(7):3382–3387. doi: 10.1073/pnas.97.7.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosenberg ES, Altfeld M, Poon SH, Phillips MN, Wilkes BM, Eldridge RL, et al. Immune control of HIV-1 after early treatment of acute infection. Nature. 2000;407(6803):523–526. doi: 10.1038/35035103. [DOI] [PubMed] [Google Scholar]
- 50.Lu W, Arraes LC, Ferreira WT, Andrieu JM. Therapeutic dendritic-cell vaccine for chronic HIV-1 infection. Nat Med. 2004;10(12):1359–1365. doi: 10.1038/nm1147. [DOI] [PubMed] [Google Scholar]
- 51.Ide F, Nakamura T, Tomizawa M, Kawana-Tachikawa A, Odawara T, Hosoya N, et al. Peptide-loaded dendritic-cell vaccination followed by treatment interruption for chronic HIV-1 infection: a phase 1 trial. J Med Virol. 2006;78(6):711–718. doi: 10.1002/jmv.20612. [DOI] [PubMed] [Google Scholar]
- 52.Rinaldo CR. Dendritic cell-based human immunodeficiency virus vaccine. J Intern Med. 2009;265(1):138–158. doi: 10.1111/j.1365-2796.2008.02047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dorrell L, Williams P, Suttill A, Brown D, Roberts J, Conlon C, et al. Safety and tolerability of recombinant modified vaccinia virus Ankara expressing an HIV-1 gag/multiepitope immunogen (MVA. HIVA) in HIV-1-infected persons receiving combination antiretroviral therapy. Vaccine. 2007;25(17):3277–3283. doi: 10.1016/j.vaccine.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 54.Gandhi RT, O'Neill D, Bosch RJ, Chan ES, Bucy RP, Shopis J, et al. A randomized therapeutic vaccine trial of canarypox-HIVpulsed dendritic cells vs. canarypox-HIV alone in HIV-1-infected patients on antiretroviral therapy. Vaccine. 2009;27(43):6088–6094. doi: 10.1016/j.vaccine.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aline F, Brand D, Pierre J, Roingeard P, Severine M, Verrier B, et al. Dendritic cells loaded with HIV-1 p24 proteins adsorbed on surfactant-free anionic PLA nanoparticles induce enhanced cellular immune responses against HIV-1 after vaccination. Vaccine. 2009;27(38):5284–5291. doi: 10.1016/j.vaccine.2009.05.028. [DOI] [PubMed] [Google Scholar]
- 56.Lori F, Calarota SA, Lisziewicz J. Nanochemistry-based immuno therapy for HIV-1. Curr Med Chem. 2007;14(18):1911–1919. doi: 10.2174/092986707781368513. [DOI] [PubMed] [Google Scholar]
- 57.El-Sadr WM, Lundgren JD, Neaton JD, Gordin F, Abrams D, Arduino RC, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355(22):2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 58.Kitahata MM, Gange SJ, Abraham AG, Merriman B, Saag MS, Justice AC, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009;360(18):1815–1826. doi: 10.1056/NEJMoa0807252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lodi S, Phillips A, Touloumi G, Geskus R, Meyer L, Thiebaut R, et al. Time from human immunodeficiency virus seroconversion to reaching CD4+ cell count thresholds <200, <350, and <500 Cells/ mm(3): assessment of need following changes in treatment guidelines. Clin Infect Dis. 2011;53(8):817–825. doi: 10.1093/cid/cir494. [DOI] [PubMed] [Google Scholar]
- 60. Writing Committee for the CASCADE Collaboration. Timing of HAART initiation and clinical outcomes in human immunodeficiency virus type 1 seroconverters. Arch Intern Med. 2011;171(17):1560–1569. doi: 10.1001/archinternmed.2011.401.This important study found that CD4+ T-cell counts less than 500 but not 500–799 cells/mm3 were associated with slower disease progression and increased mortality, which strongly supports initiation of ART in HIV-infected persons with less than 500 and the rapidity with which newly infected subjects meet current treatment guidelines
- 61.Huang X, Sara L, Fox Z, Phillips A, Johnson A, Porter K, Xu X, Wu H the Beijing PRIMO Cohort and CASCADE Seroconverter Cohorts Study Groups. Patterns of decrease in CD4 cell count after seroconversion: comparison between Beijing PRIMO cohort and CASCADE seroconverter cohorts. 6th International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention. 2011 [Google Scholar]
- 62.Friis-Moller N, Reiss P, Sabin CA, Weber R, Monforte A, El-Sadr W, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356(17):1723–1735. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- 63.Worm SW, Sabin C, Weber R, Reiss P, El-Sadr W, Dabis F, et al. Risk of myocardial infarction in patients with HIV infection exposed to specific individual antiretroviral drugs from the 3 major drug classes: the data collection on adverse events of anti-HIV drugs (D:A:D) study. J Infect Dis. 2009;201(3):318–330. doi: 10.1086/649897. [DOI] [PubMed] [Google Scholar]
- 64.Uy J, Armon C, Buchacz K, Wood K, Brooks JT. Initiation of HAART at higher CD4 cell counts is associated with a lower frequency of antiretroviral drug resistance mutations at virologic failure. J Acquir Immune Defic Syndr. 2009;51(4):450–453. doi: 10.1097/QAI.0b013e3181acb630. [DOI] [PubMed] [Google Scholar]
- 65.Freedberg KA, Losina E, Weinstein MC, Paltiel AD, Cohen CJ, Seage GR, et al. The cost effectiveness of combination antiretroviral therapy for HIV disease. N Engl J Med. 2001;344(11):824–831. doi: 10.1056/NEJM200103153441108. [DOI] [PubMed] [Google Scholar]
- 66.Schackman BR, Goldie SJ, Weinstein MC, Losina E, Zhang H, Freedberg KA. Cost-effectiveness of earlier initiation of antiretroviral therapy for uninsured HIV-infected adults. Am J Public Health. 2001;91(9):1456–1463. doi: 10.2105/ajph.91.9.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mauskopf J, Kitahata M, Kauf T, Richter A, Tolson J. HIV antiretroviral treatment: early versus later. J Acquir Immune Defic Syndr. 2005;39(5):562–569. [PubMed] [Google Scholar]
- 68.Chen RY, Accortt NA, Westfall AO, Mugavero MJ, Raper JL, Cloud GA, et al. Distribution of health care expenditures for HIV-infected patients. Clin Infect Dis. 2006;42(7):1003–1010. doi: 10.1086/500453. [DOI] [PubMed] [Google Scholar]
- 69.Quinn TC, Brookmeyer R, Kline R, Shepherd M, Paranjape R, Mehendale S, et al. Feasibility of pooling sera for HIV-1 viral RNA to diagnose acute primary HIV-1 infection and estimate HIV incidence. AIDS. 2000;14(17):2751–2757. doi: 10.1097/00002030-200012010-00015. [DOI] [PubMed] [Google Scholar]
- 70.Powers KA, Poole C, Pettifor AE, Cohen MS. Rethinking the heterosexual infectivity of HIV-1: a systematic review and meta-analysis. Lancet Infect Dis. 2008;8(9):553–563. doi: 10.1016/S1473-3099(08)70156-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pilcher CD, Tien HC, Eron JJ, Jr, Vernazza PL, Leu SY, Stewart PW, et al. Brief but efficient: acute HIV infection and the sexual transmission of HIV. J Infect Dis. 2004;189(10):1785–1792. doi: 10.1086/386333. [DOI] [PubMed] [Google Scholar]
- 72.Hollingsworth TD, Anderson RM, Fraser C. HIV-1 transmission, by stage of infection. J Infect Dis. 2008;198(5):687–693. doi: 10.1086/590501. [DOI] [PubMed] [Google Scholar]
- 73.Brenner BG, Roger M, Routy JP, Moisi D, Ntemgwa M, Matte C, et al. High rates of forward transmission events after acute/early HIV-1 infection. J Infect Dis. 2007;195(7):951–959. doi: 10.1086/512088. [DOI] [PubMed] [Google Scholar]
- 74.Pao D, Fisher M, Hue S, Dean G, Murphy G, Cane PA, et al. Transmission of HIV-1 during primary infection: relationship to sexual risk and sexually transmitted infections. AIDS. 2005;19(1):85–90. doi: 10.1097/00002030-200501030-00010. [DOI] [PubMed] [Google Scholar]
- 75.Yerly S, Vora S, Rizzardi P, Chave JP, Vernazza PL, Flepp M, et al. Acute HIV infection: impact on the spread of HIVand transmission of drug resistance. AIDS. 2001;15(17):2287–2292. doi: 10.1097/00002030-200111230-00010. [DOI] [PubMed] [Google Scholar]
- 76.Jacquez JA, Koopman JS, Simon CP, Longini IM., Jr Role of the primary infection in epidemics of HIV infection in gay cohorts. J Acquir Immune Defic Syndr. 1994;7(11):1169–1184. [PubMed] [Google Scholar]
- 77.Kretzschmar M, Dietz K. The effect of pair formation and variable infectivity on the spread of an infection without recovery. Math Biosci. 1998;148(1):83–113. doi: 10.1016/s0025-5564(97)10008-6. [DOI] [PubMed] [Google Scholar]
- 78.Abu-Raddad LJ, Longini IM., Jr No HIV stage is dominant in driving the HIV epidemic in sub-Saharan Africa. AIDS. 2008;22(9):1055–1061. doi: 10.1097/QAD.0b013e3282f8af84. [DOI] [PubMed] [Google Scholar]
- 79.Koopman JS, Jacquez JA, Welch GW, Simon CP, Foxman B, Pollock SM, et al. The role of early HIV infection in the spread of HIV through populations. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;14(3):249–258. doi: 10.1097/00042560-199703010-00009. [DOI] [PubMed] [Google Scholar]
- 80.Xiridou M, Geskus R, de Wit J, Coutinho R, Kretzschmar M. Primary HIV infection as source of HIV transmission within steady and casual partnerships among homosexual men. AIDS. 2004;18(9):1311–1320. doi: 10.1097/00002030-200406180-00010. [DOI] [PubMed] [Google Scholar]
- 81.Pinkerton SD. How many sexually-acquired HIV infections in the USA are due to acute-phase HIV transmission? AIDS. 2007;21(12):1625–1629. doi: 10.1097/QAD.0b013e32826fb6a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Prabhu VS, Hutchinson AB, Farnham PG, Sansom SL. Sexually acquired HIV infections in the United States due to acute-phase HIV transmission: an update. AIDS. 2009;23(13):1792–1794. doi: 10.1097/QAD.0b013e32832e7d04. [DOI] [PubMed] [Google Scholar]
- 83.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373(9657):48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 84.Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission. Recommendations for use of antiretroviral drugs in pregnant HIV-1-infected women for maternal health and interventions to reduce perinatal HIV transmission in the United States. 2010 http://aidsinfo.nih.gov/contentfiles/PerinatalGL.pdf.
- 85.Wheeler WH, Ziebell RA, Zabina H, Pieniazek D, Prejean J, Bodnar UR, et al. Prevalence of transmitted drug resistance associated mutations and HIV-1 subtypes in new HIV-1 diagnoses, U. S.-2006. AIDS. 2010;24(8):1203–1212. doi: 10.1097/QAD.0b013e3283388742. [DOI] [PubMed] [Google Scholar]
- 86.Wensing AM, van de Vijver DA, Angarano G, Asjo B, Balotta C, Boeri E, et al. Prevalence of drug-resistant HIV-1 variants in untreated individuals in Europe: implications for clinical management. J Infect Dis. 2005;192(6):958–966. doi: 10.1086/432916. [DOI] [PubMed] [Google Scholar]
- 87.Wittkop L, Gunthard HF, de Wolf F, Dunn D, Cozzi-Lepri A, de Luca A, et al. Effect of transmitted drug resistance on virological and immunological response to initial combination antiretroviral therapy for HIV (EuroCoord-CHAIN joint project): a European multicohort study. Lancet Infect Dis. 2011;11(5):363–371. doi: 10.1016/S1473-3099(11)70032-9. [DOI] [PubMed] [Google Scholar]
- 88.Boden D, Hurley A, Zhang L, Cao Y, Guo Y, Jones E, et al. HIV-1 drug resistance in newly infected individuals. JAMA. 1999;282(12):1135–1141. doi: 10.1001/jama.282.12.1135. [DOI] [PubMed] [Google Scholar]
- 89.Simon V, Vanderhoeven J, Hurley A, Ramratnam B, Louie M, Dawson K, et al. Evolving patterns of HIV-1 resistance to antire-troviral agents in newly infected individuals. AIDS. 2002;16(11):1511–1519. doi: 10.1097/00002030-200207260-00008. [DOI] [PubMed] [Google Scholar]
- 90.Shet A, Berry L, Mohri H, Mehandru S, Chung C, Kim A, et al. Tracking the prevalence of transmitted antiretroviral drug-resistant HIV-1: a decade of experience. J Acquir Immune Defic Syndr. 2006;41(4):439–446. doi: 10.1097/01.qai.0000219290.49152.6a. [DOI] [PubMed] [Google Scholar]
- 91.Lennox JL, DeJesus E, Lazzarin A, Pollard RB, Madruga JV, Berger DS, et al. Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naive patients with HIV-1 infection: a multicentre, double-blind randomised controlled trial. Lancet. 2009;374(9692):796–806. doi: 10.1016/S0140-6736(09)60918-1. [DOI] [PubMed] [Google Scholar]