Abstract
BACKGROUND
CYP2C9 and VKORC1 genotypes predict therapeutic warfarin dose at initiation of therapy; however, the predictive ability of genetic information after a week or longer is unknown. Experts have hypothesized that genotype becomes irrelevant once International Normalized Ratio (INR) values are available because INR response reflects warfarin sensitivity.
METHODS
We genotyped the participants in the Prevention of Recurrent Venous Thromboembolism (PREVENT) trial, who had idiopathic venous thromboemboli and began low-intensity warfarin (therapeutic INR 1.5-2.0) using a standard dosing protocol. To develop pharmacogenetic models, we quantified the effect of genotypes, clinical factors, previous doses, and INR on therapeutic warfarin dose in the 223 PREVENT participants who were randomized to warfarin and achieved stable therapeutic INRs.
RESULTS
A pharmacogenetic model using data from day 0 (before therapy initiation) explained 54% of the variability in therapeutic dose (R2). The R2 increased to 68% at day 7, 75% at day 14, and 77% at day 21, because of increasing contributions from prior doses and INR response. Although CYP2C9 and VKORC1 genotypes were significant independent predictors of therapeutic dose at each weekly interval, the magnitude of their predictive ability diminished over time: partial R2 of genotype was 43% at day 0, 12% at day 7, 4% at day 14, and 1% at day 21.
CONCLUSION
Over the first weeks of warfarin therapy, INR and prior dose become increasingly predictive of therapeutic dose, and genotype becomes less relevant. However, at day 7, genotype remains clinically relevant, accounting for 12% of therapeutic dose variability.
INTRODUCTION
Prevention and treatment of venous thromboembolism (VTE) with the oral anticoagulant warfarin is indicated for a wide variety of patients. Warfarin has a narrow therapeutic window requiring frequent monitoring of the International Normalized Ratio (INR) until a stable dose is achieved. Initiation of warfarin poses challenges for both clinicians and patients due to complex pharmacology, risks of bleeding and clotting, and great inter-subject dose variability. Typically, clinicians dose warfarin on an empiric basis--using clinical and demographic factors (e.g. age) to estimate a starting dose and INR response to make subsequent refinements. Traditionally, this trial-and-error process does not take genotype into account.
In addition to clinical and demographic factors, genetic variations in two genes affect patients’ therapeutic warfarin dose. Single nucleotide polymorphisms (SNPs) in the cytochrome P450 2C9 gene (CYP2C9) affect the rate at which patients metabolize S-warfarin, the more active enantiomer of the drug. Patients with a CYP2C9*2 and/or CYP2C9*3 SNP metabolize warfarin more slowly than a wildtype patient (CYPC29*1/*1), resulting in lower therapeutic warfarin doses.[1] Patients with haplotype A of the vitamin K epoxide reductase complex 1 (VKORC1) gene have reduced expression of the VKOR protein and concomitant increased warfarin sensitivity.[2,3] Thus, these common genetic factors often result in a patient requiring a greater or lesser dose than clinically anticipated.
Clinical dosing algorithms and genetic-based dosing algorithms have been developed to assist clinicians a priori in estimating a stable warfarin dose. Clinical algorithms account for about 17-22% (R2)[4] and genetic algorithms account for about half (R2 ~50%) of the dose variability.[5-8] Genetic-based algorithms that incorporate an INR after 3 or 4 warfarin doses are even more accurate.[9,10] However, clinical algorithms also become more accurate after several days of therapy[11,12], prompting some experts to argue that genetic information becomes irrelevant once INR response is known.[13] This uncertainty is important because currently clinicians are reluctant to consider obtaining genotype information if results will not be available within the first few days of warfarin therapy. If genotype proves to be useful beyond the first few days of therapy, it could be combined with clinical and demographic factors to create a family of dose-refinement algorithms.
To address these issues, we quantified the ability of CYP2C9*2, CYP2C9*3, and VKORC1 −1639 SNPs to predict therapeutic dose at the time of initiation of warfarin therapy and after days 7, 14, and 21 of therapy in participants of the PREVENT trial.[14] PREVENT was a randomized, double-blind, placebo-controlled trial of low-intensity warfarin (therapeutic INR 1.5-2.0) for the prevention of recurrent VTE that was terminated early after its independent Data and Safety Monitoring Board found a 64% risk reduction in recurrent VTE. PREVENT participants had an INR drawn at baseline and then weekly for four weeks, allowing us to evaluate the relevance of genotype on INR control and final therapeutic dose in the setting of protocol-driven INR monitoring and standardized dose adjustments.
METHODS
We collected blood, demographic variables, laboratory data, smoking status, and medication information from participants. The Human Subjects’ Committee approved the research protocol. All subjects gave written, informed consent.
Study Subjects
The study population consisted of the 508 participants in the PREVENT trial. Eligible subjects had experienced an idiopathic venous thromboembolic event, completed at least 3 months of standard anticoagulation therapy (INR 2-3), and returned to a baseline INR <1.2 following discontinuation of standard warfarin therapy. Potential participants were excluded if they were taking dipyridamole, ticlopidine, clopidogrel, heparin, >325 mg of aspirin, or drugs that affected the prothrombin time. After their INR had drifted to <1.2 and prior to randomization to long-term treatment with warfarin or placebo, all participants took part in an open label 28-day run-in phase to ensure compliance and to determine therapeutic warfarin dose for an INR target of 1.5-2.0. Starting with an initial dose of 3.0 mg, dose adjustments were made based on approximately weekly INR determinations using a study-specific clinical nomogram.[14] Genotyping was performed after the completion of PREVENT. Our primary analysis of the importance of genetic factors in dose prediction on days 0-21 was performed using therapeutic doses from the patients randomized to long-term warfarin (n=223). Our secondary analysis of importance of genetic factors for INR control was performed based on all patients (n=508) during the 28-day run-in phase.
Genotyping
We extracted DNA from white cell buffy coats (Gentra Systems Puregene kit, Minneapolis, MN) prepared from a 10-ml EDTA anticoagulated blood sample collected from each participant during the original PREVENT study. We used Pyrosequencing™ [15,16] to genotype for four SNPs: CYP2C9*2 [rs1799853], CYP2C9*3 [rs1057910], VKORC1 6853 [rs8050894] and −1639 [rs9923231] (listed with dbSNP reference SNP identifiers). VKORC1 SNPs −1639 and 6853 are in high linkage disequilibrium and designate haplotype A, which is associated with increased warfarin sensitivity.[2] PCR reactions and Pyrosequencing were performed as previously prescribed.[4] The genotyping platforms for the SNPs of interest are 99-100% accurate.[17]
Clinical and Genetic Variables
We used height and weight to estimate body surface area (BSA).[18] For CYP2C9*2, CYP2C9*3, and VKORC1 −1639 (A genotype and A haplotype) we coded each of the three SNPs using its own dummy variable coded as 0, 1, or 2 to quantify the number of variant alleles. We used dummy variables to code for demographic factors (sex and race), smoking status, and key medications (amiodarone, statins, aspirin). We defined therapeutic dose as the dose resulting in two in-range INR values following a week of steady dose regimen. To get a more accurate estimate of the final therapeutic dose for each patient, we excluded 285 patients from our primary analysis because they were randomized to placebo or failed to become therapeutic.
Statistical Analyses
In the primary analysis, we used multiple linear regression to test the effect of the genetic and clinical factors on percent of variability in log-transformed therapeutic dose values at four times: days 0, 7, 14, and 21 of therapy. We used backward selection to select the predictor variables and to quantify the corresponding partial R2. For this analysis, we included only those participants who were randomized to warfarin and achieved a stable therapeutic INR (n = 223); others were excluded because they lacked long-term follow-up (n = 261), did not achieve a stable therapeutic dose (n = 11), or were missing clinical or genetic factors (n = 13). In all four regression models, we adjusted for clinical factors, and starting at day 7, we also adjusted for the prior week’s average dose and most recent INR value. We then repeated the analysis in 250 bootstrapped samples to obtain the standard deviations for the R2 values.
In the secondary analyses, we used logistic and linear regression to determine the association between VKORC1 A haplotype (−1639 A genotype), CYPC29 genotype, and clinical variables with time above and then with time below the target INR range (1.5–2.0). For these analyses, we used INR data from all participants during the 28-day run-in phase. We calculated percent time each patient spent above and below therapeutic INR range between days 7 to 28 using linear interpolation.[19] The percentage of subtherapeutic time was normally distributed and was modeled by linear regression. In contrast, because most patients were never above the target INR range (“supratherapeutic”), that distribution was not normally distributed. We therefore modeled supratherapeutic time separately, using a two-staged approach: (1) we used logistic regression to compare subjects who did and did not have INR values above 2.0, then (2) in a subgroup analysis of subjects who had at least one above-range INR value we analyzed factors affecting the time spent supratherapeutic. We used backward selection to select the predictor variables. Variables with p-values <0.10 were retained in the models if they were biologically plausible, but only variables with p-values <0.05 were considered statistically significant. By examining residual errors, we confirmed the appropriateness of the linear regression models. We performed statistical analyses in SAS (Version 9.1; SAS Institute, Inc; Cary, NC).
RESULTS
After excluding 13 subjects with missing clinical or genetic information, the sample consisted of 495 participants. Mean age was 55 (range 30 – 89), 88% were Caucasian, and 47% were female (Table 1).
Table 1.
Demographic, Genetic, and Clinical Characteristics of Participants
| DEMOGRAPHIC VARIABLES | N = 495 |
| Age, mean (SD), years | 55 (13) |
| Gender | |
| female, n (%) | 234 (47%) |
| male, n (%) | 261 (53%) |
| Race | |
| Caucasian, n (%) | 434 (88%) |
| African-American, n (%) | 47 (9%) |
| Other, n (%) | 14 (3%) |
| Hispanic ethnicity, n (%) | 6 (1%) |
| GENETIC VARIABLES | |
| VKORC1 A haplotypea frequency | 37.5% |
| CYP2C9*2 allele frequency | 12.2% |
| CYP2C9*3 allele frequency | 6.4% |
| CLINICAL VARIABLES | |
| geometric mean warfarin dose, mg/day, (SD) |
4.4 (1.5) |
| body surface area, in m2 mean (SD) | 2.05 (0.27) |
| smoker, n (%) | 57 (12%) |
| takes statin, n (%) | 53 (11%) |
| takes amiodarone, n (%) | 0 (0%) |
| takes aspirin, n (%) | 97 (20%) |
SD = standard deviation; VKORC1 = vitamin K epoxide reductase complex 1. CYP = cytochrome P 450.
The VKORC1 A haplotype can be detected by −1639 G>A;
Effect of Genetic, Clinical, and Laboratory Factors on Therapeutic Dose after 0-21 Days of Therapy
As dosing history and INR response became more extensive over the initial 3 weeks of therapy, the model progressively explained more of the variance in therapeutic dose by accounting for this information. The overall R2 increased weekly (Figure 1): 54% at day 0; 68% at day 7; 75% at day 14; and 77% at day 21. Although genetic factors (VKORC1 A haplotype, CYP2C9*2, and CYP2C9*3) were significant in all models, their contribution to explaining the therapeutic dose (partial R2) declined steadily: 43% at day 0; 12% at day 7; 3.9% at day 14; and 1.4% at day 21. The contribution of INR to partial R2 also declined over time: 32% at day 7, 19% at day 14, and 5% at day 21 (Table 2). In contrast, the dosing history became progressively more predictive of therapeutic dose, easily trumping other factors over time: partial R2 18% at day 7; 50% at day 14; and 69% at day 21 (Figure 1).
Figure 1.
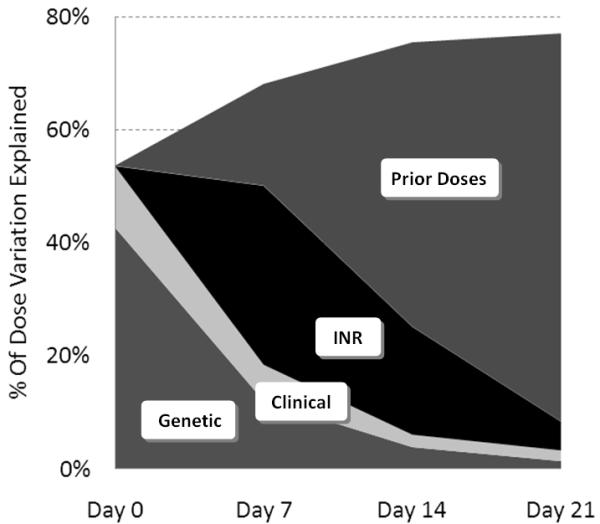
Percentage of dose variation explained at weekly time points.
Table 2.
Percent of Dose Variation Explained (partial R2) at Weekly Timepoints
| Day 0 | Day 7 | Day 14 | Day 21 | |
|---|---|---|---|---|
| Genetic | 42.8% | 12.1% | 3.9% | 1.4% |
| Clinical | 10.8% | 6.4% | 2.2% | 1.9% |
| INR | 0% | 31.7% | 19.1% | 5.1% |
| Prior Dose | 0% | 18.0% | 50.3% | 68.6% |
| TOTAL | 53.6% | 68.1% | 75.4% | 77.0% |
Time Spent Below and Above Therapeutic Range: Days 7-28
In the regression analysis, both VKORC1 and CYP2C9 genotypes had significant effect on time spent below therapeutic range. Time spent below therapeutic range decreased by 16% per VKORC1 A haplotype and 11% per CYP2C9 variant allele. The variables that increased time spent below therapeutic range were younger age (3.6% per decade, p<0.0001) and greater body surface area (3.1% per standard deviation, p=0.04). Female gender had a borderline effect (5.7% less time than in males, p=0.054). Thirty-nine percent of subjects had at least one INR above 2 during days 7-28. In the logistic regression, both VKORC1 and CYP2C9 genotypes influenced the odds of supratherapeutic dosing. The odds ratios (OR) for over-anticoagulation were 2.6 (95% confidence interval [CI] 1.9-3.5) per A haplotype of VKORC1 and 2.9 (95% CI 2.0-4.2) per CYP2C9 variant allele. The odds of over-anticoagulation also significantly increased with increasing age (OR=1.4, 95% CI 1.2-1.7 per decade, p<0.0001). Among patients with at least one supratherapeutic INR, patients with CYP2C9 variants and VKORC1 A allele spent significantly more time in the supratherapeutic range. (Effect per allele/haplotype: 5.8% and 5.6% absolute increase in supratherapeutic time for CYP2C9 and VKORC1, respectively). Older age was associated with increased supratherapeutic time (4.0% per decade, p=0.0003).
DISCUSSION
This study quantifies the decreasing predictive ability of CYP2C9 and VKORC1 genotype during the initial weeks of warfarin therapy. While the overall accuracy of the pharmacogenetic model improved over time, the incremental benefit of knowing a patient’s genotype decreased over time (Figure 1).
While some experts argue that pharmacogenetic information has a promising future for patients on warfarin[20,21], others argue that genetic information adds little incremental information once INR response is available.[22-25] Our observations suggest that both opinions may be valid. On the one hand, we found that after 7 days of warfarin, CYP2C9 and VKORC1 still account for substantial (12%) variability in therapeutic dose. Although genotype is more predictive before initiation (43%), the increased predictive ability it adds after 7 days could help clinically. In particular, some outpatients do not have their INRs checked until a week after therapy begins[26,27], so improving the accuracy of dose refinements made after 7 days might prevent adverse events. Without genetic dosing, the rate of hemorrhage is highest during the initial weeks of therapy, so pharmacogenetic dose adjustments at this time might prevent hemorrhages. In a trial of Israeli patients beginning warfarin, 95 patients randomized to pharmacogenetic dosing for the first 8 days of therapy had fewer minor hemorrhages than the 96 patients dosed using a clinical algorithm[21], but other trials of pharmacogenetic dosing have not prevented hemorrhages.[28,29]
On the other hand, we found that as INR results become available during the first weeks of therapy, genetic information provides a lower degree of predictive ability. For example, the INR during the first week of therapy will often be elevated in a patient who is VKORC1 sensitive, but dose adjustments will compensate for this effect over the subsequent weeks.[30] Here we find that after 3 weeks, the dose history trumps the value of information contained in genotype.
The results of our secondary analyses suggest that genetic information might be helpful after initiation of warfarin. When using INR data from days 7-28, CYP2C9 and VKORC1 genotypes were significant predictors of the amount of time patients spent below therapeutic range and the odds of having a supratherapeutic INR. These results suggest that weekly monitoring (as mandated in PREVENT) does not completely compensate for the presence of genetic SNPs for several weeks. This is likely because the INRs at days 7 and 14 have not yet captured the entire genetic effects. The effect of CYP2C9, in particular, may elevate the INR after the initial week of therapy [30] and not be fully reflected in the initial INR response. Because supratherapeutic INRs are associated with major hemorrhage[31], patients with VKORC1 and/or CYP2C9 variants have triple the risk of adverse events during the initial weeks of therapy.[1,32,33] This observation, along with our finding that genotype improved R2 by 12% at day 7 of therapy, suggests that additional research is needed to delineate the safety and effectiveness of pharmacogenetic-guided dosing that extends past the first few days of therapy.[9,10] In particular, multi-centered randomized trials should test the hypothesis that pharmacogenetic dosing improves laboratory and clinical outcomes over the initial weeks of therapy.
A few limitations of our study merit consideration. PREVENT participants had a low target INR (1.5–2.0), which proved effective at preventing recurrent VTE.[14] Although we did not observe an interaction between predictive ability of genotype and target INR in our prior analysis[4], the predictive ability of genotype also might vary with dosing practices and INR monitoring. A second potential limitation is that our models were developed using INR values that were obtained only once weekly. The degree that genotype correlates with dose after 1, 2, or 3 weeks of therapy may be diminished in populations who monitor their INR more frequently. A third potential limitation is the consistent indication for warfarin among patients in the PREVENT trial, as all patients were taking warfarin for prevention of recurrent VTE. While this consistency eliminates potential confounding by indication, it limits generalizability to other diseases. Because of these limitations and lack of a validation cohort, we did not develop a dose refinement algorithm for clinical use. In the future, we hope to combine the PREVENT data with data from diverse populations to develop such an algorithm.
The limitations are balanced by several strengths. One strength is that we assured accurate therapeutic dose determination by requiring two consecutive therapeutic INRs. A second strength is that PREVENT used a standard initial dose and dose adjustment protocol, thus preventing inter-clinician variability in prescribed dose. A final strength is that all participants were followed prospectively as part of a multi-centered randomized trial.
In summary, we found that SNPs causing slower warfarin metabolism and increased warfarin sensitivity account for significant variability of therapeutic warfarin dose. These SNPs are associated with increased risk of supratherapeutic INRs up to 28 days after initiation. However, the importance of genotype wanes over the initial weeks of therapy. Our findings should prompt future studies to develop and assess the clinical utility of a day 7 pharmacogenetic dosing algorithm.
Acknowledgments
Role of the Funding Sources
The study sponsor had no role in gathering or interpreting data, nor review of the manuscript.
Funded by NIH grants HL-074724, HL-097036, HL-57951, and HL-58036.
Footnotes
Addendum
The following details each author’s contribution to the study and/or manuscript: Nicholas Ferder (conception, analyzing, writing, editing, approving); Charles Eby (funding, conception, genotyping, analyzing, editing, approving); Elena Deych (funding, analyzing, writing, approving); Jenine Harris (analyzing, editing, approving); Paul Ridker (funding, conception, recruiting, editing, approving); Paul Milligan (writing, approving); Samuel Goldhaber (recruiting, editing, approving); Cristi King (genotyping, editing, approving); Tusar Giri (recruiting, editing, approving); Howard McLeod (genotyping, editing, approving); Robert Glynn (funding, conception, recruiting, editing, approving); Brian Gage (funding, conception, analyzing, writing, editing, approving).
References
- 1.Higashi MK, Veenstra DL, Kondo LM, Wittkowsky AK, Srinouanprachanh SL, Farin FM, Rettie AE. Association between CYP2C9 genetic variants and anticoagulation-related outcomes during warfarin therapy. JAMA. 2002;287:1690–8. doi: 10.1001/jama.287.13.1690. [DOI] [PubMed] [Google Scholar]
- 2.Rieder MJ, Reiner AP, Gage BF, Nickerson DA, Eby CS, McLeod HL, Blough DK, Thummel KE, Veenstra DL, Rettie AE. Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose. N Engl J Med. 2005;352:2285–93. doi: 10.1056/NEJMoa044503. [DOI] [PubMed] [Google Scholar]
- 3.Yuan HY, Chen JJ, Lee MT, Wung JC, Chen YF, Charng MJ, Lu MJ, Hung CR, Wei CY, Chen CH, Wu JY, Chen YT. A novel functional VKORC1 promoter polymorphism is associated with inter-individual and inter-ethnic differences in warfarin sensitivity. Hum Mol Genet. 2005;14:1745–51. doi: 10.1093/hmg/ddi180. [DOI] [PubMed] [Google Scholar]
- 4.Gage BF, Eby C, Johnson JA, Deych E, Rieder MJ, Ridker PM, Milligan PE, Grice G, Lenzini P, Rettie AE, Aquilante CL, Grosso L, Marsh S, Langaee T, Farnett LE, Voora D, Veenstra DL, Glynn RJ, Barrett A, McLeod HL. Use of pharmacogenetic and clinical factors to predict the therapeutic dose of warfarin. Clinical pharmacology and therapeutics. 2008;84:326–31. doi: 10.1038/clpt.2008.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schelleman H, Chen J, Chen Z, Christie J, Newcomb CW, Brensinger CM, Price M, Whitehead AS, Kealey C, Thorn CF, Samaha FF, Kimmel SE. Dosing algorithms to predict warfarin maintenance dose in Caucasians and African Americans. Clinical pharmacology and therapeutics. 2008;84:332–9. doi: 10.1038/clpt.2008.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlquist JF, Horne BD, Muhlestein JB, Lappe DL, Whiting BM, Kolek MJ, Clarke JL, James BC, Anderson JL. Genotypes of the cytochrome p450 isoform, CYP2C9, and the vitamin K epoxide reductase complex subunit 1 conjointly determine stable warfarin dose: a prospective study. Journal of thrombosis and thrombolysis. 2006;22:191–7. doi: 10.1007/s11239-006-9030-7. [DOI] [PubMed] [Google Scholar]
- 7.Zhu Y, Shennan M, Reynolds KK, Johnson NA, Herrnberger MR, Valdes R, Jr., Linder MW. Estimation of Warfarin Maintenance Dose Based on VKORC1 (−1639 G>A) and CYP2C9 Genotypes. Clin Chem. 2007;53:1199–205. doi: 10.1373/clinchem.2006.078139. [DOI] [PubMed] [Google Scholar]
- 8.Klein TE, Altman RB, Eriksson N, Gage BF, Kimmel SE, Lee MT, Limdi NA, Page D, Roden DM, Wagner MJ, Caldwell MD, Johnson JA, IWPC Estimation of the warfarin dose with clinical and pharmacogenetic data. N Engl J Med. 2009;360:753–64. doi: 10.1056/NEJMoa0809329. 360/8/753 [pii] 10.1056/NEJMoa0809329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Millican E, Lenzini P, Milligan P, Grosso L, Eby C, Deych E, Grice G, Clohisy J, Barrack R, Burnett R, Voora D, Gatchel S, Tiemeier A, Gage B. Genetic-based dosing in orthopaedic patients beginning warfarin therapy. Blood. 2007;110:1511–5. doi: 10.1182/blood-2007-01-069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lenzini PA, Grice GR, Milligan PE, Dowd MB, Subherwal S, Deych E, Eby CS, King CR, Porche-Sorbet RM, Murphy CV, Marchand R, Millican EA, Barrack RL, Clohisy JC, Kronquist K, Gatchel SK, Gage BF. Laboratory and clinical outcomes of pharmacogenetic vs. clinical protocols for warfarin initiation in orthopedic patients. J Thromb Haemost. 2008;6:1655–62. doi: 10.1111/j.1538-7836.2008.03095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lenzini P, Grice G, Milligan P, Gatchel S, Deych E, Eby C, Burnett RS, Clohisy J, Barrack R, Gage B. Optimal Dose Adjustment in Orthopaedic Patients Beginning Warfarin Therapy. Ann Pharmacother. 2007;41:1798–804. [Google Scholar]
- 12.Siguret V, Gouin I, Debray M, Perret-Guillaume C, Boddaert J, Mahe I, Donval V, Seux ML, Romain-Pilotaz M, Gisselbrecht M, Verny M, Pautas E. Initiation of warfarin therapy in elderly medical inpatients: a safe and accurate regimen. Am J Med. 2005;118:137–42. doi: 10.1016/j.amjmed.2004.07.053. [DOI] [PubMed] [Google Scholar]
- 13.Li C, Schwarz UI, Ritchie MD, Roden DM, Stein CM, Kurnik D. Relative contribution of CYP2C9 and VKORC1 genotypes and early INR response to the prediction of warfarin sensitivity during initiation of therapy. Blood. 2008 doi: 10.1182/blood-2008-09-176859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ridker PM, Goldhaber SZ, Danielson E, Rosenberg Y, Eby CS, Deitcher SR, Cushman M, Moll S, Kessler CM, Elliott CG, Paulson R, Wong T, Bauer KA, Schwartz BA, Miletich JP, Bounameaux H, Glynn RJ. N Engl J Med. 2003;348:1425–34. doi: 10.1056/NEJMoa035029. [DOI] [PubMed] [Google Scholar]
- 15.Ronaghi M, Uhlen M, Nyren P. A sequencing method based on real-time pyrophosphate. Science. 1998;281:363, 5. doi: 10.1126/science.281.5375.363. [DOI] [PubMed] [Google Scholar]
- 16.Aquilante CL, Lobmeyer MT, Langaee TY, Johnson JA. Comparison of cytochrome P450 2C9 genotyping methods and implications for the clinical laboratory. Pharmacotherapy. 2004;24:720–6. doi: 10.1592/phco.24.8.720.36074. [DOI] [PubMed] [Google Scholar]
- 17.King CR, Porche-Sorbet RM, Gage BF, Ridker PM, Renaud Y, Phillips MS, Eby C. Performance of commercial platforms for rapid genotyping of polymorphisms affecting warfarin dose. Am J Clin Path. 2008;129:876–83. doi: 10.1309/1E34UAPR06PJ6HML. 6428X1U0K1406K47 [pii] 10.1309/1E34UAPR06PJ6HML. [DOI] [PubMed] [Google Scholar]
- 18.DuBois D, DuBois E. Clinical Calorimetry; a formula to estimate the approximate surface area if height and weight be known. Arch Int med. 1916;17:863–71. [Google Scholar]
- 19.Rosendaal FR, Cannegieter SC, van der Meer FJ, Briet E. A method to determine the optimal intensity of oral anticoagulant therapy. Thrombosis and haemostasis. 1993;69:236–9. [PubMed] [Google Scholar]
- 20.Lesko LJ. The critical path of warfarin dosing: finding an optimal dosing strategy using pharmacogenetics. Clinical pharmacology and therapeutics. 2008;84:301–3. doi: 10.1038/clpt.2008.133. clpt2008133 [pii] 10.1038/clpt.2008.133. [DOI] [PubMed] [Google Scholar]
- 21.Caraco Y, Blotnick S, Muszkat M. CYP2C9 genotype-guided warfarin prescribing enhances the efficacy and safety of anticoagulation: a prospective randomized controlled study. Clinical pharmacology and therapeutics. 2008;83:460–70. doi: 10.1038/sj.clpt.6100316. [DOI] [PubMed] [Google Scholar]
- 22.Bussey HI, Wittkowsky AK, Hylek EM, Walker MB. Genetic testing for warfarin dosing? Not yet ready for prime time. Pharmacotherapy. 2008;28:141–3. doi: 10.1592/phco.28.2.141. [DOI] [PubMed] [Google Scholar]
- 23.Mannucci PM, Spreafico M, Peyvandi F. Dosing anticoagulant therapy with coumarin drugs: is genotyping clinically useful? No. J Thromb Haemost. 2008 doi: 10.1111/j.1538-7836.2008.03073.x. [DOI] [PubMed] [Google Scholar]
- 24.Garcia DA. Warfarin and pharmacogenomic testing: the case for restraint. Clinical pharmacology and therapeutics. 2008;84:303–5. doi: 10.1038/clpt.2008.131. clpt2008131 [pii] 10.1038/clpt.2008.131. [DOI] [PubMed] [Google Scholar]
- 25.Ansell J, Hirsch J, Hylek E, Jacobsn A, Crowther M, Palaretti G. Pharmacology and management of the Vitamin K Antagonists. CHEST. (8th Edition) 2008 doi: 10.1378/chest.08-0670. [DOI] [PubMed] [Google Scholar]
- 26.Janes S, Challis R, Fisher F. Safe introduction of warfarin for thrombotic prophylaxis in atrial fibrillation requiring only a weekly INR. Clin Lab Haematol. 2004;26:43–7. doi: 10.1111/j.0141-9854.2003.00579.x. [DOI] [PubMed] [Google Scholar]
- 27.Oates A, Jackson PR, Austin CA, Channer KS. A new regimen for starting warfarin therapy in out-patients. British journal of clinical pharmacology. 1998;46:157–61. doi: 10.1046/j.1365-2125.1998.00755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson JL, Horne BD, Stevens SM, Grove AS, Barton S, Nicholas ZP, Kahn SF, May HT, Samuelson KM, Muhlestein JB, Carlquist JF. Randomized trial of genotype-guided versus standard warfarin dosing in patients initiating oral anticoagulation. Circulation. 2007;116:2563–70. doi: 10.1161/CIRCULATIONAHA.107.737312. [DOI] [PubMed] [Google Scholar]
- 29.Huang SW, Chen HS, Wang XQ, Huang L, Xu DL, Hu XJ, Huang ZH, He Y, Chen KM, Xiang DK, Zou XM, Li Q, Ma LQ, Wang HF, Chen BL, Li L, Jia YK, Xu XM. Validation of VKORC1 and CYP2C9 genotypes on interindividual warfarin maintenance dose: a prospective study in Chinese patients. Pharmacogenetics and genomics. 2009 doi: 10.1097/FPC.0b013e328326e0c7. 10.1097/FPC.0b013e328326e0c7. [DOI] [PubMed] [Google Scholar]
- 30.Schwarz UI, Ritchie MD, Bradford Y, Li C, Dudek SM, Frye-Anderson A, Kim RB, Roden DM, Stein CM. Genetic determinants of response to warfarin during initial anticoagulation. N Engl J Med. 2008;358:999–1008. doi: 10.1056/NEJMoa0708078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hylek EM. Complications of oral anticoagulant therapy: bleeding and nonbleeding, rates and risk factors. Semin Vasc Med. 2003;3:271–8. doi: 10.1055/s-2003-44463. [DOI] [PubMed] [Google Scholar]
- 32.Margaglione M, Colaizzo D, D’Andrea G, Brancaccio V, Ciampa A, Grandone E, Di Minno G. Genetic modulation of oral anticoagulation with warfarin. Thrombosis and haemostasis. 2000;84:775–8. [PubMed] [Google Scholar]
- 33.Limdi NA, McGwin G, Goldstein JA, Beasley TM, Arnett DK, Adler BK, Baird MF, Acton RT. Influence of CYP2C9 and VKORC1 1173C/T genotype on the risk of hemorrhagic complications in African-American and European-American patients on warfarin. Clinical pharmacology and therapeutics. 2008;83:312–21. doi: 10.1038/sj.clpt.6100290. [DOI] [PMC free article] [PubMed] [Google Scholar]


