Abstract
Blast-related traumatic brain injury (TBI) from the Afghanistan and Iraq wars represents a significant medical concern for troops and veterans. To better understand the consequences of primary-blast injury in humans, we present a case of a Marine exposed to multiple primary blasts during his 14-year military career. The neuropsychological profile of this formerly high-functioning veteran suggested primarily executive dysfunction. Diffusion-tensor imaging revealed white-matter pathology in long fiber tracks compared with a composite fractional-anisotropy template derived from a veteran reference control group without TBI. This study supports the existence of primary blast-induced neurotrauma in humans and introduces a neuroimaging technique with potential to discriminate multiple-blast TBI.
Keywords: Blast-induced neurotrauma, Closed-head injury, Diffusion-tensor imaging, Executive control, Explosive Ordnance Disposal Service, Fractional anisotropy, Primary blast, Traumatic brain injury, White matter
Exposure to traumatic brain injury (TBI) from explosive ordnance has been a pervasive threat for military personnel deployed to Afghanistan and Iraq in support of Operation Enduring Freedom and Operation Iraqi Freedom. The rates of blast injury have increased over time in Afghanistan, with some reports suggesting that improvised explosive devices (IEDs) accounted for 75% of casualties to coalition forces in 2009 (Vanden Brook, 2009). Increasingly, military personnel may also be exposed to multiple blast injuries during their deployments (Benzinger et al., 2009; Cernak & Noble-Haeusslein, 2010). The severity of injuries is often not considered sufficient to warrant removal from duty, and therefore most personnel return to battle following a minimum of one (Zoroya, 2010) and an average of three light-duty days (Gondusky & Reiter, 2005), increasing the likelihood of exposure to multiple blasts and re-injury following return to duty. Proximity of successive brain traumas may increase risk for more serious injury and poorer clinical outcomes (Bauman et al., 2009; De Beaumont et al., 2009).
The consequences of multiple head trauma have mainly been studied in the context of sports injuries, particularly boxing (Chappell, Brown, Dalrymple-Alford, Ulug, & Watts, 2008; Di Russo & Spinelli, 2010; Zhang, Heier, Zimmerman, Jordan, & Ulug, 2006) and, more recently, football (Guskiewicz et al., 2005; Omalu, Hamilton, Kamboh, DeKosky, & Bailes, 2010). Long-term cognitive deficits are typically observed after repeated concussions, rather than a single event (Collins et al., 1999), possibly reflecting greater extent of axonal death after repeated mild head injuries (Yuen, Browne, Iwata, & Smith, 2009). Furthermore, repeated head trauma may lead to chronic traumatic encephalopathy (Clausen, McCrory, & Anderson, 2005; Drew, Templer, Schuyler, Newell, & Cannon, 1986) and increased risk of developing mild cognitive impairment with aging (Guskiewicz et al., 2005). However, little is known regarding the effects of multiple blasts on human brain tissue and function despite marked interest in the problem from the departments of Defense and Veterans Affairs (VA), as well as others (Benzinger et al., 2009; Bhattacharjee, 2008; Elder & Cristian, 2009; Polusny et al., 2011; Tanielian & Jaycox, 2008). Explosive blasts may have more complex and severe effects than sports concussions and other types of closed-head injuries due to greater acceleration and multi-directional pressure waves, causing shearing and displacement of bodily organs (Bauman et al., 2009; Chen, Smith, & Meaney, 2009; Moss, King, & Blackman, 2009; Taber, Warden, Hurley, & Hayman, 2006) and increasing the likelihood of traumatic axonal injury (Niogi et al., 2008), vascular damage, and a cascade of additional exitotoxic, neurotransmitter, and metabolic abnormalities (Cernak & Noble-Haeusslein, 2010).
Primary-blast injury results from a positive over-pressurization impulse of an expanding mass of heated gas under high pressure, giving rise to a supersonic incident shock-front or blast wave of compressed air and ensuing blast wind (Stuhmiller, Phillips, & Richmond, 1991). While a growing body of evidence is accruing supporting the presence of primary blast-induced neurotrauma in animal models (Cernak & Noble-Haeusslein, 2010), studies of the effect of primary-blast injury on human brain are scarce (Belanger, Kretzmer, Yoash-Gantz, Pickett, & Tupler, 2009; Benzinger et al., 2009; Brenner et al., 2010), and controversy remains as to whether primary blast can cause TBI in humans (Benzinger et al., 2009; Champion, Holcomb, & Young, 2009; Gaylord et al., 2008; Ling, Bandak, Armonda, Grant, & Ecklund, 2009; Moss et al., 2009; Taber et al., 2006; Wightman & Gladish, 2001). Cognitive effects resulting from primary-blast injury may be missed because visible injuries, such as those resulting from shrapnel, are typically targeted for immediate treatment (Gaylord et al., 2008; Martin, Lu, Helmick, French, & Warden, 2008; Stuhmiller et al., 1991) and occult injuries may require dedicated probing to uncover (Benzinger et al., 2009; Lew, Poole, Alvarez, & Moore, 2005). Most studies of blast injuries in humans have been unable to differentiate primary blast from secondary and tertiary injuries due to frequent co-occurrence and questionable measurement validity (Champion et al., 2009; Polusny et al., 2011). Nevertheless, clinical observation and computer modeling have led to growing consensus that primary-blast forces are capable of producing closed TBI (Bauman et al., 2009; Benzinger et al., 2009; Elder & Cristian, 2009; Finkel, 2006; Martin et al., 2008; Yilmaz & Pekdemir, 2007). Importantly, improvements in neuroimaging techniques, such as diffusion-tensor imaging (DTI), may assist in revealing subtle brain changes after blast. DTI is more sensitive to changes in the microstructure of white matter than standard structural MRI, potentially making it useful in detecting mild TBI (Bigler & Bazarian, 2010; Hartikainen et al., 2010; Kinnunen et al., 2010; Kraus et al., 2007; Lipton et al., 2009). One promising DTI measure is fractional anisotropy (FA), which assesses the directionality of water movement in neural fibers. In highly coherent and myelinated fibers, the flow of water molecules is restricted to a single direction, whereas damage to neuronal axons and myelin results in greater isotropy of water. Reduction in FA is thought to reflect axonal damage and therefore provides an index of white-matter integrity and potentially traumatic axonal injury seen after mild TBI (Kraus et al., 2007).
Recently, Warden et al. (2009) reported a case study of an Operation Iraqi Freedom soldier exposed to primary-blast injury. DTI showed altered FA in the left hemisphere relative to the right hemisphere, demonstrating intraparenchymal changes after blast. In the present case study, we complement these findings by demonstrating DTI abnormalities in a retired career-military veteran who sustained multiple blast exposures during his work in a training unit of the Explosive Ordnance Disposal Service of the U.S. Marine Corps. In contrast to the case reported by Warden et al. (2009), we compare the veteran’s FA values to an age-and sex-matched reference group without history of TBI and demonstrate a promising methodology for clinical inference from DTI, similar to z-score comparisons applied in clinical-laboratory medicine and neuropsychology, that holds potential as a diagnostic clinical test for Operation Enduring Freedom/Operation Iraqi Freedom veterans and others exposed to multiple blasts.
CASE HISTORY
J.G. is a 55-year-old, married, right-handed, Caucasian male with completion of high school (i.e., 12 years of formal education) who was referred for clinical-neuropsychological evaluation in 2008 for assessment of cognitive symptoms secondary to possible TBI. He served in the U.S. Marine Corps Explosive Ordnance Disposal Service for 14 years. The mission of the Explosive Ordnance Disposal Service is to search for, identify, evaluate, and neutralize the hazards associated with the safe disposal of munitions. Between 1979 and 1994, J.G. was deployed to various military installations around the U.S. and overseas to identify and dispose of munitions. In 1987, he was assigned to a dynamic-entry program in which he demonstrated techniques for forcibly opening inaccessible doors using explosives. The nature of this work required close proximity to high explosives upon discharge. His protective gear included earplugs and a vest with metal plates that was designed to deflect shrapnel but which may actually serve as a contact and reflecting surface, thus intensifying the blast-wave impact (Cernak & Noble-Haeusslein, 2010; Stuhmiller et al., 1991). J.G. reported that between 1987 and 1989, he conducted monthly dynamic-entry trainings in which he was exposed to approximately 5 close-range blasts per day over a 7–10-day period. He reported that he often took risks by standing closer to the blast source than was safe due to ‘bravado’. He experienced frequent headaches and ear-ringing after these trainings and reported that he had his ‘bell rung’ after several of these explosions. During one dynamic-entry training demonstration in 1987, J.G. was injured when a defective explosive discharged unexpectedly, rupturing his tympanic membranes bilaterally. He denied losing consciousness or having memory loss related to the incident but reported having difficulty with balance since that time.
In 1990, J.G. served overseas in Kuwait for 6 months during the Persian Gulf War. His duties included dismantling landmines and undetonated bombs, in addition to training troops to diffuse recovered bombs. During this time, he reported another significant blast when a landmine exploded near him. He felt disoriented after the blast. His physical injuries included shrapnel fragments to his left hand. In 1992, J.G. was involved in an explosive accident that resulted in traumatic amputation of his left arm above the elbow. He denied losing consciousness during that time and was able to describe an instantaneous awareness of the loss of his hand, implications for harm to a nearby colleague, and other events that followed the explosion. After recovering from the injury, he continued to work with explosive ordnance and tested ground contamination at water-well sites in the USA. However, he reported that in 1994, at the age of 41, he began to notice that he was unable to physically and cognitively perform the requirements of his duties due to general confusion, difficulty making decisions, and insecurity regarding his performance and risk to his fellow Marines. He was also experiencing frequent headaches and temple pain and constant ringing and pain in his ears. He decided to retire from the military and received 100% disability compensation.
Over the span of his career, J.G. reported being in the proximity of over 50 significant explosions after detonating and handling munitions, after which he would frequently experience headaches, many of which were severe. He reported that the headaches felt like ‘the worst hangover you ever had’ and would often last for several hours. However, he never lost consciousness as a result of the blasts, nor did he receive blunt or penetrating head trauma from secondary fragments, injuries from translation to the ground or into objects, or quaternary injuries, suggesting that he had sustained multiple primary-blast injuries, many of them from indoor dynamic-entry assignments expected to amplify the incident wave.
During the present evaluation, J.G. complained in interview of memory and attentional dysfunction and possible dysexecutive and apraxic problems. He described reading the same book off and on for 2.5 years because he could not remember what he had previously read. He reported difficulty remembering how to engage in well-learned tasks such as using his home computer. He noted gradual development of articulation problems and stuttering.
J.G.’s current medical history included hypertension and hyperlipidemia, which were well controlled with medication. He denied closed and open TBI from impact trauma, prior strokes or seizures, and a family history of dementia or other neurological conditions. His psychiatric history included depression and posttraumatic stress disorder (PTSD) for which he was being treated. He did not meet criteria for drug abuse or dependence. J.G. was receiving disability from the Veterans Benefits Administration and service connection through VA. He was not seeking additional benefits, nor was he in litigation. J.G. provided a signed Health Information Portability and Accountability Act (HIPAA) authorization to release the results of his clinical-neuropsychological evaluation and clinical history for research purposes. He provided informed consent to receive structural MRI and DTI per Institutional Review Board-approved procedures of the Durham VA Medical Center and Duke University Medical Center.
METHODS
Neuropsychological assessment
J.G. completed a battery of tests conducted by a neuropsychologist (JPH) over two sessions consisting of standardized measures of intellectual functioning, language, memory, attention, psychomotor speed, constructional praxis, and executive control (see Tables 1 and 2).
TABLE 1.
J.G.’s performance on memory tests
| Test measure | Raw score | Percentile |
|---|---|---|
| Verbal Memory | ||
| CVLT-II | ||
| Trials 1–5 Free Recall | 48 | 66 |
| Learning Slope Trials 1–5 | 1.7 | 84 |
| List B (interference list) | 7 | 84 |
| Short-Delay Free Recall | 10 | 69 |
| Short-Delay Cued Recall | 12 | 69 |
| Long-Delay Free Recall | 9 | 50 |
| Long-Delay Cued Recall | 9 | 31 |
| Recognition Hits | 12 | 16 |
| Recognition False Positives | 8 | 7 |
| Recognition Discriminability | 1.3 | 7 |
| WMS-III Logical Memory | ||
| Immediate Recall | 37 | 50 |
| Delayed Recall | 25 | 75 |
| Visuospatial Memory | ||
| WMS-III Spatial Span | 13 | 25 |
| BVMT-R | ||
| Total Immediate Recall | 8 | <1 |
| Learning | 7 | 96 |
| Delayed Recall | 6 | 8 |
| Percent Retained | 86% | >16 |
| Recognition Hits | 5 | 11–16 |
| Recognition False Alarms | 0 | >16 |
| Recognition Discrimination Index | 5 | 11–16 |
| ROCFT | ||
| Short Delay | 21 | 73 |
| Long Delay | 18.5 | 54 |
| Recognition | 19 | 21 |
| Copy | 35 | >16 |
CVLT-II, California Verbal Learning Test-II; WMS-III, Wechsler Memory Scale-Third Edition; BVMT-R, Brief Visuospatial Memory Test-Revised; ROCFT, Rey-Osterrieth Complex Figure Test.
TABLE 2.
J.G.’s performance on tests of executive function
| Test measure | Percentile |
|---|---|
| Processing Speed | |
| Trail Making Test Part A | 16 |
| Stroop Word Reading | 10 |
| Stroop Color Naming | 7 |
| Inhibition | |
| Stroop Color–Word Interference | 14 |
| Switching, Inhibition, Abstraction | |
| Trail Making Test Part B | 2 |
| Wisconsin Card Sorting Test | |
| Categories Achieved | 1 (raw score) |
| Perseverative Errors | 21 |
| Working Memory | |
| Letter–Number Sequencing | 5 |
| Delis–Kaplan Executive Function System | |
| Trail Making Test | |
| Condition 4: Number–Letter Switching | <1 |
| Verbal Fluency | |
| Category Switching | 9 |
| Switching Accuracy | 25 |
| Design Fluency | |
| Composite Scaled Score | 25 |
| Combined Composite Score | 25 |
| Switching vs. combined | 5 |
| Color–Word Interference | |
| Color Naming | 1 |
| Word Reading | 9 |
| Inhibition | 5 |
| Inhibition/Switching | 16 |
| Sorting Test | |
| Free Sorting Description | 1 |
| Sort Recognition | 37 |
| Combined Description | 9 |
| Twenty Questions | |
| Total Questions Asked | 16 |
| Total Weighted Achievement Score | 9 |
Brain imaging assessment
Age- and sex-matched reference subjects
J.G.’s imaging data were compared with imaging data of 10 age-matched male veterans of the U.S. military with no history of TBI or neurodegenerative disease. The reference group was recruited from the Mid-Atlantic Mental Illness Research, Education, and Clinical Center Post-Deployment Subject Registry as previously described (Dedert et al., 2009). All subjects provided written informed consent for procedures approved by the institutional review boards at the Durham VA Medical Center and Duke University Medical Center and in accordance with the 1964 Declaration of Helsinki. The reference group had a mean age of 51.6 (SD = 6.6) years and mean education of 14.6 (1.6) years; 9 subjects were right handed and 5 subjects were Caucasian. Enhancing the match with J.G., the reference group included individuals with cerebrovascular risk factors and psychiatric diagnosis. Seven of the 10 participants had cerebrovascular risk factors including hypertension, hyperlipidemia, diabetes, and hypercholesterolemia, and one participant was an active smoker. Six participants had a current psychiatric diagnosis, including four with PTSD and two with major depressive disorder, but no other psychiatric conditions were present.
MRI procedure and analysis
Conventional structural imaging and DTI data were acquired on a General Electric 3 Tesla EXCITE system with an 8-channel headcoil. High-resolution 3D structural images (FSPGR) were collected with the following parameters: 166 slices, 256 × 256 matrix, FOV = 24 cm, slice thickness = 1 mm, TR = 7.48 ms, TE = 2.98 ms, flip angle = 12°, and 1 excitation. DTI was acquired with a single-shot echo-planar imaging (EPI) technique using the following parameters: 62 slices, matrix = 128 × 128, FOV = 240 mm, slice thickness = 2 mm, 55 diffusion-gradient directions, TR = 17000 ms, TE = 77.5 ms, flip angle = 90°, and 1 excitation.
Structural and diffusion images passed visual inspection for the purpose of quality assurance. Preprocessing of DTI data was carried out using the FMRIB Diffusion Tool Box (FDT; FMRIB Centre, Oxford University, UK) to remove eddy-current distortions caused by the stretching and shearing of diffusion-weighted images by gradient coils and to correct for simple head motion. Preprocessing concluded with fitting diffusion-tensor models at each voxel to characterize the principal diffusion directions (eigenvectors), amount of diffusion in the principal directions (eigenvalues), average amount of diffusivity (mean diffusivity), and magnitude of directionality of diffusion, or FA, which served as the primary DTI measure.
J.G.’s FA values were compared with mean FA data from the reference group of age- and sex-matched controls based on a model of population-based inferential statistics. The subjects from the reference group were mutually coregistered to create a custom template resampled to a 1 × 1 × 1-mm standard image space. The coregistration ensured white-matter alignment across subjects using an intermediate degrees-of-freedom nonlinear registration with tract-based spatial statistics (TBSS; FMRIB). These normalized resampled images from the reference group were averaged to generate images of the mean and SD of FA (for each skeleton voxel). After thresholding the skeleton to exclude low FA values, indicative of non-white matter, each subject’s aligned FA image from the reference group was projected onto the mean FA skeleton. To compare J.G. to the reference group, his image was registered to the same custom template in standard image space and projected onto the reference group’s mean FA white-matter skeleton. A statistical map was computed using J.G.’s FA values that reflected the number of SDs below the mean of the reference group (z-score map).
RESULTS
Neuropsychological results
J.G. performed in the average range on measures of intelligence and language facility. His Verbal IQ assessed with the Wechsler Adult Intelligence Scale-Third Edition (WAIS-III) was 106 (66th percentile) and his Performance IQ was 105 (63rd percentile), yielding a Full Scale IQ of 106 (66th percentile). His standard score on the Wechsler Test of Adult Reading was 98 and on the Boston Naming Test he performed at the 51st percentile, both of which were also average. However, J.G.’s remaining protocol was marked by impairments in executive control, visuospatial processing, psychomotor speed and integration, and recognition memory performance (see Tables 1 and 2).
J.G.’s performance on executive function tasks represented the most salient impairment in his protocol. Particular problems were observed in the domains of sequencing, switching, and working memory. In addition, he showed signs of concrete thinking and perseveration. He was impaired on trail-making tasks that required him to switch between numbers and letters, both on Trail Making Test Part B and on Number–Letter Switching from the Delis–Kaplan Executive Function System (DKEFS). He performed in the borderline range on categories achieved on the Wisconsin Card Sorting Test, with perseverative errors in the low-average range. On Sorting from the DKEFS, J.G. was impaired in producing and describing card sortings.
No indication of insufficient effort or malingering was observed during the examination, suggesting that the test results provided an accurate presentation of J.G.’s neurocognitive functioning. Although direct measures of symptom validity were not administered, effort indicators embedded within the California Verbal Learning Test (CVLT; Sweet et al., 2000) observed on the revised CVLT-II version were consistent with sufficient effort. For instance, he performed within normal limits on the total number of words retrieved during acquisition and on long-delay free recall. J.G. was not in litigation and was already receiving full disability and health benefits from VA, and thus secondary-gain motivation appeared to be minimal if at all present.
Neuroimaging results
Structural imaging findings
J.G.’s structural scan (3D FSPGR) was inspected by a board-certified neuroradiologist to evaluate for the presence of gross neuroradiological findings (see Figure 1). A small heterogeneous mass that likely represented a benign acoustic schwannoma was visible in the right ponto-cerebellar cistern, extending into the right internal auditory canal. Acoustic schwannomas develop from the 8th cranial nerve and typically affect balance and hearing (Pollock et al., 1995) but not cognitive function.
Figure 1.
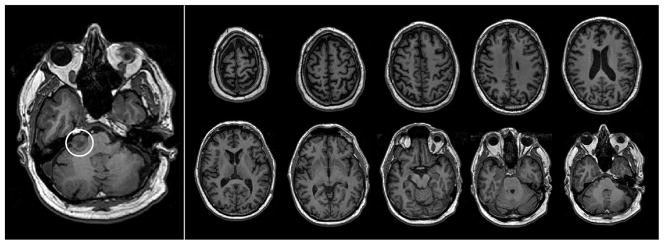
Structural MRI acquired for J.G. in June 2008 shows an acoustic schwannoma (circled in left panel) in the right ponto-cerebeller cistern, as well as age-appropriate generalized volume loss. No other abnormalities were detected on neuroradiological evaluation.
DTI findings
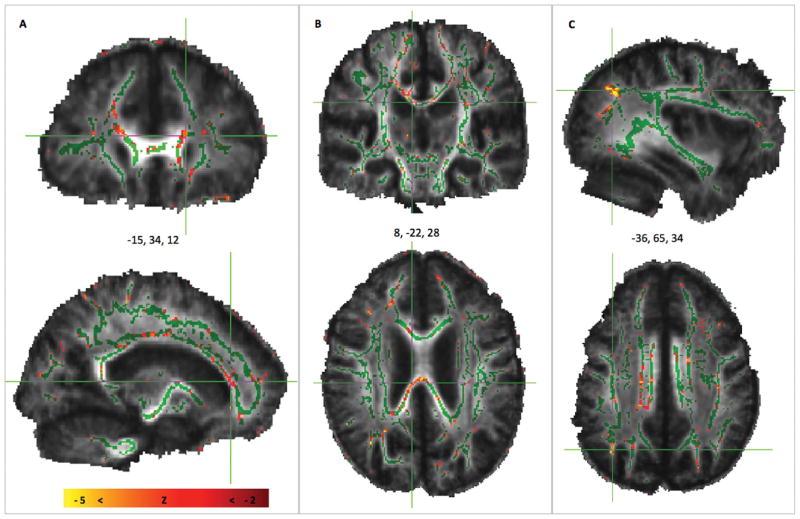
Analysis of DTI data from J.G. compared with the reference group of 10 age-matched males showed lower FA values in the patient, suggestive of white-matter injury. The z-score map shown in Figure 2 reveals significantly diminished FA (z < −2.0) in 7.52% of skeleton voxels comprising primarily major fiber bundles including the genu, body, and splenium of the corpus callosum and projections that extend bilaterally into the frontal and parietal cortices. Two separate analyses using the identical methods were performed for verification purposes. In each of these analyses, a subject randomly chosen from the reference group was assigned to constitute the test subject (just as J.G. was the test subject in the primary analysis). Both of the verification test cases yielded negative results: 0.41 and 1.72% of voxels, respectively, with z < −2.0.
Figure 2.
DTI scan from J.G. as compared with the reference group of 10 age- and sex-matched subjects with no history of TBI. The z-score map from whole-brain analysis shows voxels with FA deviations that are significantly below the mean FA of the reference group (in red and yellow voxels) that are overlaid on the mean FA skeleton (green) of the reference group. Diminished FA in J.G. is suggestive of extensive white-matter injury in major fiber bundles and extending into tracts projecting into (A) frontal cortex, (B) corpus callosum, and (C) parietal cortex.
DISCUSSION
This case report supports neurotrauma in the human brain following repetitive primary explosive blasts in a 55-year-old veteran, with evidence primarily for executive, perceptual-organization, psychomotor, visual-learning, and recognition-memory weaknesses on neuropsychological testing and white-matter structural abnormalities in corpus callosum and white-matter projections to frontal and parietal lobes visualized on DTI. His neuropsychological presentation is generally consistent with the neuroanatomic distribution of decreased FA.
Neuropsychological assessment revealed that although J.G.’s IQ is currently in the average range and may have been in the high-average range or better premorbidly from occupational history, he performed below expectation on tasks requiring processing speed, recognition memory, and, in particular, executive function, especially working memory. The deficit in executive function was evident on formal tests of sequencing, set switching, category fluency, and problem solving, in addition to behavioral observations of perseveration, concrete thinking, and word-finding difficulty during spontaneous speech and his reports of functional impairment in everyday life. The observed deficits in executive function are consistent with neuropsychological findings after repeated sports-related concussions including boxing (McLatchie et al., 1987) and football injuries (Collins et al., 1999), suggesting that repeated primary-blast injury may have similar consequences as other types of closed-head injuries.
DTI analysis revealed that patient J.G. had reduced FA in the corpus callosum and bilateral white-matter projections to frontal and parietal cortices relative to an age- and sex-matched reference sample without TBI, suggesting loss of structural integrity in these areas. This study adds to the growing literature supporting the sensitivity of DTI to detection of changes in white-matter integrity that may be associated with cognitive decline. Optimal cognitive performance is thought to rely on the structural integrity and coordinated function of distributed neural networks (Power, Fair, Schlaggar, & Petersen, 2010), whereas disruption of these pathways may impede coactivation of key regions that give rise to integrated cognition. In particular, functional and structural alterations in frontoparietal regions have been implicated in impaired executive functions, memory, and processing speed (Andrews-Hanna et al., 2007) and may explain J.G.’s neurocognitive profile and presentation. Furthermore, a reduction in anisotropy in the corpus callosum is one of the most commonly reported findings in patients with traumatic axonal injury (Hurley, McGowan, Arfanakis, & Taber, 2004) and is likely associated with alterations in interhemispheric functional connectivity (Marquez de la Plata et al., 2011). In general, these findings support the notion that cognitive dysfunction after multiple-blast injury is associated with structural changes distributed diffusely across key neural networks.
Only a handful of studies have examined blast injury using DTI, and other studies that have examined mild TBI in returning Afghanistan- and Iraq-warzone veterans have been unable to disentangle the effects of primary- versus secondary-and tertiary-blast effects (Polusny et al., 2011). Furthermore, these studies have not systematically examined the number of head injuries post-deployed personnel have reported in relation to cognitive deficit (Hoge et al., 2008). In contrast, the present study describes sustained neurotrauma after many cumulative primary blasts over a military career and compares results to a reference group of age- and sex-matched, well characterized controls without TBI history. Whereas most other studies report DTI findings across an averaged sample of individuals with TBI, limiting applicability to an individual case, our methodology is similar to that integral to normative neuropsychological and medical inference and may have utility in future clinical application to diagnostic discrimination of occult TBI (Lew et al., 2005).
Ongoing debate centers on whether primary-blast injury can cause direct damage to the brain. Some researchers suggest that stress waves from a primary blast do not affect structures with homogenous water density such as the brain (Wightman & Gladish, 2001). However, this notion has been actively challenged by the accumulating studies showing neural alterations and cognitive deficit after primary-blast injury. While the mechanism by which blast injury affects neural pathways may be different from the inertial forces typical of other closed TBIs, both types of events may result in progressive decline in axonal function and eventually lead to axonal disconnection (Büki & Povlishock, 2006; Povlishock, 1993). Supporting this idea, the animal literature is accumulating evidence for traumatic axonal injury after exposure to blast. Rats exposed to blast waves showed neural changes associated with altered axonal transport (Saljo, Bao, Haglid, & Hansson, 2000) and oxidative stress (Cernak, Wang, Jiang, Bian, & Savic, 2001). Lower FA in humans is thought to reflect axonal degradation and larger perivascular spaces, and therefore DTI is an advantageous tool in understanding neural pathology after blast.
The present study provides evidence for brain alterations after multiple primary-blast injuries. However, alternative possibilities that may produce a similar neurocognitive and neuroimaging profile are important to consider. It is unlikely that J.G.’s pattern of performance is reflective of premorbid executive dysfunction. Given the demands of the Explosive Ordnance Disposal Service, and that one mistake could have fatal consequences, the patient was likely highly organized, comfortable making rapid decisions, and had flexible thinking in order to identify different types of munitions and how to diffuse them (Madrid, William, & Holland, 1992). Due to the demanding nature of the job, Explosive Ordnance Disposal Service training-attrition rates are high, and those who succeed in graduating have completed rigorous cognitive and physical challenges (Hogan & Hogan, 1985). J.G.’s performance on hold tests in the current battery furthermore supported at least above-average premorbid intelligence (WAIS-III Information and Vocabulary scaled scores = 13). This likely premorbid profile is in striking contrast to J.G.’s current presentation of slowed processing speed, poor decision making, and reports of mild executive dysfunction and/or dyspraxia and loss of procedural knowledge. It is also unlikely that J.G.’s depressive and PTSD symptoms can explain his profile. J.G.’s deficits on neuropsychological measures do not cohere with the patterns typically seen in depressed or anxious patients. For example, comorbid depressed and anxious patients typically show greater impairment on free recall than recognition, a pattern that was reversed in the present case (Kizilbash, Vanderploeg, & Curtiss, 2002). Additionally, depressed and anxious patients do not display high numbers of false positives on recognition tasks (Kizilbash et al., 2002), whereas patients with TBI tend to show more intrusions and false positives (Crosson, Novack, Trenerry, & Craig, 1988; Wiegner & Donders, 1999) as observed in the present case. A meta-analysis of memory deficits in PTSD (Brewin, Kleiner, Vasterling, & Field, 2007) reported average effect sizes indicative of impairment primarily in the verbal domain. However, J.G.’s performance was worse in the visuospatial memory domain. Additionally, evidence exists for reduced FA in mild-TBI patients even after adjusting for stress, anxiety, and depression (Lipton et al., 2009).
Finally, a discussion of neurodegenerative and vascular disease is warranted to consider the possibility that J.G.’s deficits are due to onset of dementia not related to blast injuries, or that repetitive blast may have conferred vulnerability towards its development. Although somewhat controversial because of mixed results, some emerging evidence suggests that head injury is a risk factor for Alzheimer’s disease in men (Fleminger, Oliver, Lovestone, Rabe-Hesketh, & Giora, 2003) as well as for frontotemporal dementia (Rosso et al., 2003). However, several aspects of J.G.’s neurocognitive profile are inconsistent with early Alzheimer’s disease, the most prominent aspect of which is his intact delayed free recall on the CVLT-II, Wechsler Memory Scale-Third Edition Logical Memory, and Rey Osterrieth Complex Figure Test (see Table 1). A rule out of frontotemporal dementia is more difficult because of his demonstrated poor performance on tasks of executive function, but nevertheless J.G.’s behavioral presentation is inconsistent with this diagnosis, which is characterized by early changes in social-interpersonal conduct (e.g., decline in manners, violation of interpersonal space, disinhibited sexual and physical behavior), dysregulation of personal conduct, emotional blunting, and loss of insight. However, it will be important to follow this veteran over time to examine whether this is a case of chronic traumatic encephalopathy, a neurodegenerative condition observed in individuals that have suffered repetitive head trauma (Clausen et al., 2005; Drew et al., 1986; Guskiewicz et al., 2005). Some of the earliest signs of chronic traumatic encephalopathy include alterations in attention, memory dysfunction, and bouts of confusion (McKee et al., 2009).
It is important to acknowledge that cerebrovascular risk factors are often associated with frontal-subcortical dysfunction and that cerebrovascular complications have frequently been noted in association with blast injuries from the Operation Enduring Freedom/Operation Iraqi Freedom warzones (Armonda et al., 2006; Bauman et al., 2009; Benzinger et al., 2009; Bhattacharjee, 2008; Cernak & Noble-Haeusslein, 2010; DeWitt & Prough, 2009), suggesting a strong relationship between blast per se and vascular-system impingement. J.G. was diagnosed with hypertension and hyperlipidemia, both risk factors for cerebrovascular disease. However, he had no history of frank stroke or cardiac problems, and his hypertension and hyperlipidemia were well controlled with medications. Importantly, J.G. showed a reduction in FA relative to a reference group that had a predominantly similar cerebrovascular risk-factor history. Further studies are required to discern neural injury attributable directly to blast-pressure forces on skull and brain tissue versus effects secondary to alterations in vasculature.
CONCLUSION
This case report provides evidence that exposure to repetitive primary blasts produces similar cognitive and neural decline as exposure to multiple-concussive head injury, including executive dysfunction and white-matter pathology. Given the current geopolitical climate, military personnel and civilians will continue to be exposed to recurrent conventional and improvised high explosives. Therefore, it is important to better understand the neurocognitive and neuroanatomic consequences of multiple-blast injury and to improve techniques for diagnosing mild TBI after exposure to blast.
Acknowledgments
We would like to thank J.G. for sharing his story with us, Elizabeth Selgrade and Gregory McCarthy for helpful discussions regarding the project, and Christopher Lascola for providing a neuroradiology consult. We would also like to acknowledge the contributions of the Mid-Atlantic Mental Illness Research, Education, and Clinical Center workgroup (members include Jean C. Beckham, Patrick S. Calhoun, Rita M. Davison, A. Meade Eggleston, John A. Fairbank, Kimberly T. Green, Angela C. Kirby, Harold Kudler, Jeffrey M. Hoerle, Christine E. Marx, Scott D. Moore, Victoria Payne, Mary C. Pender, Jennifer L. Strauss, Kristy K. Straits-Tröster, and Richard D. Weiner). This work was supported by grants NIMH K23 MH084013 and K23 MH073091, VHA 101RX000389, and the VISN6 MIRECC. The views expressed herein are those of the authors and do not necessarily reflect the views of the Department of Veterans Affairs.
References
- Andrews-Hanna J, Snyder A, Vincent J, Lustig C, Head D, Raichle M, et al. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armonda RA, Bell RS, Vo AH, Ling G, DeGraba TJ, Crandall B, et al. Wartime traumatic cerebral vasospasm: Recent review of combat casualties. Neurosurgery. 2006;59:1215–1225. doi: 10.1227/01.NEU.0000249190.46033.94. [DOI] [PubMed] [Google Scholar]
- Bauman RA, Ling G, Tong L, Januszkiewicz A, Agoston D, Delanerolle N, et al. An introductory characterization of a combat-casualty-care relevant swine model of closed head injury resulting from exposure to explosive blast. Journal of Neurotrauma. 2009;26:841–860. doi: 10.1089/neu.2008.0898. [DOI] [PubMed] [Google Scholar]
- Belanger HG, Kretzmer T, Yoash-Gantz R, Pickett T, Tupler LA. Cognitive sequelae of blast-related versus other mechanisms of brain trauma. Journal of the International Neuropsychological Society. 2009;15:1–8. doi: 10.1017/S1355617708090036. [DOI] [PubMed] [Google Scholar]
- Benzinger TLS, Brody D, Cardin S, Curley KC, Mintun MA, Mun SK, et al. Blast-related brain injury: Imaging for clinical and research applications: Report of the 2008 St. Louis workshop. Journal of Neurotrauma. 2009;26:2127–2144. doi: 10.1089/neu.2009.0885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee Y. Neuroscience – Shell shock revisited: Solving the puzzle of blast trauma. Science. 2008;319:406–408. doi: 10.1126/science.319.5862.406. [DOI] [PubMed] [Google Scholar]
- Bigler E, Bazarian J. Diffusion tensor imaging: A biomarker for mild traumatic brain injury? Neurology. 2010;74:626. doi: 10.1212/WNL.0b013e3181d3e43a. [DOI] [PubMed] [Google Scholar]
- Brenner LA, Terrio H, Homaifar BY, Gutierrez PM, Staves PJ, Harwood JE, et al. Neuropsychological test performance in soldiers with blast-related mild TBI. Neuropsychology. 2010;24:160–167. doi: 10.1037/a0017966. [DOI] [PubMed] [Google Scholar]
- Brewin CR, Kleiner JS, Vasterling JJ, Field AP. Memory for emotionally neutral information in posttraumatic stress disorder: A meta-analytic investigation. Journal of Abnormal Psychology. 2007;116:448–463. doi: 10.1037/0021-843X.116.3.448. [DOI] [PubMed] [Google Scholar]
- Büki A, Povlishock J. All roads lead to disconnection? – Traumatic axonal injury revisited. Acta Neurochirurgica. 2006;148:181–194. doi: 10.1007/s00701-005-0674-4. [DOI] [PubMed] [Google Scholar]
- Cernak I, Noble-Haeusslein LJ. Traumatic brain injury: An overview of pathobiology with emphasis on military populations. Journal of Cerebral Blood Flow & Metabolism. 2010;30:255–266. doi: 10.1038/jcbfm.2009.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernak I, Wang Z, Jiang J, Bian X, Savic J. Cognitive deficits following blast injury-induced neurotrauma: Possible involvement of nitric oxide. Brain Injury. 2001;15:593–612. doi: 10.1080/02699050010009559. [DOI] [PubMed] [Google Scholar]
- Champion HR, Holcomb JB, Young LA. Injuries from explosions: Physics, biophysics, pathology, and required research focus. Journal of Trauma-Injury Infection & Critical Care. 2009;66:1468–1477. doi: 10.1097/TA.0b013e3181a27e7f. [DOI] [PubMed] [Google Scholar]
- Chappell MH, Brown JA, Dalrymple-Alford JC, Ulug AM, Watts R. Multivariate analysis of diffusion tensor imaging data improves the detection of microstructural damage in young professional boxers. Magnetic Resonance Imaging. 2008;26:1398–1405. doi: 10.1016/j.mri.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Chen YC, Smith DH, Meaney DF. In-vitro approaches for studying blast-induced traumatic brain injury. Journal of Neurotrauma. 2009;26:861–876. doi: 10.1089/neu.2008.0645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen H, McCrory P, Anderson V. The risk of chronic traumatic brain injury in professional boxing: Change in exposure variables over the past century. British Journal of Sports Medicine. 2005;39:661–664. doi: 10.1136/bjsm.2004.017046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins M, Grindel S, Lovell M, Dede D, Moser D, Phalin B, et al. Relationship between concussion and neuropsychological performance in college football players. Journal of the American Medical Association. 1999;282:964–970. doi: 10.1001/jama.282.10.964. [DOI] [PubMed] [Google Scholar]
- Crosson B, Novack T, Trenerry M, Craig P. California Verbal Learning Test (CVLT) performance in severely head-injured and neurologically normal adult males. Journal of Clinical and Experimental Neuropsychology. 1988;10:754–768. doi: 10.1080/01688638808402812. [DOI] [PubMed] [Google Scholar]
- De Beaumont L, Theoret H, Mongeon D, Messier J, Leclerc S, Tremblay S, et al. Brain function decline in healthy retired athletes who sustained their last sports concussion in early adulthood. Brain. 2009;132:695–708. doi: 10.1093/brain/awn347. [DOI] [PubMed] [Google Scholar]
- Dedert E, Green K, Calhoun P, Yoash-Gantz R, Taber K, Mumford M, et al. Association of trauma exposure with psychiatric morbidity in military veterans who have served since September 11, 2001. Journal of Psychiatric Research. 2009;43:830–836. doi: 10.1016/j.jpsychires.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWitt DS, Prough DS. Blast-induced brain injury and posttraumatic hypotension and hypoxemia. Journal of Neurotrauma. 2009;26:877–887. doi: 10.1089/neu.2007.0439. [DOI] [PubMed] [Google Scholar]
- Di Russo F, Spinelli D. Sport is not always healthy: Executive brain dysfunction in professional boxers. Psychophysiology. 2010;47:425–434. doi: 10.1111/j.1469-8986.2009.00950.x. [DOI] [PubMed] [Google Scholar]
- Drew RH, Templer DI, Schuyler BA, Newell TG, Cannon WG. Neuropsychological deficits in active licensed professional boxers. Journal of Clinical Psychology. 1986;42:520–525. doi: 10.1002/1097-4679(198605)42:3<520::aid-jclp2270420319>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Elder GA, Cristian A. Blast-related mild traumatic brain injury: Mechanisms of injury and impact on clinical care. Mount Sinai Journal of Medicine. 2009;76:111–118. doi: 10.1002/msj.20098. [DOI] [PubMed] [Google Scholar]
- Finkel MF. The neurological consequences of explosives. Journal of the Neurological Sciences. 2006;249:63–67. doi: 10.1016/j.jns.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Fleminger S, Oliver DL, Lovestone S, Rabe-Hesketh S, Giora A. Head injury as a risk factor for Alzheimer’s disease: The evidence 10 years on; a partial replication. Journal of Neurology, Neurosurgery and Psychiatry. 2003;74:857–862. doi: 10.1136/jnnp.74.7.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaylord KM, Cooper DB, Mercado JM, Kennedy JE, Yoder LH, Holcomb JB. Incidence of posttraumatic stress disorder and mild traumatic brain injury in burned service members: Preliminary report. Journal of Trauma-Injury Infection & Critical Care. 2008;64:S200–205. doi: 10.1097/TA.0b013e318160ba42. [DOI] [PubMed] [Google Scholar]
- Gondusky JS, Reiter MP. Protecting military convoys in Iraq: An examination of battle injuries sustained by a mechanized battalion during Operation Iraqi Freedom II. Military Medicine. 2005;170:546–549. doi: 10.7205/milmed.170.6.546. [DOI] [PubMed] [Google Scholar]
- Guskiewicz KM, Marshall SW, Bailes J, McCrea M, Cantu RC, Randolph C, et al. Association between recurrent concussion and late-life cognitive impairment in retired professional football players. Neurosurgery. 2005;57:719–726. doi: 10.1093/neurosurgery/57.4.719. [DOI] [PubMed] [Google Scholar]
- Hartikainen K, Wäljas M, Isoviita T, Dastidar P, Liimatainen S, Solbakk A, et al. Persistent symptoms in mild to moderate traumatic brain injury associated with executive dysfunction. Journal of Clinical and Experimental Neuropsychology. 2010;32:767–774. doi: 10.1080/13803390903521000. [DOI] [PubMed] [Google Scholar]
- Hogan J, Hogan R. Psychological and physical performance characteristics of successful explosive ordnance diver technicians. Tulsa, OK: Tulsa University; 1985. [Google Scholar]
- Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA. Mild traumatic brain injury in U.S. Soldiers returning from Iraq. New England Journal of Medicine. 2008;358:453–463. doi: 10.1056/NEJMoa072972. [DOI] [PubMed] [Google Scholar]
- Hurley RA, McGowan JC, Arfanakis K, Taber KH. Traumatic axonal injury: Novel insights into evolution and identification. Journal of Neuropsychiatry and Clinical Neurosciences. 2004;16:1–7. doi: 10.1176/jnp.16.1.1. [DOI] [PubMed] [Google Scholar]
- Kinnunen K, Greenwood R, Powell J, Leech R, Hawkins P, Bonnelle V, et al. White matter damage and cognitive impairment after traumatic brain injury. Brain. 2010 doi: 10.1093/brain/awq347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kizilbash A, Vanderploeg R, Curtiss G. The effects of depression and anxiety on memory performance. Archives of Clinical Neuropsychology. 2002;17:57–67. [PubMed] [Google Scholar]
- Kraus MF, Susmaras T, Caughlin BP, Walker CJ, Sweeney JA, Little DM. White matter integrity and cognition in chronic traumatic brain injury: A diffusion tensor imaging study. Brain. 2007;130:2508–2519. doi: 10.1093/brain/awm216. [DOI] [PubMed] [Google Scholar]
- Lew HL, Poole JH, Alvarez S, Moore W. Soldiers with occult traumatic brain injury. American Journal of Physical Medicine & Rehabilitation. 2005;84:393–398. doi: 10.1097/01.phm.0000163703.91647.a7. [DOI] [PubMed] [Google Scholar]
- Ling G, Bandak F, Armonda R, Grant G, Ecklund J. Explosive blast neurotrauma. Journal of Neurotrauma. 2009;26:815–825. doi: 10.1089/neu.2007.0484. [DOI] [PubMed] [Google Scholar]
- Lipton ML, Gulko E, Zimmerman ME, Friedman BW, Kim M, Gellella E, et al. Diffusion-tensor imaging implicates prefrontal axonal injury in executive function impairment following very mild traumatic brain injury. Radiology. 2009;252:816–824. doi: 10.1148/radiol.2523081584. [DOI] [PubMed] [Google Scholar]
- Madrid R, William B, Holland J. Artificial intelligence for explosive ordnance disposal system (AI-EOD). Paper presented at the Innovative applications of artificial intelligence; San Jose, CA (USA); Baltimore, MD (USA). 1992. Mar-Jun. [Google Scholar]
- Marquez de la Plata C, Garces J, Shokri Kojori E, Grinnan J, Krishnan K, Pidikiti R, et al. Deficits in functional connectivity of hippocampal and frontal lobe circuits after traumatic axonal injury. Archives of Neurology. 2011;68:74–84. doi: 10.1001/archneurol.2010.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin EM, Lu WC, Helmick K, French L, Warden DL. Traumatic brain injuries sustained in the Afghanistan and Iraq wars. American Journal of Nursing. 2008;108:40–47. doi: 10.1097/01.NAJ.0000315260.92070.3f. [DOI] [PubMed] [Google Scholar]
- McKee A, Cantu R, Nowinski C, Hedley-Whyte E, Gavett B, Budson A, et al. Chronic traumatic encephalopathy in athletes: Progressive tauopathy following repetitive head injury. Journal of Neuropathology and Experimental Neurology. 2009;68:709–735. doi: 10.1097/NEN.0b013e3181a9d503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLatchie G, Brooks N, Galbraith S, Hutchison J, Wilson L, Melville I, et al. Clinical neurological examination, neuropsychology, electroencephalography and computed tomographic head scanning in active amateur boxers. Journal of Neurology, Neurosurgery and Psychiatry. 1987;50:96–99. doi: 10.1136/jnnp.50.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss WC, King MJ, Blackman EG. Skull flexure from blast waves: A mechanism for brain injury with implications for helmet design. Physical Review Letters. 2009;103:1087021–1087024. doi: 10.1103/PhysRevLett.103.108702. [DOI] [PubMed] [Google Scholar]
- Niogi SN, Mukherjee P, Ghajar J, Johnson C, Kolster RA, Sarkar R, et al. Extent of microstructural white matter injury in postconcussive syndrome correlates with impaired cognitive reaction time: A 3T diffusion tensor imaging study of mild traumatic brain injury. American Journal of Neuroradiology. 2008;29:967–973. doi: 10.3174/ajnr.A0970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omalu BI, Hamilton RL, Kamboh MI, DeKosky ST, Bailes J. Chronic traumatic encephalopathy (CTE) in a National Football League player: Case report and emerging medicolegal practice questions. Journal of Forensic Nursing. 2010;6:40–46. doi: 10.1111/j.1939-3938.2009.01064.x. [DOI] [PubMed] [Google Scholar]
- Pollock B, Lunsford L, Kondziolka D, Flickinger J, Bissonette D, Kelsey S, et al. Outcome analysis of acoustic neuroma management: A comparison of microsurgery and stereotactic radiosurgery. Neurosurgery. 1995;36:215–229. doi: 10.1227/00006123-199501000-00036. [DOI] [PubMed] [Google Scholar]
- Polusny M, Kehle S, Nelson N, Erbes C, Arbisi P, Thuras P. Longitudinal effects of mild traumatic brain injury and posttraumatic stress disorder comorbidity on postdeployment outcomes in national guard soldiers deployed to Iraq. Archives of General Psychiatry. 2011;68:79. doi: 10.1001/archgenpsychiatry.2010.172. [DOI] [PubMed] [Google Scholar]
- Povlishock JT. Pathobiology of traumatically induced axonal injury in animals and man. Annals of Emergency Medicine. 1993;22:980–986. doi: 10.1016/s0196-0644(05)82738-6. [DOI] [PubMed] [Google Scholar]
- Power J, Fair D, Schlaggar B, Petersen S. The development of human functional brain networks. Neuron. 2010;67:735–748. doi: 10.1016/j.neuron.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso SM, Landweer EJ, Houterman M, Donker Kaat L, van Duijn CM, van Swieten JC. Medical and environmental risk factors for sporadic frontotemporal dementia: A retrospective case control study. Journal of Neurology, Neurosurgery and Psychiatry. 2003;74:1574–1576. doi: 10.1136/jnnp.74.11.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saljo A, Bao F, Haglid KG, Hansson HA. Blast exposure causes redistribution of phosphorylated neurofilament subunits in neurons of the adult rat brain. Journal of Neurotrauma. 2000;17:719–726. doi: 10.1089/089771500415454. [DOI] [PubMed] [Google Scholar]
- Stuhmiller JH, Phillips YY, Richmond DR. The physics and mechanisms of primary blast injury. In: Bellamy RF, Zajtchuk R, editors. Textbook of military medicine, conventional warfare: Ballistic, blast and burn injuries. Washington, DC: Office of the Surgeon General, Department of the Army; 1991. [Google Scholar]
- Sweet JJ, Wolfe P, Sattlberger E, Numan B, Rosenfeld JP, Clingerman S, et al. Further investigation of traumatic brain injury versus insufficient effort with the California Verbal Learning Test. Archives of Clinical Neuropsychology. 2000;15:105–113. [PubMed] [Google Scholar]
- Taber KH, Warden DL, Hurley RA, Hayman LA. Blast-related traumatic brain injury: What is known? Journal of Neuropsychiatry & Clinical Neurosciences. 2006;18:141–142. doi: 10.1176/jnp.2006.18.2.141. [DOI] [PubMed] [Google Scholar]
- Tanielian T, Jaycox LH. Invisible wounds of war: Psychological and cognitive injuries, their consequences, and services to assist recovery. Santa Monica, CA: RAND Corporation; 2008. [Google Scholar]
- Vanden Brook T. Casualties caused by IEDs in Afghanistan on the rise. USA Today 2009 [Google Scholar]
- Warden DL, French LM, Shupenko L, Fargus J, Riedy G, Erickson ME, et al. Case report of a soldier with primary blast brain injury. Neuroimage. 2009;47(Suppl 2):T152–153. doi: 10.1016/j.neuroimage.2009.01.060. [DOI] [PubMed] [Google Scholar]
- Wiegner S, Donders J. Performance on the California Verbal Learning Test after traumatic brain injury. Journal of Clinical and Experimental Neuropsychology. 1999;21:159–170. doi: 10.1076/jcen.21.2.159.925. [DOI] [PubMed] [Google Scholar]
- Wightman JM, Gladish SL. Explosions and blast injuries. Annals of Emergency Medicine. 2001;37:664–678. doi: 10.1067/mem.2001.114906. [DOI] [PubMed] [Google Scholar]
- Yilmaz S, Pekdemir M. An unusual primary blast injury: Traumatic brain injury due to primary blast injury. American Journal of Emergency Medicine. 2007;25:97–98. doi: 10.1016/j.ajem.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Yuen TJ, Browne KD, Iwata A, Smith DH. Sodium channelopathy induced by mild axonal trauma worsens outcome after a repeat injury. Journal of Neuroscience Research. 2009;87:3620–3625. doi: 10.1002/jnr.22161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Heier LA, Zimmerman RD, Jordan B, Ulug AM. Diffusion anisotropy changes in the brains of professional boxers. American Journal of Neuroradiology. 2006;27:2000–2004. [PMC free article] [PubMed] [Google Scholar]
- Zoroya G. Pentagon focus on brain trauma. Policy: Troops near blasts to be sidelined. USA Today 2010 Mar 2; [Google Scholar]