Abstract
Objectives
Influenza seasonality remains poorly studied in Equatorial regions. Here we assessed the seasonal characteristics and environmental drivers of influenza epidemics in French Guiana, where influenza surveillance was established in 2006.
Methods
Sentinel GPs monitored weekly incidence of Influenza-like illnesses (ILI) from January 2006 through December 2010 and collected nasopharyngeal specimens from patients for virological confirmation. Times series analysis was used to investigate relationship between ILI and climatic parameters (rainfall and specific humidity).
Results
Based on 1,533 viruses identified during the study period, we observed marked seasonality in the circulation of influenza virus in the pre-pandemic period, followed by year-round activity in the post-pandemic period, with a peak in the rainy season. ILI incidence showed seasonal autoregressive variation based on ARIMA analysis. Multivariate dynamic regression revealed that a 1mm increase of rainfall resulted in an increase of 0.33% in ILI incidence one week later, adjusting for specific humidity (SH). Conversely, an increase of 1g/kg of SH resulted in a decrease of 11% in ILI incidence 3 weeks later, adjusting for rainfall.
Conclusions
Increased rainfall and low levels of specific humidity favor influenza transmission in French Guiana.
Keywords: influenza, seasonality, tropical, climate, rainfall, humidity, times-series, ARIMA, dynamic regression
Introduction
The influenza virus causes significant morbidity and mortality worldwide. The epidemiology of influenza is characterized by epidemics that occur seasonally throughout the world every year, with occasional pandemics arising from the emergence of novel subtypes [1]. In temperate regions of the Northern Hemisphere (NH) and Southern Hemispheres (SH), there are clear seasonal variations in the occurrence of influenza activity, characterized by wintertime epidemics. In contrast in tropical regions, influenza seasonality is less defined, with high background influenza activity. Overall, the seasonality of influenza remains poorly understood globally due to the relative paucity of influenza epidemiological data from Tropical regions and the many competing mechanisms potentially explaining influenza seasonal characteristics [2].
To improve our understanding of the epidemiology of influenza in equatorial regions, we studied influenza seasonal characteristics in French Guiana, a French territory located on the northeast coast of the South American continent, where a laboratory-based influenza sentinel surveillance system was established since 2006. The objectives of the study were: (i) to analyze the seasonal characteristics of influenza in French Guiana from 2006 to 2010 using times-series techniques and (ii) to use dynamic regression analysis to investigate the impact of climatic factors (rainfall, temperature and humidity) on influenza transmission.
Patients and Methods
Study area
French Guiana (80,000 km2 for 230,000 inhabitants) is located between latitudes 2°N and 6°N, longitudes 51° and 53°W and has an equatorial climate influenced by the movements of the Atlantic intertropical convergence zone (ITCZ). The ITCZ is a belt of low pressure and a converging trade winds that encircles the Earth near the Equator. During its oscillations, the ITCZ reaches the French Guiana coastal areas twice each year, delineating a seasonal cycle with two distinct periods: a “dry season” (August to December) and a “wet season”, including a short rainy season in January and February, followed by a decrease in precipitation levels during March, and a major rainy season from April to July.
Influenza surveillance system and laboratory diagnoses
Influenza-like illness (ILI) surveillance was established in 2006 in French Guiana by the French Institute of Public Health Surveillance, based on a network of sentinel practitioners including 18 General Practitioners (GP). Participating physicians report on a weekly basis the number of visits for ILI and the total number of consultations. ILI defined as the combination of a sudden onset of fever (≥38°C) with cough or sore throat with or without general symptoms such as myalgia, prostration, headache, or malaise. The study population included every patient with ILI, regardless of age, who sought care within the sentinel GP network. The regional office of the French Institute for Public Health Surveillance recorded the weekly numbers of ILI.
Every week, from January 2006 to December 2010, all sentinel GP collected nasal swabs from their 2 first ILI patients which were sent for testing to the Pasteur Institute laboratory of virology, French Guiana. Specimens were analysed by rRT-PCR as described by the CDC.
Climatic data
Daily data on rainfall (RF), temperature and relative humidity, were compiled from seven weather stations located throughout the territory, and centralized at the regional office of Météo France in French Guiana. In this study, specific humidity (SH) in g/kg was calculated from daily averages of temperature, relative humidity and surface pressure [2] and is a proxy for absolute humidity (AH). Recent studies have suggested that absolute humidity could affect influenza survival and transmission and was linked to the timing of onset of seasonal and pandemic outbreaks [3; 4].
Statistical analysis
In a first step, we used autoregressive integrated moving average (ARIMA) times series models to describe the patterns of ILI incidence and climatic parameters (RF and SH) from 2006 to 2010. The ARIMA model is a univariate analysis, which is useful to analyse the temporal behaviour of a given series, expressed as a function of its past values, trends and sudden changes in the near past. This approach has been suggested by Box and Jenkins [5] and used to analyse times series in many epidemiological studies [6; 7]. In a second step, we used dynamic regression (DR) models to investigate the relationship between ILI incidence and climatic parameters (RF and SH) from 2006 to 2010. In brief, DR models were developed by Pankratz [8] as multivariate regression models, which are useful to analyse the association between input and output ARIMA series. Construction of ARIMA models for both the output and the input series is required before attempting to build a DR model by transfer functions. Linear transfer function (LTF) is one of several strategies used to build DR models. LTF shows how an output series (in the present case, ILI incidence) is related to the input series (climatic parameters) by taking into account possible time-lags (delay of climatic effect). This approach also takes into consideration the time structure (autocorrelation pattern) of the disturbance series. In building ARIMA and DR models, a three-stage model-building strategy was used based on model identification, model estimation and model checking. More details of the three stage model-building strategy for ARIMA models, including transformation and differentiation series, can be found elsewhere [6, 9].
Analyses were conducted using SAS software version 9.0 (SAS Institute, Cary, NC) and P values <0.05 were considered statistically significant. ILI incidence from August to October 2009 (the pandemic period) was excluded from the analysis
Results
Influenza seasonality and circulating strains
A total of 1,533 specimens were tested for influenza by real-time PCR from 2006 to 2010. Of these, 644 (42%) were positive for influenza viruses. Influenza positivity increased from 30–36% in 2006–2008 to 45–48% in 2009–2010 due to circulation of the novel pandemic influenza A(H1N1)pdm.
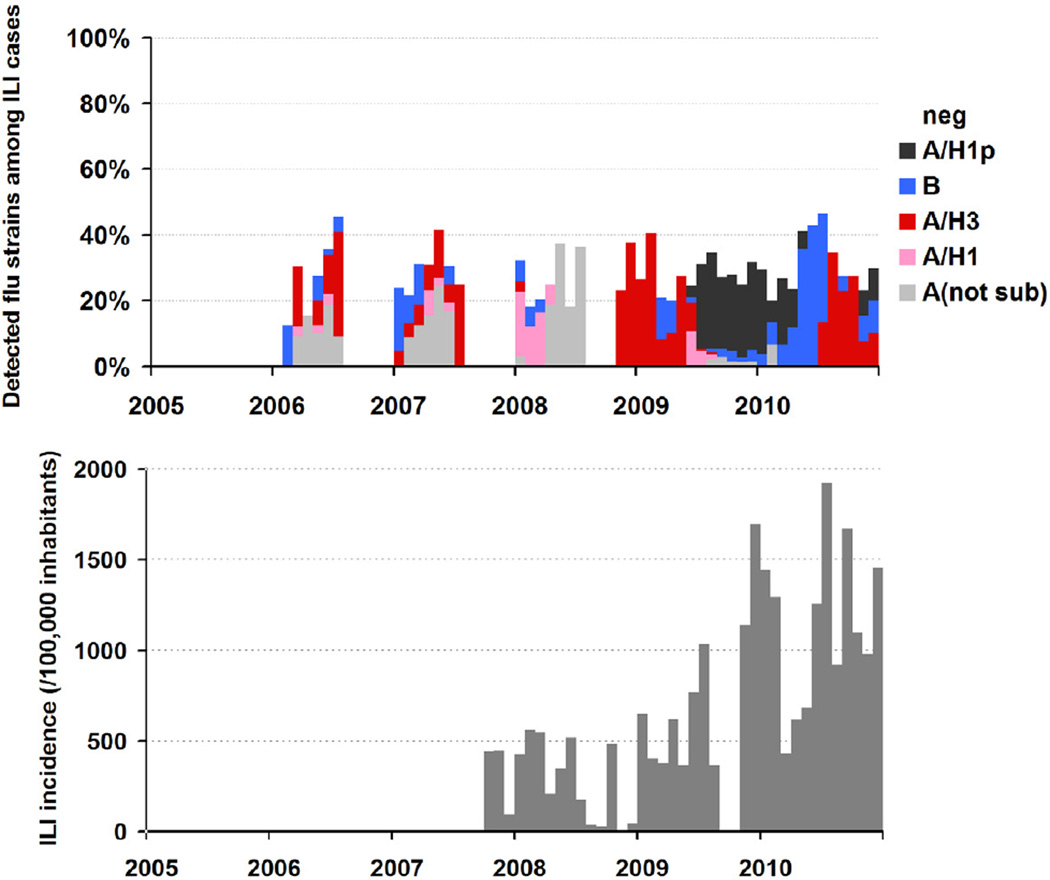
To understand the seasonality and trend of circulating influenza strains, we plotted the monthly percentage distribution of influenza-positive specimens over the total number of samples tested. A clear seasonal variation in the occurrence of influenza was seen in the pre-pandemic period from January 2006 to June 2009. Influenza activity started in January–February during the short rainy season and influenza activity increased from April to June, coinciding with the major rainy season. Since the emergence of the influenza A(H1N1)pdm in August 2009, sustained influenza activity occurred almost year-round with a major peak in the main rainy season (Figure 1a). Seasonal patterns were similar in the monthly ILI incidences, also revealing a coinciding with the major rainy season (Figure 1b).
Figure 1.
Detection of influenza strains and ILI incidence, French Guiana. Upper Panel: Proportion of influenza strains (data available from January 2006). Lower Panel: Total number of reported consultations due to ILI as informed by participating physicians (data available from Nov 2007)
During the period 2006–2010, the majority of seasonal influenza viruses circulating were of type A. In particular, Influenza A(H3N2) viruses circulated at a high level between 2006 and 2008 whereas an increasing in activity for the influenza A(H1N1) viruses was seen during the pre pandemic period. In 2010, the majority of influenza viruses circulating still of type A (60%) but we observed a high level (40%) of influenza B virus circulating (Table 1).
Table 1.
Type and Subtypes of influenza strains detected from January 2006 to December 2010, French Guiana
| Types | Subtypes A | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | Isolates tested |
Influenza positive |
Type B | Type A | H1N1 | H3N2 | H1N1p | ||||||
| n | n | % | n | % | n | % | n | % | n | % | n | % | |
| 2006 | 152 | 55 | 36 | 6 | 11 | 49 | 89 | 4 | 15 | 23 | 85 | 0 | 0 |
| 2007 | 137 | 48 | 35 | 10 | 21 | 38 | 79 | 3 | 19 | 13 | 81 | 0 | 0 |
| 2008 | 140 | 42 | 30 | 6 | 14 | 36 | 86 | 19 | 83 | 4 | 17 | 0 | 0 |
| 2009 | 871 | 390 | 45 | 18 | 5 | 372 | 95 | 23 | 6 | 68 | 19 | 263* | 74 |
| 2010 | 225 | 109 | 48 | 44 | 40 | 65 | 60 | 0 | 0 | 30 | 48 | 33 | 52 |
| Total | 1,533 | 644 | 42 | 84 | 13 | 560 | 87 | 49 | 10 | 138 | 29 | 296 | 61 |
July 31, 2009 : detection of the first laboratory-confirmed case of pandemic H1N1 in French Guiana
Description of climatic parameters and Influenza-like illness incidences patterns
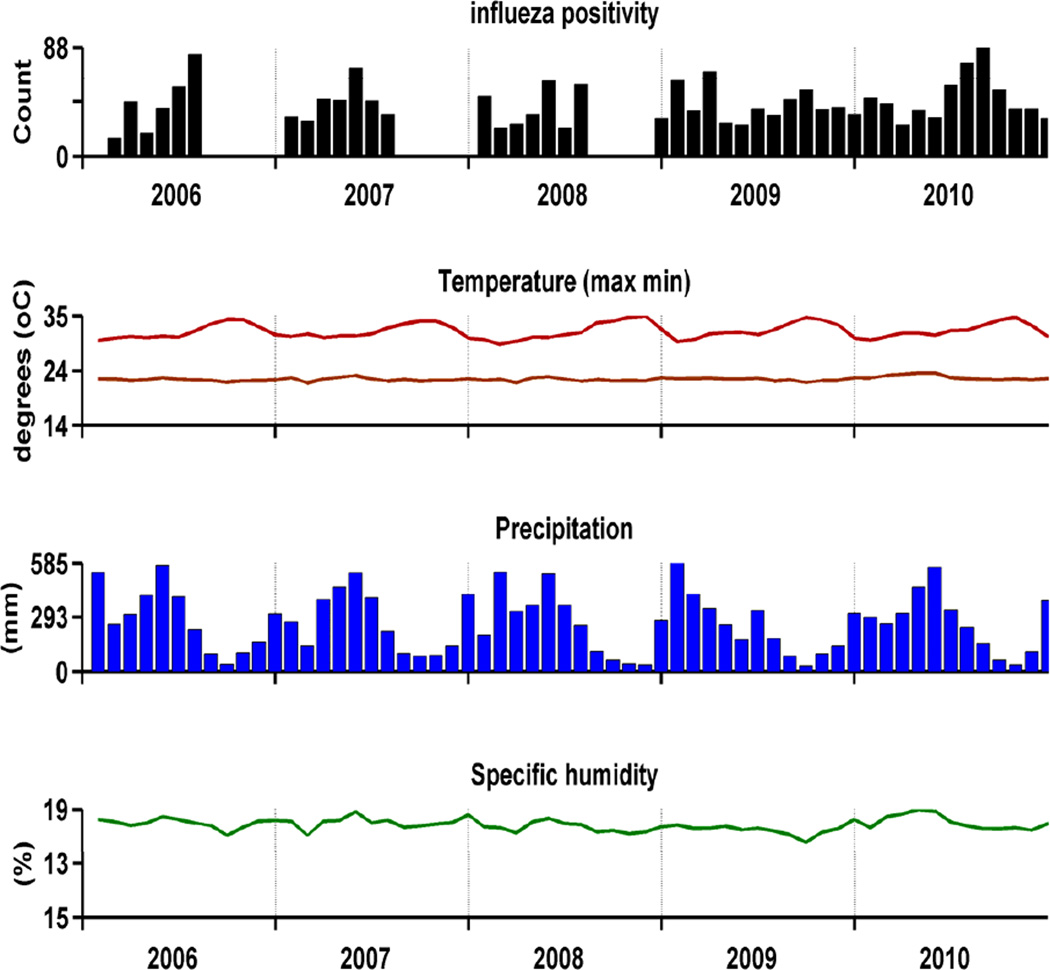
From 2006 to 2010, French Guiana experienced warm daily temperatures year-round, with little variation between the dry (22.7°C to 33.3°C) and rainy seasons (23.0°C to 31.4°C). However, there was significant variation in rainfall and SH patterns, with cumulative rainfall and SH peaking during the rainy seasons. Mean values of climatic parameters are presented in Table 2.
Table 2.
Mean values (minimum, maximuml) of climatic parameters in French Guiana, 2006–2010
| Dry Season | Short Rainy Season | Major Rainy Season | p | |
|---|---|---|---|---|
| Monthly Rainfall (in mm) | 136.5 (30.2–416.4) | 341.9 (135.1– 585.4) | 360.3 (168.8– 575.0) | <0.0001 |
| Daily maximal Temperature (in °C) | 33.2 (30.6–34.8) | 30.6 (29.5– 31.6) | 31.4 (30.7– 32.5) | <0.0001 |
| Daily minimal Temperature | 22.7 (22.3– 23.2) | 22.9 (22.2– 23.8) | 23.0 (22.5– 24.0) | <0.01 |
| Daily minimal Relative Humidity (%) | 53.7 (45.1– 68.9) | 64.9 (58.9– 70.0) | 62.6 (55.4– 68.4) | <0.0001 |
| Daily maximal Relative Humidity | 97.7 (96.4– 98.5) | 96.5 (95.4– 98.2) | 97.6 (94.7– 98.7) | <0.001 |
| Daily Specific Humidity (in g/kg)* | 16.9 (15.3– 18.2) | 17.2 (16.0– 18.3) | 17.5 (16.5– 18.8) | <0.01 |
Dry Season from August to December ; Short Rainy Season from January to March; Major Rainy Season from April to July
Specific humidity was calculated from daily mean of temperature, relative humidity and surface pressure.
An ARIMA model was built to describe the weekly evolution of both rainfall and SH during the study period. The ARIMA model for SH showed a significant seasonal autoregressive variation with a constant of 17.6 g/kg and autoregressive terms of order 2 (Table 3). Similarly, the ARIMA model for rainfall showed seasonal autoregressive variation with a constant of 56.1 mm and autoregressive terms of order 2.
Table 3.
ARIMA models for weekly ILI incidence and climatic indicators, French Guiana, 2006–2010
| Series | Term | Ordera | Parameterb (SE) | T-ratio | p | R2 | SBIC | RSME |
|---|---|---|---|---|---|---|---|---|
| ILI | ||||||||
| Constant | 81.39 (19.9) | 4.09 | <0.0001 | |||||
| AR | 1 | 0.54 (0.07) | 7.32 | <0.0001 | 0.57 | 1303 | 43.5 | |
| AR | 2 | 0.25 (0.07) | 3.36 | <0.001 | ||||
| Seasonal Autoregressive | 52 | 0.28 (0.10) | 2.78 | <0.001 | ||||
| Rainfall | ||||||||
| Constant | 56.1 (9.0) | 6.2 | <0.001 | |||||
| AR | 1 | 0.47 (0.07) | 6.3 | <0.001 | 0.43 | 1218 | 33.9 | |
| AR | 2 | 0.28 (0.07) | 3.7 | <0.001 | ||||
| Seasonal Autoregressive | 52 | −0.21 (0.08) | −2.3 | <0.005 | ||||
| Specific Humidity | ||||||||
| Constant | 17.16 (0.21) | 79.33 | <0.0001 | |||||
| AR | 1 | 0.42 (0.07) | 5.87 | <0.0001 | 0.52 | 462 | 0.58 | |
| AR | 2 | 0.35 (0.07) | 4.87 | <0.0001 | ||||
| Seasonal Autoregressive | 52 | 0.12 (0.09) | 1.23 | 0.2 |
Delay before effect is observed (weeks).
Size and direction of the effect.
AR: autoregressive term, representing disturbances. SBIC: Schwartz-bayesian index criterion; RSME: root mean square error; ILI : Influenza-like illness
The ARIMA model for weekly ILI incidence showed a 1-year seasonal autoregressive variation with a mean of 81 ILI/100,000 inhabitants and two significant autoregressive terms of order 2.
Association between climate and ILI incidence
Dynamic regression was used to study the relationship between ILI incidence and climatic drivers. Both rainfall and SH were included as input series in the ILI model, which showed seasonal variation. An increase of 1 mm in rainfall resulted in a significant increase of 0.33% in ILI incidence one week later (p<0.01), after adjustment for SH. Similarly, an increase of 1g/kg in SH resulted in a significant decrease of 11% in ILI incidence 3 weeks later, after adjustment for RF (Table 4). Using the SBIC criteria for model selection, the final model suggested that both rainfall and specific humidity contributed to ILI incidence patterns, with the full model explaining 61% of the variance in ILI fluctuations.
Table 4.
Multivariate dynamic regression analysis of weekly ILI and climatic drivers, French Guiana, 2006–2010
| Series | Term | Ordera | Parameter (SE)b | T-ratio | p | R2 | SBIC | RSME |
|---|---|---|---|---|---|---|---|---|
| Model 1 | ||||||||
| Constant | 287.71 (98.71) | 2.91 | 0.004 | |||||
| AR | 1 | 0.54 (0.07) | 7.26 | <0.001 | ||||
| AR | 2 | 0.27 (0.07) | 3.55 | <0.001 | 0.58 | 1284.3 | 43.3 | |
| Seasonal Autoregressive | 52 | 0.27 (0.10) | 2.64 | <0.05 | ||||
| Specific humidity | 3 | −11.97 (0.8) | −2.14 | <0.05 | ||||
| Model 2 | ||||||||
| Constant | 62.09 (21.52) | 2.88 | <0.001 | |||||
| AR | 1 | 0.59 (0.07) | 7.86 | <0.0001 | ||||
| AR | 2 | 0.21 (0.07) | 2.78 | <0.001 | 0.61 | 1286.9 | 41.7 | |
| Seasonal Autoregressive | 52 | 0.32 (0.10) | 3.17 | <0.001 | ||||
| Rainfall | 1 | 0.34 (0.09) | 3.98 | 0.0001 | ||||
| Model 3 | ||||||||
| Constant | 253.54 (93.6) | 2.71 | <0.005 | |||||
| AR | 1 | 0.59 (0.07) | 7.86 | <0.0001 | 0.61 | 1274.6 | 41.4 | |
| AR | 2 | 0.22 (0.07) | 2.82 | <0.005 | ||||
| Seasonal Autoregressive | 52 | 0.31 (0.10) | 3.04 | 0.002 | ||||
| Rainfall | 1 | 0.33 (0.09) | 3.94 | 0.0001 | ||||
| Specific humidity | 3 | −11.13 (5.3) | −2.11 | <0.005 |
Delay before effect is observed (weeks).
Size and direction of the effect.
AR: autoregressive term, representing disturbances. SBIC: Schwartz-bayesian index criterion; RSME: root mean square error; ILI : Influenza-like illness
Discussion
Before 2006, there was little surveillance for influenza in French Guiana. In response to an important dengue epidemic in 2006 and a rising global concern for avian influenza, a GP sentinel network was set up, allowing detection of influenza activity in a previously understudied area. Our study provides the first description of influenza virus activity in French Guiana. This study complements previous influenza surveillance reports in this equatorial region [10; 11]. We observed that influenza activity closely follows the rainy season, with moderate activity in January–February, followed by more intense activity in April–July when major precipitations occur.
Based on dynamic regression (DR) models, we showed that rainfall and specific humidity were independently associated with influenza-like-illness incidence, with influenza activity increasing with increasing rainfall and decreasing specific humidity at a short time scale of 1–3 weeks. Multivariate DR analysis, a powerful method of times series analysis, has some advantages over classical regression analysis (logistic or linear), in particular by taking into account autocorrelated observations derived from surveillance systems. Furthermore, the DR model allows quasi-experimental analysis with the possibility to show how ILI incidence (an output series) is related to the climatic parameters (input series) by taking into account delay of climatic effect [6; 8–9; 12].
Our data nicely complements earlier reports of influenza activity in the equatorial regions of Peru and North Brazil [10; 11]. Interestingly, in the region of Iquitos in Northern Peru, influenza activity does not display pronounced seasonal patterns, with occasional peaks in March–April coinciding with the peak of rainfall. Indeed, Iquitos experiences year-round precipitations and lacks a dry season, in contrast to the more marked fluctuations of rainfall and influenza activity in French Guiana. In Belem, Northern Brazil, which is located just across the border from French Guiana, influenza activity patterns resemble those described in the present study, with a marked seasonal cycle in the first half of the year coinciding with the rainy season [13]. A gradient of influenza seasonality and timing has been described from North to South Brazil, suggesting that environmental factors drive a wave-like pattern of influenza transmission from equatorial to subtropical regions over a 3-month period [14]. The Brazilian travelling wave pattern is consistent with the timing of influenza activity in French Guiana, which is intermediate between the influenza seasons in temperate areas of the Northern and Southern Hemisphere.
There has been much interest in recent years in understanding the circulation of influenza viruses globally, assessing the evidence for persistence of viruses between seasons, especially in tropical areas which may serve as source populations for new variants [15 – 19]. Such questions are best resolved by phylogenetic analysis of virus sequence data, and so far genetic evidence for a tropical source population and viral persistence have proven elusive [20]. It would be particularly interesting to analyse the genetic makeup of influenza viruses circulating in French Guiana and assess whether they are more closely related to those circulating in mainland France, a country with which there is high connectivity, or to viruses circulating in neighbouring locations, including Peru and Brazil. In particular, the early period of influenza activity in January–February in French Guiana coincides with the typical winter influenza season in France, and could reflect seasonal seeding of influenza viruses from the mainland. Unfortunately, we did not have genetic information to assess this possibility. Influenza viruses circulating during the rainy seasons in 2006, 2007 and 2008 in French Guiana were antigenically similar to viruses circulating in mainland France during the winters of 2005, 2006 and 2007 (data not shown).
Our DR analysis revealed a link between influenza, rainfall and specific humidity, both of which have been suggested as potential seasonal drivers of influenza activity in previous work [4]. Laboratory experiments and epidemiological data from temperate regions suggest that low levels of absolute humidity favours influenza virus survival and transmission [3]. The association found in French Guiana data is consistent with this hypothesis, suggesting that absolute humidity, may also play a role in tropical settings, independently of the rainfall. In addition, in middle and low-latitude sites, influenza activity has been shown to coincide with the rainy season [10; 21 – 23]. These regions are characterized by pronounced dry and rainy seasons, with a large amplitude range in precipitations (from 25 mm in the dry season to 300 mm in the rainy season). It has been hypothesized in these regions that rainfall leads to more time spent indoors, thus increasing exposure to other people and in turn increasing the transmission of influenza.
A recent global model for influenza seasonality suggests that if specific humidity goes below a threshold of 12 g/kg, influenza will occur during the colder and drier months of the year (winter in temperate regions)[24]. In mid and low-latitude locations with a distinct rainy season (peak specific humidity>12g/kg), influenza activity will coincide with the rainy season. In contrast, in low latitudes regions which experience limited fluctuations in precipitations, with monthly averages constrained to a narrow range of 150–300 mm year-round, influenza is predicted to circulate year-round, such reported in Iquitos, Peru, and Singapore. Seasonal patterns in influenza activity in French Guiana are consistent with this global model given the pronounced rainy season and the fact that specific humidity never decreases below 12 g/kg in this location.
Our ILI incidence analysis excluded data for the pandemic period 2009 because in many locations, pandemic influenza activity did not respect typical seasonal patterns. Interestingly, pandemic influenza occurred during the dry season (August to November) in French Guiana and was not particularly delayed, with the detection of the first laboratory-confirmed case of H1N1pdm in a traveller returning from New York on July 31, 2009 and peak activity occurring in week 37, perhaps because of high connectivity with North America via the French West Indies. This is in contrast to the particularly late onset of pandemic activity in equatorial Africa [22–23; 25] and in Northeast Brazil [26]. We note that in 2010, influenza activity had not reverted to the usual pattern of rainy-season activity in French Guiana, perhaps due to perturbations in the circulation of influenza viruses due to interferences between subtypes, as seen in other locations (Hong-Kong). Furthermore, there is no clear intrinsic seasonality difference between influenza viruses in experimental models [27] although some epidemiologic studies have suggested slightly different peak timings of activity [28].
An improved sentinel GP surveillance since the even of the pandemic virus may explain the high level of influenza activity seen in French Guiana since 2010.
Our study is subject to a number of caveats. Most importantly perhaps, our dataset was relatively short, focusing on 5 years of surveillance. Future studies based on longer surveillance records will help confirm or infirm the seasonal characteristics of influenza in French Guiana and their association with climatic factors. Further, our sample size for the number of positive viruses was quite low at the monthly and weekly time scale, limiting the potential for time series analysis of lab-confirmed influenza data. Our climate data was based on 7 weather stations scattered throughout the coastal band, which we took to be a proxy for the area under study. Near 90% of the French Guiana surface of 80,000 km2 is Amazonian rain forest and the remaining 10% in the northeast is a coastal band where 90% of the population lives. Hence these 7 stations correspond to locations where most of the population lives, and presumably where influenza transmission occurs.
In conclusion, we provide the first report describing influenza seasonality patterns in French Guiana based on sentinel reports for ILI and laboratory-confirmation of influenza infections. We show that the influenza season broadly coincides with the rainy season, and that influenza activity is statistically linked with increasing rainfall and decreasing levels of specific humidity at a short time scale. We note differences in the epidemiology of influenza in French Guiana with that in other locations of the equatorial Amazonian region. Indeed, influenza activity in French Guiana predates the influenza season in the neighbouring city of Belem, Brazil, by a 1–2 months, and seasonality is more pronounced in French Guiana than in Iquitos, Peru. Overall, our results are broadly consistent with a recently proposed global model of influenza seasonality, stipulating that influenza activity coincides with the rainy season in low latitude regions with marked annual fluctuations in rainfall or humidity. Additional epidemiological data from under sampled tropical and equatorial regions, together with time series analysis of climate, will help confirm or infirm this model and tease out the biological mechanism responsible for influenza seasonality. Equally importantly, such seasonal analysis will help optimize influenza vaccination strategies in tropical regions and more broadly.
Supplementary Material
Figure 2.
Influenza detection per month by the sentinel GP network and climatic data from weather stations provided by the regional office of Météo France, French Guiana.
Acknowledgments
We thank for their helpful in the data collecting:
-
-
All GP participating in the Sentinel Network
-
-
The regional office of Météo-France in Matoury, French Guiana (M. Yves Bhurdop)
-
-
Regional office of the French Institute of Public Health Surveillance (C. Flamand, V. Ardillon and L. Carvalho)
We thank for his helpful in the figure design Dr WJ Alonso from Fogarty International Centre, National Institutes of Health, Bethesda, USA
Financial Disclosure:
This research was conducted in the context of the Multinational Influenza Seasonal Mortality Study (MISMS), an on-going international collaborative effort to understand influenza epidemiological and evolutionary patterns, led by the Fogarty International Center, National Institutes of Health. Funding for this project comes from the Office of Global Affairs’ International Influenza Unit in the Office of the Secretary of the Department of Health and Human Services.
This work has benefited from an "Investissement d’Avenir" grant managed by Agence Nationale de la Recherche (CEBA, ref. ANR-10-LABX-0025).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors declare that no competing interests exist
References
- 1.Simonsen L. The global impact of influenza on morbidity and mortality. Vaccine. 1999;30:S3–S10. doi: 10.1016/s0264-410x(99)00099-7. [DOI] [PubMed] [Google Scholar]
- 2.Tamerius J, Nelson MI, Zhou SZ, Viboud C, Miller MA, Alonso WJ. Global influenza seasonality: reconciling patterns across temperate and tropical regions. Environ Health Perspect. 2011;119(4):439–445. doi: 10.1289/ehp.1002383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaman J, Kohn M. Absolute humidity modulates influenza survival, transmission, and seasonality. Proc Natl Acad Sci USA. 2009;106(9):3243–3248. doi: 10.1073/pnas.0806852106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaman J, Pitzer VE, Viboud C, Grenfell BT, Lipsitch M. Absolute humidity and the seasonal onset of influenza in the continental United States. PLoS Biol. 2010;8(3) doi: 10.1371/journal.pbio.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Box G, Jenkins G. Time series analysis: forecasting and control. 2nd edn. San Francisco, CA: Holden Day; 1976. [Google Scholar]
- 6.Mahamat A, Lavigne JP, Fabbro-Peray P, Kinowski JM, Daurès JP, Sotto A. Evolution of fluoroquinolone resistance among Escherichia coli urinary tract isolates from a French university hospital: application of the dynamic regression model. Clin Microbiol Infect. 2005;11:301–306. doi: 10.1111/j.1469-0691.2005.01098.x. [DOI] [PubMed] [Google Scholar]
- 7.Quénel P, Dab W. Influenza A and B epidemic criteria based on time-series analysis of health services surveillance data. Eur J Epidemiol. 1998;14:275–285. doi: 10.1023/a:1007467814485. [DOI] [PubMed] [Google Scholar]
- 8.Pankratz A. Forecasting with dynamic regression models. New York: Wiley; 1991. [Google Scholar]
- 9.Mahamat A, MacKenzie FM, Brooker K, Monnet DL, Daures JP, Gould IM. Impact of infection control interventions and antibiotic use on hospital MRSA: a multivariate interrupted time-series analysis. Int J Antimicrob Agents. 2007;30:169–176. doi: 10.1016/j.ijantimicag.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Moura FE, Perdigão AC, Siqueira MM. Seasonality of influenza in the tropics: a distinct pattern in northeastern Brazil. Am J Trop Med Hyg. 2009;81:180–183. [PubMed] [Google Scholar]
- 11.Laguna-Torres VA, Gómez J, Ocaña V, Aguilar P, Saldarriaga T, Chavez E, et al. Influenza-like illness sentinel surveillance in Peru. PLoS One. 2009;4(7):e6118. doi: 10.1371/journal.pone.0006118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shardell M, Harris AD, El-Kamary SS, Furono JP, Miller RR, Perencevich EN. Statistical analysis and application of quasi-experiments to antimicrobial resistance intervention studies. Clin Infect Dis. 2007;45:901–907. doi: 10.1086/521255. [DOI] [PubMed] [Google Scholar]
- 13.Alonso WJ, Viboud C, Simonsen L, Hirano EW, Daufenbach LZ, Miller MA. Seasonality of influenza in Brazil: a traveling wave from the Amazon to the subtropics. Am J Epidemiol. 2007;165:1434–1442. doi: 10.1093/aje/kwm012. [DOI] [PubMed] [Google Scholar]
- 14.de Mello WA, de Paiva TM, Ishida MA, et al. The dilemma of influenza vaccine recommendations when applied to the tropics: the Brazilian case examined under alternative scenarios. PLoS One. 2009;4(4):e5095. doi: 10.1371/journal.pone.0005095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Viboud C, Boëlle PY, Pakdaman K, Carrat F, Valleron AJ, Flahault A. Influenza epidemics in the United States, France, and Australia, 1972–1997. Emerg Infect Dis. 2004;10:32–39. doi: 10.3201/eid1001.020705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rambaut A, Pybus OG, Nelson MI, Viboud C, Taubenberger JK, Holmes EC. The genomic and epidemiological dynamics of human influenza A virus. Nature. 2008;453(7195):615–619. doi: 10.1038/nature06945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Russell CA, Jones TC, Barr IG, Cox NJ, Garten RJ, Gregory V, et al. The global circulation of seasonal influenza A (H3N2) viruses. Science. 2008;320(5874):340–346. doi: 10.1126/science.1154137. [DOI] [PubMed] [Google Scholar]
- 18.Cheng X, Tan Y, He M, Lam TT, Lu X, Viboud C, et al. Epidemiological Dynamics and Phylogeography of Influenza Virus in Southern China. J Infect Dis. 2013;207(1):106–114. doi: 10.1093/infdis/jis526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bahl J, Nelson MI, Chan KH, Chen R, Vijaykrishna D, Halpin RA, et al. Temporally structured metapopulation dynamics and persistence of influenza A H3N2 virus in humans. Proc Natl Acad Sci USA. 2011;108(48):19359–19364. doi: 10.1073/pnas.1109314108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nelson MI, Lemey P, Tan Y, Vincent A, Lam TT, Detmer S, et al. Spatial dynamics of human-origin H1 influenza A virus in North American swine. PLoS Pathog. 2011;7(6):e1002077. doi: 10.1371/journal.ppat.1002077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dosseh A, Ndiaye K, Spiegel A, Sagna M, Mathiot C. Epidemiological and virological influenza survey in Dakar, Senegal : 1996–1998. Am J Trop Med Hyg. 2000;62:639–634. doi: 10.4269/ajtmh.2000.62.639. [DOI] [PubMed] [Google Scholar]
- 22.Lutwama JJ, Bakamutumaho B, Kayiwa JT, Chiiza R, Namagambo B, Katz MA, Geissler AL. Clinic- and hospital-based sentinel influenza surveillance, Uganda 2007–2010. J Infect Dis. 2012;206(Suppl 1):S87–S93. doi: 10.1093/infdis/jis578. [DOI] [PubMed] [Google Scholar]
- 23.Nyatanyi T, Nkunda R, Rukelibuga J, Palekar R, Muhimpundu MA, Kabeja A, et al. Influenza sentinel surveillance in Rwanda, 2008–2010. J Infect Dis. 2012;206(Suppl 1):S74–S79. doi: 10.1093/infdis/jis574. [DOI] [PubMed] [Google Scholar]
- 23.Tamerius J, et al. Plos Path. 2013 In Press. [Google Scholar]
- 25.Nzussouo NT, Michalove J, Diop, Njouom R, Monteiro MdeL, Adje HK, et al. Delayed 2009 Pandemic Influenza A Virus Subtype H1N1 Circulation in West Africa, May 2009-April 2010. J Infect Dis. 2012;206(Suppl 1):S101–S107. doi: 10.1093/infdis/jis572. [DOI] [PubMed] [Google Scholar]
- 26.Schuck-Paim C, Viboud C, Simonsen L, Miller MA, Moura FE, Fernandes RM, et al. Were equatorial regions less affected by the 2009 influenza pandemic? The Brazilian experience. PLoS One. 2012;7(8):e41918. doi: 10.1371/journal.pone.0041918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pica N, Chou YY, Bouvier NM, Palese P. Transmission of influenza B viruses in the Guinea Pig. J. Virol. 2012;86(8):4279. doi: 10.1128/JVI.06645-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Finkelman BS, Viboud C, Koelle K, Ferrari MJ, Bharti N, Grenfell BT. Global patterns in seasonal activity of influenza A/H3N2, A/H1N1, and B from 1997 to 2005: viral coexistence and latitudinal gradients. Plos One. 2007;2(12):e1296. doi: 10.1371/journal.pone.0001296. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.