Abstract
In a wide range of taxa, including baboons, close social bonds seem to help animals cope with stress and enhance long-term reproductive success and longevity. Current evidence suggests that female baboons may benefit from establishing and maintaining highly individuated relationships with a relatively small number of partners. Here, we extend previous work on the stability of female baboons’ social relationships in three different ways. First, we assess the stability of females’ social relationships in two distinct and geographically distant sites using the same method. Second, we conduct simulations to determine whether females’ social relationships were more stable than expected by chance. Third, we examine demographic sources of variance in the stability of close social bonds. At both sites, females’ relationships with their most preferred partners were significantly more stable than expected by chance. In contrast, their relationships with less preferred partners were more ephemeral, often changing from year to year. While nearly all females experienced some change in their top partners across time, many maintained relationships with top partners for several years. Females that lived in smaller groups and had more close kin available had more stable social relationships than those that lived in larger groups and had fewer close kin available.
Keywords: baboon, bond stability, female bond, Papio hamadryas ursinus, reproductive success, stress
A growing body of evidence suggests that close social bonds with same-sex partners help animals cope with stress and enhance long-term reproductive success and longevity. Female rats, Rattus norvegicus, that have well-balanced affiliative relationships exhibit lower glucocorticoid levels than those with less symmetric relationships (Yee et al. 2008). In chacma baboons, Papio hamadryas ursinus, or P. ursinus, grooming relationships enhance females’ ability to cope with various sources of stress, such as the immigration of potentially infanticidal males or instability of the dominance hierarchy (Engh et al. 2006a; Crockford et al. 2008; Wittig et al. 2008). Females have lower glucocorticoid levels during months in which they focus their grooming on a small number of partners than in months in which they distribute grooming to a wider subset of group members (Crockford et al. 2008). Females also display marked increases in glucocorticoid levels after a preferred partner dies or disappears from the group (Engh et al. 2006b).
These findings are complemented by evidence suggesting that social bonds confer fitness advantages on individuals (reviewed in: Silk 2007; Seyfarth & Cheney 2012). For example, female house mice, Mus musculus, which often share nests with other females and rear their pups communally, reproduce more successfully when they are allowed to choose their nestmates than when nestmates are assigned randomly (Weidt et al. 2008). Well-balanced social relationships also enhance the longevity of female rats (Yee et al. 2008). Female yellow baboons (Papio hamadryas cynocephalus, Papio cynocephalus) living in the Amboseli basin of Kenya that are more socially integrated into their groups have higher survivorship among their infants than females that are less socially integrated (Silk et al. 2003). Similarly, female chacma baboons in the Moremi Reserve of the Okavango Delta of Botswana that maintained strong bonds with other adult females had higher survivorship among their offspring than females with weaker social bonds (Silk et al. 2009). Moreover, in Moremi, females that had stronger and more consistent relationships with preferred partners lived longer than other females in their group (Silk et al. 2010a). Positive correlations between sociality and reproductive success have also been documented in female horses, Equus equus (Cameron et al. 2009) and bottlenose dolphins, Tursiops truncatus (Frère et al. 2010), as well as male Assamese macaques, Macaca assamensis (Schülke et al. 2010).
These findings suggest that females gain benefits from establishing and maintaining highly individuated relationships with a relatively small number of partners, rather than interacting less selectively with a wider range of social partners. This has important implications for the proximate mechanisms through which social bonds confer benefits and the ultimate forces that shape the dynamics of close social bonds among female baboons, and by extension, other species that form highly individuated social bonds.
However, there is presently some debate about the temporal stability of social relationships among female baboons. We have previously shown that female yellow baboons in Amboseli and female chacma baboons in Moremi maintain consistent preferences for favoured partners across time. For example, adult females form their strongest ties to their own mothers, and their mothers will be among their top three partners as long as their mothers remain alive (Silk et al. 2006a,b, 2010b). In contrast, chacma baboons in South Africa are reported to show little temporal consistency in female partner choice from month to month (Henzi et al. 2009; but see Seyfarth 1977) or across years (Barrett & Henzi 2002).
Here, we extend our previous analyses to assess the stability of female baboons’ social relationships in three ways. First, we assessed the stability of females’ social relationships in two distinct and geographically distant sites (Amboseli and Moremi) using the same method. This allowed us to assess the generality of our results across populations. Second, we conducted simulations in which females’ partners were chosen at random each year from the set of available females; then, we determined whether female social relationships were more stable than expected by chance, by comparing these simulated patterns of partner stability with observed patterns.
Third, we examined demographic sources of variance in partner stability. Previous analyses revealed that female baboons form the strongest relationships with their mothers and sisters, and with agemates (Silk et al. 2006a,b, 2010b). However, the availability of these preferred types of partners could affect the stability of partner preferences and could explain apparent differences between social groups or populations of female baboons in the formation of stable relationships. That is, it is possible that baboons have stable partner ‘preferences’, but the opportunity to sustain relationships with preferred partners from one year to the next is limited by demographic factors. The stability of close social bonds may reflect a compromise between the preferences that guide partner choice and demographic factors that constrain the ability of individuals to exercise those preferences. The number of adult females within a group is likely to affect the stability of females’ associations because the chance of having the same top partner from one year to the next by chance is higher when there are fewer potential partners than when there are many potential partners. Because females form the strongest bonds with close kin, particularly their mothers and daughters, the availability of close kin is also likely to influence the stability of social bonds. Finally, if females compete for access to preferred partners, then we might expect high-ranking females, which generally have priority of access to resources, to have more stable social bonds than lower-ranking females.
METHODS
Amboseli
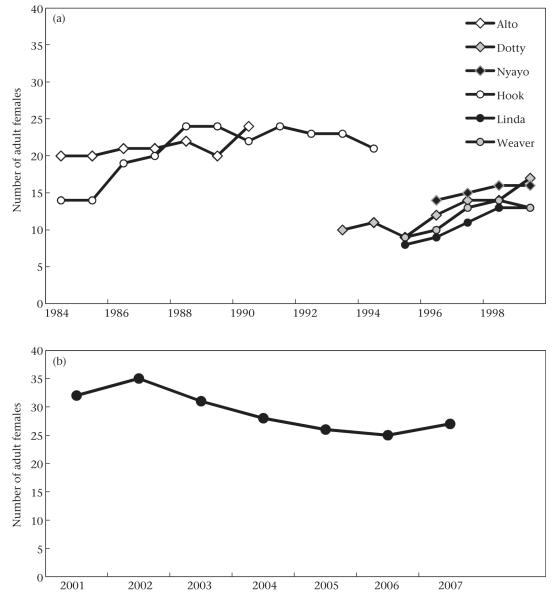
The study site is located in the Amboseli Basin, which straddles the border between Kenya and Tanzania and lies at the foot of Mount Kilimanjaro. Data are derived from observations of 137 adult female baboons living in six well-habituated groups over a 16-year period (Table 1). Alto’s group has been monitored since 1971, and Hook’s group has been monitored since 1980. Both of these groups shifted their ranges in response to deteriorating ecological conditions and subsequently fissioned (Fig. 1a). Alto’s group fissioned into Dotty’s group and Nyayo’s group (for more details about the study groups, see Altmann & Alberts 2003). The fission took several years to complete and was followed by a period of limited behavioural (but not demographic) data collection in the newly formed groups. As a consequence of the paucity of focal behavioural observations during this period, focal samples were available for only three females both in Alto’s group and in one of the daughter groups. Hook’s group fissioned into Linda’s group and Weaver’s group, but there was no lull in focal behavioural observations during the fission. Fourteen females were observed both in Hook’s group and in one of the two daughter groups. All procedures were approved by the Institutional Animal Care and Use Committee of Duke University (Protocol number A028-12-02).
Table 1.
Numbers of adult female baboons observed in each group in Amboseli and Moremi
| Group | Years analysed |
Average number of adult females present each year |
Total number of females in sample |
|---|---|---|---|
| Amboseli | |||
| Alto | 5 | 21.6 | 34 |
| Dotty | 7 | 12.3 | 21 |
| Nyayo | 4 | 15.3 | 16 |
| Hook | 11 | 20.9 | 38 |
| Linda | 5 | 10.8 | 14 |
| Weaver | 5 | 12.4 | 14 |
| Moremi | 7 | 29.1 | 49 |
Figure 1.
Composition of baboon study groups. Number of adult females in (a) Amboseli and (b) Moremi study groups each year.
Grooming and proximity maintenance are widely considered to provide meaningful measures of social relationships among nonhuman primates and they make up the major components of female baboons’ social time. Focal samples conducted on adult females provided information about grooming partners and spatial proximity. Females were sampled on a random schedule during daylight hours. At 60 s intervals within each 10 min focal sample, the activity of the focal female and the identity of her nearest neighbour within 5 m were recorded.
Dyads that had high rates of grooming also had high rates of association, so we used the frequency of grooming and proximity to compute a composite sociality index (CSIA) for each dyad in each year:
The first term in the numerator is the adjusted frequency of grooming for dyad ij in year y divided by the mean adjusted frequency of grooming for all dyads in group x in year y. The second term in the numerator is the adjusted frequency of proximity for dyad ij divided by the mean frequency of proximity for all dyads in group x in year y. The CSIA measures the extent to which each dyad deviated from other dyads. The mean of the CSIA is defined as 1, but the values can range from 0 to infinity. High values of the CSIA represent dyads that had stronger social bonds than the average female dyad in the group in the same year, and low values of the CSIA represent dyads that had weaker social bonds (for more details about observation and analysis procedures, see Silk et al. 2006a,b).
Moremi
The study site is located in the Moremi Game Reserve in the Okavango Delta of Botswana. The data are derived from focal samples on 49 adult females collected over a 7-year period in one well-habituated group (Table 1). The number of adult females in the study group ranged from 25 to 35 (Fig.1b). For more details about the study site and the history of the study population, see Cheney &Seyfarth (2007) and Cheney et al. (2004). All procedures were approved by the Animal Care and Use Committee of the University of Pennsylvania (Protocol number 19001).
During 10 min focal samples, observers recorded all approaches, vocalizations and social interactions involving focal females using a common protocol. The onset and termination of all grooming bouts was also recorded to obtain information about the total amount of time spent grooming. The frequency of female–female approaches and groom-presents, and the number and duration of grooming bouts were correlated, so we constructed a composite index (CSIM) for each female–female dyad in each year using basically the same procedure outlined above. The CSIM was based on the rate of approaches, groom-presents and grooming initiations, and the duration of grooming within dyads:
The first term in the numerator is the adjusted frequency of approaches for dyad ij in year y divided by the mean adjusted frequency of approaches for all dyads in group x in year y. The second term in the numerator is the adjusted frequency of grooming initiations for dyad ij in year y divided by the mean adjusted frequency of grooming initiations for all dyads in group x in year y. The third term is the adjusted frequency of groom-presents for dyad ij divided by the mean frequency of groom-presents for all dyads in group x in year y. (Groom-presents are recorded when one individual positions her hindquarters in front of another individual.) The fourth term represents the proportion of time spent grooming by dyad ij divided by the mean proportion of time spent grooming by all dyads. The CSIM measures the extent to which each dyad deviated from other dyads; high values represent dyads that had stronger social bonds than the average female dyad in the group in the same year, and low values represent dyads that had weaker social bonds (for more details about observation and analysis procedures, see Silk et al. 2010b).
Analysis
Partner stability index (PSI): stability of partner choice
For each female that was present for at least 2 years, we used the values of the composite sociality index (CSIA, CSIM) to rank-order her partners in each year. (For 17 females in the Amboseli population that were observed before and after group fission, we computed separate PSI values for them in each group.) The top-ranked partner (i.e. the one with the highest CSI) was ranked 1. Then, for each female we tabulated the number of different partners that she had within a range of ranks (e.g. rank orders 1–3, 4–6, 7–9) across years. For example, a Moremi female, DO, was present for 4 years (Table 2). One female was among DO’s top three partners in 3 of the 4 years; three others were among her top partners in 2 years; and three were among her top partners in only 1 year. In all, seven different females were among DO’s top three partners over the 4-year period.
Table 2.
Example for computing the partner stability index of adult female baboons
| Female DO | Top three partners |
||
|---|---|---|---|
| 1 | 2 | 3 | |
| Year 1 | GI | BL | AZ |
| Year 2 | BL | LX | CO |
| Year 3 | BA | AZ | TS |
| Year 4 | BA | AZ | LX |
For each female, we assessed her partner stability index using the following formula:
where N is the number of years in which partner rank orders were evaluated, S is the number of rank slots being evaluated, and U is the observed number of unique partners. To conform to previous analyses of females’ relationships with their top partners, S = 3 in all analyses reported here. The value of the partner stability index (PSI) varies from 1 (females that had the same three partners in each year) to 0 (females that had a completely different set of top three partners in each year). For example, for female DO in Table 2, N = 4, S = 3 and U = 7, and PSI = 0.56.
Simulation of partner associations across years
We also performed a simulation to compare the observed values of the partner stability index against expected values if females chose their partners within a given range of ranks (e.g. partners rank-ordered 1–3) at random. For each focal female in each year that she was present, S different partners were selected at random from the available set of adult females, which included all females present in the group in the given year except the focal female herself. Then, the simulated value of the PSI was computed. This process was repeated for each female 10 000 times to generate a distribution of simulated values of the PSI for each focal female. The distribution of simulated PSI values provides the likelihood of observing a value as high as the observed value of the PSI by chance. The distribution of simulated PSI values for female DO is shown in Table 3.
Table 3.
Distribution of simulated values of the partner stability index for female DO
| Number of unique partners |
Partner stability index |
Frequency | Cumulative probability |
|---|---|---|---|
| 3 | 1.0000 | 0 | 0 |
| 4 | 0.8889 | 0 | 0 |
| 5 | 0.7778 | 0 | 0 |
| 6 | 0.6667 | 2 | 0.0002 |
| 7 | 0.5556 | 52 | 0.0054 |
| 8 | 0.4444 | 290 | 0.0344 |
| 9 | 0.3333 | 1524 | 0.1868 |
| 10 | 0.2222 | 3329 | 0.5197 |
| 11 | 0.1111 | 3510 | 0.8707 |
| 12 | 0.0000 | 1293 | 1 |
The simulation generates values of the partner stability index (PSI) under the assumption that partners are chosen at random from among the available females in the group each year. For female DO, who was present in the group for 4 years, the minimum number of unique partners was 3 and the maximum number was 12. Only 54 runs of the simulation generated a simulated PSI value that was as low as the observed value, 7 (in bold).
Statistical Analyses
In the analyses presented below, the unit of analysis is the individual female and the primary outcome variable is the value of the PSI. Paired t tests were used to compare the values of the PSI with simulated values and to compare values of the PSI across partner rank ranges (e.g. partners rank-ordered 1–3 versus partners rank-ordered 4–6). Data from the Amboseli and the Moremi populations were analysed separately. For the analyses of the sources of variation in the PSI among the Amboseli females, we used multilevel regression models (GLMM; Baayen 2008) to control for effects of group membership and identity of females (N = 17) that were observed in more than one group. For the Moremi study group, we used a generalized linear model (GLM) with robust standard errors. All statistical analyses were conducted with Stata 11.0 (StatCorp 2009, College Station, TX, U.S.A.). Where appropriate, we report means ± SE. Two-tailed tests of significance were used throughout.
RESULTS
Amboseli
Most preferred partners
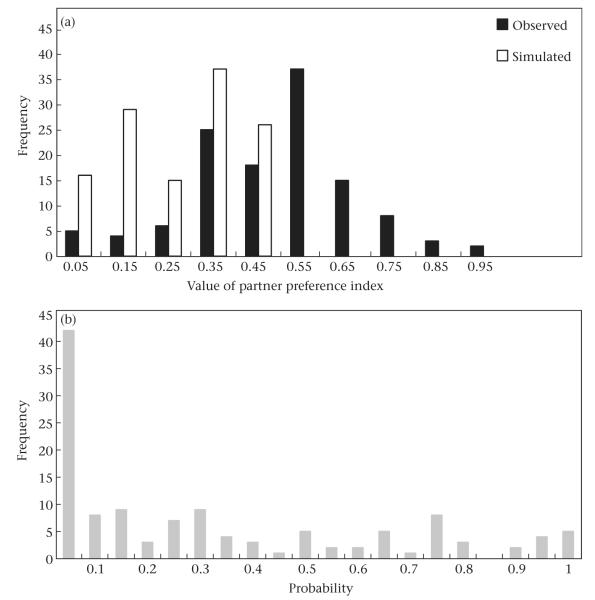
We computed the stability of females’ preferences for their three most preferred partners in the six Amboseli study groups. Values of the PSI spanned the full range from 0 to 1, with a mean across groups of 0.47 ± 0.02 (Fig. 2a). The distribution of observed values differed substantially from that of simulated values, as simulated values always fell between 0 and 0.5 (mean - ± SE = 0.32 ± 0.01; Fig. 2a). For individual females, observed PSI values were higher than simulated values in nearly all cases (83%; paired t test: t122 = 10.16, P < 0.001). The distribution of probabilities associated with observed values of PSI was skewed to the left with a mode at P < 0.05 (Fig. 2b).
Figure 2.
Partner stability index (PSI) of adult female baboons in the Amboseli study groups. (a) Distribution of observed (black bars) and simulated (white bars) PSI values. (b) Distribution of probability levels associated with observed PSI values. The distribution of simulated PSI values is shifted further to the left than the distribution of observed PSI values, which indicates that females’ relationships with their top partners were generally more stable than expected by chance.
A substantial fraction of females had significantly more stable relationships with their most preferred partners than would be expected if they chose their partners at random. However, for other females, there was little consistency in the identity of their top partners from one year to the next. Females that lived in groups that contained fewer adult females had significantly higher PSI values (Table 4). In addition, females that had more close kin (mothers and daughters) in the group had significantly more stable social bonds than females that lived with fewer close kin. Neither a female’s dominance rank nor her number of sisters affected the value of the PSI, and the addition of these terms did not improve model fit.
Table 4.
Sources of variation in the partner stability index (PSI) of adult female baboons in Amboseli
| Predictor variable | Coefficient | SE | Z | P |
|---|---|---|---|---|
| Number of adult females | −0.0184 | 0.0045 | −4.12 | <0.001 |
| Number of mothers and daughters | 0.0524 | 0.0248 | 2.11 | 0.0350 |
Less preferred partners
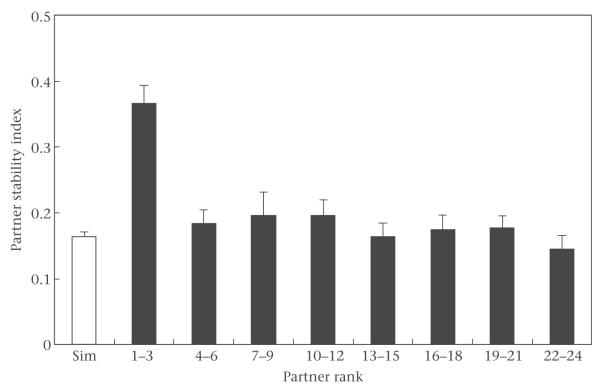
Females had more stable relationships (higher PSI values) with their three most preferred partners than with their less preferred partners (Fig. 3). PSI values were significantly higher for partners with preference rank orderings of 1–3 than for partners with preference rank orderings of 4–6 (paired t test: t118 = 6.15, P < 0.0001), and PSI values for partners with preference rank orderings of 4–6 were significantly higher than PSI values for partners with rank orderings of 7–9 (t112 = 3.86, P = 0.0002). PSI values for partners with rank orderings of 7–9 did not differ from those for partners with rank orderings of 10–12 (t110 = 0.0050, P = 0.9960; sample sizes decline for lower rank orderings because of multiple tied values of the PSI).
Figure 3.
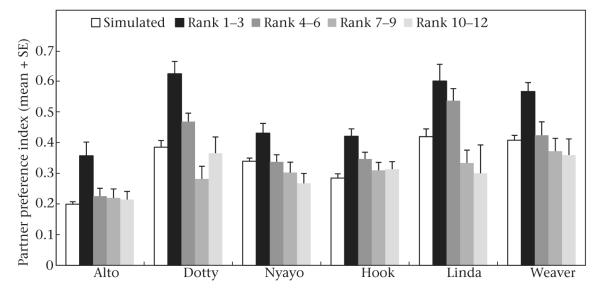
Mean + SE simulated and observed partner stability index (PSI) values for different ranges of preference rank orderings of adult female baboons in each of the six Amboseli groups.
Moremi
Most preferred partners
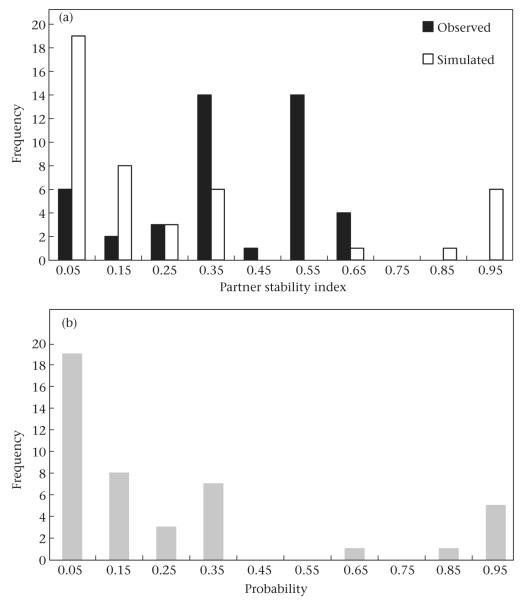
The pattern of partner choice in the Moremi study group was similar to that in the Amboseli groups. In Moremi, the mean value of the PSI for the three most preferred partners was 0.37 ± 0.03 (range 0–0.67, N = 44; Fig. 4a). As in the Amboseli groups, the distribution of observed values was offset from the distribution of simulated values (mean ± SE = 0.16 ± 0.01, range 0–0.22; Fig. 4a), and a substantial majority of females had higher PSI values than expected by chance (82%; paired t test: t43 = 7.86, P < 0.0001). Probabilities associated with observed values of PSI were skewed to the left with a mode at P < 0.05 (Fig. 4b).
Figure 4.
Partner stability index (PSI) for adult female baboons in the Moremi study group. (a) Distribution of observed and simulated PSI values. (b) Distribution of probability levels associated with observed PSI values. The distribution of simulated PSI values is shifted further to the left than the distribution of observed PSI values, which indicates that females’ relationships with their top partners were generally more stable than expected by chance.
Females that lived in the group when it contained fewer adult females had higher PSI values than females that lived in the group when it contained more adult females (Table 5). In addition, females that lived with more mothers and daughters had higher PSI values than other females. Other variables (presence of sisters, female dominance rank, sampling effort) did not improve model fit.
Table 5.
Sources of variation in the partner stability index (PSI) of adult female baboons in Moremi
| Predictor variable | Coefficient | SE | Z | P |
|---|---|---|---|---|
| Number of adult females | −0.0248 | 0.0090 | −2.75 | 0.006 |
| Number of mothers and daughters | 0.1484 | 0.0321 | 4.62 | <0.001 |
Less preferred partners
Females had significantly more stable relationships with partners with rank orderings of 1–3 than with partners with rank orderings of 4–6 (t43 = 5.79, P < 0.0001; Fig. 5). However, there were no significant differences in the stability of females’ relationships with partners with rank orderings of 4–6 and partners with lower rank orderings (P > 0.20 in all comparisons).
Figure 5.
Mean + SE simulated (open bar) and observed (solid bars) partner stability index (PSI) values for adult female baboons in the Moremi study group.
DISCUSSION
These analyses demonstrate that, in both Amboseli and Moremi, female baboons’ relationships with their most preferred partners (top partners) were significantly more stable than expected by chance. In contrast, their relationships with less preferred partners were more ephemeral, changing from year to year. While nearly all females experienced some change in their top partners across time, many maintained relationships with top partners for several years. The extent of stability in females’ relationships with their top partners was a function of the presence of mothers and daughters and the size of the groups in which they lived.
Females that coresided with their mothers and daughters for longer stretches of time had more stable relationships with their top partners. Previous analyses showed that females had very stable preferences for close kin, particularly their mothers and daughters (Silk et al. 2006b, 2010b). Thus, it is not surprising to find that the extent of stability in females’ relationships with top partners was influenced by the availability of their most preferred partners.
Females living in larger groups had significantly less stable relationships with their top partners than females living in smaller groups. This was true even when demographic factors, like the death of a close partner, were controlled for (Cheney et al. 2012). There are several possible explanations for this finding. Females in larger groups may face more competition for access to preferred partners (Seyfarth 1977, 1980), and this may produce more variability in partner choice across time. Moreover, females living in larger groups may find it beneficial to divide their attentions among a wider set of partners (Henzi et al. 1997a,b), and this will also reduce the consistency of partner choice across years. In addition to these social factors, demographic factors that constrain partner choice may play a more important role in large groups than in small groups. In Moremi, rates of female mortality are highest in years when the number of adult females is highest (Cheney et al. 2012). Thus, females that lived in the group when it was larger may have lost more favoured partners from one year to the next than females that lived in the group when it was smaller.
The methods presented here provide a way to quantify the extent of stability in social relationships across time and to compare observed patterns to those expected to occur by chance. The partner stability index provides an objective basis for comparisons of the stability of social bonds. For example, while we have argued that female baboons in Amboseli and Moremi form strong and enduring bonds with preferred partners, Henzi & Barrett (2002) have argued that females in De Hoop, South Africa, have unstable relationships. However, PSI values among females in De Hoop (Barrett & Henzi 2002) did not differ substantially from those among females in Moremi (Silk et al. 2010b). This finding is in contrast to the qualitative descriptions of social bond differences between these populations and underscores the value of a quantitative method for assessing the stability of relationships across time, populations and taxa.
These methods may be useful in comparative analyses of the fitness consequences of social bonds in primates and other taxa. Strong social bonds confer reproductive advantages in a number of mammalian species outside the primate order, including house mice, horses and bottlenose dolphins, but we do not know whether all of these animals form enduring relationships with particular partners, or whether the stability of close social bonds enhances fitness. In other cases, enduring associations between individuals have been documented (e.g. chimpanzees, Pan troglodytes: Gilby & Wrangham 2008; Mitani 2009; elephants, Loxodonta africana: Archie et al. 2006; Indian Ocean bottlenose dolphins, Tursiops aduncus: Connor et al. 2000), but the adaptive consequences of long-term associations have not been documented. The methods we have developed may provide the foundation for research on these questions.
Acknowledgments
S.C.A. and J.A. thank the Office of the President of Kenya and the Kenya Wildlife Service for permission to work in Amboseli. The staff of Amboseli National Park provided valuable cooperation. The members of the pastoralist communities of Amboseli and Longido and the Institute for Primate Research in Nairobi provided local sponsorship. We thank R. S. Mututua, S. N. Sayialel and J. K. Warutere for their expert assistance in data collection in Amboseli. We are grateful to the National Science Foundation and the National Institute on Aging for financial support of the Amboseli Baboon Project from several grants over the years (NIA R01AG034513-01 and P01-AG031719, and NSF IBN-9985910, IBN-0322613, BCS-0323553, IOS 0919200).
R.M.S. and D.L.C. thank the Office of the President and the Department of Wildlife and National Parks of the Republic of Botswana for the permission to conduct research in the Moremi Reserve. A. Mokopi, M. Mokopi, M. Heesen, C. Shaw, W. Smith and E. Wikberg provided valuable help with data collection and logistics in the field. J. Fischer, S. Johnson, D. Kitchen, R. Palombit and D. Rendall contributed to the long-term records. Field research was supported by grants from the National Geographic Foundation, the Research Foundation of the University of Pennsylvania, the Institute for Research in Cognitive Science at the University of Pennsylvania and the National Institutes of Health (HD-29433; MH62249). R.M.S., D.L.C. and J.B.S. thank J. Beehner, T. Bergman, C. Crockford, A. Engh, L. Mosovitz and R. Wittig for access to behavioural data collected between 2001 and 2007. J.B.S. thanks her coauthors for the opportunity to work in Moremi and Amboseli and to analyse the long-term data. Her fieldwork in Moremi was partially funded by a grant from the National Science Foundation (BCS-9213586), and her work in Amboseli was funded by the LSB Leakey Foundation, the National Geographic Society and the US National Science Foundation (BCS-0003245).
References
- Altmann J, Alberts SC. Intraspecific variability in fertility and offspring survival in a nonhuman primate: behavioral control of ecological and social sources. In: Wachter KW, Bulatao RA, editors. Offspring: the Biodemography of Fertility and Family Behavior. National Academy; Washington, D.C.: 2003. pp. 140–169. [Google Scholar]
- Archie EA, Moss CJ, Alberts SC. Ties that bind: genetic relatedness predictions the fission and fusion of social groups in wild African elephants. Proceedings of the Royal Society B. 2006;273:513–522. doi: 10.1098/rspb.2005.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baayen RH. Analyzing Linguistic Data. A Practical Introduction to Statistics. Cambridge University Press; Cambridge: 2008. [Google Scholar]
- Barrett L, Henzi SP. Constraints on relationship formation among female primates. Behaviour. 2002;139:263–289. [Google Scholar]
- Cameron EZ, Setsaas TH, Linklater WL. Social bonds between unrelated females increase reproductive success in feral horses. Proceedings of the National Academy of Sciences, U.S.A. 2009;106:13850–13853. doi: 10.1073/pnas.0900639106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney DL, Seyfarth RM. Baboon Metaphysics: the Evolution of a Social Mind. University of Chicago Press; Chicago: 2007. [Google Scholar]
- Cheney DL, Seyfarth RM, Fischer J, Beehner JC, Bergman TJ, Johnson SE, Kitchen DM, Palombit RA, Rendall D, Silk JB. Factors affecting reproduction and mortality among baboons in the Okavango Delta, Botswana. International Journal of Primatology. 2004;25:401–428. [Google Scholar]
- Cheney DL, Silk JB, Seyfarth RM. Evidence for intra-sexual selection in wild female baboons. Animal Behaviour. 2012 doi: 10.1016/j.anbehav.2012.03.010. published online 3 April 2012, doi:10.1016/j.anbehav.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor RC, Wells R, Mann J, Read A. The bottlenose dolphin: social relationships in a fission–fusion society. In: Mann J, Connor R, Tyack P, Whitehead H, editors. Cetacean Societies: Field Studies of Whales and Dolphins. University of Chicago Press; Chicago: 2000. pp. 91–126. [Google Scholar]
- Crockford C, Wittig RM, Whitten PL, Seyfarth RM, Cheney DL. Social stressors and coping mechanisms in wild female baboons (Papio hamadryas ursinus) Hormones and Behaviour. 2008;53:254–265. doi: 10.1016/j.yhbeh.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Engh AL, Beehner JC, Bergman TJ, Whitten PL, Hoffmeier R, Seyfarth RM, Cheney DL. Female hierarchy instability, male immigration and infanticide increase glucocorticoid levels in female chacma baboons. Animal Behaviour. 2006a;71:1227–1237. [Google Scholar]
- Engh AL, Beehner JC, Bergman TJ, Whitten PL, Hoffmeier R, Seyfarth RM, Cheney DL. Behavioural and hormonal responses to predation in female chacma baboons (Papio hamadryas ursinus) Proceedings of the Royal Society B. 2006b;273:707–712. doi: 10.1098/rspb.2005.3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frère CH, Krutzen M, Mann J, Connor RC, Bjeder L, Sherwin WB. Social and genetic interactions drive fitness variation in a free-living dolphin population. Proceedings of the National Academy of Sciences, U.S.A. 2010;107:19949–19954. doi: 10.1073/pnas.1007997107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilby IC, Wrangham RW. Association patterns among wild chimpanzees (Pan troglodytes schweinfurthii) reflect sex differences in cooperation. Behavioral Ecology and Sociobiology. 2008;62:1831–1842. [Google Scholar]
- Henzi SP, Barrett L. Infants as a commodity in a baboon market. Animal Behaviour. 2002;63:915–921. [Google Scholar]
- Henzi SP, Lycett JE, Piper SE. Fission and troop size in a mountain baboon population. Animal Behaviour. 1997a;53:525–535. [Google Scholar]
- Henzi SP, Lycett JE, Weingrill T. Cohort size and the allocation of social effort by female mountain baboons. Animal Behaviour. 1997b;54:1235–1243. doi: 10.1006/anbe.1997.0520. [DOI] [PubMed] [Google Scholar]
- Henzi SP, Lusseau D, Weingrill T, van Schaik CP, Barrett L. Cyclicity in the structure of female baboon social networks. Behavioral Ecology and Sociobiology. 2009;63:1015–1021. [Google Scholar]
- Mitani JC. Male chimpanzees form enduring and equitable social bonds. Animal Behaviour. 2009;77:633–640. [Google Scholar]
- Schülke O, Bhagavatula J, Vigilant L, Ostner J. Social bonds enhance reproductive success in male macaques. Current Biology. 2010;20:2207–2210. doi: 10.1016/j.cub.2010.10.058. [DOI] [PubMed] [Google Scholar]
- Seyfarth RM. A model of social grooming among adult female monkeys. Journal of Theoretical Biology. 1977;65:671–698. doi: 10.1016/0022-5193(77)90015-7. [DOI] [PubMed] [Google Scholar]
- Seyfarth RM. The distribution of grooming and related behaviours among adult female vervet monkeys. Animal Behaviour. 1980;28:798–813. [Google Scholar]
- Seyfarth RM, Cheney DL. The evolutionary origins of friendship. Annual Review of Psychology. 2012;63:153–177. doi: 10.1146/annurev-psych-120710-100337. doi:10.1146/annurev-psych-120710-100337. [DOI] [PubMed] [Google Scholar]
- Silk JB. The adaptive value of sociality in mammalian groups. Philosophical Transactions of the Royal Society B. 2007;362:539–559. doi: 10.1098/rstb.2006.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JB, Alberts SC, Altmann J. Social bonds of female baboons enhance infant survival. Science. 2003;302:1331–1334. doi: 10.1126/science.1088580. [DOI] [PubMed] [Google Scholar]
- Silk JB, Altmann J, Alberts SC. Social relationships among adult female baboons (Papio cynocephalus) I. Variation in the strength of social bonds. Behavioral Ecology and Sociobiology. 2006a;61:183–195. [Google Scholar]
- Silk JB, Alberts SC, Altmann J. Social relationships among adult female baboons (Papio cynocephalus) II. Variation in the quality and stability of social bonds. Behavioral Ecology and Sociobiology. 2006b;61:197–204. [Google Scholar]
- Silk JB, Beehner JC, Berman TJ, Crockford C, Engh AL, Moscovice LR, Wittig RM, Seyfarth RM, Cheney DL. The benefits of social capital: close social bonds among female baboons enhance offspring survival. Proceedings of the Royal Society B. 2009;276:3099–3104. doi: 10.1098/rspb.2009.0681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JB, Beehner JC, Berman TJ, Crockford C, Engh AL, Moscovice LR, Wittig RM, Seyfarth RM, Cheney DL. Strong and consistent social bonds enhance the longevity of female baboons. Current Biology. 2010a;20:1359–1361. doi: 10.1016/j.cub.2010.05.067. [DOI] [PubMed] [Google Scholar]
- Silk JB, Beehner JC, Berman TJ, Crockford C, Engh AL, Moscovice LR, Wittig RM, Seyfarth RM, Cheney DL. Female chacma baboons form strong, equitable, and enduring social bonds. Behavioral Ecology and Sociobiology. 2010b;61:197–204. doi: 10.1007/s00265-010-0986-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidt A, Hofmann SE, Konig B. Not only mate choice matters: fitness consequences of social partner choice in female house mice. Animal Behaviour. 2008;75:801–808. [Google Scholar]
- Wittig RM, Crockford C, Lehmann J, Whitten P, Seyfarth RM, Cheney DL. Focused grooming networks and stress alleviation in wild female baboons. Hormones and Behavior. 2008;54:170–177. doi: 10.1016/j.yhbeh.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee JR, Cavigelli SA, Delgado B, McClintock MK. Reciprocal affiliation among adolescent rats during a mild group stressor predicts mammary tumors and lifespan. Psychosomatic Medicine. 2008;70:1050–1059. doi: 10.1097/PSY.0b013e31818425fb. [DOI] [PMC free article] [PubMed] [Google Scholar]