Abstract
Object
This study investigates a potential novel application of a selective cathepsin B and L inhibitor in experimental intracerebral hemorrhage (ICH) in rats.
Methods
Adult male Wistar rats (n = 40) received an ICH by stereotactic injection of 100 µL of autologous blood or sham via needle insertion into the right striatum. The rats were treated with a selective cathepsin B/L inhibitor (CP-1) or sterile saline intravenously at 2 and 4 hours after injury. Modified neurological severity score (mNSS) and corner turn tests were performed at 1, 4, 7, and 14 days after ICH. The rats were sacrificed at 3 and 14 days post-ICH for immunohistology to analyze tissue loss, neurogenesis, angiogenesis, and apoptosis.
Results
CP-1-treated animals demonstrated significantly reduced apoptosis as well as tissue loss compared to controls (p < 0.05 for each). Neurological function as assessed by mNSS and corner turn tests showed improvement after CP-1 treatment at 7 and 14 days (p < 0.05). Angiogenesis and neurogenesis parameters demonstrated improvement after CP-1 treatment compared to controls (p < 0.05) at 14 days.
Conclusion
This study is the first report of treatment of ICH with a selective cathepsin B/L inhibitor. Cathepsin B/L inhibition has been shown to be beneficial after cerebral ischemia, likely by its upstream regulation of the other prominent cysteine proteases, calpains and caspases. While ICH may not induce a major component of ischemia, the cellular stress in the border zone may activate these proteolytic pathways. The observation that cathepsin B/L blockage is efficacious in this model is provocative for further investigation.
Keywords: Intracerebral hemorrhage, cysteine protease inhibitors, cathepsin B, cathepsin L
Spontaneous intracerebral hemorrhage (ICH) represents 10–15% of all strokes, and it exerts a much worse prognosis than injury caused by ischemic stroke.9 The size of the hematoma formed in the brain parenchyma from the rupture of a cerebral vessel is a significant predictor of poor outcome.40 Inflammation and toxicity triggered by extravascular blood products increase the cerebral damage observed in the early phases after hematoma formation and also lead to worsening edema around the clot.41 Effective surgical and drug approaches for clinical practice have been elusive.9,10,14
Cathepsins B and L are intracellular/lysosomal and secreted cysteine proteases that have been implicated in the process of neuronal cell death after experimental global and focal cerebral ischemia and after experimental spinal cord injury.1,6,,11,16,17,34,38 The levels of these cathepsins are significantly increased and relocate from the lysosomes to the cytoplasm following global cerebral ischemia.. Cathepsin B is one of the causative factors of microglia-induced neuronal apoptosis.32,34 In our previous study, CP-1, a non-toxic cysteine protease inhibitor which is selective for cathepsins B and L, but not the calpains or caspases, was shown to be effective at reducing infarct volume and improving functional scores when administered intravenously to rats after 2 hours of middle cerebral artery occlusion (MCAO) and reperfusion.35
The role of cathepsins in ICH has not been studied. However, results from models of cerebral ischemia suggest that inhibition of cathepsin B and L may be efficacious in preventing neuronal cell death. In this study, we investigated the ability of CP-1 to promote functional and histological recovery after ICH.
Material and Methods
Animal Surgical Procedures
All studies were approved by the Institutional Animal Care and Use Committee. 40 male Wistar rats (Charles River; Wilmington, MA) weighing between 270 and 320 g were selected for this study. An ICH lesion was induced by injecting 100 µl of nonheparinized autologous blood, which was obtained from the femoral vein and placed into a 1cc syringe with a 26½G needle, into the right striatum (3.5 mm lateral to midline, 0.5 mm anterior to bregma, depth 5.5 mm below the surface to midline) at a steady infusion rate of 10 µl per minute. Sham-injured animals were subjected to the same manipulations as ICH rats but no blood was injected.
Cysteine protease inhibitors
CP-1, Cbz-Phe-Ser(OBzl)-CHN2 (Cbz, carbobenoxy; OBzl, O-benzyl), is lipophilic and its molecular mass is 500.5. This inhibitor is chosen based on its relative selectivity for cathepsins B and L. The compound was synthesized and purified according to the method of Shaw et al.36 After synthesis, CP-1 was chromatographed on silica gel with chloroform/methanol (49:1, v/v) and recrystallized from boiling ethyl acetate (mp 125–126 °C). The purity of the compound was checked by High-performance liquid chromatography (HPLC), Thin-layer chromatography (TLC), elemental analysis, infrared spectroscopy, proton nuclear magnetic resonance (1H NMR) and mass spectrometry.4 The effectiveness of CP-1 in vitro was determined by protease inhibition kinetic measurements on purified cathepsin B, cathepsin L, calpain 1 and calpain 2 as described previously.2,3,7,8
The dosing of CP-1 after experimental ICH was based on in vivo dose-response studies that we had previously performed in Wistar rats subjected to focal cerebral ischemia.3,35 CP-1 at 0.35 mg/kg significantly reduced cathepsin B activity in the brain in vivo.1 In the current study, CP-1 was initially dissolved in 100% dimethyl sulfoxide (DMSO), and was blended into a serial dilution of 0.1 mM in 1% DMSO in sterile saline. CP-1 was given intravenously (0.175 mg/kg) at 2 and 4 hours after surgery (total dosage =0.35 mg/kg, while control animals received a same volume infusion of 1% DMSO in sterile saline.
Experimental Groups
This study was randomly divided into four groups: 1) CP-1 group: ICH rats (n = 16) were treated with the selective cathepsin B/L inhibitor; 2) Control group: ICH rats (n = 16) were treated with 1% DMSO in sterile saline; 3) Sham-CP-1 group: Sham rats (n = 4) were treated with CP-1; and 4) Sham-control group: Sham rats (n = 4) were treated with sterile saline. Six rats in the first group and six rats in the second group were killed at Day 3 for TUNNEL staining. The rest of the animals received daily injections of bromodeoxyuridne (BrdU) 100 mg/kg (Sigma, St. Louis, MO) intraperitoneally starting at 24 hours after injury and subsequently for 13 consecutive days. These rats were evaluated for neurological function as described below. After 14 days post-ICH or post-sham procedure the rats from groups 1–4 were sacrificed for immunohistology.
Neurological Functional Studies
Functional outcomes were measured using both the modified neurological severity score (mNSS)12 and the corner turn test43 by an observer blinded to the individual treatment status of the animals. Functional outcomes for each rat were tested at Days 1, 7 and 14 after ICH. The mNSS is used to assess motor, sensory, balance and reflex skills, with higher scores implying greater neurological injury, while the corner turn test measures the number of times that an animal turns to the right or left when placed in a corner.
Histology and Immunohistochemistry
At 14 days postoperatively, the animals were anesthetized with an intraperitoneal injection of ketamine and xylazine and subsequently perfused transcardially with PBS followed by 4% paraformaldehyde. Brain tissues were excised, fixed in formalin and sliced into a series of 2-mm- thick blocks. Each block was processed and embedded in paraffin. A series of adjacent 6-um-thick coronal sections were cut with a microtome from each block. A total of 7 sections from each animal were processed and stained with hematoxylin and eosin (H&E). These sections were tracked using an image analysis system (Data Translation, Marlboro, MA). The intact striatal area of the ipsilateral hemisphere was subtracted from the striatal area of the contralateral hemisphere, and the tissue loss volume was presented as a volume percentage of the lesion compared with the contralateral striatum.
The brain tissue residing between +0.1 – 0.86 mm of the bregma on the third block was the most severely injured and therefore every 40th coronal section from the third block for a total of 6 sections was specifically selected for immunohistochemical staining with the same antibody. Immunohistochemical staining was used for BrdU (1:100; Boehringer Mannheim, Indianapolis, IN), TUJ1 (1:5,000; BABCO, Berkeley, CA), Synaptophysin (1:1000; Millipore, Billerica, MA), and vWF (1:400, Dako, Carpinteria, Calif), as previously described.13,27 Double staining was performed to colocalize the BrdU-reactive cells expressing TUJ1 or VWF, as previously described.13,27 All immunostainings were performed at the same time with the omission of primary antibody and the use of pre-immune serum for quality control of the immunoassay procedure. To identify cellular apoptosis, terminal deoxynucleotidyl nick-end labeling (TUNEL) was performed using an ApopTag peroxidase in situ apoptosis detection Kit (Millipore, Billerica, MA).
Quantification
For semiquantitative measurements, all slides were digitized under a 20× objective lens (Olympus BX40; Olympus Optical Co, Tokyo, Japan) by using a 3D-CCD color video camera (model DXC-970MD; Sony Corp, Tokyo, Japan) interfaced with an image analysis system (Imaging Research, Inc., St. Catharine’s, ON, Canada). The numbers of BrdU-positive cells and the percentage of TUJ1-positive signals were counted in the SVZ; the percentage of synaptophysin-positive signals compared with the corresponding contralateral side and the numbers of apoptosis cells were calculated in the subcortical striatum; and the perimeters of vWF positive vessels were measured in the peri-hemorrhagic striatum. The cells with BrdU (brown stained) that colocalized to the nucleus (hematoxylin stained) were counted as BrdU-positive cells, and the cells stained with Tunnel that showed dark brown, rounded or oval apoptotic bodies were counted as Tunnel-positive cells.
Statistical Analysis
Statistical analysis of neurological functional scores, areas of ICH-related tissue losses, and immunohistochemical results were all performed using the independent Student t-test. Data were presented as the mean ± standard error, and p values < 0.05 were considered significant. All measurements were performed by observers blinded to individual treatments.
Results
Neurobehavioral tests
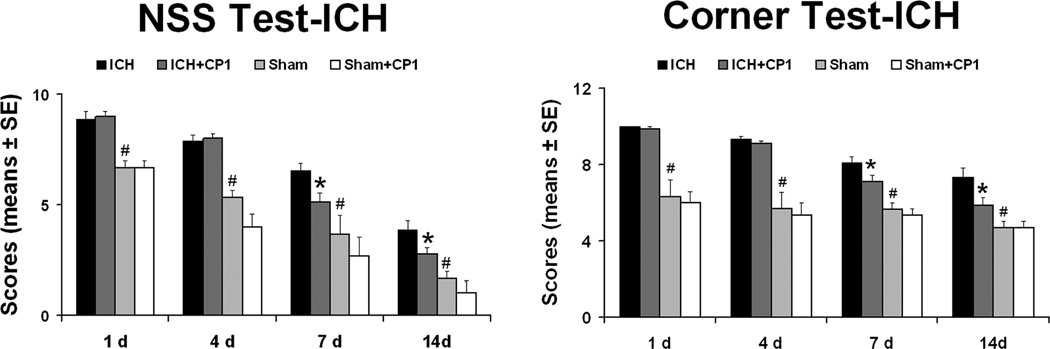
Figure 1 presented the results for the cornering and mNSS tests. All ICH rats displayed similar and marked neurological impairments at Day 1 after injury compared to the sham-operated groups. Before the first week post-ICH there were still no significant differences observed between the control group and CP-1 group. At 1 and 2 weeks post-ictus cornering scores demonstrated that the CP-1 group improved significantly compared to the control group. Similarly, improvement of neurological function assessed using mNSS was significant at 1 and 2 weeks post-ICH compared to the control group; however, no significant differences were observed in mNSS and corner turn test results between the sham-control group and sham-CP1 group during the two weeks of experiments.
Fig. 1.
CP-1 improves neurological outcome. Quantitative bar graph results of NSS and corner turn test of control and treatment groups are presented in the left and middle panels, respectively. Statistical significance levels are: *p < 0.05 CP-1 group compared with control group. #p < 0.05 control group compared with sham control group. @ p < 0.05 sham control group compared with sham CP-1 group.
Tissue loss measurements
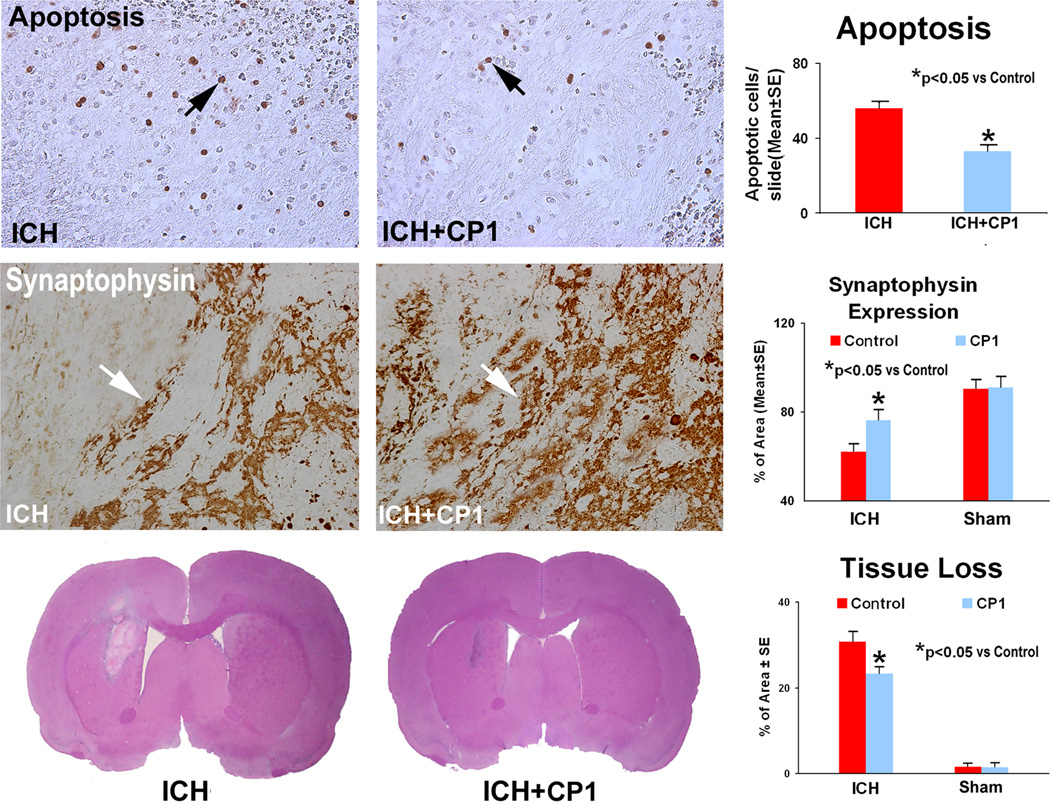
Using this model of ICH, we have demonstrated consistent results measured by MRI and immunohistological methods. After breakdown or resorption of the hematoma, there is an area of tissue loss in this model.24,33 The areas of striatal tissue loss as a percentage of the normal contralateral side obtained from H&E histology were as follows: sham-control group, 1.56 ± 0.85%; sham-CP-1 group, 1.48 ± 1.09%; control group, 30.67 ± 2.46%; and CP-1 group, 23.22 ± 1.61% (Fig. 4). Statistically, the CP-1 group showed significantly reduced tissue loss compared to control group at 2 weeks after ICH.
Fig. 4.
Immunostaining for TUNEL and synaptophysin along the boundary area around the hematoma and tissue loss at 2 weeks after ICH. The number of apoptotic cells is reduced in the CP-1 treatment group compared to the control group. A higher percentage per area of cells positively reacting to synaptophysin is seen in the sections derived from CP-1-treated versus control rats. Quantitative immunoreactivities for both groups are presented as bar graphs on the right side of each panel. H&E staining showing representative sections and bar graphs of quantitative striatal tissue loss percentages in the ICH region relative to the contralateral normal region of four groups are shown in the bottom panels.
Cell Proliferation and Differentiation
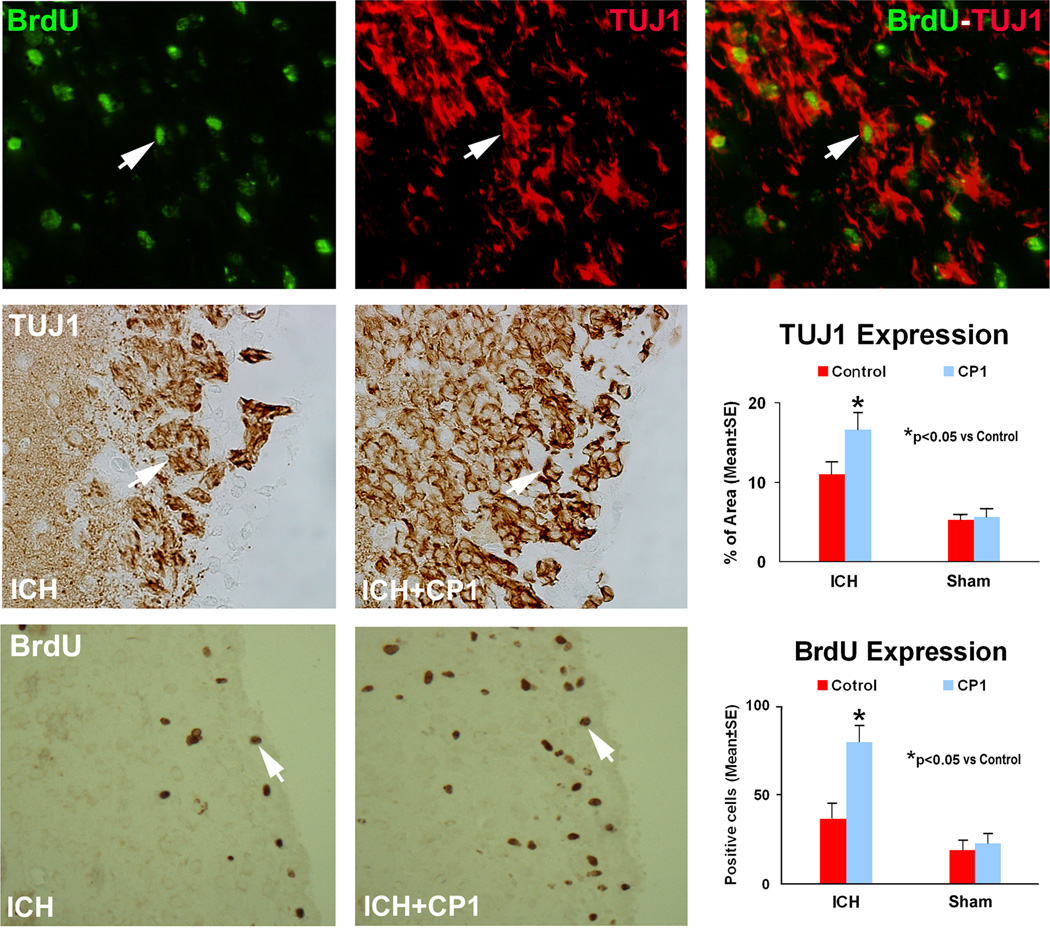
Endogenous cell proliferation and differentiation are believed to contribute to neurological functional recovery after ICH. To test if post-ICH treatment with CP-1 may have an effect on this process, BrdU and TUJ1 immunostainings were performed. The ipsilateral subventricular zone (SVZ) demonstrated significant increase in the number of newly formed cells in the CP-1-treated groups at 2 weeks post-ICH, compared to the control group (p < 0.05) (Fig. 2). CP-1 treatment similarly significantly enhanced the TUJ1 expression over the control group. A small number of BrdU-positive cells were colabeled with TUJ1, suggesting that only limited, newly formed cells represent neurogenesis. In each hemispheric section of the sham-operated rats and in the contralateral hemisphere of ICH rats, there were limited positive signals for BrdU and TUJ1 stainings, and no significant difference between the two sham groups.
Fig. 2.
Immunostaining of the control and treatment sections showing the quantitative immunoreactivities of BrdU and TUJ1. Quantitative immunoreactivities for both groups are presented as bar graphs on the right side of each panel. Colocalization of BrdU and TUJ1 in a subpopulation of cells near the injured region of the combination group is shown on the upper panel. Arrows indicate cells positively stained for both BrdU and TUJ1.
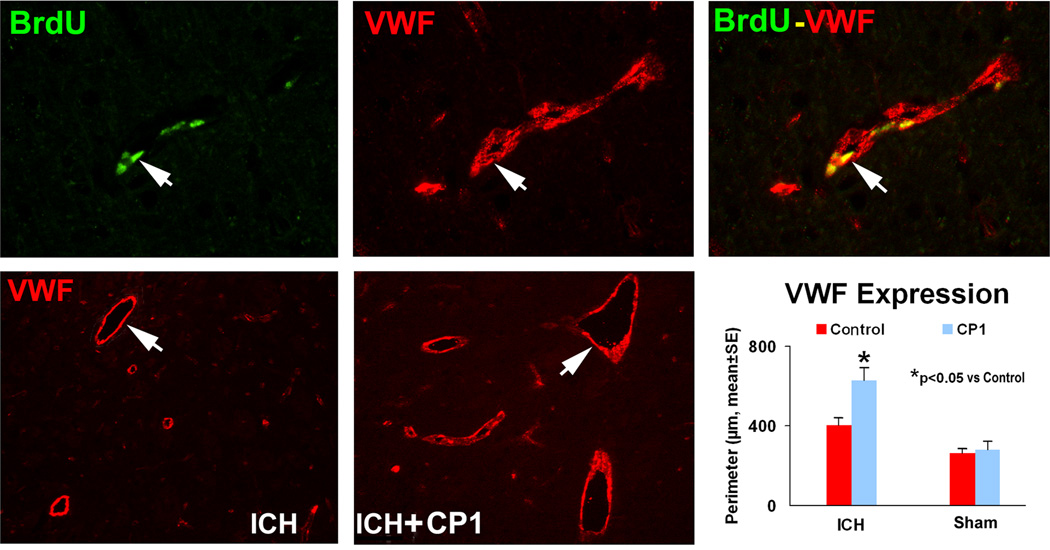
Angiogenesis
Angiogenesis is described as the sprouting of new blood vessels from pre-existing vessels. Cerebral vascular perimeters (e.g., von Willibrand factor) were significantly increased in the CP1 treatment group compared with the control group (p < 0.05) (Fig. 3). Double immunostaining revealed a subpopulation of cells that express a vascular marker while still proliferating, suggesting that this subpopulation of cells positive for vascular phenotype is newly formed during the recovery stage.
Fig. 3.
Representative immunostaining and quantitative immunoreactivities of VWF and BrdU in the ipsilateral hemisphere at 2 weeks after ICH. Upper panels show BrdU (left) and VWF (center) immunostaining and colocalization of BrdU-VWF (right) for a subpopulation of cells located near the injured site for a CP-1-treated animal. Arrows indicate cells that stained positively for both BrdU and VWF. VWF results are shown in the lower panels for control (left) and CP-1 (center) treatment groups, respectively. Quantitative immunoreactivities for both groups are presented as bar graphs in the lower right panel.
Apoptosis
TUNEL staining showed apoptotic cells with typical dark brown, rounded or oval apoptotic bodies (Fig. 4). Scattered apoptotic cells were present throughout the damaged tissue, the vast majority of which were located along the boundary zone of the hematoma. The number of apoptotic cells is significantly reduced in the ipsilateral hemisphere in the CP-1 treatment groups compared to the control group (p < 0.05).
Discussion
Accumulating evidence has suggested a prominent role for cathepsins B and L in the pathogenesis of brain ischemia.1,22,25,30,35 Cathepsins B and L have been shown to be upregulated in microvessels and adjacent glial cells or neurons after brain ischemia.18 Broad specificity cysteine protease inhibitors and selective cathepsins B and L inhibitors have been shown to promote neurological recovery following stroke.35,39 While ICH may not have a major component of ischemia, the cellular stress along the border zone may activate these proteolytic pathways. The findings from our current study suggest that early treatment with CP-1 after ICH improves neurological function and reduces tissue loss, supporting the hypothesis that cathepsins B and L play an important role in ICH.
The underlying damaging effects of cathepsins following brain injury are partially associated with apoptosis and necrotic cell death.34,42 Microglial-secreted cathepsin B induces neuronal apoptosis and activates the proinflammatory caspases 11 and 1.19,25,32 Furthermore, cathepsin B can cleave the Bcl-2 family member Bid, which may lead to cytochrome-c release from the mitochondria and subsequent caspase activation.21 Cathepsin L is also implicated in apoptosis.26 In line with these findings, the selective inhibitor for cathepsins B/L reduced the number of apoptotic cells at 2 weeks post-ICH, which may contribute to neurological recovery. Regarding necrotic cell death, we described previously that cathepsin B/L inhibition reduces loss of tissue after cerebral ischemia: one of the postulated mechanisms being reduction of the lysosome-mediated cell destruction.34 In the cerebral hemorrhage model 10–20% of cell death is due to necrosis from direct injury from the clot. This suggests that CP-1 may improve post ICH recovery by interfering with the cathepsin B/L-induced apoptosis as well as the necrotic cell death pathway.10,20,41
Neurogenesis has been demonstrated in animal models and adult human brain after ICH,5,13 and this may contribute to brain repair and functional recovery13,44 either by cell replacement or by indirect mechanisms such as cytoprotective or trophic factors. Inflammatory processes, which also play a vital role in secondary brain injury following the initial hematoma post ICH, may block neurogenesis.15,29 Cathepsins represent one of the important factors involved in necrosis and the inflammatory cascade,42 and inhibition of cathepsins B and L by CP-1 in the current study enhanced endogenous neurogenesis after ICH. Therefore, we postulate that CP-1 may facilitate endogenous neurogenesis because it interferes with the inflammatory enzymatic cascades. Cystatin C, a potent endogenous inhibitor of lysosomal proteinases including cathepsins B and L, has also been shown to stimulate self-renewing, multipotent neural stromal cells in vitro and neurogenesis in vivo.31 CP-1 may be functioning with a similar mechanism as cystatin C in regards to neurogenesis.
Neurological functional recovery has been attributed to synaptic reorganization after stroke and brain trauma.13,27 Synaptophysin expression is an indicator of synaptogenesis.28 In our study, synaptophysin density is significantly increased in the boundary zone around the hematoma region 2 weeks after CP-1 treatment, which suggests that CP-1 treatment may either directly protect the synapses from damage or enhance new synaptic formation.
An interesting finding is that CP-1 treatment at the acute period promotes endogenous angiogenesis following ICH. This may relate to the neuroprotective effect of CP-1, whereby cell survival and recovery is promoted by cathepsin B/L inhibitor during the first day after injury. Cathepsin cysteine proteases, however, have been reported to be effectors of invasive growth and angiogenesis during multistage tumorigenesis.23 Moreover, deficiency of these cysteine proteases can impair microvessel growth. The main effect of cathepsins is to penetrate the extracellular matrix to form vessel sprouts. After MCAO, new capillary buds start to appear in the ischemic bed 3 days post-injury, and angiogenesis is induced after 7 days of focal cerebral ischemia.37 Following acute treatment of ICH in our study, CP-1 is expected to limit the initial cathepsin surge after injury. However, cathepsin activity is restored subacutely by the creation of new enzyme, thereby being available to promote neurological recovery with angiogenesis at the later timepoints. Whether CP-1 has a direct effect on angiogenesis after ICH cannot be determined by the current study.
Conclusion
This is the first study demonstrating that post-ICH administration of a cysteine protease inhibitor selective for cathepsins B and L significantly reduces tissue loss and improves neurological function in the rat. One likely mechanism is neuroprotection as demonstrated by decreased neuronal apoptosis following CP-1 treatment, substantiating the involvement of cathepsins B and/or L in neuronal cell death after ICH. This suggests that the selective inhibitor improves cellular survival along the ICH border independently from direct effects on the calpain or caspase enzyme system. In addition to the observed neuroprotective effect of CP-1, the ICH region also shows post-treatment evidence of neuronal recovery with neuroplasticity and angiogenesis.
Acknowledgment
Special thanks to Susan MacPhee-Gray for editorial assistance.
Sources of Funding
Supported by National Institute of Health grant RO1 NS058581-01 (D.M.S.).
Footnotes
Disclosure
The authors do not report any conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
References
- 1.Anagli J, Abounit K, Stemmer P, Han Y, Allred L, Weinsheimer S, et al. Effects of cathepsins b and l inhibition on postischemic protein alterations in the brain. Biochem Biophys Res Commun. 2008;366:86–91. doi: 10.1016/j.bbrc.2007.11.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anagli J, Hagmann J, Shaw E. Affinity labelling of the Ca2+-activated neutral proteinase in intact human platelets. Biochem J. 1993;289:93–99. doi: 10.1042/bj2890093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anagli J, Hagmann J, Shaw E. Investigation of the role of calpain as a stimulus-response mediator in human platelets using New Synthetic Inhibitors. Biochem J. 1991;274:497–502. doi: 10.1042/bj2740497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angliker H, Anagli J, Shaw E. Inactivation of calpain by peptidyl fluoro-methylketones. J Med Chem. 1992;35:216–220. doi: 10.1021/jm00080a003. PMID: 1732539. [DOI] [PubMed] [Google Scholar]
- 5.Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 6.Benchoua A, Braudeau J, Reis A, Couriaud C, Onteniente B. Activation of proinflammatory caspases by cathepsin b in focal cerebral ischemia. J Cereb Blood Flow Metab. 2004;24:1272–1279. doi: 10.1097/01.WCB.0000140272.54583.FB. [DOI] [PubMed] [Google Scholar]
- 7.Betts R, Anagli J. The β- and γ-CH2 of B27-WT’s Leu11 and Ile18 side chains play a direct role in calpain inhibition. Biochemistry. 2004;43:2596–2604. doi: 10.1021/bi0359832. [DOI] [PubMed] [Google Scholar]
- 8.Betts R, Weinsheimer S, Blouse G, Anagli J. Structural determinants of the valpain inhibitory activity of calpastatin peptide B27-WT. J Biol Chem. 2003;278:7800–7809. doi: 10.1074/jbc.M208350200. [DOI] [PubMed] [Google Scholar]
- 9.Broderick J, Connolly S, Feldmann E, Hanley D, Kase C, Krieger D, et al. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: A guideline from the American Heart Association/American Stroke Association stroke council, high blood pressure research council, and the quality of care and outcomes in research interdisciplinary working group. Circulation. 2007;116:e391–e413. doi: 10.1161/CIRCULATIONAHA.107.183689. [DOI] [PubMed] [Google Scholar]
- 10.Bullock R, Mendelow AD, Teasdale GM, Graham DI. Intracranial haemorrhage induced at arterial pressure in the rat. Part 1: Description of technique, icp changes and neuropathological findings. Neurol Res. 1984;6:184–188. doi: 10.1080/01616412.1984.11739687. [DOI] [PubMed] [Google Scholar]
- 11.Chaitanya GV, Babu PP. Activation of calpain, cathepsin-b and caspase-3 during transient focal cerebral ischemia in rat model. Neurochem Res. 2008;33:2178–2186. doi: 10.1007/s11064-007-9567-7. [DOI] [PubMed] [Google Scholar]
- 12.Chen J, Sanberg PR, Li Y, Wang L, Lu M, Willing AE, et al. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke. 2001;32:2682–2688. doi: 10.1161/hs1101.098367. [DOI] [PubMed] [Google Scholar]
- 13.Chen J, Zhang ZG, Li Y, Wang Y, Wang L, Jiang H, et al. Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Ann Neurol. 2003;53:743–751. doi: 10.1002/ana.10555. [DOI] [PubMed] [Google Scholar]
- 14.Cheung RT. Update on medical and surgical management of intracerebral hemorrhage. Rev Recent Clin Trials. 2007;2:174–181. doi: 10.2174/157488707781662751. [DOI] [PubMed] [Google Scholar]
- 15.Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci U S A. 2003;100:13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellis RC, Earnhardt JN, Hayes RL, Wang KK, Anderson DK. Cathepsin b mrna and protein expression following contusion spinal cord injury in rats. J Neurochem. 2004;88:689–697. doi: 10.1046/j.1471-4159.2003.02197.x. [DOI] [PubMed] [Google Scholar]
- 17.Ellis RC, O'Steen WA, Hayes RL, Nick HS, Wang KK, Anderson DK. Cellular localization and enzymatic activity of cathepsin b after spinal cord injury in the rat. Exp Neurol. 2005;193:19–28. doi: 10.1016/j.expneurol.2004.11.034. [DOI] [PubMed] [Google Scholar]
- 18.Fukuda S, Fini CA, Mabuchi T, Koziol JA, Eggleston LL, Jr, del Zoppo GJ. Focal cerebral ischemia induces active proteases that degrade microvascular matrix. Stroke. 2004;35:998–1004. doi: 10.1161/01.STR.0000119383.76447.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gan L, Ye S, Chu A, Anton K, Yi S, Vincent VA, et al. Identification of cathepsin b as a mediator of neuronal death induced by a beta-activated microglial cells using a functional genomics approach. J Biol Chem. 2004;279:5565–5572. doi: 10.1074/jbc.M306183200. [DOI] [PubMed] [Google Scholar]
- 20.Gong C, Boulis N, Qian J, Turner DE, Hoff JT, Keep RF. Intracerebral hemorrhage-induced neuronal death. Neurosurgery. 2001;48:875–882. doi: 10.1097/00006123-200104000-00037. discussion 882-873. [DOI] [PubMed] [Google Scholar]
- 21.Guicciardi ME, Deussing J, Miyoshi H, Bronk SF, Svingen PA, Peters C, et al. Cathepsin b contributes to tnf-alpha-mediated hepatocyte apoptosis by promoting mitochondrial release of cytochrome c. J Clin Invest. 2000;106:1127–1137. doi: 10.1172/JCI9914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hill IE, Preston E, Monette R, MacManus JP. A comparison of cathepsin b processing and distribution during neuronal death in rats following global ischemia or decapitation necrosis. Brain Res. 1997;751:206–216. doi: 10.1016/s0006-8993(96)01403-5. [DOI] [PubMed] [Google Scholar]
- 23.Joyce JA, Baruch A, Chehade K, Meyer-Morse N, Giraudo E, Tsai FY, et al. Cathepsin cysteine proteases are effectors of invasive growth and angiogenesis during multistage tumorigenesis. Cancer Cell. 2004;5:443–453. doi: 10.1016/s1535-6108(04)00111-4. [DOI] [PubMed] [Google Scholar]
- 24.Karki K, Knight RA, Han Y, Yang D, Zhang J, Ledbetter KA, et al. Simvastatin and atorvastatin improve neurological outcome after experimental intracerebral hemorrhage. Stroke. 2009;40 doi: 10.1161/STROKEAHA.108.544395. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kingham PJ, Pocock JM. Microglial secreted cathepsin b induces neuronal apoptosis. J Neurochem. 2001;76:1475–1484. doi: 10.1046/j.1471-4159.2001.00146.x. [DOI] [PubMed] [Google Scholar]
- 26.Li W, Kornmark L, Jonasson L, Forssell C, Yuan XM. Cathepsin l is significantly associated with apoptosis and plaque destabilization in human atherosclerosis. Atherosclerosis. 2009;202:92–102. doi: 10.1016/j.atherosclerosis.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 27.Lu D, Goussev A, Chen J, Pannu P, Li Y, Mahmood A, Chopp M. Atorvastatin reduces neurological deficit and increases synaptogenesis, angiogenesis, and neuronal survival in rats subjected to traumatic brain injury. J Neurotrauma. 2004;21:21–32. doi: 10.1089/089771504772695913. [DOI] [PubMed] [Google Scholar]
- 28.Masliah E, Fagan AM, Terry RD, DeTeresa R, Mallory M, Gage FH. Reactive synaptogenesis assessed by synaptophysin immunoreactivity is associated with gap-43 in the dentate gyrus of the adult rat. Exp Neurol. 1991;113:131–142. doi: 10.1016/0014-4886(91)90169-d. [DOI] [PubMed] [Google Scholar]
- 29.Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- 30.Nitatori T, Sato N, Kominami E, Uchiyama Y. Participation of cathepsins b, h, and l in perikaryal condensation of ca1 pyramidal neurons undergoing apoptosis after brief ischemia. Adv Exp Med Biol. 1996;389:177–185. doi: 10.1007/978-1-4613-0335-0_22. [DOI] [PubMed] [Google Scholar]
- 31.Pirttila TJ, Lukasiuk K, Hakansson K, Grubb A, Abrahamson M, Pitkanen A. Cystatin c modulates neurodegeneration and neurogenesis following status epilepticus in mouse. Neurobiol Dis. 2005;20:241–253. doi: 10.1016/j.nbd.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 32.Schotte P, Van Criekinge W, Van de Craen M, Van Loo G, Desmedt M, Grooten J, et al. Cathepsin b-mediated activation of the proinflammatory caspase-11. Biochem Biophys Res Commun. 1998;251:379–387. doi: 10.1006/bbrc.1998.9425. [DOI] [PubMed] [Google Scholar]
- 33.Seyfried DM, Han Y, Yang D, Ding J, Savant-Bhonsale S, Shukairy MS, et al. Mannitol enhances delivery of marrow stromal cells to the brain after experimental intracerebral hemorrhage. Brain Res. 2008;1224:12–19. doi: 10.1016/j.brainres.2008.05.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seyfried D, Han Y, Zheng Z, Day N, Moin K, Rempel S, et al. Cathepsin b and middle cerebral artery occlusion in the rat. J Neurosurg. 1997;87:716–723. doi: 10.3171/jns.1997.87.5.0716. [DOI] [PubMed] [Google Scholar]
- 35.Seyfried DM, Veyna R, Han Y, Li K, Tang N, Betts RL, et al. A selective cysteine protease inhibitor is non-toxic and cerebroprotective in rats undergoing transient middle cerebral artery ischemia. Brain Res. 2001;901:94–101. doi: 10.1016/s0006-8993(01)02289-2. [DOI] [PubMed] [Google Scholar]
- 36.Shaw E, Wikstrom P, Ruscica J. An exploration of the primary specificity site of cathepsin b. Arch Biochem Biophys. 1983;222:424–429. doi: 10.1016/0003-9861(83)90540-4. [DOI] [PubMed] [Google Scholar]
- 37.Shen F, Su H, Fan Y, Chen Y, Zhu Y, Liu W, et al. Adeno-associated viral-vector-mediated hypoxia-inducible vascular endothelial growth factor gene expression attenuates ischemic brain injury after focal cerebral ischemia in mice. Stroke. 2006;37:2601–2606. doi: 10.1161/01.STR.0000240407.14765.e8. [DOI] [PubMed] [Google Scholar]
- 38.Tsubokawa T, Solaroglu I, Yatsushige H, Cahill J, Yata K, Zhang JH. Cathepsin and calpain inhibitor e64d attenuates matrix metalloproteinase-9 activity after focal cerebral ischemia in rats. Stroke. 2006;37:1888–1894. doi: 10.1161/01.STR.0000227259.15506.24. [DOI] [PubMed] [Google Scholar]
- 39.Tsubokawa T, Yamaguchi-Okada M, Calvert JW, Solaroglu I, Shimamura N, Yata K, et al. Neurovascular and neuronal protection by e64d after focal cerebral ischemia in rats. J Neurosci Res. 2006;84:832–840. doi: 10.1002/jnr.20977. [DOI] [PubMed] [Google Scholar]
- 40.Wagner KR, Broderick JP. Hemorrhagic stroke: Pathophysiological mechanisms and neuroprotective treatments. In: Lo EH, Marwah J, editors. Neuroprotection. Scottsdale: Prominent Press; 2002. pp. 471–508. [Google Scholar]
- 41.Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006;5:53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]
- 42.Yamashima T, Oikawa S. The role of lysosomal rupture in neuronal death. Prog Neurobiol. 2009 doi: 10.1016/j.pneurobio.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 43.Zhang L, Schallert T, Zhang ZG, Jiang Q, Arniego P, Li Q, et al. A test for detecting long-term sensorimotor dysfunction in the mouse after focal cerebral ischemia. J Neurosci Methods. 2002;117:207–214. doi: 10.1016/s0165-0270(02)00114-0. [DOI] [PubMed] [Google Scholar]
- 44.Zhang RL, Zhang ZG, Chopp M. Ischemic stroke and neurogenesis in the subventricular zone. Neuropharmacology. 2008;55:345–352. doi: 10.1016/j.neuropharm.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]