Abstract
Highly active antiretroviral therapy (HAART) decreases plasma viremia below the limits of detection in the majority of HIV-infected individuals, thus serving to slow disease progression. However, HAART targets only actively replicating virus and is unable to eliminate latently infected, resting CD4+ T cells. Such infected cells are potentially capable of reinitiating virus replication upon cessation of HAART, thus leading to viral rebound. Agents that would eliminate these reservoirs, when used in combination with HAART, could thus provide a strategy for the eradication of HIV. Prostratin is a preclinical candidate that induces HIV expression from latently infected CD4+ T cells, potentially leading to their elimination through a virus-induced cytopathic effect or host anti-HIV immunity. Here, we report the synthesis of a series of designed prostratin analogs and report in vitro and ex vivo studies of their activity relevant to induction of HIV expression. Members of this series are up to 100-fold more potent than the preclinical lead (prostratin) in binding to cell-free PKC, and in inducing HIV expression in a latently infected cell line and prostratin-like modulation of cell surface receptor expression in primary cells from HIV-negative donors. Significantly, selected members were also tested for HIV induction in resting CD4+ T cells isolated from infected individuals receiving HAART and were found to exhibit potent induction activity. These more potent agents and by extension related tunable analogs now accessible through the studies described herein should facilitate research and preclinical advancement of this strategy for HIV/AIDS eradication.
Keywords: HIV latency, NF-κB, PKC-δ, bryostatin
HIV/AIDS is a catastrophic pandemic (1). Over 34 million people worldwide are living with HIV (2). Over 2.7 million new infections were estimated for 2010. In the same year, 1.8 million infected individuals died of the disease. Current therapy for the treatment of HIV involves administration of a combination of antiretroviral agents, collectively referred to as highly active antiretroviral therapy (HAART), which serves to reduce HIV plasma viremia of many infected individuals to undetectable levels, thereby slowing disease progression (3). HAART, however, is not curative. Reservoirs of HIV-infected cells persist in infected individuals receiving HAART despite years to decades of therapy. As a result, interruption of HAART could potentially lead to plasma viral rebound that putatively is supplied by the latent provirus in infected CD4+ T cells and other persistent HIV reservoirs (4, 5). As such, HAART must be used chronically, leading to concerns regarding side effects, compliance, cost, and the generation of viral resistance to the drugs.
The most extensively studied persistent HIV reservoirs identified to date are latently infected, resting memory CD4+ T cells (6). These long-lived cells are activated when presented with a specific antigen or by cytokine stimulation, leading to concomitant bursts of viral production. It is estimated that an HIV-positive individual harbors about 1 million such cells, which, with an estimated half-life of 44 mo for elimination, would take over 70 y to be naturally depleted (7).
Increasing research attention has been directed at the development of strategies that would eliminate the latent viral reservoir, which with concomitant HAART would provide for HIV eradication or a functional cure. For this approach, it has been proposed that certain agents might be used in combination with HAART to induce HIV activation and therefore depletion of reservoir cells through a cytopathic effect associated with virus production. It is also possible that a combination of inducers and HIV-specific agents would provide a complementary or synergistic strategy for clearance of infected cells (8, 9). Immunotoxins, for example, consisting of an antibody to target the HIV envelope protein on the surface of productively infected cells (10) and toxins to kill the cell, represent one strategy to eliminate reservoirs more effectively in conjunction with latency activating agents. Several agents have been evaluated for their capacity to activate HIV from latency, including general immune activators such as IL-2 and anti-CD3 antibody, and histone deacetylase inhibitors (valproic acid). Although promising, these inducing agents suffer from toxicity, lack of potency, or both (11). Despite the advances over the last decade in our understanding of factors contributing to HIV latency, there has been a paucity of novel small-molecule agents that potently induce reservoir clearance. Very recently, however, it was demonstrated that administration of suberoylanilide hydroxamic acid to HIV-infected subjects with undetectable viremia led to induction of HIV expression in vivo (12). In addition, prostratin and more recently bryostatin and its analogs have also emerged as lead candidates in such studies directed at reservoir clearance (13–15).
The natural product prostratin 3 has figured prominently in studies directed at purging latently infected, resting CD4+ T cells and is currently in preclinical development (15, 16). Although prostratin was originally isolated from Pimelea prostrata by Hecker and coworkers in the 1970s (17), interest in its therapeutic potential was intensified in the early 1990s when it was found by Cox and a team of National Institutes of Health scientists to be the active component of the Samoan medicinal plant Homalanthus nutans (18). Prostratin is thought to elicit its biological effects wholly or in part by binding to the diacylglycerol binding domain of protein kinase C (PKC), leading to its activation, translocation to cellular membranes, and downstream signaling (19). Unlike the structurally related phorbol esters, prostratin is not a tumor promoter or an irritant, and furthermore, it protects against the tumor-promoting effects of these agents (20, 21). In addition to having selective inhibitory activity against specific cancer cell lines (22), prostratin has been found to have unique activity against HIV.
Prostratin had been shown to elicit three distinct effects relevant to HIV treatment. It causes down-regulation of CD4, C-X-C chemokine receptor type 4 (CXCR4), and in some cases C-C chemokine receptor type 5 (CCR5), thereby protecting CD4+ T cells from HIV-1 entry (23, 24). Other cell surface receptors, such as the early activation marker CD69, are up-regulated, whereas the late activation marker CD25 is little affected by prostratin treatment (25). In acutely infected cells, prostratin enhances cell survival possibly due to cytostatic effects (18). Most importantly, in latently infected cells, prostratin stimulates viral replication putatively through PKC-mediated phosphorylation of IκB kinase, which enables release and penetration of the transcription factor NF-κB into the cell nucleus (26), where its binding to the HIV LTR leads to viral gene expression and subsequent replication of the virus.
Until recently, research on prostratin has relied exclusively on plant sources that produce prostratin but generally in only variable and low isolation yields (27). In 2008, we reported a step-economical (five steps) synthesis of prostratin from phorbol 5, a readily available constituent of croton oil (28). This synthesis not only provides a reliable and scalable supply of prostratin but it also allows access to derivatives including nonnatural analogs needed to establish the structural basis for its activity and thereby to identify superior candidates. Herein, we describe an adaptation of the reported synthesis designed to rapidly access prostratin analogs and disclose the biological activities of these agents pertinent to latency induction, including PKC binding, modulation of cell surface receptor levels in cells from healthy donors, and induction of latent virus in a model cell line and in cells obtained from patients on suppressive therapy. These agents outperform prostratin, with some being 100-fold more potent than the current lead clinical candidate.
Results and Discussion
Designed Analog Synthesis.
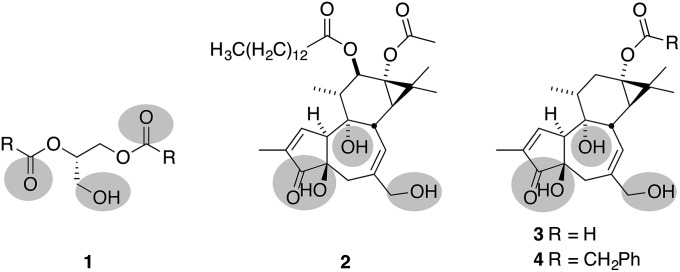
Previously, we proposed that the PKC affinities exhibited by tigliane natural products (e.g., phorbol esters) could be attributed to a subset of hydrogen bond donors and acceptors, with priority given to the oxygens at C20, C3/C4, and C9, which colocate spatially with similar donors and acceptors in the endogenous ligand, diacylglycerol (1) (Fig. 1) (29). In this model, the lipids of the CD-ring system are thought to influence membrane insertion (both depth and orientation) of the bound PKC–ligand complex and thus indirectly the conformation, affinity, and catalytic function of the complex. This analysis is consistent with the observation that 12-deoxyphorbol-13-phenylacetate (DPP) (4) is 10-fold more potent than prostratin in PKC binding assays (19), 20–40 times more potent at inducing viral production in latently infected cell lines (30), and at least five times more potent at inducing viral expression from human peripheral blood mononuclear cells (huPBMCs) from HAART-suppressed patients (31). With this understanding, we sought to investigate whether modifications at C13 could be used to achieve more potent analogs with better pharmacological properties. To achieve this goal, we designed a diversification strategy in which a readily available precursor can be chemoselectively derivatized at C13 and the resultant product deprotected in one step to rapidly produce analogs. Overall, this now successful strategy provides unique access to a wide range of analogs for research and clinical advancement from a readily available and renewable source, i.e., phorbol derived from croton oil.
Fig. 1.
Pharmacophore model highlighting triad of oxygenation proposed for binding to the PKC-C1 regulatory domain in diacylglycerol (1), phorbol-12-myristate-13-acetate (2), prostratin (3; R = CH3), and DPP (4; R = CH2Ph).
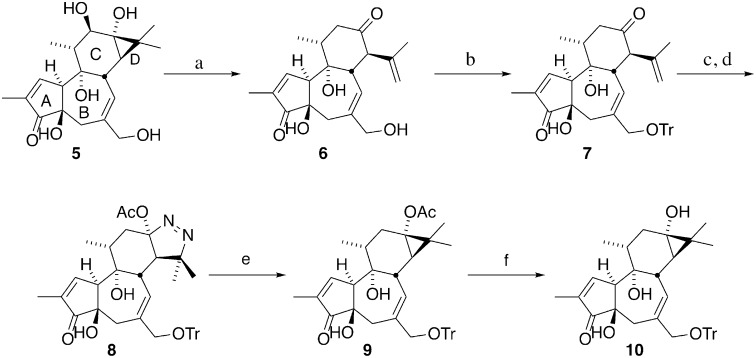
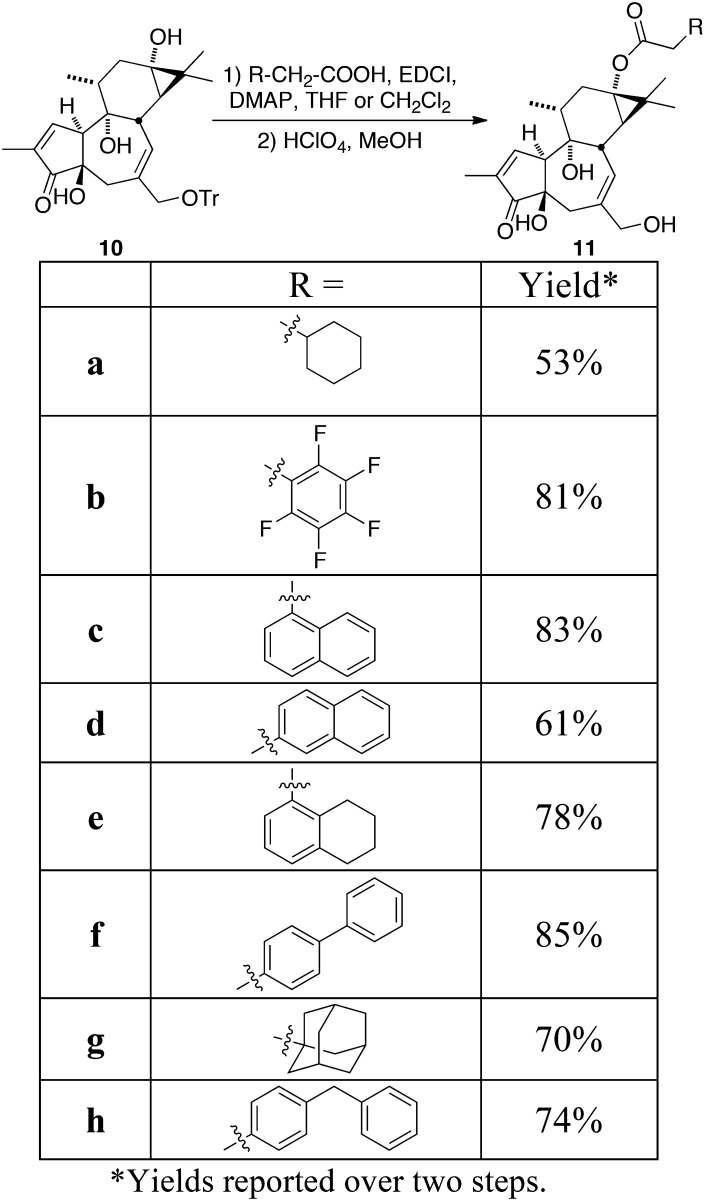
Our synthetic route to this diversifiable precursor is given in Fig. 2. Previous work in our group addressed the problem of prostratin supply by converting phorbol (5), a relatively abundant natural product, to prostratin in five synthetic steps (28). Drawing on this strategy, the synthesis described herein is designed to furnish a stable late-stage intermediate containing the desired free C13 hydroxyl (10), which can be converted to analogs in only two steps (Fig. 2). In this sequence, phorbol is subjected to acid hydrolysis, which effectively removes the undesired C12 hydroxyl group, and cleaves the cyclopropane ring to afford crotophorbolone (6). The isopropenyl moiety resulting from the opening of the D-ring provides the needed functional handle to reclose this ring. At this point, the C20 hydroxyl group of crotophorbolone is converted to a trityl ether, thereby serving to remove the more reactive C20 alcohol from competing with the C13 hydroxyl group for derivatization. Tritylation also renders compounds in the synthetic route soluble in a broader variety of solvents than the parent alcohols. Treatment of the ketone (7) with hydrazine hydrate forms a hydrazone, which upon heating with diisopropylethyl amine in toluene cyclizes to an air-sensitive pyrazoline. After cyclization is complete, the reaction mixture is cooled to −78 °C and treated with a solution of lead tetraacetate in dichloromethane to afford the oxidized cyclic diazene (8) in an isolated yield of up to 59% over two steps from trityl-crotophorbolone. The use of toluene in combination with diisopropylethyl amine instead of pyridine allows the cyclization and oxidation reactions to be conducted in one flask, thus shortening the synthetic route. Diazene (8) is then photolyzed to extrude nitrogen, providing C20-O-trityl-prostratin (9). Saponification of the C13 acetate moiety using barium hydroxide in methanol reveals the desired C13 alcohol (10), the diversification point for analog synthesis. Steglich esterification with various carboxylic acids and subsequent C20 deprotection affords a range of prostratin analogs in two steps from the diversification point 10. The structures produced in this study are summarized in Fig. 3. It is worth noting that the current sequence introduces the photolytic extrusion of nitrogen before esterification of C13, thereby avoiding problems that could arise with photolabile C13 esters if photoextrusion were done after C13 ester introduction. Additionally, this diversification sequence requires only two simple steps (selective C13 esterification and C20 hydrolysis) from the diversification point 10, whereas the original sequence involved a more restrictive introduction of C13 through oxidative formation of a C13–O bond.
Fig. 2.
Synthesis of C20-O-trityl-prostratin-ol (10). Reagents and conditions: (a) H2SO4, H2O, 90 °C, 40% yield; (b) trityl chloride, pyridine, 50 °C, 94–96% yield; (c) N2H4•H2O, AcOH, MeOH; (d) iPr2NEt, toluene, 140 °C, then Pb(OAc)4, CH2Cl2, −78 °C → room temperature, 50–59% yield over two steps; (e) hν (350 nM), EtOAc, 64% yield; and (f) Ba(OH)2•8H2O, MeOH, 89% yield.
Fig. 3.
Summary of analogs synthesized.
PKC Binding Affinity.
Given that PKC is proposed to mediate latent virus activation, we evaluated the PKC affinities of the analogs relative to the lead compounds, prostratin and DPP, by testing for binding to PKC-δ (Table 1) using a standard radiolabeled ligand displacement assay. In this assay, prostratin and DPP exhibit PKC binding affinities of 21.8 and 3.1 nM, respectively, consistent with previous reports (19). Significantly, each analog bound PKC-δ with a higher affinity than either prostratin or DPP, all exhibiting Ki values of less than 2 nM. Two analogs (11b and 11c) showed a binding affinity in the picomolar potency range, corresponding to a 33-fold increase in potency compared with the current clinical lead, prostratin. A correlation between PKC-δ binding affinity and cLogP is apparent, with maximal binding affinity corresponding to a cLogP range of 3.47 and 3.75. As prostratin operates via a PKC-dependent pathway for the induction of the latent virus, these studies show that variation in the C13 position can be used to improve potency while providing options for controlling metabolism and biodistribution.
Table 1.
Summary of biological activities for the natural products and analogs
| Analog | Side chain | PKC-δ Ki, nM* | U1 EC50, nM† | CD69 EC50, nM‡ | cLogP§ |
| Prostratin | Acetate | 21.8 (13.6–35.1) | >1,000 | 398.7 | 1.20 |
| DPP | Phenyl acetate | 3.1 (1.1–8.7) | 163.2 (122.5–217.6) | 33.8 | 2.66 |
| 11a | Cyclohexyl acetate | 1.2 (0.77–1.9) | 127.4 (94.1–172.5) | 33.2 | 3.40 |
| 11b | Pentafluorophenyl acetate | 0.61 ± 0.03¶ | 19.5 (11.4–33.6) | 32.5 | 3.47 |
| 11c | 1-Naphthyl acetate | 0.65 (0.39–1.1) | 10.2 (4.8–21.9) | 3.2 | 3.75 |
| 11d | 2-Naphthyl acetate | 1.2 (0.63–2.2) | 7.3 (3-17.9) | 3.9 | 3.75 |
| 11e | (5,6,7,8)Tetrahydro-1-naphthyl acetate | 1.3 (0.7–2.7) | 25.6 (10-60) | ND | 4.00 |
| 11f | Biphenyl acetate | 1.75 (0.87–3.5) | 9.0 (3.5–23) | 26.8 | 4.23 |
| 11g | Adamantyl acetate | 1.5 (0.82–3.1) | 13.1 (4.8–35.4) | 24.3 | 4.29 |
| 11h | p-Benzyl phenyl acetate | 1.8 (0.88–3.7) | 5.8 (3.3–10) | ND | 4.44 |
PKC-δ affinities from competitive binding assay using recombinant human protein; results are from a single triplicate dilution experiment, and values in parentheses indicate 95% confidence intervals unless otherwise noted.
U1 EC50 from triplicate stimulation studies incubating cells with compound for 48 h, p24 levels in culture supernatants read by ELISA; values in parentheses indicate 95% confidence intervals.
CD69 induction tested in primary resting CD4+ T cells from HIV-negative donors and analyzed by flow cytometry.
Average of two runs; error is reported as SEM.
Latent HIV Induction.
Having scalable and tunable access now to analogs that possess higher affinities for PKC than prostratin and DPP, we next evaluated whether they could induce latent HIV in vitro. For this purpose, the latently infected U1 cell line, a derivative of the promonocytic U937 cell line was selected. These cells harbor two copies of HIV provirus with a TAT defect that prevents viral production as the cells replicate. Upon treatment with prostratin or other inducing agents, these cells produce new virions, which can be quantified by an ELISA for the viral p24 gag protein. Similar to exposure to prostratin, when U1 cells are treated with the analogs, significant levels of p24 production are observed after a 48-h incubation (SI Appendix, Fig. S1). All of our compounds produced p24 with an EC50 in the nanomolar to single-digit nanomolar range (Table 1). In agreement with the PKC binding assay, all of our compounds outperformed prostratin and DPP in the U1 cell assay, with our most potent compounds being over 20-fold more potent than DPP and greater than 130-fold more potent than the clinical lead, prostratin. These findings demonstrate that increased PKC binding affinity correlates with increased viral induction. These studies further show that unnatural prostratin analogs can activate latent HIV with potencies that significantly exceed the natural system and clinical lead.
Cell Surface Receptors Expression.
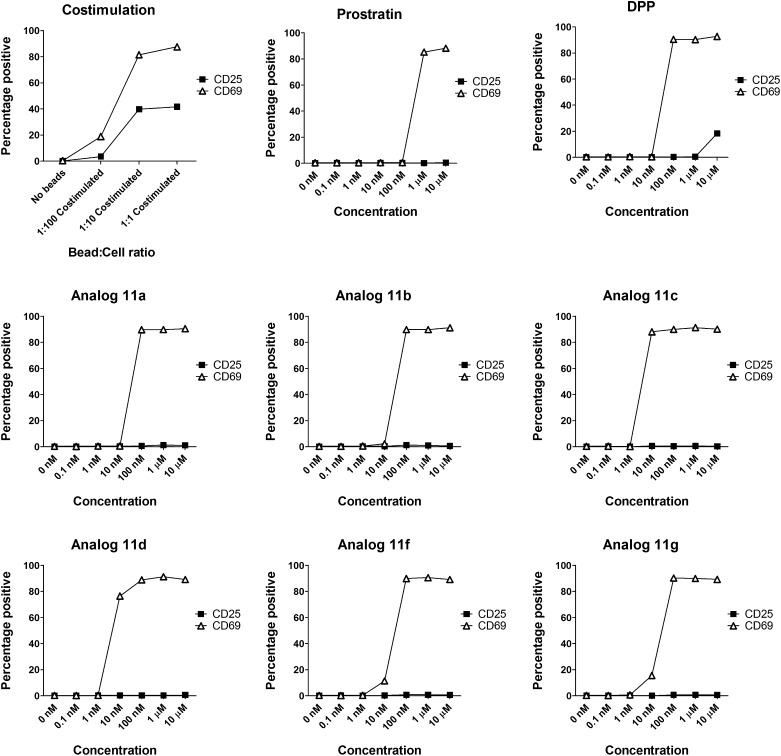
Given the encouraging PKC affinities and U1 cell performance of our analogs, attention was next turned to the performance of these compounds in primary CD4+ T cells from healthy individuals. Prostratin is proposed to work via an NF-κB–related pathway; therefore, it was of interest to know whether our compounds could activate NF-κB in CD4+ T cells. We selected the cell surface receptor CD69 for study as a surrogate marker for NF-κB activation, given that CD69 has three known NF-κB binding sites in its promoter region (34), and is up-regulated when CD4+ T cells are stimulated with prostratin (25). Primary resting CD4+ cells were isolated from HIV-negative donors, exposed to our compounds for 48 h, and evaluated for cell surface expression of CD69 by flow cytometery. As expected from the binding data, our analogs induced the expression of CD69 with one to two orders of magnitude greater potency than did prostratin (Fig. 4). Importantly, our compounds induce CD69 activation in greater than 90% of the cells tested after only a single dose, suggesting that prolonged exposure or repeated dosing could effect activation of all cells harboring latent virus as preferred for an eradication strategy.
Fig. 4.
Treatment of resting CD4+ T cells with costimulation (via bead-based antibody ligation of CD3 and CD28), natural products, or analogs. Results are from a single experiment and are representative of two to four similar experiments.
Following demonstration of CD69 up-regulation on CD4+ T cells, we then examined the effect of our compounds on other activation markers to determine whether the activation is selective. Importantly, although our compounds induce expression of CD69, an early activation marker, they do not induce the high expression levels of the late activation marker CD25 that is a characteristic of costimulated T cells, suggesting that our compounds generate lower overall levels of cellular activation than costimulation does (Fig. 4).
Treatment of uninfected CD4+ T cells with prostratin can reduce HIV infection by transcriptional and/or translational down-regulation of CD4 and other cell surface receptors used by HIV to enter the cell (23, 24). To further assess the prostratin-like activity of our compounds on the expression of cell surface markers, we examined the level of CD4 expression on resting CD4+ T cells. Supporting the other trends that we have observed, our compounds caused the down-regulation of CD4 and did so at concentrations of 10–100 times lower than prostratin (data shown in SI Appendix, Fig. S2). Based on the three cell surface markers observed in resting CD4+ T cells from healthy donors, our compounds display prostratin-like activity, but with superior potency.
HIV Induction in Resting CD4+ T Cells from Infected Individuals Receiving HAART.
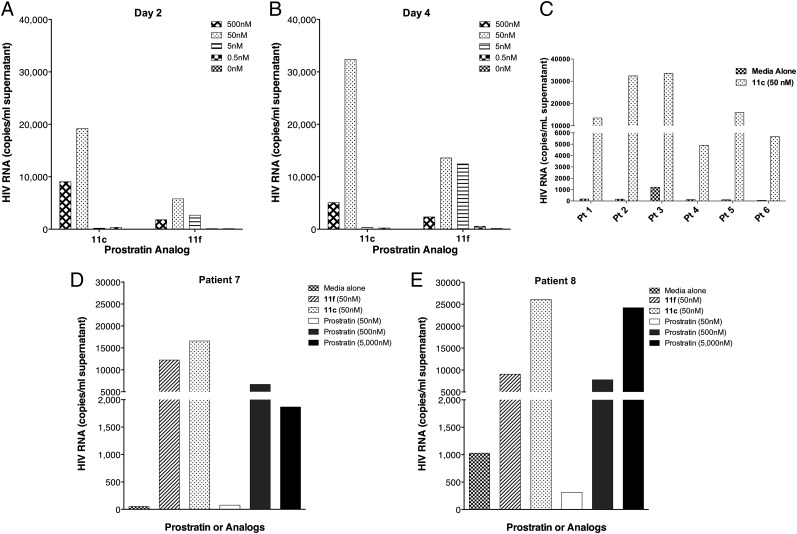
Given the positive results obtained in cell lines mimicking HIV latency and CD4+ T cells obtained from healthy donors, our next focus was the immunologic and virologic effects of our compounds on CD4+ T cells from infected individuals receiving HAART. Resting CD4+ T cells were isolated from the blood of HIV-infected individuals who were on suppressive HAART, and treated with either analog 11c or 11f. The cells were evaluated for induction of virion-associated HIV RNA at 2 and 4 d following treatment. Both compounds were exceptionally potent in this assay, with 11c exhibiting high levels of virion production at both time points at 50 nM (Fig. 5). Although compound 11f induced lower overall levels of viral RNA expression, at day 2, viral induction was clearly evident even at 5 nM concentration. In a side-by-side comparison with the clinical lead, prostratin, both 11c and 11f induced high levels of viral expression at 50 nM, whereas 50 nM prostratin was indistinguishable from the control. Prostratin, although active at 500 nM, required a 5 μM dose to induce viral expression to the same extent as 11c at 50 nM. Encouraged by these results, we examined 11c in resting CD4+ T cells isolated from multiple patients, and found that it demonstrated consistently high levels of HIV production across the board. Variability from patient to patient could either be due to differential sensitivity to our compounds or in varying number of latently infected cells that could give rise to HIV particles. This finding is highly significant because it shows that the activity of these compounds observed in immortal cell line models of HIV latency translates to potent activation from latently infected primary cells from HIV-positive donors.
Fig. 5.
Summary of viral induction from huPBMC from fully suppressed HIV-positive donors. (A) Amount of viral induction after 2 d of treatment with compounds 11c and 11f at four different concentrations. (B) Amount of viral induction after 4 d of treatment with compounds 11c and 11f at four different concentrations. (C) Amount of viral induction after 3 d of exposure to compound 11c (50 nM) in samples from six different patients. (D and E) Amount of viral induction after 3 d of exposure to compound 11c (50 nM), 11f (50 nM), and prostratin (multiple doses).
Conclusion
Prostratin represents a clinical lead for HIV eradication, an as-yet-unachieved goal. Here, we report a highly efficient, scalable, and diversifiable synthetic route to prostratin analogs. These analogs exhibit superior potency over prostratin, the parent compound and current clinical lead for an eradication strategy. Based on a renewable resource, croton oil, from which phorbol is readily obtained, the synthetic route developed allows for great variation in accessing C13 analogs, requiring only two simple and selective steps (esterification and hydrolysis) from an advanced intermediate. The analogs prepared in this way exhibited higher affinities to a representative PKC isoform and mode of action target than does prostratin. They induced expression of HIV in a latently infected model cell line, again with potencies better than prostratin. When treated with our compounds, resting CD4+ T cells from healthy donors consistently showed the same patterns of cell surface marker modulation as is elicited by prostratin, but with up to 100-fold greater potency. Significantly, a lead analog was also found to potently induce the expression of latent virus from PBMCs isolated from HIV-infected individuals receiving HAART at concentrations in which prostratin elicits no effect. This study provides a versatile synthetic route to analogs of the clinical lead prostratin and several analogs with activities superior to the current clinical lead. These analogs, their promising activities, and the potential of this synthesis to access related systems as needed to evaluate preclinical performance open a broad range of opportunities for research and for the advancement of this approach to HIV eradication.
Materials and Methods
Additional supplemental figures, synthetic procedures and characterization data for compounds along with protocols for PKC-δ binding affinity, U1 cell stimulation, surface receptor expression analysis in primary CD4+ T cells, and HIV induction in CD4+ T cells from infected individuals receiving HAART can be found in SI Appendix.
Supplementary Material
Acknowledgments
We thank Prof. Lynette Cegelski for support with biological materials and equipment. Support of this work through National Institutes of Health Grants AI70010 (to J.A.Z.) and R01 CA031841 and R01 CA031845 (to P.A.W.), and University of California, Los Angeles, Center for AIDS Research Grant AI28697 is gratefully acknowledged. This research was also supported in part by the Intramural Research Program of the National Institute Allergy and Infectious Diseases. Further support through fellowships by Novartis (to E.J.B.), Fulbright Commission (to C.G.), American Cancer Society (to L.V.H.), and Alexander von Humboldt Foundation (to R.K.) is gratefully acknowledged.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1302634110/-/DCSupplemental.
References
- 1.Fauci AS, et al. HIV vaccine research: The way forward. Science. 2008;321(5888):530–532. doi: 10.1126/science.1161000. [DOI] [PubMed] [Google Scholar]
- 2.Joint UUNPO. 2011. UNAIDS World AIDS Day Report 2011 (UNAIDS, Geneva) Available at www.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2011/jc2216_worldaidsday_report_2011_en.pdf. Accessed January 28, 2013.
- 3.Flexner C. 2011. Antiretroviral agents and treatment of HIV infection. Goodman & Gilman's The Pharmacological Basis of Therapeutics, eds Brunton LL, Chabner BA, Knollmann BC (McGraw-Hill, New York), 12th Ed. Available at www.accessmedicine.com/content.aspx?aID=16679561. Accessed August 30, 2011.
- 4.Davey RT, Jr, et al. HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proc Natl Acad Sci USA. 1999;96(26):15109–15114. doi: 10.1073/pnas.96.26.15109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chun TW, et al. Relationship between pre-existing viral reservoirs and the re-emergence of plasma viremia after discontinuation of highly active anti-retroviral therapy. Nat Med. 2000;6(7):757–761. doi: 10.1038/77481. [DOI] [PubMed] [Google Scholar]
- 6.Coiras M, López-Huertas MR, Pérez-Olmeda M, Alcamí J. Understanding HIV-1 latency provides clues for the eradication of long-term reservoirs. Nat Rev Microbiol. 2009;7(11):798–812. doi: 10.1038/nrmicro2223. [DOI] [PubMed] [Google Scholar]
- 7.Finzi D, et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med. 1999;5(5):512–517. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- 8.Parker CG, Domaoal RA, Anderson KS, Spiegel DA. An antibody-recruiting small molecule that targets HIV gp120. J Am Chem Soc. 2009;131(45):16392–16394. doi: 10.1021/ja9057647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marsden MD, Xu J, Hamer D, Zack JA. Short communication: Activating stimuli enhance immunotoxin-mediated killing of HIV-infected macrophages. AIDS Res Hum Retroviruses. 2008;24(11):1399–1404. doi: 10.1089/aid.2008.0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berger EA, Pastan I. Immunotoxin complementation of HAART to deplete persisting HIV-infected cell reservoirs. PLoS Pathog. 2010;6(6):e1000803. doi: 10.1371/journal.ppat.1000803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kulkosky J, Bray S. HAART-persistent HIV-1 latent reservoirs: Their origin, mechanisms of stability and potential strategies for eradication. Curr HIV Res. 2006;4(2):199–208. doi: 10.2174/157016206776055084. [DOI] [PubMed] [Google Scholar]
- 12.Archin NM, et al. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature. 2012;487(7408):482–485. doi: 10.1038/nature11286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehla R, et al. Bryostatin modulates latent HIV-1 infection via PKC and AMPK signaling but inhibits acute infection in a receptor independent manner. PLoS One. 2010;5(6):e11160. doi: 10.1371/journal.pone.0011160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeChristopher BA, et al. Designed, synthetically accessible bryostatin analogues potently induce activation of latent HIV reservoirs in vitro. Nat Chem. 2012;4(9):705–710. doi: 10.1038/nchem.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gulakowski RJ, McMahon JB, Buckheit RW, Jr, Gustafson KR, Boyd MR. Antireplicative and anticytopathic activities of prostratin, a non-tumor-promoting phorbol ester, against human immunodeficiency virus (HIV) Antiviral Res. 1997;33(2):87–97. doi: 10.1016/s0166-3542(96)01004-2. [DOI] [PubMed] [Google Scholar]
- 16. AIDS Research Alliance, Our Progress in Developing Prostratin. Available at http://aidsresearch.org/cure-research/our-progress. Accessed January 28, 2013.
- 17.Cashmore AR, et al. The structure of prostratin: A toxic tetracyclic diterpene ester from Pimelea prostrata. Tetrahedron Lett. 1976;17(20):1737–1738. [Google Scholar]
- 18.Gustafson KR, et al. A nonpromoting phorbol from the samoan medicinal plant Homalanthus nutans inhibits cell killing by HIV-1. J Med Chem. 1992;35(11):1978–1986. doi: 10.1021/jm00089a006. [DOI] [PubMed] [Google Scholar]
- 19.Kazanietz MG, et al. Characterization of ligand and substrate specificity for the calcium-dependent and calcium-independent protein kinase C isozymes. Mol Pharmacol. 1993;44(2):298–307. [PubMed] [Google Scholar]
- 20.Zayed S, Sorg B, Hecker E. Structure activity relations of polyfunctional diterpenes of the tigliane type, VI. Irritant and tumor promoting activities of semisynthetic mono and diesters of 12-deoxyphorbol. Planta Med. 1984;50(1):65–69. [PubMed] [Google Scholar]
- 21.Szallasi Z, Krsmanovic L, Blumberg PM. Nonpromoting 12-deoxyphorbol 13-esters inhibit phorbol 12-myristate 13-acetate induced tumor promotion in CD-1 mouse skin. Cancer Res. 1993;53(11):2507–2512. [PubMed] [Google Scholar]
- 22. Developmental Therapeutics Program, G150 Mean Graph for Compound 623310 NCI Cancer Screen Current Data. Available at http://dtp.nci.nih.gov/branches/btb/ivclsp.html. Accessed September 18, 2008.
- 23.Hezareh M, et al. Mechanisms of HIV receptor and co-receptor down-regulation by prostratin: Role of conventional and novel PKC isoforms. Antivir Chem Chemother. 2004;15(4):207–222. doi: 10.1177/095632020401500404. [DOI] [PubMed] [Google Scholar]
- 24.Kulkosky J, et al. Prostratin: Activation of latent HIV-1 expression suggests a potential inductive adjuvant therapy for HAART. Blood. 2001;98(10):3006–3015. doi: 10.1182/blood.v98.10.3006. [DOI] [PubMed] [Google Scholar]
- 25.Korin YD, Brooks DG, Brown S, Korotzer A, Zack JA. Effects of prostratin on T-cell activation and human immunodeficiency virus latency. J Virol. 2002;76(16):8118–8123. doi: 10.1128/JVI.76.16.8118-8123.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trushin SA, et al. Human immunodeficiency virus reactivation by phorbol esters or T-cell receptor ligation requires both PKCalpha and PKCtheta. J Virol. 2005;79(15):9821–9830. doi: 10.1128/JVI.79.15.9821-9830.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson HE, Banack SA, Cox PA. Variability in content of the anti-AIDS drug candidate prostratin in Samoan populations of Homalanthus nutans. J Nat Prod. 2008;71(12):2041–2044. doi: 10.1021/np800295m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wender PA, Kee JM, Warrington JM. Practical synthesis of prostratin, DPP, and their analogs, adjuvant leads against latent HIV. Science. 2008;320(5876):649–652. doi: 10.1126/science.1154690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wender PA, Koehler KF, Sharkey NA, Dell’Aquila ML, Blumberg PM. Analysis of the phorbol ester pharmacophore on protein kinase C as a guide to the rational design of new classes of analogs. Proc Natl Acad Sci USA. 1986;83(12):4214–4218. doi: 10.1073/pnas.83.12.4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bocklandt S, Blumberg PM, Hamer DH. Activation of latent HIV-1 expression by the potent anti-tumor promoter 12-deoxyphorbol 13-phenylacetate. Antiviral Res. 2003;59(2):89–98. doi: 10.1016/s0166-3542(03)00034-2. [DOI] [PubMed] [Google Scholar]
- 31.Kulkosky J, et al. Expression of latent HAART-persistent HIV type 1 induced by novel cellular activating agents. AIDS Res Hum Retroviruses. 2004;20(5):497–505. doi: 10.1089/088922204323087741. [DOI] [PubMed] [Google Scholar]
- 32.Tetko IV, et al. Virtual computational chemistry laboratory—design and description. J Comput Aided Mol Des. 2005;19(6):453–463. doi: 10.1007/s10822-005-8694-y. [DOI] [PubMed] [Google Scholar]
- 33.VCCLAB 2005. ALOGPS 2.1. Available at http://www.vcclab.org. Accessed March 1, 2012.
- 34.López-Cabrera M, et al. Transcriptional regulation of the gene encoding the human C-type lectin leukocyte receptor AIM/CD69 and functional characterization of its tumor necrosis factor-alpha-responsive elements. J Biol Chem. 1995;270(37):21545–21551. doi: 10.1074/jbc.270.37.21545. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.