Hematopoiesis is maintained and sustained throughout life by rare hematopoietic stem cells (HSCs). The hematopoietic system is organized in a hierarchy with the primitive HSCs at the top, generating mature progeny through a series of intermediate and progressively restricted progenitor cells. Normal blood cell development is a tightly controlled process, regulated by transcription factors and epigenetic regulators internally and by cytokines and cellular interactions in the bone marrow microenvironment. Perturbation of the critical molecular regulators is associated often with malignancy of the hematopoietic system, such as leukemia. A key transcriptional regulator in HSCs, the mixed lineage leukemia (MLL) gene, is commonly mutated in both myeloid and lymphoid leukemia (1). MLL is the mammalian ortholog of Drosophila melanogaster Trithorax and widely expressed in multiple organs and tissues including the hematopoietic system and neuronal and vascular systems (2–4). It belongs to a family of histone methyltransferases, where the MLL protein specifically methylates histone H3 on lysine 4 (H3K4), a mark associated with active transcription. MLL is included in a larger multiprotein complex containing protein with chromatin modification and remodeling functions (5). The MLL gene is frequently rearranged through chromosomal translocations, most notably in >70% of cases of infant leukemia and in 10% of all acute myelogeneous leukemia (AML) and acute lymphoblastic leukemia (ALL) (5, 6). The presence of MLL rearrangement in leukemia is generally associated with a poor prognosis. The chromosomal translocations result in MLL fusion proteins that lead to aberrant target gene expression. Remarkably, the chromosomal translocations result in production of more than 50 different MLL fusion proteins composed of the N terminus of the MLL protein fused with diverse partners (5, 6). Given the shared N-terminal domain, it is likely that MLL fusion proteins target many of the same gene loci as the WT full-length MLL protein. Indeed, the most established targets for both the normal MLL protein and MLL fusion proteins are the Homeobox (HOX) genes. HOX genes are critical transcription factors in the establishment of cell identity and patterning of body structures during early embryonic development. In patients, overexpression of HOX transcription factors is a hallmark of leukemia caused by MLL rearrangement (7, 8). Much work has been done to unravel the molecular network in leukemia caused by MLL fusion proteins, whereas the normal MLL protein and its molecular targets in hematopoiesis are less well characterized. Elucidation of the MLL gene expression network and its regulation in a nonpathogenic setting may improve our understanding of the mechanisms by which rearranged MLL causes leukemia. In PNAS, Artinger et al. (9) identify the transcriptional network of MLL in the hematopoietic system and show that it extends beyond Hox genes to include several genes implicated in HSC function (Fig. 1).
Fig. 1.
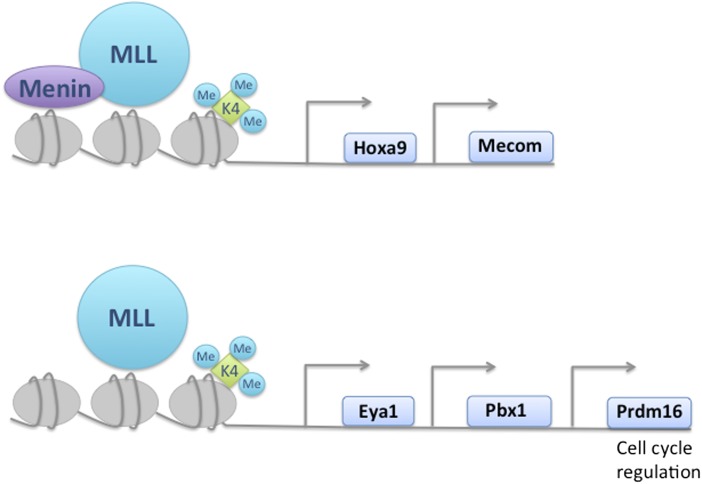
The molecular targets of MLL in hematopoietic stem and progenitor cells. Five transcription factors were identified to be directly regulated by MLL in hematopoietic stem and progenitor cells. The gene activation of Hoxa9 and Mecom is dependent on the MLL cofactor Menin, whereas Eya1, Pbx1, and Prdm16 are cofactor independent. MLL controls HSC maintenance and proliferation through Prdm16 by regulating cell cycle.
Prior studies have demonstrated an essential role for the MLL protein in maintenance and self-renewal of HSCs. Complete loss of Mll in the developing mouse is embryonic lethal. KO embryos die between embryonic days 12.5 and 16.5 and have reduced numbers of HSCs. Conditional deletion of Mll in adult mice leads to bone marrow failure within 3 wk. In both settings, Mll-deficient HSCs are at a great disadvantage when challenged in a competitive transplantation setting and exhibit selective loss of quiescent HSCs through increased cell cycling rather than cell death (10, 11).
Artinger and colleagues perform a global gene expression study comparing Mll-deleted and WT stem and progenitor cells (HSPCs). Microarray analysis identified 1,607 up- and 328 down-regulated genes. A large proportion of the up-regulated genes was associated with proliferation and cell cycling, as expected from previous studies. Notably, transcriptional regulators represented the largest annotated group of the down-regulated genes. Of these, the authors identify five genes (Mecom, Prdm16, Pbx1, Eya1, and Hoxa9) that were consistently down-regulated. Not surprisingly, most of the genes identified have been implicated previously in HSC maintenance and proliferation (12–15), consistent with a role for MLL in HSC function.
The N-terminal portion of MLL interacts physically with several proteins, including Menin, a tumor suppressor protein containing a sequence-specific DNA binding motif. Menin is essential for regulation of Hoxa9 by MLL in leukemogenesis (5). To interrogate the direct interaction of MLL with the identified transcriptional regulators, Artinger et al. first assess the presence of MLL at possible targets by ChIP analyses. As with the Hoxa9 locus, all of the identified gene loci were bound by the MLL protein. However, unlike Hoxa9, targeting of MLL to the gene loci was independent of the cofactor Menin, with the exception of Mecom. Next, to examine the interaction of the HSC associated transcriptional regulators, the authors perform a series of tissue culture overexpression studies in WT and Mll-deleted HSPCs. Although, forced expression of individual transcription factors affected the gene expression of the other regulators, most did not restore gene expression to WT levels in Mll-deficient cells, indicating independent and diverse functions of these proteins. In the setting of transplantation, Prdm16 was the only transcriptional regulator, apart from Hoxa9, that reversed the effects of HSPC depletion caused by Mll deficiency. In accordance with a role
Artinger et al. identify the transcriptional network of MLL in the hematopoietic system and show that it extends beyond Hox genes to include several genes implicated in HSC function.
of MLL in HSC self-renewal and proliferation, the authors show that reexpression of Prdm16 corrected the deficiency by restricting the hyperproliferation of HSPCs. Unlike Hoxa9, Prdm16 is not overexpressed in MLL-rearranged leukemia. This implicates a targeting mechanism as a possible difference between WT MLL and MLL fusion proteins. It has been suggested that different fusion proteins recruit or activate distinct transcriptional components. For example, biochemical studies have demonstrated that many MLL fusion proteins bind to protein complexes normally involved in transcriptional elongation. One subset of MLL fusion proteins associates with H3K79 methyltransferase Dot1L, resulting in active transcription of key target genes including Hox genes. Dot1L has been shown to be necessary for leukemogenesis (16). As such, overexpression of genes in the Mll regulatory network may be dependent not only on different target specificities of MLL fusion proteins but also on factors in complex with the fusion proteins.
The study by Artinger et al. is important because it has been recognized that the outcome of leukemogenesis from MLL fusion proteins is highly dependent on the cell of origin. MLL-rearranged leukemia cells originating from HSCs have distinct gene expression and methylation profiles combined with a higher resistance to chemotherapeutic agents compared with leukemia cells derived from myeloid progenitors. The gene expression profile of MLL leukemic stem cells originating from HSCs is enriched in genes associated with poor prognosis MLL-rearranged AML, including Evi-1, which was also identified by Artinger and colleagues (17). These findings highlight the value of further exploration of the regulatory network of normal Mll in hematopoietic stem cells.
Footnotes
The authors declare no conflict of interest.
See companion article on page 12000.
References
- 1.Orkin SHS, Zon LIL. Hematopoiesis: An evolving paradigm for stem cell biology. Cell. 2008;132(4):631–644. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gu YY, et al. The t(4;11) chromosome translocation of human acute leukemias fuses the ALL-1 gene, related to Drosophila trithorax, to the AF-4 gene. Cell. 1992;71(4):701–708. doi: 10.1016/0092-8674(92)90603-a. [DOI] [PubMed] [Google Scholar]
- 3.Tkachuk DC, Kohler S, Cleary ML. Involvement of a homolog of Drosophila trithorax by 11q23 chromosomal translocations in acute leukemias. Cell. 1992;71(4):691–700. doi: 10.1016/0092-8674(92)90602-9. [DOI] [PubMed] [Google Scholar]
- 4.Butler LH, Slany R, Cui X, Cleary ML, Mason DY. The HRX proto-oncogene product is widely expressed in human tissues and localizes to nuclear structures. Blood. 1997;89(9):3361–3370. [PubMed] [Google Scholar]
- 5.Krivtsov AV, Armstrong SA. MLL translocations, histone modifications and leukaemia stem-cell development. Nat Rev Cancer. 2007;7(11):823–833. doi: 10.1038/nrc2253. [DOI] [PubMed] [Google Scholar]
- 6.Marschalek R. Mechanisms of leukemogenesis by MLL fusion proteins. Br J Haematol. 2011;152(2):141–154. doi: 10.1111/j.1365-2141.2010.08459.x. [DOI] [PubMed] [Google Scholar]
- 7.Faber J, et al. HOXA9 is required for survival in human MLL-rearranged acute leukemias. Blood. 2009;113(11):2375–2385. doi: 10.1182/blood-2007-09-113597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armstrong SA, et al. MLL translocations specify a distinct gene expression profile that distinguishes a unique leukemia. Nat Genet. 2002;30(1):41–47. doi: 10.1038/ng765. [DOI] [PubMed] [Google Scholar]
- 9.Artinger EL, et al. An MLL-dependent network sustains hematopoiesis. Proc Natl Acad Sci USA. 2013;110:12000–12005. doi: 10.1073/pnas.1301278110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jude CD, et al. Unique and independent roles for MLL in adult hematopoietic stem cells and progenitors. Cell Stem Cell. 2007;1(3):324–337. doi: 10.1016/j.stem.2007.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McMahon KA, et al. Mll has a critical role in fetal and adult hematopoietic stem cell self-renewal. Cell Stem Cell. 2007;1(3):338–345. doi: 10.1016/j.stem.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Aguilo FF, et al. Prdm16 is a physiologic regulator of hematopoietic stem cells. Blood. 2011;117:5057–5066. doi: 10.1182/blood-2010-08-300145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ficara F, Murphy MJ, Lin M, Cleary ML. Pbx1 regulates self-renewal of long-term hematopoietic stem cells by maintaining their quiescence. Cell Stem Cell. 2008;2(5):484–496. doi: 10.1016/j.stem.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, et al. PR-domain-containing Mds1-Evi1 is critical for long-term hematopoietic stem cell function. Blood. 2011;118(14):3853–3861. doi: 10.1182/blood-2011-02-334680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chuikov S, Levi BP, Smith ML, Morrison SJ. Prdm16 promotes stem cell maintenance in multiple tissues, partly by regulating oxidative stress. Nat Cell Biol. 2010;12(10):999–1006. doi: 10.1038/ncb2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernt KM, et al. MLL-rearranged leukemia is dependent on aberrant H3K79 methylation by DOT1L. Cancer Cell. 2011;20(1):66–78. doi: 10.1016/j.ccr.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krivtsov AV, et al. Cell of origin determines clinically relevant subtypes of MLL-rearranged AML. Leukemia. 2013;27(4):852–860. doi: 10.1038/leu.2012.363. [DOI] [PMC free article] [PubMed] [Google Scholar]