Abstract
Reactive Mn(IV) oxide minerals are ubiquitous in the environment and control the bioavailability and distribution of many toxic and essential elements and organic compounds. Their formation is thought to be dependent on microbial enzymes, because spontaneous Mn(II) to Mn(IV) oxidation is slow. Several species of marine Bacillus spores oxidize Mn(II) on their exosporium, the outermost layer of the spore, encrusting them with Mn(IV) oxides. Molecular studies have identified the mnx (Mn oxidation) genes, including mnxG, encoding a putative multicopper oxidase (MCO), as responsible for this two-electron oxidation, a surprising finding because MCOs only catalyze single-electron transfer reactions. Characterization of the enzymatic mechanism has been hindered by the lack of purified protein. By purifying active protein from the mnxDEFG expression construct, we found that the resulting enzyme is a blue (absorption maximum 590 nm) complex containing MnxE, MnxF, and MnxG proteins. Further, by analyzing the Mn(II)- and (III)-oxidizing activity in the presence of a Mn(III) chelator, pyrophosphate, we found that the complex facilitates both electron transfers from Mn(II) to Mn(III) and from Mn(III) to Mn(IV). X-ray absorption spectroscopy of the Mn mineral product confirmed its similarity to Mn(IV) oxides generated by whole spores. Our results demonstrate that Mn oxidation from soluble Mn(II) to Mn(IV) oxides is a two-step reaction catalyzed by an MCO-containing complex. With the purification of active Mn oxidase, we will be able to uncover its mechanism, broadening our understanding of Mn mineral formation and the bioinorganic capabilities of MCOs.
Keywords: biomineralization, biogeochemistry, microbial redox, metal cycling
Mn(IV) oxide minerals are nearly ubiquitous in aquatic and terrestrial environments and are recognized as playing an important role in global biogeochemical cycles (1–4). Manganese is the second most abundant redox active transition metal in the Earth’s crust and is readily mobilized as Mn(II) from igneous and metamorphic rock at near-neutral pH through reductive dissolution of Mn(III, IV) oxides or leaching from Mn(II)-bearing minerals. Mn(IV) is the thermodynamically favorable oxidation state under oxic conditions, so it follows that Mn(IV) oxides would be found in diverse environments such as metal-contaminated streams (5), hydrothermal mounds near oceanic spreading centers (6), and on up to 30% of the Pacific ocean floor, where they occur as ferromanganese nodules, microconcretions, coatings, and crusts (7). In addition to being nearly ubiquitous, they have high surface area that provides reactive sites for sorptive and redox reactions and are one of the strongest natural oxidants in the environment. Because of these properties Mn cycling is tightly linked with other elemental cycles such as S, C, N, P, Fe, and trace elements and radionuclides through scavenging and oxidation reactions (4).
In the environment, microorganisms are believed to have major control of the formation of Mn oxide minerals, yet little is known about this mechanism. These Mn-oxidizing microbes encrust themselves in Mn oxides for an unknown physiological purpose, although protection from predation, oxidative stress, or UV damage or a mechanism to access recalcitrant carbon from organic matter are plausible hypotheses (4). Multicopper oxidases (MCOs) have been implicated as the Mn oxidase in several model Mn-oxidizing bacteria including Leptothrix species (8), Pseudomonas putida (9), Pedomicrobium species (10), and diverse marine spore-forming Bacillus species whose spores are capable of oxidizing Mn(II) (11–18). Thus, MCOs are involved in Mn oxidation by a variety of organisms that are recognized to be important agents in Mn oxidation in a range of terrestrial and aquatic environments including soils, sediments, and freshwater and marine systems; however, purification of these enzymes in quantities sufficient for biochemical characterization has not been possible. The Bacillus species Mn oxidizing system encoded by the mnx operon (19, 20) is a relevant model for studying mechanisms by which a bacterial MCO catalyzes the unprecedented two-electron oxidation of its substrate, Mn(II), to form a Mn(IV) oxide mineral.
Enzymatic Mn oxidation on the Bacillus spore surface overturns the widely held perception that bacterial spores are inactive, dormant cells. In fact, the Bacillus exosporium is made up of a highly ordered matrix of proteins and sugars responsible for interacting with the environment and conferring pathogenesis (21). Many attempts have been made to purify the Mn-oxidizing exosporium protein, but it is in low abundance and difficult to solubilize. Exosporium preparation and extraction takes about 2 wk and yields very little, impure protein, whereas the expression system described here produces about 2 mg of purified Bacillus sp. strain PL-12 Mn(II) oxidase per liter of Escherichia coli culture in 5 d.
Results and Discussion
Direct molecular evidence for the oxidation in the Bacillus exosporium demonstrated the presence of MnxF and MnxG in an active SDS PAGE gel band (22), but coexpressing these genes in E. coli did not produce active protein. However, active protein was obtained when the mnxDEFG operon construct was expressed without a tag in E. coli by inducing at 17 °C and loading with 2 mM CuSO4 under microaerobic conditions. (Preliminary experiments indicate that mnxD can be omitted from the construct.) After lysis, E. coli proteins were removed by trypsin proteolytic digestion or by heat precipitation at 70 °C for 20 min. The active heat-stable and trypsin cleavage-resistant Mn oxidase was purified by a series of native chromatography steps: hydrophobic interaction, gel filtration, and ion exchange. Enzymatic Mn(II)-oxidizing activity was demonstrated by the formation of brown Mn(IV) oxides after the addition of MnCl2 to protein in solution or run on a nondenaturing SDS PAGE (without thiol reductant or boiling sample pretreatments). The presence of oxidized Mn was confirmed by a colorimetric assay, in which Mn(III) and Mn(IV) react with leucoberbelin blue (LBB) to turn the solution blue [absorption maximum (Abs) 618 nm].
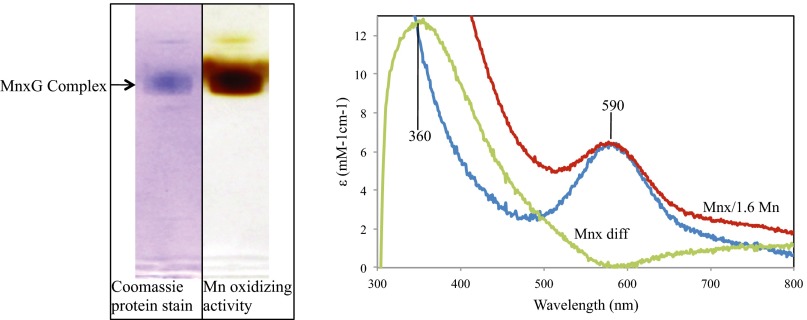
To elucidate which mnxDEFG operon gene products were in the purified active protein, we ran an in-gel Mn oxidation assay (13) in nondenaturing SDS PAGE to select an active protein band for tandem mass spectrometry (MS/MS) identification (Fig. 1, Left). MS/MS identified 100 unique peptides from MnxG (48.3% coverage), 10 from MnxE (48.2% coverage), and 5 from MnxF (23.3% coverage) (Fig. S1 and Table S1), a surprising result because no MCO has been previously purified as a multiprotein complex. The molecular weight of the purified MnxG complex determined by size-exclusion chromatography and a molecular weight calibration curve is ∼230 kDa, suggesting the presence of one full-length MnxG (138 kDa) and an oligomer of six to eight MnxE and MnxF subunits (12 kDa each) of unknown stoichiometry. MnxE and MnxF have no conserved sequence homology to known proteins. However, a BLAST search indicated that MnxE, MnxF, and MnxG all have homologs in the spore-forming bacteria Cellulosilyticum lentocellum, a cellulose-degrading Clostridium, and Desulfotomaculum kuznetsovii, a thermophilic methylotrophic sulfate reducer. It is unknown whether these organisms can oxidize Mn, but because MnxE and MnxF are only conserved among spore-forming bacteria, they may be required for activity, stability, and/or localization of MnxG-like MCOs to the spore surface or exosporium.
Fig. 1.
Characterization of purified Mnx oxidase. (Left) SDS PAGE of purified oxidase diluted into Laemmli buffer and Coomassie-stained (lane 1) or incubated with Mn(II) (lane 2), showing MnO2 formation by the protein. The arrow indicates a single native purified protein band. (Right) UV-visible spectrum before (blue trace, 590-nm band) and after incubation with threefold excess MnCl2 and dialysis (red trace). The difference spectrum (green) shows Mn(IV) (360-nm band) bound to the protein (1.6 Mn/mol protein).
The MnxG sequence predicts it to be a large, six-domain MCO, similar to human ceruloplasmin, a ferroxidase (19). MCOs contain four copper atoms that couple the oxidation of phenolic or metal substrates to the sequential reduction of O2 to H2O. These four copper atoms reside in the blue type 1 (Abs ∼600 nm) center and the trinuclear center containing one type 2 and two type 3 (Abs ∼330 nm) Cu atoms. In addition to these canonical Cu atoms, MCOs can also bind Cu in extra T1 sites and in labile regulatory sites (23, 24). Our purified MnxG complex is blue-colored (Abs 590 nm) (Fig. 1, Right) and has a copper occupancy of ∼6.4 Cu per mole of MnxG complex by inductively coupled plasma-optical emission spectroscopy (ICP-OES) analysis.
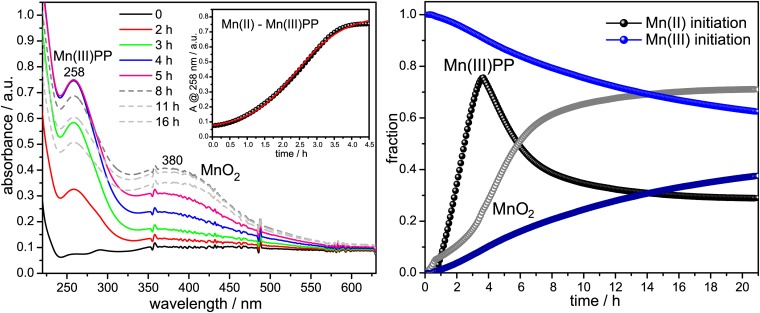
To determine the course of MnO2 formation, we monitored changes in the UV-visible absorption spectrum when Mn(II) or Mn(III) was allowed to react with oxygenated buffer in the presence of purified enzyme and the Mn(III) chelator, pyrophosphate (PP) (Fig. 2). When the reaction was initiated with Mn(II), a 258-nm peak, due to Mn(III)–PP, rises and then falls as a 360-nm peak, due to colloidal MnO2, rises. The initial time course of the rising Mn(III)–PP peak is sigmoidal, suggesting cooperative, allosteric substrate binding. When Mn(III)–PP initiates the reaction, the Mn(III)–PP peak decays as the MnO2 forms (Fig. 2). These results corroborate the earlier findings using Bacillus exosporium that Mn(II) oxidation to MnO2 is catalyzed by two single electron transfers (25, 26).
Fig. 2.
Mn(II) and Mn(III) initiated Mn oxidation assays. (Left) UV-visible absorption spectra of purified oxidase in Hepes buffer, pH 7.5, in the presence of sodium pyrophosphate (PP) and O2, taken at the indicated time points after the addition of 0.1 mM MnCl2. (Right) Time courses followed by Mn(III)–PP and MnO2 species during Mn oxidation by purified oxidase and obtained from a linear least squares fit analysis of the time-resolved spectra (23). After the addition of MnCl2, the 258-nm absorption band due to Mn(III)–PP rises sigmoidally (Left, Inset) and then decays (Right, black points) as the 380-nm absorption band due to MnO2 rises (Right, gray points). Addition of 0.1 mM Mn(III) is followed by a steady decay of the 258-nm band and a concomitant rise of the 360-nm absorption band.
Because formation of mononuclear Mn(IV) in a protein environment would be energetically prohibitive, we have proposed a polynuclear mechanism, in which electron transfer from Mn(III) would be driven by the formation of bridging oxides (25). Consistent with such a mechanism, we found that when threefold excess Mn(II) was titrated into purified enzyme, dialyzed overnight, and concentrated, the solution displayed a 360-nm difference absorption band (Fig. 1, Right) and LBB reactivity, suggestive of a polynuclear Mn(IV) species. ICP-OES analysis gave a stoichiometry of 1.6 Mn per mole of MnxG complex. This result suggests that Mn(II) oxidation results in a protein-bound dinuclear or trinuclear Mn(IV) oxo complex that can nucleate MnO2 formation.
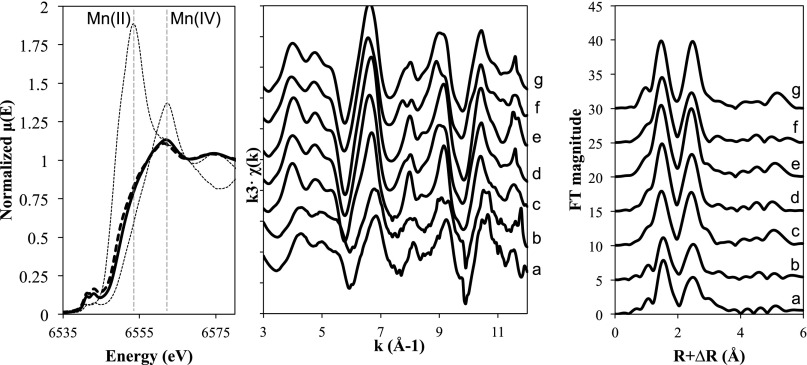
The Mn oxidation state and the structure of the MnO2 minerals made by purified Bacillus sp. PL-12 Mn oxidase were analyzed using X-ray absorption spectroscopy and compared with those previously reported for intact purified Bacillus sp. SG-1 spores (18, 27–29). X-ray absorption near-edge spectra (XANES) of solid phases of two different concentrations of purified Mn oxidase, 5 and 50 μg·L-1, demonstrated that both preparations oxidized Mn(II) to Mn(IV) (Fig. 3, Left). The absorbance maximum for these two preparations occurs near the white line of Mn(IV) at 6,562 eV. Linear combination fitting of the data confirm a predominance of Mn(IV) in the samples with 20–30% Mn(II) and Mn(III) present, mostly as Mn(II), as evidenced by the shoulder at 6,553 eV.
Fig. 3.
Mn K-edge XANES (Left) and EXAFS (Center) and Fourier transforms (Right) of oxides formed by two different amounts of MnxG complexes after incubation with Mn(II). (Left) XANES spectra of 50 µg·L−1 (solid bold line) and 5 µg·L−1 (dashed bold line) protein incubated in 100 µM Mn(II). XANES of MnCl2·4H2O and δ-MnO2 are provided as indicators for Mn(II) and Mn(IV) with maximum absorbance features at 6,553 and 6,562 eV, respectively. Mn K-edge EXAFS (Center) and Fourier transforms (Right) of the two different concentrations of MnxG complex, 50 (a) and 5 (b) μg·L−1. EXAFS of selected Mn oxides, biogenic Mn oxides formed by spores of Bacillus sp. SG-1 in NaCl solution (c), biogenic Mn oxides formed by spores of Bacillus sp. SG-1 in CaCl2 solution (d), δ-MnO2 (e), triclinic birnessite (f), and hexagonal birnessite (g) are shown for comparison.
Measurements of the Mn K-edge extended X-ray absorption fine structure (EXAFS) were also performed on these preparations (Fig. 3, Right). In previous studies, it was shown that Mn oxides formed by spores of Bacillus sp. SG-1 in NaCl solution were similar to δ-MnO2 (18), whereas in CaCl2 solution biogenic Mn oxides exhibited features similar to triclinic or orthogonal manganates (28). Qualitatively, EXAFS of Mn oxides formed by purified Mn oxidase were similar to the spore product but with some differences between the Mn oxides depending on the amount of protein used. Specifically, EXAFS of Mn oxides formed with 50 μg·L−1 purified protein showed single antinodes at 8.0 and 9.3 Å−1, whereas Mn oxides produced from 5 μg·L−1 protein showed double antinodes (Fig. 3, Right). Single antinodes can be found in hexagonal layer symmetry, which includes δ-MnO2, hexagonal birnessites, and biogenic Mn oxides from Bacillus sp. SG-1 in NaCl, whereas double antinodes at 8.0 and 9.3 Å−1 can be observed in orthogonal layer symmetry such as triclinic birnessites and biogenic Mn oxides formed by spores of Bacillus sp. SG-1 in CaCl2 (28–31). Fourier transforms show that Mn oxides from purified Mn oxidase have most of the features observed in Bacillus sp. SG-1 spore-generated Mn oxides in both NaCl and CaCl2 solution [i.e., first peak (Mn–O) and second peak (edge-sharing Mn–Mn)] and amplitude of multiple-scattering peak at 5.2 Å.
The Bacillus exosporium MnxG complex represents a distinct chapter in biomineralization. It is the only example of an MCO catalyzing two energetically distinct metal oxidation steps, as part of bio-oxide formation. The requirement of two small accessory proteins for function is also unprecedented. Now that a method is in hand to produce quantities of purified Mn oxidase, the unique mechanism of Mn(II) oxidation and MnO2 biomineralization can be elucidated through crystallographic and spectroscopic methods. Given that MCOs seem to be the enzymes responsible for Mn oxidation in a variety of environmentally conspicuous organisms (8–12, 14, 22), this insight will be invaluable in understanding how Mn cycling occurs and how it affects other geochemical cycles. The enzyme may also have useful biotechnological applications in the fields of biomaterials, enzyme catalysts, bioremediation, metal recovery, and bioenergy.
Materials and Methods
mnxDEFG Gene Cloning.
Bacillus sp. PL-12 genomic DNA was isolated with DNeasy kit (Qiagen). mnxDEFG was amplified with Pfu Phusion high-fidelity DNA polymerase (New England Biosciences) using forward primer 5′-GCTAGCATGCGTCATTCGGATTATTTGAAAAATTTGT-3′ and reverse primer, 5′-CTCGAGTTATGCCTTTTCTTCATTGTCCCACCCC-3′, including mnxG stop codon and bolded restriction enzyme sites. This amplicon was subcloned into pJet (Invitrogen) before insertion into pTXB1 (New England Biosciences) with NheI and XhoI (New England Biosciences) restriction enzymes to excise from the entry vector and ligate into the expression vector.
Native Protein Expression and Purification in E. coli.
One liter of LB was inoculated from 10 mL of E. coli BL12(DE3) (pTXB1/mnxDEFG) with 100 μg/mL ampicillin, 0.2 mM CuSO4, and 10 mM Tris⋅HCl (pH 7.5) added, grown until an OD600 ∼0.5, chilled to 17 °C, and induced with 0.1 mM isopropyl β-d-1-thiogalactopyranoside for 18 h (140 rpm). The shaking function was turned off for 22 h with CuSO4 added to 2 mM. The harvested cells were lysed into hydrophobic interaction chromatography start buffer (20 mM Tris-HCl, 1.25 M NaCl) supplemented with 10 mM CaCl2, 1 mM CuSO4, and EDTA-free protease inhibitor mixture (Sigma) by two rounds of French press at 1,000 psi. The crude extract was clarified by 20 min of incubation at 70 °C. The oxidase was purified by collecting active fractions of chromatography steps supplemented with 50 µM CuSO4, Phenyl Sepharose 6 Fast Flow (high sub), HiPrep 16/60 Sephacryl S-300 High Resolution, and HiTrap Q HP (GE Healthcare Life Sciences), where it was isolated from a single peak. Protein was quantified using the bicinchoninic acid reagent (Thermo Scientific), using the size-exclusion chromatography-determined molecular weight of the purified complex of 230 kDa. Purified protein copper content was determined with a Perkin-Elmer Optima 2000 DV inductively coupled plasma optical emission spectrometer. UV-visible absorption spectrum was collected on Varian Cary 50 spectrophotometer.
Mass Spectrometry Analysis.
Purified protein was run on Tris Glycine 4–15% SDS gel (Bio-Rad) in adjacent wells. After electrophoresis, the lanes were separated and stained in Imperial protein stain (Pierce) or Silver stain (Pierce) and assayed for Mn oxidation (13). The Imperial-stained band that corresponded to the Mn oxidation active band was excised by razor blade and submitted to LC-MS at the Oregon Health and Science University proteomics shared resource center. The gel slice was reduced with 10 mM DTT and alkylated with 55 mM iodoacetamide before an overnight digestion with trypsin at a protease-to-protein ratio of greater than 5:1. All solutions were in 100 mM ammonium bicarbonate. For analysis on the mass spectrometer each protein digest was analyzed by LC-MS using an Agilent 1100 series capillary LC system (Agilent Technolgies Inc.) and an LTQ Velos linear ion trap mass spectrometer (ThermoFisher). Bacillus sp. PL-12 MnxD, MnxE, MnxF, and MnxG were appended to E. coli sequences downloaded from the UniProt website (November 2011). The database sequences and their reversed sequences were appended to 179 common contaminant sequences and their reversed forms. We used the sequence-reversed database to estimate error thresholds (32). The database processing was performed with Python scripts available at www.ProteomicAnalysisWorkbench.com. Bacillus sp. PL-12 MnxG and MnxE were identified with a protein false-discovery rate of less than 1%. MnxF was identified with a false-discovery rate of approximately 20%, and then subsequently confirmed by manual validation of the MS2 spectra using Scaffold version 3.0 (www.proteomesoftware.com/products/scaffold/). Further mass spectrometry details can be found in Supporting Information.
Mn(III)–PP Trapping.
UV visible spectral measurements of Mn oxidation reactions with purified oxidase and Na pyrophosphate were performed and fitted as described previously with Bacillus sp. SG-1 exosporium (25).
X-Ray Absorption Spectroscopy.
Mn oxide minerals were harvested as follows. Five micrograms of purified protein was added to 100 mL or 1 L of 10 mM Hepes (pH 7.8), 50 mM NaCl, and 100 µM MnCl2. The reaction was shaken overnight at 30 °C. The 100-mL reaction was allowed to settle for about 3 d and the 1-L reaction was allowed to settle about 2 h before the oxides were siphoned off the bottom, centrifuged 5,000 × g for 10 min, then centrifuged again 15,000 × g for 1 min in microfuge tubes. The oxides were allowed to air-dry before packing into Al sample holders. Samples were secured with kapton tape with lexan covers between the samples and kapton tape to prevent any reduction of Mn induced by the beam during data collection (28). Mn K-edge EXAFS was collected on transmission mode at Stanford Synchrotron Radiation Lightsource beamline 4-1 with a Si(220) double-crystal monochromator and detuned 60%. Energy calibration was done using the pre-edge feature of potassium permanganate (6,543.34 eV). Samples were run at 77°K using a liquid nitrogen cryostat. Background subtraction and normalization was done using Athena (33).
Supplementary Material
Acknowledgments
We thank Kelly Chacón and Ninian Blackburn’s lab for assistance with equipment and Sam Webb (Stanford Synchrotron Radiation Lightsource, SSRL) for the spectra of model reference materials. We also acknowledge the laboratory assistance of Gloria Kim and Katherine Osterlund, who were undergraduate interns supported by the US National Science Foundation through the Science and Technology Center for Coastal Margin Observation and Prediction. We thank Larry David and John Klimek at the Oregon Health and Science University Proteomics Shared Resource for analyzing the protein samples and assistance with their method details. This work was supported by the Oregon Opportunity Fund and US National Science Foundation Grants OCE-1154307 and OCE-1129553. Portions of this research were carried out at the SSRL, a Directorate of Stanford Linear Accelerator Center National Accelerator Laboratory and an Office of Science User Facility operated for the US Department of Energy Office of Science by Stanford University.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1303677110/-/DCSupplemental.
References
- 1.Ulrich HJ, Stone AT. Oxidation of chlorophenols adsorbed to manganese oxide surfaces. Environ Sci Technol. 1989;23:421–428. [Google Scholar]
- 2.Stone AT, Morgan JJ. Reduction and dissolution of manganese(III) and manganese(IV) oxides by organics. 1. Reaction with hydroquinone. Environ Sci Technol. 1984;18(6):450–456. doi: 10.1021/es00124a011. [DOI] [PubMed] [Google Scholar]
- 3.Stone AT, Morgan JJ. Reduction and dissolution of manganese(III) and manganese(IV) oxides by organics: 2. Survey of the reactivity of organics. Environ Sci Technol. 1984;18(8):617–624. doi: 10.1021/es00126a010. [DOI] [PubMed] [Google Scholar]
- 4.Tebo BM, et al. Biogenic manganese oxides: Properties and mechanisms of formation. Annu Rev Earth Planet Sci. 2004;32:287–328. [Google Scholar]
- 5.Bilinski H, Giovanoli R, Usui A, Hanžel D. Characterization of Mn oxides in cemented streambed crusts from Pinal Creek, Arizona U.S.A., and in hot-spring deposits from Yuno-Taki Falls, Hokkaido, Japan. Am Mineral. 2002;87:580–591. [Google Scholar]
- 6.Davis RE, Stakes DS, Wheat CG, Moyer CL. Bacterial variability within an iron-silica-manganese-rich hydrothermal mound located off-axis at the Cleft Segment, Juan de Fuca Ridge. Geomicrobiol J. 2009;26:570–580. [Google Scholar]
- 7.Post JE. Manganese oxide minerals: Crystal structures and economic and environmental significance. Proc Natl Acad Sci USA. 1999;96(7):3447–3454. doi: 10.1073/pnas.96.7.3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corstjens PLAM, De Vrind JPM, Goosen T, De Vrind-De Jong EW. Identification and molecular analysis of the Leptothrix discophora SS-1 mofA gene, a gene putatively encoding a manganese-oxidizing protein with copper domains. Geomicrobiol J. 1997;14:91–108. [Google Scholar]
- 9.Geszvain K, McCarthy JK, Tebo BM. Elimination of manganese(II,III) oxidation in Pseudomonas putida GB-1 by a double knockout of two putative multicopper oxidase genes. Appl Environ Microbiol. 2013;79(1):357–366. doi: 10.1128/AEM.01850-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ridge JP, et al. A multicopper oxidase is essential for manganese oxidation and laccase-like activity in Pedomicrobium sp. ACM 3067. Environ Microbiol. 2007;9(4):944–953. doi: 10.1111/j.1462-2920.2006.01216.x. [DOI] [PubMed] [Google Scholar]
- 11.Dick GJ, Lee YE, Tebo BM. Manganese(II)-oxidizing Bacillus spores in Guaymas Basin hydrothermal sediments and plumes. Appl Environ Microbiol. 2006;72(5):3184–3190. doi: 10.1128/AEM.72.5.3184-3190.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee Y. Microbial Oxidation of Cobalt: Characterization and Its Significance in Marine Environments. San Diego: Univ of California; 1994. p. 159. [Google Scholar]
- 13.Francis CA, Tebo BM. Enzymatic manganese(II) oxidation by metabolically dormant spores of diverse Bacillus species. Appl Environ Microbiol. 2002;68(2):874–880. doi: 10.1128/AEM.68.2.874-880.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Francis CA, Casciotti KL, Tebo BM. Localization of Mn(II)-oxidizing activity and the putative multicopper oxidase, MnxG, to the exosporium of the marine Bacillus sp. strain SG-1. Arch Microbiol. 2002;178(6):450–456. doi: 10.1007/s00203-002-0472-9. [DOI] [PubMed] [Google Scholar]
- 15. Morgan JJ (2000) Manganese in natural waters and earth’s crust: Its availability to organisms. Metal Ions in Biological Systems. Vol. 37 Manganese and Its Role in Biological Processes, eds Sigel A, Sigel H (Marcel Dekker, New York), pp 1–33. [PubMed]
- 16.Hastings D, Emerson S. Oxidation of manganese by spores of a marine Bacillus: Kinetic and thermodynamic considerations. Geochim Cosmochim Acta. 1986;50:1819–1824. [Google Scholar]
- 17.Tebo BM, Ghiorse WC, Van Waasbergen LG, Siering PL, Caspi R. Bacterially mediated mineral formation: Insights into manganese(II) oxidation from molecular genetic and biochemical studies. Rev Mineral. 1997;35:259–266. [Google Scholar]
- 18.Bargar JR, Tebo BM, Villinski JE. In situ characterization of Mn(II) oxidation by spores of the marine Bacillus sp. strain SG-1. Geochim Cosmochim Acta. 2000;64:2775–2778. [Google Scholar]
- 19.van Waasbergen LG, Hildebrand M, Tebo BM. Identification and characterization of a gene cluster involved in manganese oxidation by spores of the marine Bacillus sp. strain SG-1. J Bacteriol. 1996;178(12):3517–3530. doi: 10.1128/jb.178.12.3517-3530.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Waasbergen LG, Hoch JA, Tebo BM. Genetic analysis of the marine manganese-oxidizing Bacillus sp. strain SG-1: Protoplast transformation, Tn917 mutagenesis, and identification of chromosomal loci involved in manganese oxidation. J Bacteriol. 1993;175(23):7594–7603. doi: 10.1128/jb.175.23.7594-7603.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kailas L, et al. Surface architecture of endospores of the Bacillus cereus/anthracis/thuringiensis family at the subnanometer scale. Proc Natl Acad Sci USA. 2011;108(38):16014–16019. doi: 10.1073/pnas.1109419108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dick GJ, Torpey JW, Beveridge TJ, Tebo BM. Direct identification of a bacterial manganese(II) oxidase, the multicopper oxidase MnxG, from spores of several different marine Bacillus species. Appl Environ Microbiol. 2008;74(5):1527–1534. doi: 10.1128/AEM.01240-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindley PF, et al. An X-ray structural study of human ceruloplasmin in relation to ferroxidase activity. J Biol Inorg Chem. 1997;2:454–463. [Google Scholar]
- 24.Roberts SA, et al. A labile regulatory copper ion lies near the T1 copper site in the multicopper oxidase CueO. J Biol Chem. 2003;278(34):31958–31963. doi: 10.1074/jbc.M302963200. [DOI] [PubMed] [Google Scholar]
- 25.Soldatova AV, Butterfield CN, Tebo BM, Spiro TG. Multicopper oxidase involvement in both Mn(II) and Mn(III) oxidation during bacterial formation of MnO2. J Biol Inorg Chem. 2012;17(8):1151–1158. doi: 10.1007/s00775-012-0928-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Webb SM, Dick GJ, Bargar JR, Tebo BM. Evidence for the presence of Mn(III) intermediates in the bacterial oxidation of Mn(II) Proc Natl Acad Sci USA. 2005;102(15):5558–5563. doi: 10.1073/pnas.0409119102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bargar JR, et al. Biotic and abiotic products of Mn(II) oxidation by spores of the marine Bacillus sp. strain SG-1. Am Mineral. 2005;90:143–154. [Google Scholar]
- 28.Webb SM, Tebo BM, Bargar JR. Structural influences of sodium and calcium ions on the biogenic manganese oxides produced by the marine Bacillus sp., strain SG-1. Geomicrobiol J. 2005;22:181–193. [Google Scholar]
- 29.Webb SM, Tebo BM, Bargar JR. Structural characterization of biogenic Mn oxides produced in seawater by the marine Bacillus sp. strain SG-1. Am Mineral. 2005;90:1342–1357. [Google Scholar]
- 30.Gaillot AC, et al. Structure of synthetic K-rich birnessite obtained by high-temperature decomposition of KMnO4. I. Two-layer polytype from 800 °C experiment. Chem Mater. 2003;15:4666–4678. [Google Scholar]
- 31.Manceau A, et al. Natural speciation of Zn at the micrometer scale in a clayey soil using X-ray fluorescence, absorption, and diffraction. Geochim Cosmochim Acta. 2004;68:2467–2483. [Google Scholar]
- 32.Elias JE, Gygi SP. Target-decoy search strategy for increased confidence in large-scale protein identifications by mass spectrometry. Nat Methods. 2007;4(3):207–214. doi: 10.1038/nmeth1019. [DOI] [PubMed] [Google Scholar]
- 33.Ravel B, Newville M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J Synchrotron Radiat. 2005;12(Pt 4):537–541. doi: 10.1107/S0909049505012719. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.