Abstract
Oral feed-based passive immunization can be a promising strategy to prolong maternal lactogenic immunity against postweaning infections. Enterotoxigenic Escherichia coli (ETEC)-caused postweaning diarrhea in piglets is one such infection that may be prevented by oral passive immunization and might avert recurrent economic losses to the pig farming industry. As a proof of principle, we designed anti-ETEC antibodies by fusing variable domains of llama heavy chain-only antibodies (VHHs) against ETEC to the Fc part of a porcine immunoglobulin (IgG or IgA) and expressed them in Arabidopsis thaliana seeds. In this way, four VHH-IgG and four VHH-IgA antibodies were produced to levels of about 3% and 0.2% of seed weight, respectively. Cotransformation of VHH-IgA with the porcine joining chain and secretory component led to the production of light-chain devoid, assembled multivalent dimeric, and secretory IgA-like antibodies. In vitro analysis of all of the antibody-producing seed extracts showed inhibition of bacterial binding to porcine gut villous enterocytes. However, in the piglet feed-challenge experiment, only the piglets receiving feed containing the VHH-IgA–based antibodies (dose 20 mg/d per pig) were protected. Piglets receiving the VHH-IgA–based antibodies in the feed showed a progressive decline in shedding of bacteria, significantly lower immune responses corroborating reduced exposure to the ETEC pathogen, and a significantly higher weight gain compared with the piglets receiving VHH-IgG producing (dose 80 mg/d per pig) or wild-type seeds. These results stress the importance of the antibody format in oral passive immunization and encourage future expression of these antibodies in crop seeds.
Keywords: molecular farming, mucosal immunity, nanobody, enteric infections, antibiotic replacement
Like humans, young mammals inherit a battery of protective immunoglobulins transplacentally and after birth through the maternal milk, which protects them from infections during the first phase of their life. Exceptionally, however, economically important farm animals, such as pigs, horses, sheep, and cows, acquire their passive systemic immunity only after birth by colostral uptake, and their passive mucosal gastro-intestinal immunity during the whole period of lacteal uptake (1). After weaning, the latter is lost, rendering the animals vulnerable to gastrointestinal infections. In this phase, antibiotics become an important arsenal against common bacterial infections. However, given the risk of introducing antibiotic-resistant strains, suitable alternatives are needed (2, 3).
One such globally occurring gastrointestinal infection is the piglet postweaning diarrhea (PWD) caused by enterotoxigenic Escherichia coli (ETEC). The ETEC-related PWD in piglets is an important cause of economic losses, which result from either piglet death in case of acute ETEC infections, or poor weight gain observed in surviving piglets (3, 4). The ETEC strains bearing F4 fimbriae (F4+ETEC) are most often isolated from diseased piglets. Attachment of F4 fimbriae via adhesin FaeG to specific F4 receptors (F4Rs) on the pig intestinal brush border is the first step in elicitation of infection. Colonization of the gut is followed by secretion of one or more toxins (LT, STa, or STb), leading to acute diarrhea (3). F4+ETEC strains can bear three variants of the FaeG adhesin: FaeGab, FaeGac, or FaeGad, each having a conserved “a” epitope and one of the respective variable epitopes “b,” “c,” or “d” (5). Considerable efforts have been invested in developing vaccines against the F4+ETEC, however, with limited success. It has been established that to prevent this enteric infection, mucosal immunity is needed and oral vaccination with FaeG has been successful in raising protective secretory IgAs at the intestinal surface (6). However, development of oral vaccines is hurdled by the prospects of being neutralized by the preexisting maternal antibodies in the consumed milk, and gastric digestion of vaccines before priming of the immune system (6). Moreover, vaccines do not provide immediate protection on administration, because they require time to induce antibodies at the intestinal mucosal surface (6).
As an alternative, we envisaged a strategy to prolong the passive immunity postweaning by producing anti-F4+ETEC antibodies in seeds that can be incorporated into the starter feed of weaned piglets. The seeds would provide an antibody production platform with ease of storage at high concentrations in a confined space, and convenience of oral administration, which is particularly advantageous for large herds of piglets (7, 8). More importantly, the crushed seed matrix might protect the antibodies from gastric digestion by outcompeting proteases, as demonstrated in the case of in-pea-seed–produced anti-Eimeria antibodies administered in chicken fodder (9). As a proof of concept, we developed anti-F4+ETEC antibodies in seeds of Arabidopsis thaliana, transformation of which is easier than that of feed crops, and sufficient antibody-producing seeds can be up-scaled in greenhouses in a relatively short time.
To engineer a robust anti-F4+ETEC antibody, we used the antigen-binding variable domain of the llama heavy chain-only antibody (VHH), as it can survive harsh chemical and temperature conditions yet remain functional (10). In addition, the third complementarity determining region (CDR3) of VHHs often forms a convex loop, which can interact with deep antigenic clefts (11), and together with CDR1 provides VHHs an enhanced repertoire of antigen binding paratopes (12).
The VHHs were grafted onto porcine Fc to ensure multiple valences, necessary for agglutination (cross-linking) of the bacteria and to prevent bacterial attachment to receptors on gut villous enterocytes (13). Another advantage of using VHH-Fc is that only one gene has to be transformed to produce a functional homodimer. Four isolated anti- F4+ETEC VHHs were fused to the Fc fragment of porcine IgG3 and IgAb, and expressed under the control of the seed-specific β-phaseolin promoter (14).
The predominant antibody at the mucosal surfaces is the secretory IgA (SIgA); the polyvalent antigen-binding domains and the secretory component (SC) impart higher efficacy, retention time, and protection from proteolytic degradation (15). Although progress is being made in the recombinant production of SIgAs in mammalian cells or from plasma-purified polymeric IgAs reconstituted as SIgAs, their mass production is still challenging (15, 16). Plants, on the other hand, have been proven to be an effective expression system for production of SIgAs. Ma et al. (17) expressed an SIgA in tobacco plants by successively crossing lines expressing the four elements: the heavy and light chains, the joining (J) chain to express dimeric IgA (dIgA), and the SC. Today, the only recombinant SIgA approved for marketing is CaroRx, an anti-Dental caries antibody manufactured in tobacco plants (18).
Aiming to tap the benefits of SIgA, but refraining from the complex hetero-decameric SIgA structure of ∼400 kDa, we coexpressed the porcine J chain and SC, along with the VHH-IgA chain, and could demonstrate in-seed production of unique simplified (light-chain devoid) VHH-IgA-based dimeric (dVHH-IgA) and secretory IgA (sVHH-IgA) antibodies.
Here, we describe the antibody construction, its in-seed production, and efficacy in preventing F4+ETEC infection when fed to weaned piglets. The positive results demonstrate the feasibility of in-seed–based oral passive immunization and highlight the need for apt antibody design.
Results
Anti-F4+ETEC VHHs Agglutinate All Serotypes of F4+ETEC Bacteria in Multivalent Format.
The predominantly identified FaeGac adhesin, which is associated with acute diarrhea in piglets (19), was used to immunize a llama. Using phage-display technology, the VHHs from the llama immune library were panned against the antigens FaeGac and FaeGad for isolating VHHs specific for conserved epitopes of FaeG variants. This process enabled the selection of a panel of 79 VHHs, which specifically recognized FaeG as well as purified F4 fimbriae in respective ELISAs. The sequence of all of the VHHs showed the occurrence of the typical structurally conserved framework regions in which three distinct hypervariable CDRs were embedded (12). Based on the deduced amino acid sequence and the divergence in CDR3, the 79 VHH sequences could be divided into three clades. Four VHHs representing these three clades were selected and named V1, V2, V3, and V4 (Fig. S1A). The VHHs V3 and V4 belonged to the third clade and differed by a single Lys-100-Arg amino acid substitution within the CDR3 loop (Fig. S1 A and C).
Furthermore, the four VHHs in a multivalent format, being covalently coated on magnetic beads, showed specific agglutination with the three serotypes of F4+ETEC bacteria and not with the negative control (E. coli strain K514) or with the nonspecific fimbriae control (F18 fimbriae) (Fig. S1B).
Production of High Accumulating VHH-IgG Bivalent Antibodies in Arabidopsis Seeds.
The native sequence of each of the four anti-F4+ETEC monovalent VHHs was grafted on the hinge of the codon-optimized sequence of porcine IgG3 Fc, to produce divalent VHH-IgG fusion antibodies, named V1G, V2G, V3G, and V4G. Among the porcine IgGs, IgG3 has the longest hinge (23 amino acids) with three cysteine residues and has been predicted to be resistant to peptic degradation (20).
The VHH-IgG antibody constructs under the control of β-phaseoline promoter cloned within the pPhasGW vector (14) (see Fig. S3A) were introduced in Arabidopsis via floral dip transformation. The accumulation of VHH-IgG was evaluated in the T2 seeds via a high-throughput ELISA setup with immobilized FaeGac (Fig. S2 A–D). Within the 24 transformants screened for each VHH-IgG antibody, the range of variation in functional VHH-IgG production was the highest for V2G, followed by V1G, but V3G and V4G antibodies had a relatively low range of variation in accumulation levels (Fig. S2 A–D). Based on their relative accumulation of functional antibodies, the respective transformants were empirically classified as high, medium, and low expressors for each VHH-IgG. Further characterization of seed extracts from eight to nine transformants (five highest-, two medium-, and at least one low-expressing transformant) for each VHH-IgG antibody by SDS/PAGE under reducing conditions revealed a heterologous band migrating at ∼49 kDa. This band was confirmed to be VHH-IgG (theoretically ∼39.79 kDa) by immunoblot using the anti-pig IgG-specific polyclonal serum (Fig. S2 A–D). The higher molecular weight is likely because of glycosylation. The immunoblots also showed the occurrence of ∼33 and ∼15 kDa proteolytic fragments, which were more conspicuous in high expressors (Fig. S2E). Under nonreducing conditions, the VHH-IgG antibodies migrated at ∼80 kDa, thus confirming that VHH-IgG chains form bivalent homodimers in planta (Fig. S2F). Additionally, lower molecular-weight dimers of ∼60 kDa were seen in immunoblots developed from nonreduced SDS/PAGE, which could be the union of the proteolytically cleaved IgG3 Fc chains (Fig. S2F).
The relative accumulation of this ∼49-kDa VHH-IgG antibody chain was in concordance with the functional ELISA results, implying that most of the protein was functional against FaeGac (Fig. 1 and Fig. S2). The four VHH-IgGs in bivalent format also recognize FaeGab and FaeGad variants in a similar ELISA setup. Using purified V2G produced in Nicotiana benthamiana leaves as standards on Coomassie-stained SDS/PAGE and immunoblots, the accumulation level of the different antibodies in seeds was deduced from the band intensity with ImageLab (Fig. 1).
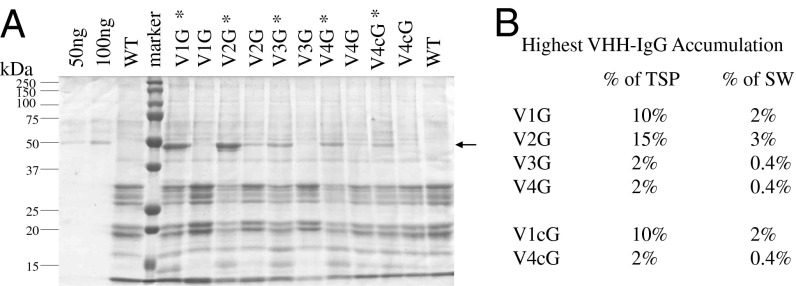
Fig. 1.
Comparison of the four VHH-IgG accumulation levels. Seed protein extracts from the highest (*) and the lowest VHH-IgG accumulating Arabidopsis transformants from constructs V1G, V2G, V3G, V4G, and codon-optimized V4cG separated on a Coomassie-stained 12% SDS/PAGE (A). The arrow indicates the ∼49 kDa VHH-IgG band. WT stands for wild-type seed extract. Ten micrograms of seed extract has been loaded in each lane, except the first two lanes, with 50 ng and 100 ng of purified antibody V2G. (B) Highest accumulation of VHH-IgG expressed as percent of total soluble protein (TSP) and seed weight (SW).
To evaluate if higher accumulation can be attained by additional codon optimization of the VHH domain, the VHH (llama) codon use within the highly expressed antibody V1G and the lowly expressed V4G was exchanged with the codon preference of Arabidopsis seed storage proteins. However, this process did not result in a change in the level of antibody accumulation. The codon-optimized version V1cG showed 10% total soluble protein accumulation, and V4cG accumulated to 2% of the total soluble protein, each similar to their respective unoptimized counterparts V1G and V4G (Fig. 1 and Fig. S2E).
In-Seed Assembled Functional VHH-IgA–Based Monomeric, Dimeric, and Secretory IgA Antibodies.
To produce VHH-IgA–based monomeric (bivalent), dimeric (tetravalent), and secretory IgA (tetravalent) formats (Fig. 2), the four anti-F4+ETEC VHHs were grafted on the codon-optimized Fc part of the porcine IgAb. The porcine IgAb has a short hinge of two amino acids (Asp-Pro), which is suggested to lack bacterial IgA protease site and, hence, is deemed to be suitable for the orogastric environment (21). The resultant four VHH-IgA fusions were named V1A, V2A, V3A, and V4A. Each T-DNA bearing the VHH-IgA coding sequence was cotransformed with individual T-DNAs bearing the coding sequence of the porcine J chain and the SC, each under the control of the β-phaseolin promoter (Fig. S3A) and primary transformants with different insertions of the three elements (VHH-IgA, J chain, and SC) were identified (Fig. S3B). Fifty plants with only the VHH-IgA insertion were classified as monomeric VHH-IgA– (mVHH-IgA) expressing plants, 31 plants with a VHH-IgA and J chain insertion as dimeric VHH-IgA (dVHH-IgA), and 11 plants with a VHH-IgA, J chain, and SC insert as secretory VHH-IgA (sVHH-IgA) (Fig. S3B). The respective individual transformants were denoted by letters “m,” “d,” or “s” before the transformant’s name.
Fig. 2.
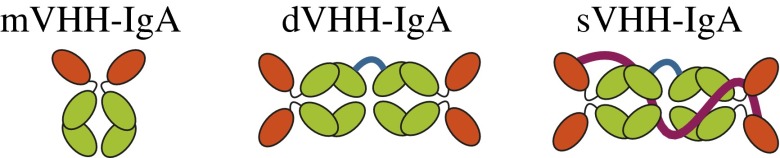
Schematic representation of VHH-IgA based monomeric (mVHH-IgA), dimeric (dVHH-IgA), and secretory IgA (sVHH-IgA). The VHH domain is indicated in red, fused to the porcine IgA Fc via a hinge in which CH2 and CH3 domains are indicated in green. The porcine joining chain is colored in blue and the secretory component is indicated in magenta.
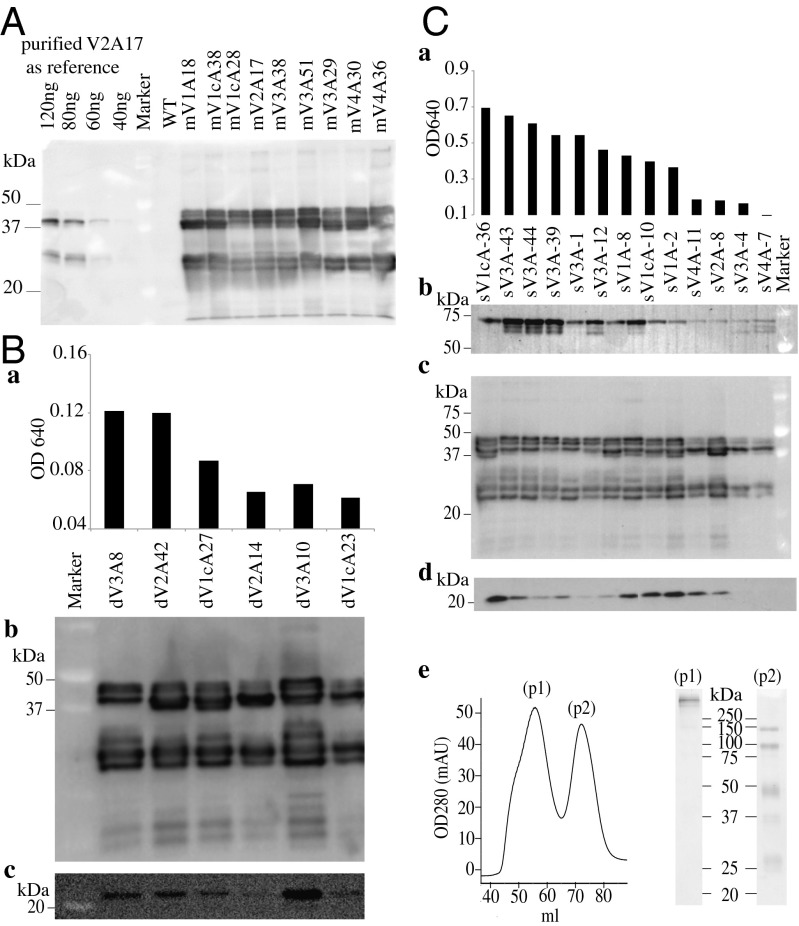
The accumulation level of functional FaeG-binding monomeric IgA (bivalent) was similar for all four mVHH-IgAs constructs, as detected by ELISA using polyclonal anti–porcine-IgA antibody (Fig. S3C). Among the 50 mVHH-IgA–expressing T2 seed stocks, nine (three mV1A, one mV2A, three mV3A, and two mV4A) were selected for further up-scaling and characterization (Fig. 3A). Immunoblot analysis under reducing conditions showed four heterologous bands of ∼37 to ∼50 kDa, instead of the expected single ∼39 kDa VHH-IgA band (Fig. 3A). Additionally, lower molecular-weight bands of ∼20 and ∼27 kDa were observed, which could presumably be the result of in-seed proteolysis (Fig. 3A). The four bands from ∼37 to ∼50 kDa were found to bind FaeG in an immunoprecipitation assay (Fig. S4B). Thus, the summation of these four band intensities should correspond to the expression level of functional VHH-IgA antibodies, which was determined to be about 0.5% of the seed weight (SW) in all of the lines examined (Fig. S5). Glycostaining with periodic acid Schiff’s reagent and endoglycosidase treatment with PNGase F and EndoH demonstrate that the two high molecular-weight bands bear N-linked glycans (Fig. S4A, bands i and ii). Analysis under nonreducing conditions shows dimers of ∼70 and ∼90 kDa. The ∼70-kDa dimer could perhaps be because of proteolytic truncation of the carboxylic end of VHH-IgA chains. In summary, differential glycosylation along with proteolytic truncation probably leads to the observed four bands.
Fig. 3.
Characterizing in-seed–produced monomeric VHH-IgA (A), dimeric VHH-IgA (B), and secretory VHH-IgA (C) antibodies. Immunoblots developed with anti-porcine IgA under reducing conditions of all transformants expressing VHH-IgA chains (A, B, b and C, c) show four bands about the expected molecular weight of 37 kDa and lower molecular weight (proteolyzed) bands around 25–20 kDa. The numbers above each line refer to the names of the transformants. WT stands for wild-type seed extract. In the dimeric VHH-IgA–producing transformants (B), the relative accumulation of functional assembled dVHH-IgA detected via anti-J chain ELISA is shown in (B, a). The corresponding accumulation of each of the two constituent elements (i.e., VHH-IgA and J chain) is shown in the immunoblots (B, b) and (B, c), respectively. Transformants with three different T-DNAs to produce secretory VHH-IgA (C) were analyzed by a functional anti-SC ELISA (C, a) to determine the proportion of FaeG-binding, SC-bearing VHH-IgA complexes. Immunoblots show the proportion of SC (C, b), VHH-IgA (C, c), and J chain (C, d) in respective transformants. Size-exclusion chromatography of the affinity-purified assembled complexes in extract sV1cA36, showing two peaks (p1, assembled sVHH-IgA; p2, partial assembly) analyzed under nonreducing coomassie stained SDS/PAGE (C, e).
Dimeric functional complexes of VHH-IgA + J chain were detected in 6 of 32 dVHH-IgA T2 seed extracts using the immobilized FaeG ELISA and a monoclonal antibody against the J chain (Fig. 3 B, a). The accumulation trend of the assembled VHH-IgA + J chain complex (Fig. 3 B, a) in the six seed extracts was reflected also in the accumulation of the J chain (Fig. 3 B, c) detected via immunoblot under reduced conditions. The summation of the four VHH-IgA band (∼37 to ∼50 kDa) intensities was also comparably similar and calculated to be about 0.5% of the SW (Fig. 3 B, b and Fig. S5). Seemingly, the proportion of assembled VHH-IgA + J chain might depend on the relative ratio of VHH-IgA: J chain molecules expressed and the stoichiometry on their posttranslational modifications.
FaeG-binding functional sVHH-IgA complexes bearing the SC were detected in 9 of the 15 sVHH-IgA T2 seed extracts, using monoclonal anti-SC antibody (Fig. 3 C, a). The high molecular-weight assembled complexes could be detected in immunoblots under nonreducing conditions and were also demonstrated by size-exclusion chromatography of the affinity-purified IgAs (using ligand Staphylococcus aureus superantigen-like protein 7/agarose) from the sV1cA36 seeds (Fig. 3 C, e, peak p1, and Fig. S6). To understand the observed variation in the proportion of assembled sVHH-IgA, the relative accumulation of the three constituent elements was determined via immunodetection under reduced conditions from the same blot after successive probing and stripping with anti-IgA, anti-SC, and anti-J chain antibodies. The results showed that VHH-IgA expression was identical in all nine lines and showed typical VHH-IgA bands (Fig. 3 C, c) as detected for mVHH-IgA and dVHH-IgA. The J-chain expression levels were variable (Fig. 3 C, d), and the amount of SC (Fig. 3 C, b) correlated with the relative expression of assembled sVHH-IgA determined by ELISA (Fig. 3 C, a). Additionally, heterogeneity in SC’s N-link glycosylation was noticed (Fig. 3 C, b). In totality, the finding implies that the molar accumulation of each of the three elements (VHH-IgA, J chain, and SC) in sVHH-IgA has an effect on the final accumulation of assembled sVHH-IgA antibodies. Thus, a fraction of VHH-IgA assembles as sVHH-IgA, but the remainder might assemble as monomeric or dimeric complexes. We could identify the coexistence of dVHH-IgA in three of the sVHH-IgA seed extracts (sV1cA36, sV1A8, and sV1A2). ELISA-based quantitation with purified V2A antibody indicated that the assembled sVHH-IgA in line V2A8 roughly accounts for about 50% of the VHH-IgA produced.
Seed-Produced Anti-F4+ETEC Antibodies Inhibit Bacterial Binding to Gut Villous Enterocytes.
The inhibitory function of the plant-produced antibodies was first tested in an in vitro villus adhesion assay, in which all seed extracts producing the VHH-IgG and mVHH-IgA inhibited the bacterial attachment to the porcine gut villous enterocytes by at least 50%, but wild-type seed extracts did not inhibit the bacterial villus interaction (Fig. S7A). In addition, mVHH-IgA, dVHH-IgA, or sVHH-IgA also showed inhibition (59%, 66%, and 61%, respectively) (Fig. S7B).
Protective Effect of Oral Feed-Based Passive Immunization in Challenged Piglets.
The capacity of seed-produced antibodies in preventing the F4+ETEC infection when administered orally in feed was evaluated in 21 F4-seronegative and F4R-positive piglets identified from a local pig farm (experimental scheme in Fig. 4A). These piglets were from different sows and bound to have genetic variability, affecting the outcome of the experiment, of which the F4R status is particularly important. The mucin-4 gene polymorphism test increases the likelihood of identifying the F4R-positive piglets; however, the phenotypic expression of receptors largely varies, as it is believed to involve gene epistasis (22). Hence, the piglets were randomly assorted into four groups and the phenotypic expression of F4R was confirmed after euthanasia.
Fig. 4.
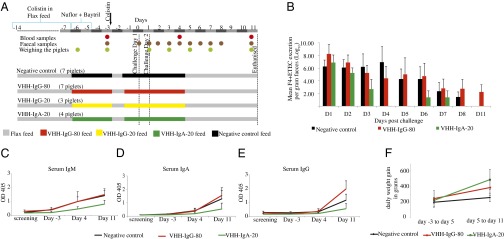
VHH-IgA feed reduces F4+ETEC infection in challenged piglets. (A) Schematic overview of the feed-challenge experiment with reference to the time line (D0 = day of the first challenge); feed regimen and time points of samples taken are indicated. (B) Shedding profile of F4+ETEC detected in feces expressed as the mean log10 for each group. Anti-F4+ETEC serum IgM (C), IgA (D), and IgG (E) responses averaged for the different piglets within each group. The error bars represent SD within the group. (F) Comparative weight gain during (day −3 to day 5) and after the experimental diet (day 5 to day 11) in the three groups represented as average daily weight gain calculated in grams.
For each of the four groups, the respective experimental feed was provided in the feeding vessel installed in the pens. Piglet nos. 1–7 constituted the negative control group, receiving feed with milled wild-type Arabidopsis seeds. The next seven piglets (nos. 8–14) received an 80-mg mixture of four VHH-IgG antibodies in feed per pig per day (VHH-IgG-80 group), and piglet nos. 15–17 received the same VHH-IgG mixture but at a dose of 20 mg per pig per day (VHH-IgG-20 group). The oligoclonal mix of all of the VHH-IgA based antibody formats in the piglet feed at a dose of 20 mg per pig per day was fed to piglet nos. 18–21 of the VHH-IgA-20 group. All piglets were challenged consecutively on the second (day 0) and third (day 1) day after providing the experimental feed (Fig. 4A) and the bacterial excretion was followed for a period of 11 d (Fig. 4B).
Compared with the control group, in which shedding of bacteria was observed until about 8 d postchallenge, the piglets of the VHH-IgA-20 group only shed F4+ETEC during the first 3 d postchallenge. When interpreting data of the individual piglets (Table S1), we observed that three of four piglets in the VHH-IgA-20 group showed a high excretion of F4+ETEC [>5.4 (log10)] on day 1, and bacteria could not be detected anymore in fecal samples of day 4. In contrast, three piglets of the control group, with similarly high excretion of F4+ETEC on day 1, continued to shed F4+ETEC until day 7 or 8. In the other piglets of the control group that did not show high excretion of F4+ETEC [<5.4 (log10)] on day 1, the initial infection rate was rather low, which can explain the shorter excretion period. Susceptibility of piglets is also influenced by the factors, such as the gut microbiota and stomach physiology, at the moment of experimental infection, which can attribute to variation in F4+ETEC infection. Nevertheless, high shedding of bacteria by 40–50% of piglets in a group leads to maintenance of higher infection pressure in a pigpen. Notably, in the VHH-IgA-20 group a small amount of F4+ETEC (100 bacteria per gram feces) reappeared on days 6 and 7 after discontinuing the VHH-IgA feeding regimen. This finding stresses the importance of a continued prophylactic treatment during the F4+ETEC critical window period (i.e., first 2 wk after weaning), after which piglets are less susceptible to ETEC infections.
The seven piglets of the VHH-IgG-80 group showed prolonged shedding of challenged bacteria in titers higher than those of the control piglets. The VHH-IgG-20 group showed an early decline in bacterial shedding; however, postmortem analysis revealed that these piglets had a low expression of the F4 receptor and are therefore naturally insensitive to F4+ETEC. Hence, this group was not included in our interpretation (see Table S1 for F4R status of all piglets). The evolution of bacterial shedding in the course of time within the VHH-IgG-80, VHH-IgA-20, and negative control group was significant (P = 0.019).
The prolonged shedding profile of the control and the VHH-IgG-80 group correlates with the observed increase of anti-F4 fimbriae antibodies in the blood samples of day 11. Indeed, the VHH-IgA-20 group showed only a weak F4-specific immune response compared with the control and VHH-IgG-80 group. The evolution of the Ig seroconversion rate (all isotypes) observed for the piglets in the VHH-IgA-20 group over time was significantly (P = 0.003) lower than that in the VHH-IgG-80 and control groups (Fig. 4 C–E). The evolution of anti–F4-IgM levels in serum over time between the VHH-IgG-80, VHH-IgA-20, and negative control group from 3 d before the challenge (day −3) till the day of euthanasia (day 11) showed a tendency of being significant (P > 0.05) (Fig. 4C), but the difference was statistically significant at day −3 (P = 0.044) and day 11 (P = 0.025). The evolution of serum anti–F4-IgG (Fig. 4E) and anti–F4-IgA (Fig. 4D) levels between these three groups from day −3 till the day 11 was significant (P < 0.01 and P = 0.01, respectively). This result suggests that the in-seed–made VHH-IgA-based antibodies at its 20 mg/d dose provided passive protection at the gut mucosal surface by preventing the interaction of the pathogenic bacteria with the host cells, thus avoiding priming of the immune system.
The piglet weight-gain parameters corroborated the results for the VHH-IgA-20 group. The daily weight gain of all piglets in the three groups (negative control, VHH-IgG-80, and VHH-IgA-20) was similar during the challenge period (day −3 to day 5); however, after changing this experimental feed to the basal flax feed from day 5 to day 11, piglets that were previously on VHH-IgA-20 feed had the highest weight gain, followed by VHH-IgG-80 feed and the negative control (Fig. 4F). This observed evolution of weight gain for the three groups over time was highly significant (P = 0.006).
Discussion
Oral passive immunization can lead to immediate mucosal protection at the gastric surface, and can prevent enteric infections (16). The oral route is more suitable for mass administration of antibodies for humans, as well as for farm animals compared with the needle-based route. Particularly in case of PWD, specific antibodies against noninvasive E. coli are needed at the intestinal mucosal surface, to prevent ETEC attachment, colonization, and subsequent secretion of toxins. Oral passive immunization, however, runs the risk of antibody degradation in the harsh gastric environment before being effective (23). Another factor is the choice of antibody isotypes; unlike IgG, antibody isotypes IgA and IgM in their polymeric and secretory formats are better suited for mucosal surfaces. With the aim to develop an oral passive immunization-based approach to prevent the F4+ETEC infection-related PWD in piglets, we expressed antibodies in seeds. To address the second issue of apt antibody isotype choice, we embarked on a nonconventional strategy by designing unique light chain-devoid, simple, and robust chimeric antibodies by grafting llama anti-F4+ETEC VHHs on pig Fc. Based on the strategy, four anti-F4+ETEC VHHs, when expressed as a fusion to porcine IgG3 or IgAb in Arabidopsis seeds, led to the production of divalent antibodies. Furthermore, cotransformation of VHH-IgA with the J chain- and SC-produced multivalent dVHH-IgAs and sVHH-IgAs. Although all of the assembled antibody formats precisely recognized the antigen and inhibited the attachment of the F4+ETEC bacteria in vitro, the results of the piglet feed-challenge experiment provide the proof of efficacy of oral passive immunization.
In the feed-challenge experiment, the dose for antibodies in feed formulation was calculated based on a previous neonatal challenge model where protection was achieved by monoclonal anti-F4+ETEC antibodies (murine IgG1) at a concentration of 8 mg/100 mL of milk (24). From the observed consumption of milk by neonatal piglets until weaning (4 wk of age) and the ratio of weight gain to feed consumption during the first and second week after weaning, we estimated that if not weaned, 4- to 5-wk-old piglets would consume a minimum of 1 L milk per day, corresponding to ∼80 mg of antibody per piglet per day. Because a substantial portion of the VHH-IgA treatment mixture contains the tetravalent constructs, we opted to use four-times less VHH-IgA (i.e., 20 mg) compared with the VHH-IgG dose (80 mg). Thus, three treatment groups receiving (i) 80 mg of VHH-IgG per day, (ii) 20 mg of VHH-IgA per day, and (iii) 20 mg of VHH-IgG per day were included. However, piglets in the VHH-IgG-20 group showed low F4R expression, which is required for bacterial attachment and establishment of infection, making it difficult to draw comparisons between the two oligoclonal mixtures of VHH-IgG– and VHH-IgA–based feeds.
The rapid decline in shedding of the challenge strain in the sensitive piglets (F4R-positive) of the VHH-IgA-20 group to below the detection level of less than 100 bacteria per gram of feces at day 4, strongly suggests that the VHH-IgA-20 diet reduced or even prevented bacterial attachment, colonization, and hence multiplication. This process resulted in an overall reduced infection pressure in the pigpen, which is essential for achieving a herd-passive immunity and limiting transmission in farm conditions.
The observed high bacterial shedding and high seroconversion of the VHH-IgG-80 group suggests that the VHH-IgG fusion might not always be best for oral passive immunization. An explanation for this may be the presence of the Fc neonatal receptor (FcRn). While studying the porcine FcRn, Stirling et al. noticed almost a promiscuous transport of orally administered bovine IgG into the pig’s blood circulation, through the porcine FcRn, which is expressed in the gut of juvenile as well as adult pigs (25). The faster seroconversion and prolonged bacterial shedding observed in the VHH-IgG-80 group could be a result of the interaction of the porcine VHH-IgG3 with the FcRn, resulting either in clearance of the VHH-IgG by transport across the gut epithelium or in facilitating bacterial attachment.
From the sequence analysis, porcine IgG3 has been predicted to bind the FcRn receptor with the highest affinity than the other five porcine IgG subclasses (20); given this, the interaction of VHH-IgG to FcRn in the gut could be very likely. Alternatively, the VHH-IgG might have been rendered ineffective in the gastric environment. If the hypothesis of FcRn-IgG3 interaction would be true, it would be an interesting way for receptor-mediated uptake of vaccine antigens (e.g., antigen-IgG3 Fc fusion) in the gut’s epithelium to elicit mucosal immunity.
The stability of antibody constructs in gut might have also favored efficacy of VHH-IgA-20 feed over VHH-IgG-80 feed. The association of the SC with human plasma-derived polymeric IgAs was shown to prevent their degradation in mouse intestinal washes for more than 16 h (15). Presumably, the seed-expressed SC, associated to the VHH-IgA, also attributes to the VHH-IgA’s stability in the piglet gut. Higher accumulation of assembled sVHH-IgA would thus be desirable. Modulating the expression ratios of all of the elements (VHH-IgA, J chain, and SC) might influence higher assembly, as it has been recently demonstrated in case of reconstituted SIgA from plasma-purified dIgA + human colostrum-derived SC, that the association is equimolar and excess of SC remains unassembled (15). Additionally, the observed differential glycosylation and in-seed proteolysis of VHH-IgA might also affect the stoichiometric assembly of antibodies; however, this aspect of posttranslational modifications might differ in the seed tissue of other plant species.
To deliver a proof of commercial feasibility for oral passive immunization of piglets in the future, the VHH-IgA–based antibodies would need to be transformed in a feed crop like soybean or pea. In field conditions, both in large-scale pig farms as well as small-scale subsistence farming, the ability of antibody-containing feed to rapidly limit bacterial shedding would help in decreasing the overall infection pressure within the herd and in reducing the dependency on antibiotics. The future valorization of these results into a product will depend on the final cost of the VHH-IgA–based feed. Although the production cost of such feed would be low, the final market sale price would also have to incur for the expensive GMP regulatory testing and registration steps.
In addition, the simplified sVHH-IgA–based molecules could be evaluated for human passive mucosal immunization, for example against cholera, which is based on similar pathogenic mechanisms as weaning diarrhea. For such an application, sVHH-IgA can be produced in confined cGMP-compliant infrastructures. Production of VHH-IgA–based dVHH-IgA and sVHH-IgA might be an alternative to commercial production of dIgA and SIgA, thereby empowering to unleash the potential for passive mucosal immunization in veterinary and human medicine.
In conclusion, we have shown the merit of designer VHH-IgA–based multivalent antibodies for immediate protection by oral, feed-based passive immunization.
Materials and Methods
Generation of Anti-FaeG VHHs.
The anti-FaeG VHHs were generated, as described previously (26), by immunizing llama with purified FaeGac, with the exception that the VHH displaying phage particles were panned in parallel against immobilized FaeGac and FaeGad. Sequence of the selected four VHHs (V1–V4) and the codon-optimized versions (V1c, V4c) have been deposited in GenBank database (accession nos. KC848502–KC848507).
VHH-ETEC Agglutination Assay.
The VHHs were covalently linked to magnetic beads (Dynabeads M-270 carboxylic acid), making them multivalent, and mixed with bacterial cells as follows: C585-80 expressing F4ad fimbriae, C95-72 expressing F4ac, C1023-78 expressing F4ab, the negative control strain K514 expressing no fimbriae or K514-PIH120 (transformed with plasmid PIH120) expressing F18 fimbriae on a glass slide, after which the agglutination was noted.
Construction, Expression, and Characterization of VHH-Fc Fusion-Based Antibodies.
All of the VHH-Fc fusions and the SC were cloned into the pPhasGW vector (14) with the N-terminal signal peptide of the Arabidopsis seed storage 2S2 protein and a C-terminal endoplasmic retention tag (KDEL). The J chain was cloned with the same regulatory elements into a multisite gateway vector with the Bar gene for selection. Transformants were obtained by floral dip. The soluble seed protein content was measured using the Bio-Rad DC protein assay kit. The accumulation of assembled functional antibodies was determined by FaeGac immobilized ELISA. The characterization and quantification of the seed-produced antibodies was performed via SDS/PAGE and immunoblotting. More details can be found in the SI Materials and Methods.
Glycan Analysis.
The periodic acid Schiff’s staining of the glycoproteins was performed using the Glycoprotein staining kit (Thermo Scientific). The mobility shift in SDS/PAGE was assessed on N-linked Glycosidase treatment with Endo H and or PNGase F.
Piglet Villous Binding Inhibition Test.
This test was performed as described by Coddens et al. (27); more details are in SI Materials and Methods.
Feed Formulation and Piglet Feed-Challenge Experiment.
From the determined antibody expression level in seeds, the respective experimental feed were formulated (Table S2), details of which are in SI Materials and Methods. Based on a previous piglet challenge model (28), the feed-challenge experiment (Fig. 4A) was developed, which is described in detail in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank E. Pardon for mentoring the VHH isolation experiments; M. De Kerpel, G. De Smet, S. Brabant, R. Cooman, P. Almgren, T. Goetstouwers, E. Van Lerberge, J. Nolf, H. Hoffmeisterova, and N. Keirse for their practical assistance; G. Angenon and B. Van Droogenbroeck for use of their greenhouse facilities; and G. Lomonossoff for providing the pEAQ vectors. V.V. received the “Bijzonder Onderzoeksfonds” predoctoral fellowship of the Ghent University during the first 3 y, and sponsorship from the Vlaams Instituut voor Biotechnologie (VIB) for the fourth year. The animal challenge experiment was funded by a starTT grant from Industrieel Onderzoeksfonds of Ghent University.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. KC848502–KC848507).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1301975110/-/DCSupplemental.
References
- 1.Hurley WL, Theil PK. Perspectives on immunoglobulins in colostrum and milk. Nutrients. 2011;3(4):442–474. doi: 10.3390/nu3040442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adjiri-Awere A, Van Lunen TA. Subtherapeutic use of antibiotics in pork production: Risks and alternatives. Can J Anim Sci. 2005;85(2):117–130. [Google Scholar]
- 3.Fairbrother JM, Nadeau É, Gyles CL. Escherichia coli in postweaning diarrhea in pigs: An update on bacterial types, pathogenesis, and prevention strategies. Anim Health Res Rev. 2005;6(1):17–39. doi: 10.1079/ahr2005105. [DOI] [PubMed] [Google Scholar]
- 4.van Beers-Schreurs HM, Vellenga L, Wensing T, Breukink HJ. The pathogenesis of the post-weaning syndrome in weaned piglets: A review. Vet Q. 1992;14(1):29–34. doi: 10.1080/01652176.1992.9694322. [DOI] [PubMed] [Google Scholar]
- 5.Verdonck F, Cox E, Goddeeris BM. F4 fimbriae expressed by porcine enterotoxigenic Escherichia coli, an example of an eccentric fimbrial system? J Mol Microbiol Biotechnol. 2004;7(4):155–169. doi: 10.1159/000079825. [DOI] [PubMed] [Google Scholar]
- 6.Melkebeek V, Goddeeris BM, Cox E. ETEC vaccination in pigs. Vet Immunol Immunopathol. 2013;152(1-2):37–42. doi: 10.1016/j.vetimm.2012.09.024. [DOI] [PubMed] [Google Scholar]
- 7.Lau OS, Sun SSM. Plant seeds as bioreactors for recombinant protein production. Biotechnol Adv. 2009;27(6):1015–1022. doi: 10.1016/j.biotechadv.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Van Droogenbroeck B, et al. Aberrant localization and underglycosylation of highly accumulating single-chain Fv-Fc antibodies in transgenic Arabidopsis seeds. Proc Natl Acad Sci USA. 2007;104(4):1430–1435. doi: 10.1073/pnas.0609997104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zimmermann J, et al. Antibody expressing pea seeds as fodder for prevention of gastrointestinal parasitic infections in chickens. BMC Biotechnol. 2009;9:79. doi: 10.1186/1472-6750-9-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muyldermans S. Single domain camel antibodies: Current status. J Biotechnol. 2001;74(4):277–302. doi: 10.1016/s1389-0352(01)00021-6. [DOI] [PubMed] [Google Scholar]
- 11.Transue TR, De Genst E, Ghahroudi MA, Wyns L, Muyldermans S. Camel single-domain antibody inhibits enzyme by mimicking carbohydrate substrate. Proteins. 1998;32(4):515–522. doi: 10.1002/(sici)1097-0134(19980901)32:4<515::aid-prot9>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 12.Muyldermans S, Cambillau C, Wyns L. Recognition of antigens by single-domain antibody fragments: The superfluous luxury of paired domains. Trends Biochem Sci. 2001;26(4):230–235. doi: 10.1016/s0968-0004(01)01790-x. [DOI] [PubMed] [Google Scholar]
- 13.Harmsen MM, et al. Escherichia coli F4 fimbriae specific llama single-domain antibody fragments effectively inhibit bacterial adhesion in vitro but poorly protect against diarrhoea. Vet Microbiol. 2005;111(1-2):89–98. doi: 10.1016/j.vetmic.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 14.Morandini F, et al. Non-food/feed seeds as biofactories for the high-yield production of recombinant pharmaceuticals. Plant Biotechnol J. 2011;9(8):911–921. doi: 10.1111/j.1467-7652.2011.00605.x. [DOI] [PubMed] [Google Scholar]
- 15.Longet S, et al. Human plasma-derived polymeric IgA and IgM antibodies associate with secretory component to yield biologically active secretory-like antibodies. J Biol Chem. 2013;288(6):4085–4094. doi: 10.1074/jbc.M112.410811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corthésy B. Recombinant secretory immunoglobulin A in passive immunotherapy: Linking immunology and biotechnology. Curr Pharm Biotechnol. 2003;4(1):51–67. doi: 10.2174/1389201033378020. [DOI] [PubMed] [Google Scholar]
- 17.Ma JK, et al. Generation and assembly of secretory antibodies in plants. Science. 1995;268(5211):716–719. doi: 10.1126/science.7732380. [DOI] [PubMed] [Google Scholar]
- 18.Paul M, Ma JKC. Plant-made pharmaceuticals: Leading products and production platforms. Biotechnol Appl Biochem. 2011;58(1):58–67. doi: 10.1002/bab.6. [DOI] [PubMed] [Google Scholar]
- 19.González EA, Vázquez F, Ignacio Garabal J, Blanco J. Isolation of K88 antigen variants (ab, ac, ad) from porcine enterotoxigenic Escherichia coli belonging to different serotypes. Microbiol Immunol. 1995;39(12):937–942. doi: 10.1111/j.1348-0421.1995.tb03296.x. [DOI] [PubMed] [Google Scholar]
- 20.Butler JE, Wertz N, Deschacht N, Kacskovics I. Porcine IgG: Structure, genetics, and evolution. Immunogenetics. 2009;61(3):209–230. doi: 10.1007/s00251-008-0336-9. [DOI] [PubMed] [Google Scholar]
- 21.Brown WR, et al. The hinge deletion allelic variant of porcine IgA results from a mutation at the splice acceptor site in the first C alpha intron. J Immunol. 1995;154(8):3836–3842. [PubMed] [Google Scholar]
- 22.Rasschaert K, Verdonck F, Goddeeris BM, Duchateau L, Cox E. Screening of pigs resistant to F4 enterotoxigenic Escherichia coli (ETEC) infection. Vet Microbiol. 2007;123(1-3):249–253. doi: 10.1016/j.vetmic.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 23.Reilly RM, Domingo R, Sandhu J. Oral delivery of antibodies. Future pharmacokinetic trends. Clin Pharmacokinet. 1997;32(4):313–323. doi: 10.2165/00003088-199732040-00004. [DOI] [PubMed] [Google Scholar]
- 24.de Geus B, Harmsen M, van Zijderveld F. Prevention of diarrhoea using pathogen specific monoclonal antibodies in an experimental enterotoxigenic E. coli infection in germfree piglets. Vet Q. 1998;20(Suppl 3):S87–S89. [PubMed] [Google Scholar]
- 25.Stirling CMA, et al. Characterization of the porcine neonatal Fc receptor—Potential use for trans-epithelial protein delivery. Immunology. 2005;114(4):542–553. doi: 10.1111/j.1365-2567.2004.02121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korotkov KV, Pardon E, Steyaert J, Hol WGJ. Crystal structure of the N-terminal domain of the secretin GspD from ETEC determined with the assistance of a nanobody. Structure. 2009;17(2):255–265. doi: 10.1016/j.str.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coddens A, et al. Recognition of blood group ABH type 1 determinants by the FedF adhesin of F18-fimbriated Escherichia coli. J Biol Chem. 2009;284(15):9713–9726. doi: 10.1074/jbc.M807866200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cox E, Schrauwen E, Cools V, Houvenaghel A. Experimental induction of diarrhea in newly-weaned piglets. J Vet Med. 1991;38(6):418–426. doi: 10.1111/j.1439-0442.1991.tb01030.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.