Abstract
The properties of 5α-reductase have been compared in genital skin fibroblasts cultured from five patients from three families (Los Angeles, Dallas, and Dominican Republic) in which hereditary male pseudohermaphroditism has been established to result from deficient conversion of testosterone to dihydrotestosterone.
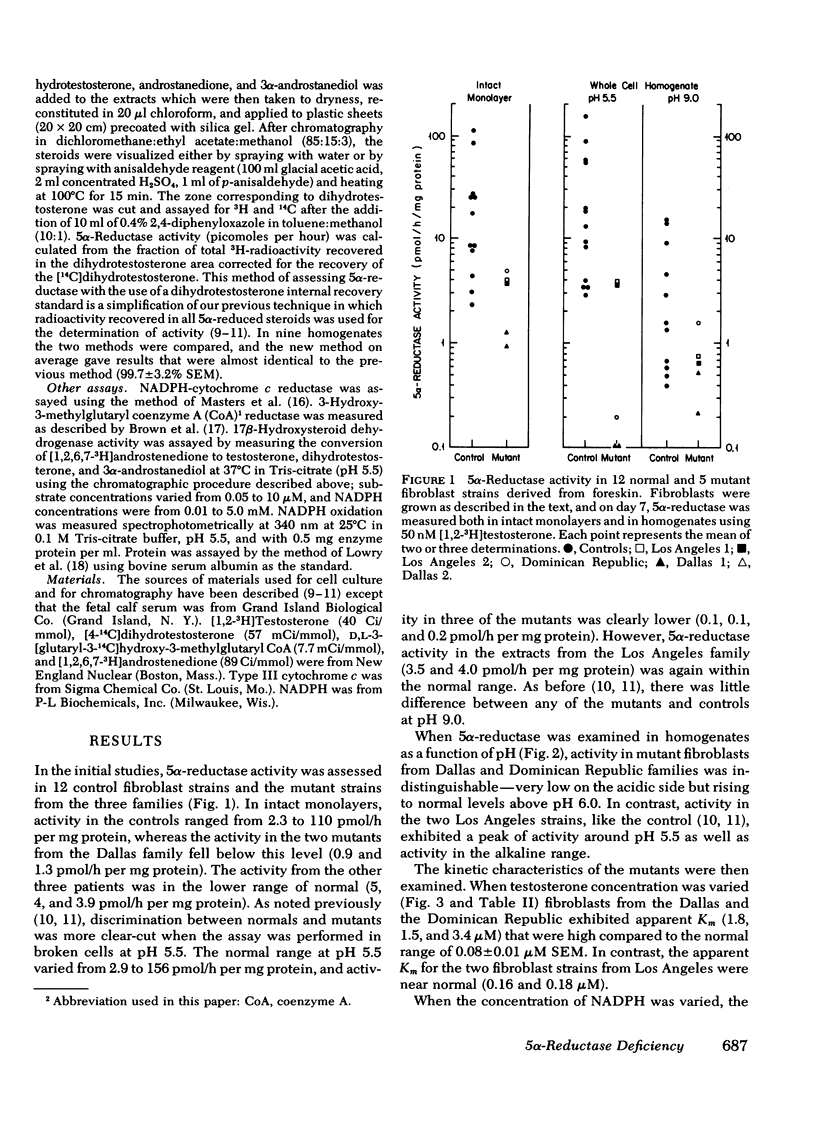
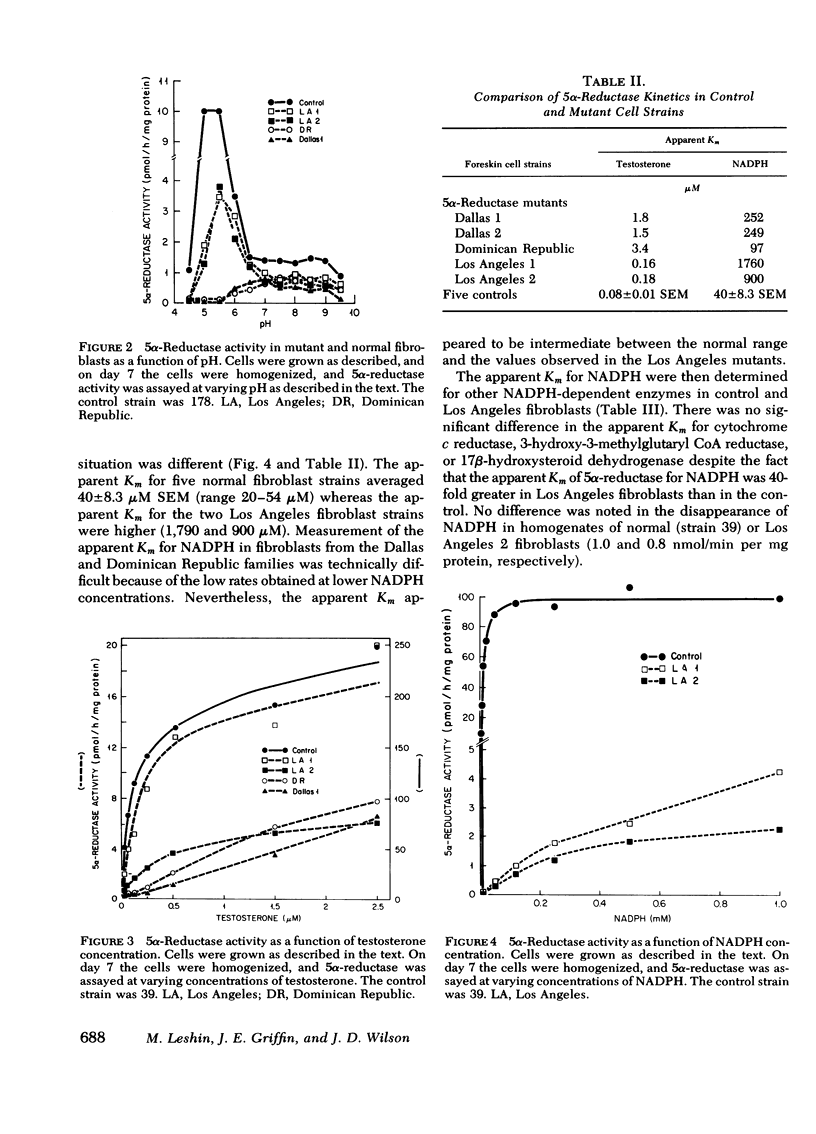
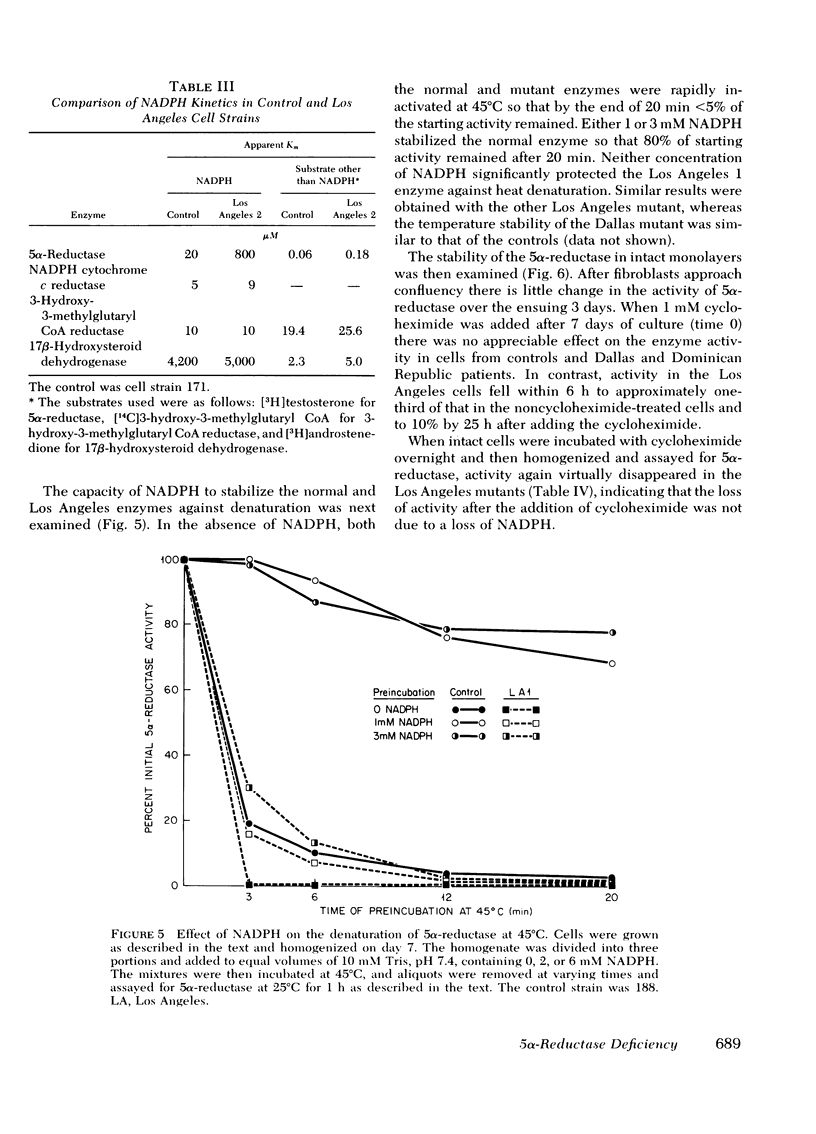
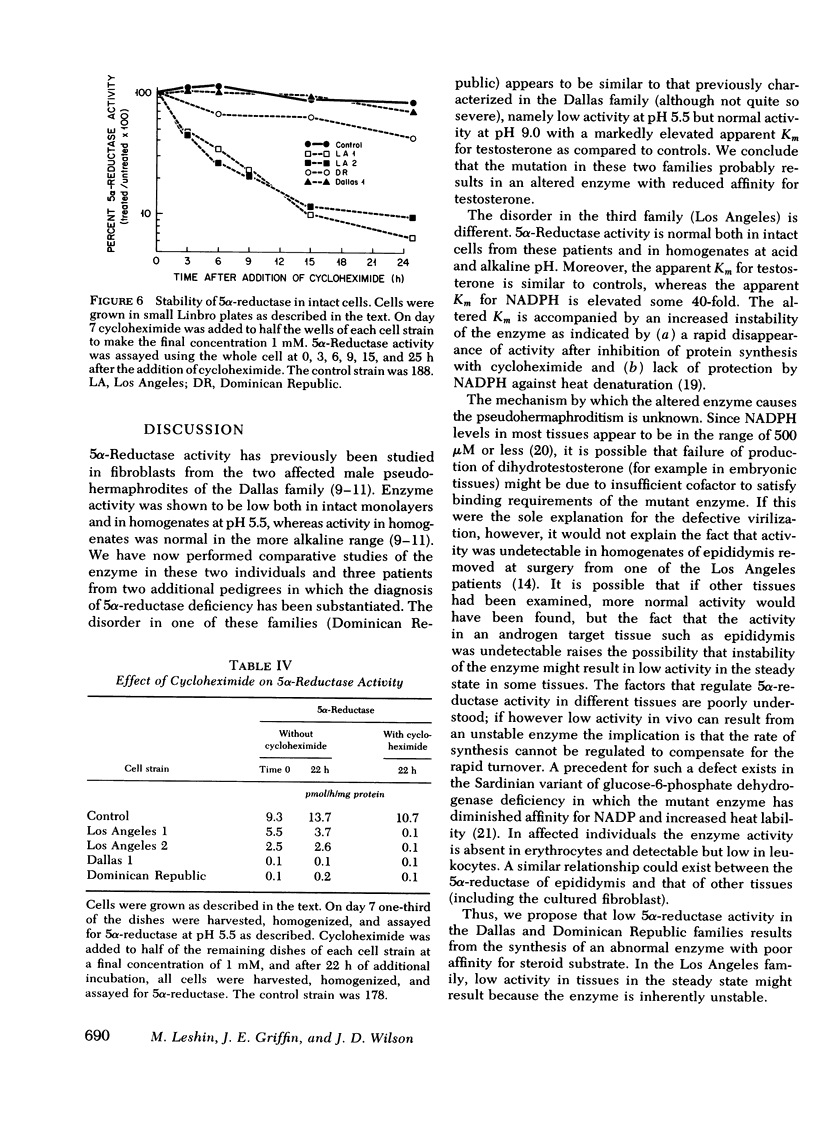
Despite the fact that 5α-reductase was immeasurable in a homogenate of epididymis removed from one of the Los Angeles patients, 5α-reductase activity was normal in intact fibroblasts and fibroblast extracts from both patients from the Los Angeles family. Although the apparent Km for testosterone was also near normal, the apparent Km for NADPH in these mutants is elevated some 40-fold above normal. Furthermore, the enzyme is not protected against denaturation at 45°C by concentrations of NADPH that stabilize normal 5α-reductase, and in intact fibroblasts from these patients (but not from controls), enzyme activity decreases promptly when protein synthesis is inhibited. We conclude that the mutation in this family results in an unstable enzyme.
In contrast 5α-reductase activity in fibroblast extracts from a patient from the Dominican Republic family is similar to that previously described in two members of the Dallas family, namely total enzyme activity is low at the optimal pH for the normal reaction, and the apparent Km for testosterone is some 20-fold higher than that of the controls. We conclude that the mutations in the Dallas and Dominican Republic families are similar and result in low activity of the enzyme as the result of a decreased affinity for testosterone.
Thus, two distinct types of mutations can produce male pseudohermaphroditism due to deficient dihydrotestosterone formation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonsignore A., Fornaini G., Leoncini G., Fantoni A., Segni P. Characterization of leukocyte glucose 6-phosphate dehydrogenase in Sardinian mutants. J Clin Invest. 1966 Dec;45(12):1865–1874. doi: 10.1172/JCI105491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Dana S. E., Goldstein J. L. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J Biol Chem. 1974 Feb 10;249(3):789–796. [PubMed] [Google Scholar]
- Burch H. B., Bradley M. E., Lowry O. H. The measurement of triphosphopyridine nucleotide and reduced triphosphopyridine nucleotide and the role of hemoglobin in producing erroneous triphosphopyridine nucleotide values. J Biol Chem. 1967 Oct 10;242(19):4546–4554. [PubMed] [Google Scholar]
- Griffin J. E., Punyashthiti K., Wilson J. D. Dihydrotestosterone binding by cultured human fibroblasts. Comparison of cells from control subjects and from patients with hereditary male pseudohermaphroditism due to androgen resistance. J Clin Invest. 1976 May;57(5):1342–1351. doi: 10.1172/JCI108402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperato-McGinley J., Guerrero L., Gautier T., Peterson R. E. Steroid 5alpha-reductase deficiency in man: an inherited form of male pseudohermaphroditism. Science. 1974 Dec 27;186(4170):1213–1215. doi: 10.1126/science.186.4170.1213. [DOI] [PubMed] [Google Scholar]
- JOST A. The role of fetal hormones in prenatal development. Harvey Lect. 1961;55:201–226. [PubMed] [Google Scholar]
- Jost A. A new look at the mechanisms controlling sex differentiation in mammals. Johns Hopkins Med J. 1972 Jan;130(1):38–53. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Moore R. J., Griffin J. E., Wilson J. D. Diminished 5alpha-reductase activity in extracts of fibroblasts cultured from patients with familial incomplete male pseudohermaphroditism, type 2. J Biol Chem. 1975 Sep 25;250(18):7168–7172. [PubMed] [Google Scholar]
- Moore R. J., Wilson J. D. Extraction of the reduced nicotinamide adenine dinucleotide phosphate:delta 4-3-ketosteroid-5-alpha oxidoreductase of rat prostate with digitonin and potassium chloride. Biochemistry. 1974 Jan 29;13(3):450–456. doi: 10.1021/bi00700a009. [DOI] [PubMed] [Google Scholar]
- Moore R. J., Wilson J. D. Steroid 5alpha-reductase in cultured human fibroblasts. Biochemical and genetic evidence for two distinct enzyme activities. J Biol Chem. 1976 Oct 10;251(19):5895–5900. [PubMed] [Google Scholar]
- NOWAKOWSKI H., LENZ W. Genetic aspects in male hypogonadism. Recent Prog Horm Res. 1961;17:53–95. [PubMed] [Google Scholar]
- Opitz J. M., Simpson J. L., Sarto G. E., Summitt R. L., New M., German J. Pseudovaginal perineoscrotal hypospadias. Clin Genet. 1972;3(1):1–26. doi: 10.1111/j.1399-0004.1972.tb01721.x. [DOI] [PubMed] [Google Scholar]
- Peterson R. E., Imperato-McGinley J., Gautier T., Sturla E. Male pseudohermaphroditism due to steroid 5-alpha-reductase deficiency. Am J Med. 1977 Feb;62(2):170–191. doi: 10.1016/0002-9343(77)90313-8. [DOI] [PubMed] [Google Scholar]
- Siiteri P. K., Wilson J. D. Testosterone formation and metabolism during male sexual differentiation in the human embryo. J Clin Endocrinol Metab. 1974 Jan;38(1):113–125. doi: 10.1210/jcem-38-1-113. [DOI] [PubMed] [Google Scholar]
- Simpson J. L., New M., Peterson R. E., German J. Pseudovaginal perineoscrotal hypospadias (PPSH) in sibs. Birth Defects Orig Artic Ser. 1971 May;7(6):140–144. [PubMed] [Google Scholar]
- Walsh P. C., Madden J. D., Harrod M. J., Goldstein J. L., MacDonald P. C., Wilson J. D. Familial incomplete male pseudohermaphroditism, type 2. Decreased dihydrotestosterone formation in pseudovaginal perineoscrotal hypospadias. N Engl J Med. 1974 Oct 31;291(18):944–949. doi: 10.1056/NEJM197410312911806. [DOI] [PubMed] [Google Scholar]
- Wilson J. D. Dihydrotestosterone formation in cultured human fibroblasts. Comparison of cells from normal subjects and patients with familial incomplete male pseudohermaphroditism, Type 2. J Biol Chem. 1975 May 10;250(9):3498–3504. [PubMed] [Google Scholar]
- Wilson J. D. Sexual differentiation. Annu Rev Physiol. 1978;40:279–306. doi: 10.1146/annurev.ph.40.030178.001431. [DOI] [PubMed] [Google Scholar]



