Abstract
When positioned into the integrin α-6 gene, an Hoxd9lacZ reporter transgene displayed parental imprinting in mouse embryos. While the expression from the paternal allele was comparable with patterns seen for the same transgene when present at the neighboring HoxD locus, almost no signal was scored at this integration site when the transgene was inherited from the mother, although the Itga6 locus itself is not imprinted. The transgene exhibited maternal allele-specific DNA hypermethylation acquired during oogenesis, and its expression silencing was reversible on passage through the male germ line. Histone modifications also corresponded to profiles described at known imprinted loci. Chromosome conformation analyses revealed distinct chromatin microarchitectures, with a more compact structure characterizing the maternally inherited repressed allele. Such genetic analyses of well-characterized transgene insertions associated with a de novo-induced parental imprint may help us understand the molecular determinants of imprinting.
Keywords: epigenetic, architecture, enhancer, Hox genes
Genomic imprinting is a phenomenon observed in mammals and marsupials, where some genes are expressed in a parent-of-origin–specific manner, with one of two alleles being stably silenced during its passage through the germ line (1). As such, imprinting can be considered as a regulatory process to control gene expression, and the dysfunction of this mechanism in humans leads to severe conditions, such as Angelman and Prader–Willi syndromes. Although this allelic silencing is, thus, admittedly of great benefit for the organism, both the evolutionary and ontogenetic origins of this parental-specific gene dosage remain unclear. Imprinted genes are usually associated with differentially methylated regions, a DNA mark that acts as the imprint to distinguish the parental alleles. In the mouse, many imprinted genes seem to cluster together at particular genomic loci under the regulation of a single major differentially methylated region called the imprinting control region (ICR). The ICR may, thus, control the imprinting for the full gene cluster, which can contain both maternally and paternally expressed genes as well as genes transcribed irrespective of parental origin (2, 3).
Several mechanisms have been proposed to account for imprint-associated gene silencing. One possibility is to directly trigger monoallelic DNA methylation followed by a repressive chromatin environment over one allele, whereas the other allele remains active. Gene transcription may be another mechanism used when the ICR contains the promoter for a noncoding RNA, which may target methylation and repressive histone modifications to imprinted genes (4–7). Imprinting may also rely on the presence of an insulator within the ICR, which restricts the activity of shared enhancers to either one of the target genes (8). The specificity of DNA methyltransferases (9) for ICRs and the maintenance of these marks throughout development at a time when the embryo undergoes genome-wide demethylation (10, 11) is poorly understood. Although some chromatin modifications can prevent de novo DNA methylation, others facilitate the recruitment of DNA methyltransferases (12–16). DNA or chromatin marks at ICRs are acquired in the germ line and persist after fertilization in the developing embryo; eventually, they are erased during gametogenesis. This epigenetic reprogramming ensures a proper transmission of imprinted loci to the following generation, with appropriate parent-of-origin–specific gene expression. Defects in these processes have been reported to lead to severe congenital syndromes as well as cancer and obesity (17).
The HoxD locus is a cluster of genes with important functions during embryonic patterning. These genes are regulated through both local and long-range mechanisms, including regulatory sequences located within large flanking centromeric gene deserts. Although no imprinted region has been identified on this large genomic landscape on mouse chromosome 2 (18–20), some reports mention that the human syntenic region 2q31 may contain imprinted genes involved in bipolar affective disorder (21) and obesity (22). By studying long-range gene regulation at this locus, we noticed that a lacZ reporter transgene inserted into the Itga6 gene displayed a clear parent-of-origin transcription. Transgene-induced imprinting was previously reported in several cases but generally in a position-independent manner (23–27). Here, we show that lacZ expression is silenced when the transgene is inherited from the mother and that this silencing is position-dependent, because the same transgene relocated nearby was not imprinted. We also show that the two alleles adopt different 3D conformations, and we describe how this local imprint impacts on the enhancer activity and expression of neighbor genes.
Results
Allele-Specific and Position-Dependent Expression of a lacZ Reporter Transgene.
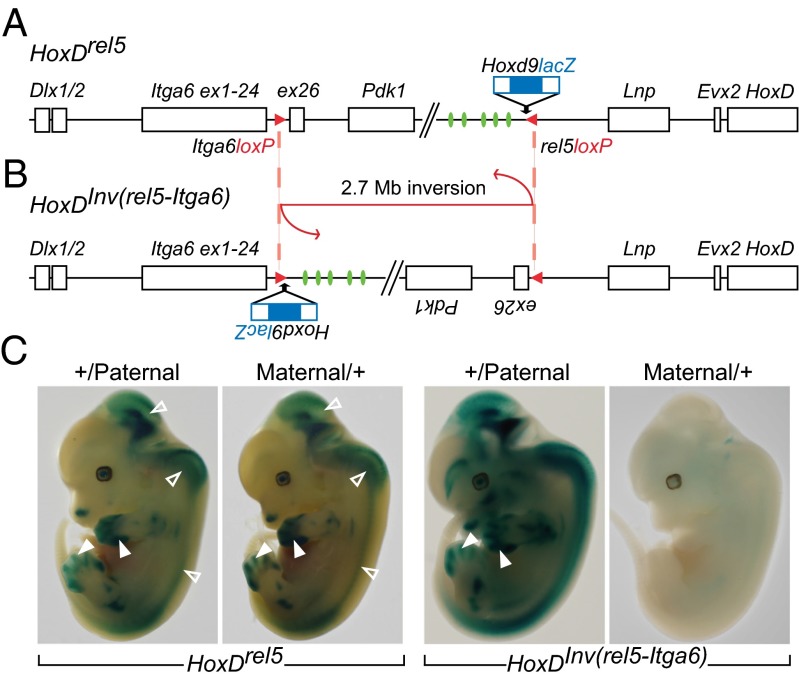
The mouse HoxD gene cluster is flanked by a centromeric gene desert (28) containing remote enhancer sequences necessary to control Hoxd gene transcription in developing digits (29). To investigate this complex regulation, we undertook a targeted transgenic approach, where an Hoxd9lacZ reporter transgene was inserted into several sites upstream of HoxD to capture regulatory influences. Whenever this transgene was inserted within 1 Mb of centromeric DNA, lacZ expression in developing digits was observed, showing its capacity to respond to the influence of the strong enhancers located nearby (30). We induced a large inversion by using a loxP site contained in the Hoxd9lacZ transgene targeted to the rel5 position (Fig. 1A) and another loxP site located in the Itga6 locus (31). This 2.7-Mb large inversion [HoxDInv(rel5-Itga6)] repositioned both the lacZ reporter transgene and the whole centromeric gene desert into the Itga6 locus (Fig. 1B). Because enhancer sequences were inverted, lacZ activity was still scored in presumptive digits (Fig. 1C), whereas the endogenous, noninverted Hoxd13 gene was severely down-regulated, because it was disconnected from the inverted enhancers (29).
Fig. 1.
Allele-specific expression of a lacZ transgene positioned inside the Itga6 locus. (A) Scheme of the HoxDrel5 allele. An Hoxd9lacZ transgene (blue) was inserted into the rel5 site along with a loxP site (red). On the same chromosome, another loxP site with an opposite orientation is present within the Itga6 gene (red) substituting for a deletion of exon 25 (31). (B) Scheme of the same locus after the HoxDInv(rel5-Itga6) inversion was induced. As a result, the Hoxd9lacZ transgene is repositioned within Itga6 2.7 Mb away from its original insertion site. Digit enhancer sequences (green) active on HoxD cluster genes are inverted along with the reporter transgene. (C) Whole-mount staining of β-gal activities of embryos heterozygous for the transgene (Left) before and (Right) after the inversion. Embryos carrying either a paternally or a maternally inherited transgene are marked +/Paternal or Maternal/+, respectively. In Left, β-gal reporter activities show strong and comparable patterns independent of parental inheritance. Staining is observed in the brain and the spinal cord (open arrowheads) as well as in the developing digits in response to the nearby-located enhancers. Right shows the imprinting of the Hoxd9lacZ transgene after inversion when inherited from the mother with an almost complete absence of staining. In HoxDInv(rel5-Itga6) +/Paternal fetuses, staining persists in both the CNS and the digits (arrowheads), with some expression sites likely imposed by the unique transgene environment.
When males were used to propagate the transgene, a strong lacZ staining was scored. In contrast, when the transgene was transmitted through the mother, F1 progenies surprisingly revealed either a very weak or no lacZ staining at all (Fig. 1C, Right). Females and males carrying the HoxDInv(rel5-Itga6) allele were, thus, crossed to WT mice, and 309 embryos were stained between embryonic day 10.5 (E10.5) and E13.5. Of 87 embryos with the transgene inherited from the father (+/Paternal), 76 (87%) embryos showed the expected signal, whereas only 8 of 92 (9%) embryos carrying a transgene transmitted by the female (Maternal/+) displayed the expected lacZ activity (Fig. S1), thus suggesting that the Hoxd9lacZ transgene was maternally imprinted at this locus. This silencing on the maternal chromosome was not always complete, and some embryos escaped to different extents; 33% of Maternal/+ transgenic animals showed intermediate expression of the lacZ gene from little staining to almost no repression at all with a β-gal pattern comparable with +/Paternal transgenic animals (Fig. S1). These escapers, which were often littermates, usually showed distinct reduced patterns of expression, with clonal patches of cells and streaks showing lacZ activity. Likewise, some +/Paternal transgenic animals showed little or no expression but with a much lower frequency (12%).
When located at its rel5 position, before the inversion, the transgene showed no expression bias (Fig. 1C, Left), indicating that the imprinting was site-specific. We next assayed whether imprinting was transgene-specific by introducing another transgene into Itga6. We used an Hoxd11lacZ transgene inserted upstream of Hoxd13 (32) to induce an inversion repositioning the transgene within the exact same breakpoint in the Itga6 locus [HoxDInv(TgHd11lacNsi-Itga6)] (33) (Fig. S2). As for Hoxd9lacZ, the inverted Hoxd11lacZ was strongly repressed when inherited from the mother, while active when inherited from the father. Before inversion, imprinting was again not observed (Fig. S2). We assessed whether the parent-of-origin trait of these transgenes was dependent on the integration site by analyzing other rearrangements where a lacZ transgene was inserted at various positions within the HoxD centromeric landscape. None of them revealed any parental-specific expression (Fig. S2) (29, 32, 34–36). Altogether, these results indicated that maternal repression was position-dependent and only occurred at the Itga6 locus.
lacZ-Specific and Itga6 Locus-Specific Imprinting.
We next investigated whether the lacZ sequence was required to initiate maternal imprinting and analyzed the HoxDInv(HoxDRVIII-Itga6) inversion, which carries no transgene but breaks the HoxD cluster between Hoxd11 and Hoxd10 and thus, relocates the native Hoxd11 gene into the Itga6 locus (Fig. S3) (37). As a consequence, Hoxd11 was placed at the same position as the imprinted Hoxd11lacZ transgene but without any lacZ sequence. We crossed mice carrying this inversion with mice lacking Hoxd11 (38) to compare the expression of Hoxd11 after either paternal or maternal inheritance. In situ hybridization and quantitative RT-PCR (RT-qPCR) quantifications revealed no significant variation in Hoxd11 expression levels when derived from either parental allele (Fig. S3). Therefore, the presence of lacZ sequence was required to elicit maternal repression.
Because exogenous sequences may acquire the imprinted status of their insertion sites or potential imprinted genes located nearby (39), we assessed the allelic expression of the Itga6 gene by RT-qPCR. E12.5 digits mRNA was collected from either +/Paternal or Maternal/+ HoxDInv(rel5-Itga6) embryos. For each embryo, remaining body parts were separately stained for β-gal activity to ascertain that limbs from escaper embryos were not included. Because this modified allele carries an in cis deletion of exon 25 (31), we distinguished the parental origin of the Itga6 alleles by using this polymorphism (Fig. S4). We used specific primers for this exon and did not detect any difference in expression levels between the paternal and maternal copies of Itga6 (Fig. S4). This result suggested that the Itga6 gene is not imprinted (at least for transcripts including exon 25), although the repression of both the Hoxd9lacZ and Hoxd11lacZ transgenes, when coming from the mother, was specific to this locus.
Methylation Status of the Transgene and Chromatin Marks.
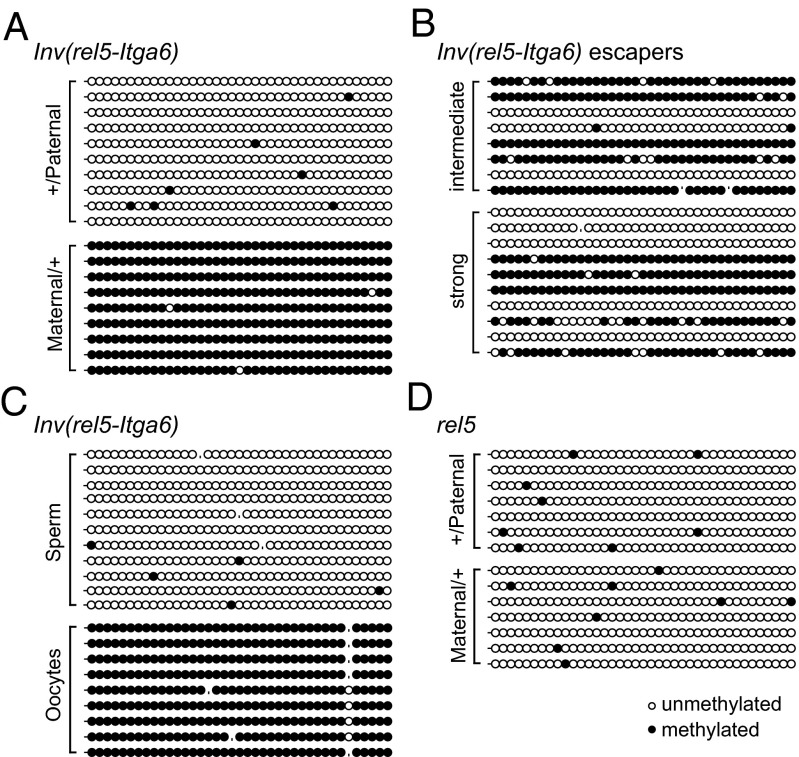
Both DNA methylation and the methylation of specific histone residues are associated with imprinting. Transgenes can drive parent-of-origin–dependent methylation (25), and repressed genes are generally methylated at their promoters. We compared DNA methylation profiles of Maternal/+ vs. +/Paternal HoxDInv(rel5-Itga6) heterozygous embryos by performing bisulfite sequencing on DNA isolated from developing digits using a set of primers specific for the transgene. DNA methylation over this region corresponded to the activity of the transgene; the maternally inherited transgene showed almost 100% of methylation, whereas the paternal copy showed no methylation at all (Fig. 2A). Maternal escaper embryos showed a variable but lower rate of methylation (Fig. 2B). We also looked at the DNA methylation in germ cells of animals heterozygous for the transgene, and methylation was scored in oocytes but not sperm cells, illustrating a genuine maternal imprinting (Fig. 2C). The germ cells samples displayed the expected control patterns of methylation for H19 and Snprn (Fig. S5) (40). In addition, differential methylation was (as expected) not detected on the transgene in noninverted HoxDrel5 animals (Fig. 2D), and the analysis of the Hoxd11 gene without the lacZ component when positioned within the Itga6 locus [HoxDInv(HoxDRVIII-Itga6)] (Fig. S5) also showed no difference in DNA methylation patterns.
Fig. 2.
Transgene imprinting is associated with differential allelic DNA methylation acquired in the germ line. (A) Methylation profiles on DNA from E12.5 presumptive digits of nonescaper HoxDInv(rel5-Itga6) Maternal/+ or +/Paternal embryos using transgene-specific primers (Table S1). (B) Same analysis using escaper embryos. The methylation rate is strongly reduced, with variations between samples reflecting the variable expression of the transgene. (C) Analysis of oocytes and sperm from the inverted allele shows germ line-acquired methylation. (D) Differential methylation over the transgene was not found in noninverted animals.
We investigated the trimethylation of H3K9, which usually covers repressive heterochromatin. This mark was described at most known ICRs (41) along with other specific inactive marks, such as H4K20me3 (42). We performed ChIP on HoxDInv(rel5-Itga6) heterozygous mutant brains and digits using antibodies directed against these repressive marks as well as H3K27me3. A comparison between maternally and paternally inherited transgenes showed an enrichment of both H3K9me3 and H4K20me3 over the Hoxd9 promoter and lacZ on the repressed allele compared with the active allele in both tissues (Fig. S6). The transgene was not active in the brain and covered by repressive H3K27me3 marks in +/Paternal heterozygous embryos, whereas H3K9me3 decorated the transgene in Maternal/+ embryos (Fig. S6). These results emphasized that maternal imprinting likely occurred in all embryonic cells. Studies of the offspring from a second generation of Hoxd9lacZ heterozygous mice showed that the imprint was reversible after passage through the germ line, such that males who had received a silent allele from their mother gave rise to lacZ-positive progeny (12 of 12), whereas female mice transmitted a silenced transgene (3 of 3). These epigenetic marks were, thus, correctly reset at each generation, such as for endogenous imprinted genes.
Allele-Specific 3D Conformations.
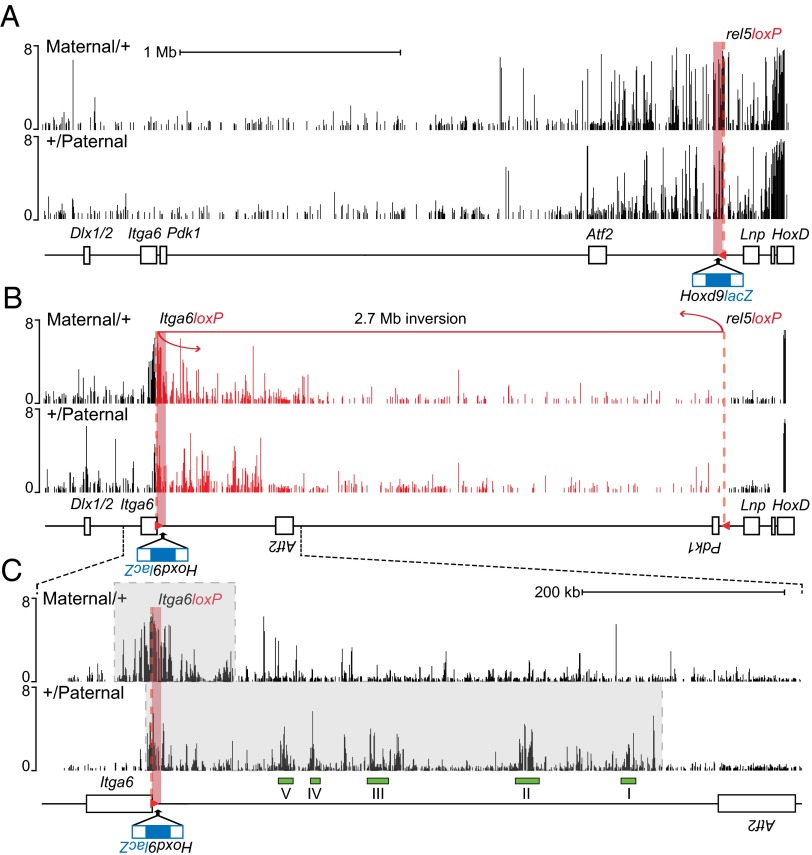
We could assess potential allelic differences in 3D architectures by using the lacZ as a bait in chromosome conformation capture experiments. We previously showed that the enhancer elements controlling Hoxd13 transcription in digits physically interact with the HoxD cluster (29) and that some of these contacts exist in tissues where Hox genes are inactive, suggesting the presence of a preformed regulatory structure independent from the state of activation (29, 43) and matching a reported topological domain (44, 45). We could, thus, analyze the 3D conformation of this locus in either transcriptionally active or silenced states in the same cellular population. Allele-specific chromosome conformation capture was first performed on digits dissected from HoxDrel5 embryos (i.e., specimen carrying the transgene before inversion). In these animals, the lacZ pattern correlates with the WT Hoxd13 expression domain, regardless of parental origin. Primers within the lacZ reporter gene were used to identify interacting DNA sequences. As expected, most of these interactions involved sequences located within the gene desert centromeric to HoxD, and strong contacts were scored with the various enhancers previously described to control Hoxd13 transcription in this embryonic tissue (Fig. 3A). The interaction profiles were indistinguishable when established from either a paternally or a maternally inherited transgene.
Fig. 3.
Allele-specific conformations induced by the Hoxd9lacZ transgene when positioned into the Itga6 locus. (A) The lacZ sequence is used as a bait for the 4C analysis (red square). Allele-specific chromosome conformation captures on chip from E12.5 HoxDrel5-dissected digits show similar interaction profiles for the maternal and paternal alleles. Contacts are mostly located in the centromeric gene desert flanking the HoxD cluster (from Atf2 to HoxD). (B) Same experiment using HoxDInv(rel5-Itga6) embryos showing allele-specific profiles of lacZ interactions. Inversion breakpoints are indicated with a dashed red line. For comparison, the segment corresponding to the inverted 2.7-Mb fragment (red) was manually inverted to represent the WT order in the DNA sequence. (C) Enlargement of the region shown under B with interactions on both side of the lacZ (gray boxes). Only the paternal allele interacts with previously identified digits enhancers (I–V, green rectangles). The y axis represents log2 ratio of the 4C normalized to input DNA.
We next used the same bait to assess the spatial conformation of the locus in HoxDInv(rel5-Itga6) inverted animals. In +/Paternal heterozygous embryos, the Hoxd9lacZ exogenous DNA contacted again all of the regulatory islands, which had been inverted along with the transgene (Fig. 3 B and C). In contrast, the interactions with either HoxD cluster genes or other digits enhancers located between the HoxD cluster and the rel5 inversion breakpoint were absent as expected. However, new contacts were gained in the other side of the integration site, reflecting spontaneous interactions between the transgene and its novel genomic environment. We then looked at chromosome conformation when the inverted allele was inherited from the mother (i.e., when lacZ staining was not detected in digits). The silenced allele showed a more compact domain of interactions (ca. 70-kb large and equally distributed on both sides of the breakpoint), suggesting a tight folding of the transgene with closely neighboring DNA unlike the paternally inherited allele, where the bulk of contacts was mostly biased to the inverted gene desert and its enhancers (Fig. 3B). Noteworthy, the contacts established between the paternally inherited transgene with the digit enhancers were no longer observed in the maternally inherited copy (Fig. 3C). In fact, this allelic difference in chromosome conformations was more marked than the difference observed at the same HoxD locus between active and inactive tissues (29), suggesting that imprinted loci may generally display distinct allelic conformations associated with the two alleles (45).
Regulatory Side Effects of Imprinting.
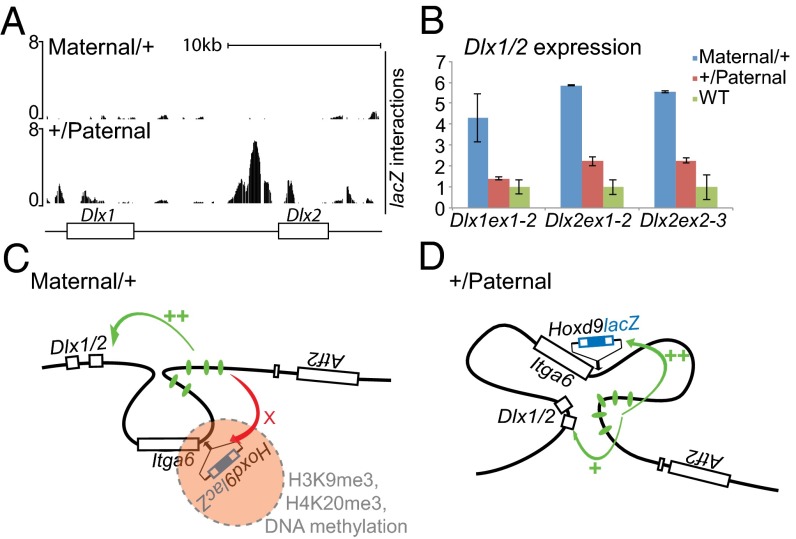
The comparison between interaction profiles on both paternal and maternal HoxDInv(rel5-Itga6) inverted alleles revealed an unexpected effect. In digit cells, the active paternal Hoxd9lacZ transgene established significant contacts with the Dlx1/Dlx2 locus, which is located ca. 300 kb centromeric to the Itga6 breakpoint (Fig. 4A). In the maternal allele, the imprinted configuration prevented these contacts from occurring, which was seen by the absence of any 4C signal over the Dlx genes. In this case, the compaction of the locus isolated the transgene from both the digit enhancers (Fig. 3C) and the Dlx locus. Dlx genes are expressed during limb development mostly in the apical ectodermal ridge (i.e., where Hoxd genes are not transcribed). Thus, we checked if the contacts between the lacZ transgene and the Dlx locus initiated in the +/Paternal inversion would lead to a transcriptional enhancement of Dlx genes in digits after the inversion had positioned the digit enhancers closer to the Dlx locus than in the WT situation (Fig. 1 A and B).
Fig. 4.
Impact of chromosome conformation on neighbor genes regulation. (A) Dlx1 and Dlx2 located 300 kb upstream of the loxP site within Itga6 (Fig. 1B). Allele-specific 4C shows strong interactions between Dlx genes and the transgene when active (+/Paternal) in contrast to the silenced state (Maternal/+). (B) RT-qPCR on mRNA from E12.5 HoxDInv(rel5-Itga6) digits (here n = 2). Dlx genes are up-regulated in both the paternal and maternal inverted alleles but with a more robust increase in Maternal/+ embryos. (C and D) Hypothetical conformations of the inverted Hoxd9lacZ allele when (C) maternally or (D) paternally inherited. (C) On maternal repression, chromatin compaction prevents interactions from occurring between the digits enhancers present in the inversion and the transgene. As a consequence, these regulatory elements are hijacked by the Dlx locus. (D) In contrast, in the active allele, the transgene interacts with the digits enhancers, thus reducing the interaction with the Dlx locus.
We assessed the expression of both Dlx1 and Dlx2 by RT-qPCR in digits dissected from E12.5 embryos. In the paternally transmitted inversion, an increase in both Dlx1 and Dlx2 mRNAs levels was observed; these genes became regulated in digits along with the lacZ transgene, which was anticipated from the 4C profiles. This twofold increase was significant but not dramatic (Fig. 4B), indicating that the digit enhancers may preferentially target the Hoxd9lacZ transgene. Unexpectedly, however, the transcriptional enhancement of Dlx genes was reenforced when the inverted allele was inherited from the mother, despite the absence of 4C contacts between the lacZ reporter transgene and the Dlx locus. In this case, the increase in transcripts levels was between four- and sixfold (Fig. 4B). This result suggested that, because the imprinting prevented contacts from occurring between the transgene and the enhancers, these enhancers were free to be partially reallocated to the Dlx locus.
Discussion
Parental imprinting leads to the nonexpression of either the maternal or the paternal allele, generally in all cells of the organism, and thus, it is an efficient regulatory mechanism to properly dose gene products. Among the various approaches used to understand the molecular bases of this epigenetic control, the analysis of imprinted transgenes can be instrumental (23–27). The imprinting that we describe here displays the hallmarks associated with this process. The silent version of the transgene was highly methylated at both the DNA and the particular residues of histone, two features ensuring correct allelic expression. Allele-specific silencing was reversible, because the passage through the opposite germ line could reset the imprint, and the epigenetic nature of the repression was confirmed by the analysis of oocytes and sperm, showing maternal germ line-specific DNA methylation.
Sequence-Specific and Position-Dependent Imprinting.
However, this imprinting was locus-dependent, because the same transgene, which was maternally repressed when inserted at the Itga6 locus, was readily expressed by both alleles when located at neighbor sites within a ca. 2-Mb-long DNA interval. Another lacZ-containing transgene also showed imprinting when moved into Itga6, showing that imprinting at this site was promoter-independent. Also, when the same DNA sequence without the lacZ gene was positioned at this site, imprinting was not observed. From this information, we conclude that, in this instance, (i) the imprinting is triggered by the lacZ sequence, and (ii) this sequence must be positioned into the Itga6 locus, which is itself not imprinted. The fact that the lacZ sequence may cause DNA methylation is not surprising (46), and a generic mechanism at work in the female germ line may recognize this sequence and deposit an imprint. However, this process does not explain the locus specificity reported in this work, a feature suggesting that particular DNA regions may be more prone than others to be imprinted if the right DNA sequences are there. Because some genes expressed from both alleles have been described inside imprinted clusters (47), another locus located nearby Itga6 could be imprinted and impact on a transgene inserted in the vicinity. Also, the biallelic expression of Hoxd11 when relocated into Itga6 may be sustained by the neighboring of Hoxd13, which was inverted along with all its digit enhancers. A robust transcription of this latter gene remains in this inversion (48), which may somehow counteract the imprinting process.
Allele-Specific Chromosome Conformation and Side Effects.
By using the lacZ reporter transgene as a viewpoint, we assessed the interaction profiles of this exogenous DNA in an allele-specific manner and showed the formation of clearly distinct, parent-of-origin interaction domains, which are similar to what was reported on the X chromosome (45, 49). In the imprinted maternal allele, contacts were mostly observed around the insertion site, suggesting a compaction of the chromatin over a ca. 140-kb large domain centered on the lacZ reporter transgene. The size of this domain indicates the extent of endogenous DNA that can be directly affected by the presence of the imprinted piece of DNA. Whether these increased contacts were caused by the spreading of epigenetic marks (H3K9me3, H4K20me3, and DNA methylation) from the lacZ reporter gene to flanking sequences remains to be assessed. On the repressed allele, although some contacts were still observed between the inverted gene desert and the transgene, interactions with the digits enhancers were lost, although their topological relationship with the target Hoxd9lacZ promoter remained unchanged after inversion. This observation was expected, because the imprinted transgene was no longer transcribed in these cells. Interestingly, however, this result showed that even such a robust regulatory mechanism (29), which normally sustains one of the strongest transcriptional outcomes during development (50), is unable to elicit any response from the reporter transgene when imprinted, thus illustrating the remarkable efficiency of the inherited repressive configuration.
The loss of contacts between digits enhancers and the transgene in the imprinted allele likely favored a reallocation of these regulatory elements to the Dlx1/2 locus (Fig. 4C). Some contacts between this latter locus and the lacZ transgene were observed in the paternal, nonimprinted allele in association with a slight gain of expression of both genes in digits. We hypothesize that, after inversion, the Dlx1/2 locus was included into a novel regulatory landscape under the control of digit enhancers. In the paternal allele, however, most of the contacts established by the lacZ transgene were biased to the inverted gene desert (Figs. 3 and 4D). In the imprinted allele, the transgene and surrounding sequences were excluded from this landscape because of the repressive configuration, and hence, the digit enhancers increased their interactions with the Dlx locus (Fig. 4).
Whether such enhancer reallocations routinely occur around imprinted loci remains an open question. Although the induced side effects may be functionally neutral in many instances, the potential of imprinting as a way to globally regulate enhancer–promoter interactions over large distances should not be overlooked. High-throughput studies have revealed that chromosomes are organized into domains of preferential regulatory interactions (44, 45), and our results suggest that the presence of an imprinted locus within such a domain may lead to a significant reorganization, which may not be visible until allele-specific approaches are considered in the definition of such domains (45, 49). In any case, the potential role of imprinting in organizing enhancer–promoter interactions may be difficult to evaluate, because its benefit may occur in restricted cell populations that are not yet amenable to genome-wide studies.
Variability in Transgene Silencing.
The imprinting reported here shows an unusual frequency of escapers with both the maternal and paternal alleles. Maternal escapers (escaping repression) came as a significant number and displayed patterns of variegation different from one another. For example, some embryos displayed staining in a given tissue but not in another tissue rather than having a general increase of the β-gal staining everywhere. Such a tissue-specific variability can hardly be explained by the sole defect in the initial imprinting process and must involve cellular-specific component. In this view, it is possible that enhancers can sometimes win over repressive configuration and still elicit a transcriptional response from an imprinted locus. This mechanism may be helped by a somehow weaker repression, which would derive from the initial imprinting process, and such escapers may, thus, reflect the combination of both factors. The study of such escapers over several generations will be of interest in this respect.
Paternal escapers were less frequent, but 1 of 10 embryos did not express (or weakly express) lacZ. The low frequency and variability of these escapers made their study difficult. One possibility is that this repression was caused by other mechanisms used to silence exogenous DNA. In fact, should the same frequency of nonimprinted but silenced embryos be present within the pool of maternally imprinted embryos, they would remain unnoticed. Alternatively, the presence of the transgene may induce either maternal or paternal imprinting but with different frequencies. Finally, it is also conceivable that, after inversion, the enhancer–promoter interactions were weakened because of a new genomic topology leading to the weak or nonexpression of the transgene in some cases. In this context, one should remember that the 4C approach shows an average of contacts taken at a given time for a pool of cells, and hence, it merely reflects a trend, thereby diluting potential distinct configurations formed at low frequency.
Material and Methods
Mouse Strains, in Situ Hybridization, and lacZ Staining.
All strains were described before: HoxDrel5 (29), HoxDInv(rel5-Itga6) (29), HoxDTgHd11lacNsi (32), HoxDInv(TgHd11lacNsi-Itga6) (33), HoxDInv(HoxDRVIII-Itga6) (37), HoxDDelRXIIDel(13-8) (38), HoxDTpSB1 (36), HoxDDel(TpSB1-Atf2) (29), and HoxDInv(TpSB1-Itga6) (51). Genotyping conditions are found in the references mentioned above. Mice heterozygous for the inversion HoxDInv(HoxDRVIII-Itga6) were crossed with animals carrying a deletion from Hoxd8 to Hoxd13 [HoxDDelRXIIDel(13-8) or ∆]. Whole-mount in situ hybridization and lacZ staining were performed using standard protocols. The Hoxd11 in situ hybridization mouse probe has been described previously (52).
Bisulfite Sequencing.
Genomic DNA was isolated from E12.5 digits, and β-gal activity was assessed on the remaining parts of the embryos to discard escapers. Germ cells were collected directly in 40 µL DNA lysis buffer of 33 mM Tris⋅HCl (pH 8), 1 mM EDTA, and 10 mg/mL SDS; 2.5 µL sperm were squeezed from vas deferens, and oocytes (160 and 212 per tube) were collected from superovulated females (3–6 wk postpartum). Sodium bisulfite conversion and cleanup for methylation analysis of either 1 µg DNA (not digested) from digits or total volume of germ cells in lysis buffer were performed using the QIAGEN EpiTect Bisulfite Kit. For the analysis of the transgene, single-round PCR amplification was performed using specific unbiased primers (Table S1) for the converted DNA. H19 and Snrpn were used as controls (40). Analysis of clones was performed using the BISMA web interface (53) or the BiqAnalyzer software (54). Clones were accepted at a conversion rate of 95%, and detection of clonal molecules based on nonconverted cytosine or base error was used to avoid clones originating from same template DNA. For oocytes, the conversion rate was around 80%. Lollipop methylation plots were generated with BiqAnalyzer.
RNA Extraction, RT-qPCR, and ChIP.
E12.5 digits were stored at −80 °C in RNAlater RNA Stabilization Reagent (Ambion) before genotyping. RNA was extracted using the QIAGEN RNeasy Plus Micro Kit after tissue disruption and homogenization. Reverse-transcription qPCR analyses were performed using the primers listed in Table S1. ChIP–RT-qPCR (55) was carried out on E12.5 digits and brains of nonescaper embryos. One-half of a forebrain or pools of six pairs of distal limbs fixed for 15 min in 1% formaldehyde in PBS were used for each ChIP using 4 µL anti-H3K9me3 (pAb-056-050; Diagenode), H3K27me3 (17–622; Millipore), or H4K20me3 (ab9053; abcam) antibodies and immunoprecipated with EZview Red Protein G/A Affinity Gel Beads from Sigma (primers for RT-qPCR are in Table S1).
Chromosome Conformation Capture and Tiling Array Analysis.
4C analysis was as described (29) using E12.5 digits; 2% formaldehyde-fixed nuclei from collagenase-dissociated tissues were stored at −80 °C before genotyping. Pools of 10 pairs of mutant digits were used for each 4C analysis using DpnII restriction enzyme to identify interacting partners of the lacZ sequence from the transgene (primers are in Table S1), allowing for an allele-specific conformation capture. 4C libraries were fragmented, and then, they were labeled and hybridized on chromosome 2 Affymetrix tiling arrays. For HoxDInv(rel5-Itga6) heterozygous embryos, two independent experiments were conducted for both parental transmissions. Tiling Analysis Software from Affymetrix was used to extract and analyze raw hybridization using gDNA input to normalize (29). Log2 data were plotted on University of California Santa Cruz genome browser after genome conversion (mm5 to mm9).
Supplementary Material
Acknowledgments
We thank B. Mascrez and S. Gitto for their help with mice as well as P. Schorderet for his advice for the ChIP and all members of the D.D. laboratories for discussions and reagents. We also thank M. Docquier, C. Barraclough, and D. Chollet from the National Centre of Competence in Research genomic platform, the D. Trono laboratory for sharing primers, and Anne Ferguson-Smith for her advice and helpful comments on the manuscript. This work was supported by funds from the University of Geneva; the Ecole Polytechnique Fédérale, Lausanne; the Swiss National Research Fund; and the European Research Council (ERC).
Footnotes
The authors declare no conflict of interest.
Data deposition: The sequence reported in this paper has been deposited in the GEO database (accession no. GSE48148).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1310704110/-/DCSupplemental.
References
- 1.Ferguson-Smith AC, Surani MA. Imprinting and the epigenetic asymmetry between parental genomes. Science. 2001;293(5532):1086–1089. doi: 10.1126/science.1064020. [DOI] [PubMed] [Google Scholar]
- 2.da Rocha ST, Edwards CA, Ito M, Ogata T, Ferguson-Smith AC. Genomic imprinting at the mammalian Dlk1-Dio3 domain. Trends Genet. 2008;24(6):306–316. doi: 10.1016/j.tig.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 3.Ferguson-Smith AC. Genomic imprinting: The emergence of an epigenetic paradigm. Nat Rev Genet. 2011;12(8):565–575. doi: 10.1038/nrg3032. [DOI] [PubMed] [Google Scholar]
- 4.Kacem S, Feil R. Chromatin mechanisms in genomic imprinting. Mamm Genome. 2009;20(9-10):544–556. doi: 10.1007/s00335-009-9223-4. [DOI] [PubMed] [Google Scholar]
- 5.Chotalia M, et al. Transcription is required for establishment of germline methylation marks at imprinted genes. Genes Dev. 2009;23(1):105–117. doi: 10.1101/gad.495809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pauler FM, Koerner MV, Barlow DP. Silencing by imprinted noncoding RNAs: Is transcription the answer? Trends Genet. 2007;23(6):284–292. doi: 10.1016/j.tig.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edwards CA, Ferguson-Smith AC. Mechanisms regulating imprinted genes in clusters. Curr Opin Cell Biol. 2007;19(3):281–289. doi: 10.1016/j.ceb.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 8.Verona RI, Mann MRW, Bartolomei MS. Genomic imprinting: Intricacies of epigenetic regulation in clusters. Annu Rev Cell Dev Biol. 2003;19:237–259. doi: 10.1146/annurev.cellbio.19.111401.092717. [DOI] [PubMed] [Google Scholar]
- 9.Kaneda M, et al. Essential role for de novo DNA methyltransferase Dnmt3a in paternal and maternal imprinting. Nature. 2004;429(6994):900–903. doi: 10.1038/nature02633. [DOI] [PubMed] [Google Scholar]
- 10.Reik W, Dean W, Walter J. Epigenetic reprogramming in mammalian development. Science. 2001;293(5532):1089–1093. doi: 10.1126/science.1063443. [DOI] [PubMed] [Google Scholar]
- 11.Rougier N, et al. Chromosome methylation patterns during mammalian preimplantation development. Genes Dev. 1998;12(14):2108–2113. doi: 10.1101/gad.12.14.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ooi SKT, et al. DNMT3L connects unmethylated lysine 4 of histone H3 to de novo methylation of DNA. Nature. 2007;448(7154):714–717. doi: 10.1038/nature05987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feil R. Epigenetics: Ready for the marks. Nature. 2009;461(7262):359–360. doi: 10.1038/461359a. [DOI] [PubMed] [Google Scholar]
- 14.Zhao Q, et al. PRMT5-mediated methylation of histone H4R3 recruits DNMT3A, coupling histone and DNA methylation in gene silencing. Nat Struct Mol Biol. 2009;16(3):304–311. doi: 10.1038/nsmb.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ciccone DN, et al. KDM1B is a histone H3K4 demethylase required to establish maternal genomic imprints. Nature. 2009;461(7262):415–418. doi: 10.1038/nature08315. [DOI] [PubMed] [Google Scholar]
- 16.Jia D, Jurkowska RZ, Zhang X, Jeltsch A, Cheng X. Structure of Dnmt3a bound to Dnmt3L suggests a model for de novo DNA methylation. Nature. 2007;449(7159):248–251. doi: 10.1038/nature06146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Butler MG. Genomic imprinting disorders in humans: A mini-review. J Assist Reprod Genet. 2009;26(9-10):477–486. doi: 10.1007/s10815-009-9353-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Williamson CMBA, et al. (2010) Mouse Imprinting Data and References. Available at http://www.har.mrc.ac.uk/research/genomic_imprinting/. Accessed May 10, 2013.
- 19.Luedi PP, et al. Computational and experimental identification of novel human imprinted genes. Genome Res. 2007;17(12):1723–1730. doi: 10.1101/gr.6584707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luedi PP, Hartemink AJ, Jirtle RL. Genome-wide prediction of imprinted murine genes. Genome Res. 2005;15(6):875–884. doi: 10.1101/gr.3303505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cichon S, et al. A genome screen for genes predisposing to bipolar affective disorder detects a new susceptibility locus on 8q. Hum Mol Genet. 2001;10(25):2933–2944. doi: 10.1093/hmg/10.25.2933. [DOI] [PubMed] [Google Scholar]
- 22.Guo Y-F, et al. Assessment of genetic linkage and parent-of-origin effects on obesity. J Clin Endocrinol Metab. 2006;91(10):4001–4005. doi: 10.1210/jc.2006-0549. [DOI] [PubMed] [Google Scholar]
- 23.Swain JL, Stewart TA, Leder P. Parental legacy determines methylation and expression of an autosomal transgene: A molecular mechanism for parental imprinting. Cell. 1987;50(5):719–727. doi: 10.1016/0092-8674(87)90330-8. [DOI] [PubMed] [Google Scholar]
- 24.Surani MA, Reik W, Allen ND. Transgenes as molecular probes for genomic imprinting. Trends Genet. 1988;4(3):59–62. doi: 10.1016/0168-9525(88)90040-6. [DOI] [PubMed] [Google Scholar]
- 25.Sasaki H, et al. Inherited type of allelic methylation variations in a mouse chromosome region where an integrated transgene shows methylation imprinting. Development. 1991;111(2):573–581. doi: 10.1242/dev.111.2.573. [DOI] [PubMed] [Google Scholar]
- 26.Allen ND, et al. Transgenes as probes for active chromosomal domains in mouse development. Nature. 1988;333(6176):852–855. doi: 10.1038/333852a0. [DOI] [PubMed] [Google Scholar]
- 27.DeLoia JA, Solter D. A transgene insertional mutation at an imprinted locus in the mouse genome. Dev Suppl. 1990;1990:73–79. [PubMed] [Google Scholar]
- 28.Lee AP, Koh EGL, Tay A, Brenner S, Venkatesh B. Highly conserved syntenic blocks at the vertebrate Hox loci and conserved regulatory elements within and outside Hox gene clusters. Proc Natl Acad Sci USA. 2006;103(18):6994–6999. doi: 10.1073/pnas.0601492103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Montavon T, et al. A regulatory archipelago controls Hox genes transcription in digits. Cell. 2011;147(5):1132–1145. doi: 10.1016/j.cell.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 30.Kondo T, Duboule D. Breaking colinearity in the mouse HoxD complex. Cell. 1999;97(3):407–417. doi: 10.1016/s0092-8674(00)80749-7. [DOI] [PubMed] [Google Scholar]
- 31.Gimond C, et al. Cre-loxP-mediated inactivation of the alpha6A integrin splice variant in vivo: Evidence for a specific functional role of alpha6A in lymphocyte migration but not in heart development. J Cell Biol. 1998;143(1):253–266. doi: 10.1083/jcb.143.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van der Hoeven F, Zákány J, Duboule D. Gene transpositions in the HoxD complex reveal a hierarchy of regulatory controls. Cell. 1996;85(7):1025–1035. doi: 10.1016/s0092-8674(00)81303-3. [DOI] [PubMed] [Google Scholar]
- 33.Tschopp P, Duboule D. A regulatory ‘landscape effect’ over the HoxD cluster. Dev Biol. 2011;351(2):288–296. doi: 10.1016/j.ydbio.2010.12.034. [DOI] [PubMed] [Google Scholar]
- 34.Spitz F, et al. Large scale transgenic and cluster deletion analysis of the HoxD complex separate an ancestral regulatory module from evolutionary innovations. Genes Dev. 2001;15(17):2209–2214. doi: 10.1101/gad.205701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Montavon T, Duboule D. Landscapes and archipelagos: Spatial organization of gene regulation in vertebrates. Trends Cell Biol. 2012;22(7):347–354. doi: 10.1016/j.tcb.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 36.Ruf S, et al. Large-scale analysis of the regulatory architecture of the mouse genome with a transposon-associated sensor. Nat Genet. 2011;43(4):379–386. doi: 10.1038/ng.790. [DOI] [PubMed] [Google Scholar]
- 37.Spitz F, Herkenne C, Morris MA, Duboule D. Inversion-induced disruption of the Hoxd cluster leads to the partition of regulatory landscapes. Nat Genet. 2005;37(8):889–893. doi: 10.1038/ng1597. [DOI] [PubMed] [Google Scholar]
- 38.Tarchini B, Huynh THN, Cox GA, Duboule D. HoxD cluster scanning deletions identify multiple defects leading to paralysis in the mouse mutant Ironside. Genes Dev. 2005;19(23):2862–2876. doi: 10.1101/gad.351105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones MJ, Lefebvre L. An imprinted GFP insertion reveals long-range epigenetic regulation in embryonic lineages. Dev Biol. 2009;336(1):42–52. doi: 10.1016/j.ydbio.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Market-Velker BA, Zhang L, Magri LS, Bonvissuto AC, Mann MRW. Dual effects of superovulation: Loss of maternal and paternal imprinted methylation in a dose-dependent manner. Hum Mol Genet. 2010;19(1):36–51. doi: 10.1093/hmg/ddp465. [DOI] [PubMed] [Google Scholar]
- 41.Dindot SV, Person R, Strivens M, Garcia R, Beaudet AL. Epigenetic profiling at mouse imprinted gene clusters reveals novel epigenetic and genetic features at differentially methylated regions. Genome Res. 2009;19(8):1374–1383. doi: 10.1101/gr.089185.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McEwen KR, Ferguson-Smith AC. Distinguishing epigenetic marks of developmental and imprinting regulation. Epigenetics Chromatin. 2010;3(1):2–225. doi: 10.1186/1756-8935-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Noordermeer D, et al. The dynamic architecture of Hox gene clusters. Science. 2011;334(6053):222–225. doi: 10.1126/science.1207194. [DOI] [PubMed] [Google Scholar]
- 44.Dixon JR, et al. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature. 2012;485(7398):376–380. doi: 10.1038/nature11082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nora EP, et al. Spatial partitioning of the regulatory landscape of the X-inactivation centre. Nature. 2012;485(7398):381–385. doi: 10.1038/nature11049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chevalier-Mariette C, et al. CpG content affects gene silencing in mice: Evidence from novel transgenes. Genome Biol. 2003;4(9):R53. doi: 10.1186/gb-2003-4-9-r53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bartolomei MS. Genomic imprinting: Employing and avoiding epigenetic processes. Genes Dev. 2009;23(18):2124–2133. doi: 10.1101/gad.1841409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soshnikova N, Duboule D. Epigenetic temporal control of mouse Hox genes in vivo. Science. 2009;324(5932):1320–1323. doi: 10.1126/science.1171468. [DOI] [PubMed] [Google Scholar]
- 49.Splinter E, et al. The inactive X chromosome adopts a unique three-dimensional conformation that is dependent on Xist RNA. Genes Dev. 2011;25(13):1371–1383. doi: 10.1101/gad.633311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Montavon T, Le Garrec J-F, Kerszberg M, Duboule D. Modeling Hox gene regulation in digits: Reverse collinearity and the molecular origin of thumbness. Genes Dev. 2008;22(3):346–359. doi: 10.1101/gad.1631708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Montavon T, Thevenet L, Duboule D. Impact of copy number variations (CNVs) on long-range gene regulation at the HoxD locus. Proc Natl Acad Sci USA. 2012;109(50):20204–20211. doi: 10.1073/pnas.1217659109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gérard M, et al. In vivo targeted mutagenesis of a regulatory element required for positioning the Hoxd-11 and Hoxd-10 expression boundaries. Genes Dev. 1996;10(18):2326–2334. doi: 10.1101/gad.10.18.2326. [DOI] [PubMed] [Google Scholar]
- 53.Rohde C, Zhang Y, Reinhardt R, Jeltsch A. BISMA—fast and accurate bisulfite sequencing data analysis of individual clones from unique and repetitive sequences. BMC Bioinformatics. 2010;11:230. doi: 10.1186/1471-2105-11-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bock C, et al. BiQ Analyzer: Visualization and quality control for DNA methylation data from bisulfite sequencing. Bioinformatics. 2005;21(21):4067–4068. doi: 10.1093/bioinformatics/bti652. [DOI] [PubMed] [Google Scholar]
- 55.Lee TI, Johnstone SE, Young RA. Chromatin immunoprecipitation and microarray-based analysis of protein location. Nat Protoc. 2006;1(2):729–748. doi: 10.1038/nprot.2006.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.