Abstract
Systemic therapy with anti-VEGF drugs such as bevacizumab is widely used for treatment of human patients with various solid tumors. However, systemic impacts of such drugs in host healthy vasculatures remain poorly understood. Here, we show that, in mice, systemic delivery of an anti-VEGF or an anti–VEGF receptor (VEGFR)-2 neutralizing antibody caused global vascular regression. Among all examined tissues, vasculatures in endocrine glands, intestinal villi, and uterus are the most affected in response to VEGF or VEGFR-2 blockades. Thyroid vascular fenestrations were virtually completely blocked by VEGF blockade, leading to marked accumulation of intraendothelial caveolae vesicles. VEGF blockade markedly increased thyroid endothelial cell apoptosis, and withdrawal of anti-VEGF resulted in full recovery of vascular density and architecture after 14 d. Prolonged anti-VEGF treatment resulted in a significant decrease of the circulating level of the predominant thyroid hormone free thyroxine, but not the minimal isoform of triiodothyronine, suggesting that chronic anti-VEGF treatment impairs thyroid functions. Conversely, VEGFR-1–specific blockade produced virtually no obvious phenotypes. These findings provide structural and functional bases of anti-VEGF–specific drug-induced side effects in relation to vascular changes in healthy tissues. Understanding anti-VEGF drug-induced vascular alterations in healthy tissues is crucial to minimize and even to avoid adverse effects produced by currently used anti-VEGF–specific drugs.
Keywords: angiogenesis, vascular homeostasis, vessel regression, antiangiogenic therapy, off-tumor targets
The antiangiogenic concept for treatment of solid tumors was proposed by Judah Folkman 41 y ago (1). In this hypothetic paper, Folkman wrote that “one approach to the initiation of ‘anti-angiogenesis’ would be the production of an antibody against tumor angiogenic factor.” Today, the most commonly used anti-VEGF drug, bevacizumab (avastin) (2), is a humanized neutralizing antibody against tumor-derived VEGF, validating Folkman’s early prediction.
Tumors produce various angiogenic factors and cytokines to induce angiogenesis that is essential for tumor growth. Among these tumor-derived factors, VEGF, also called VEGF-A, is a key angiogenic factor that induces angiogenesis, vascular permeability, and tortuosity (3, 4). VEGF displays these broad vascular functions via binding and activation of its specific receptors, VEGFR-1 and VEGFR-2, mainly expressed in vascular endothelial cells, although other cell types may also express these receptors (5–8). Abundant experimental data demonstrate that VEGFR-2 is the primary functional receptor that transduces both angiogenic and vascular permeability signals whereas VEGFR-1 may function as a decoy receptor (9, 10). It should be emphasized that, under physiological and pathological conditions, VEGFR-2 is expressed not only in angiogenic vessels, but also in quiescent vasculatures in various tissues (11). The broad distribution of VEGFRs in quiescent vasculatures in various healthy tissues suggests that the VEGF–VEGFR signaling system is essential for maintenance of vascular homeostasis. In some tissues, such as endocrine organs including adrenal glands, thyroid, and pancreatic islets, the endothelium in these vasculatures remains fenestrated and VEGF is crucial for maintenance of vascular fenestrations (12–14). Kamba et al. reported that systemic delivery of tyrosine kinase inhibitors (TKIs) containing the anti-VEGFR component caused vascular regression in various tissues (15). However, TKIs have broad targets, and it was unclear whether blocking VEGFR signaling was solely responsible for the vascular phenotype.
In the present study, we chose to use VEGF- and VEGFR-specific blockades that do not target other signaling systems. Additionally, we have also distinguished the significance of VEGFR-1– and VEGFR-2–mediated signaling pathways in maintenance of vascular homeostasis in various tissues and organs. Given the fact that bevacizumab is the most commonly used antiangiogenic drug in oncology (16–21), our findings using these specific blockades may be directly translated into clinical relevance concerning the global impact of these drugs in cancer patients and the structural basis of adverse effects. Thus, the information provided in our study could be potentially useful for development of new therapeutic strategies to minimize or avoid adverse effects of anti-VEGF–based therapeutics.
Results
Impact of Anti-VEGF Blockades on Vasculature in Endocrine Organs.
To study the impact of VEGF-specific blockades on vasculatures in various healthy tissues, we used three specific anti-VEGF agents known to block VEGF-induced biological activities: a rabbit anti-mouse neutralizing monoclonal antibody (BD0801) (22); a rat anti-mouse VEGFR-1 neutralizing monoclonal antibody (MF-1) (23–25); and a rat anti-mouse VEGFR-2 neutralizing monoclonal antibody (DC101) (23–25). These antibodies were systemically delivered for 2 wk to healthy mice using doses known to block tumor angiogenesis (22–25). Because bevacizumab (a humanized anti-human VEGF neutralizing monoclonal antibody) is widely being used for treatment of various human cancers (2, 17–21) and ramucirumab (a humanized anti-human VEGFR-2 neutralizing monoclonal antibody) together with docetaxel-based chemotherapy is planned for a phase III trial for treatment of non-small cell lung carcinoma (26), systemic treatment with the same agents designed in our study would be clinically relevant.
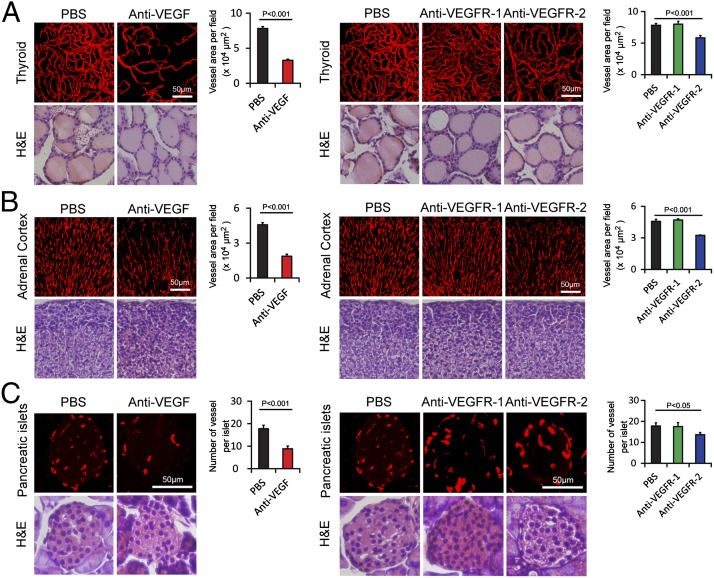
We first analyzed vasculatures in endocrine organs that are known to express relatively high levels of VEGF (27). Markedly, nearly 60% reduction of vascular density in thyroid was observed in response to anti-VEGF blockade (Fig. 1A). A similar degree of vascular reduction occurred in adrenal cortex (Fig. 1B). Conversely, the number of microvessels in the adrenal medulla did not alter in response to VEGF or VEGFR-2 treatment (see Figs. S5 and S6). In pancreatic islets, ∼50% reduction of microvessel density in response to VEGF blockade treatment was observed (Fig. 1C). However, none of these tissues showed obvious structural changes upon anti-VEGF treatment as detected by hematoxylin/eosin histological staining. These data demonstrate that VEGF plays a pivotal role in maintenance of vascular homeostasis in these endocrine organs.
Fig. 1.
Impact of anti-VEGF blockades on vasculature in endocrine organs. (A) CD31+ thyroid microvessels (red). H&E staining was used to reveal tissue structures. Vessel areas were quantified (20× magnification, n = 8 fields per group). (B) Endomucin+ adrenal cortex microvessels were detected in paraffin-embedded tissues (red). H&E staining was used to reveal tissue structures. Vessel areas were quantified (20× magnification, n = 8 fields per group). (C) Endomucin+ pancreatic islet microvessels were detected in paraffin-embedded tissues (red). H&E staining was used to reveal tissue structures. Vessel numbers were quantified (20× magnification, n = 8 fields per group). Data are presented as means ± SEM.
To define the receptor type that is involved in maintenance of VEGF-dependent vascular homeostasis, VEGFR-1– and VEGFR-2–specific blockades were systemically delivered to mice. Consistent with the known receptor functions, the VEGFR-2–specific blockade produced a similar vascular regression activity in these endocrine organs (Fig. 1 A–C). However, delivery of the VEGFR-1 blockade resulted in virtually no repressive effects on the vasculature in these tissues. These data show that VEGFR-2 is the critical receptor that mediates the VEGF-dependent maintenance function in these endocrine organs.
Vascular Response in Gastrointestinal Tracts and the Female Reproductive System.
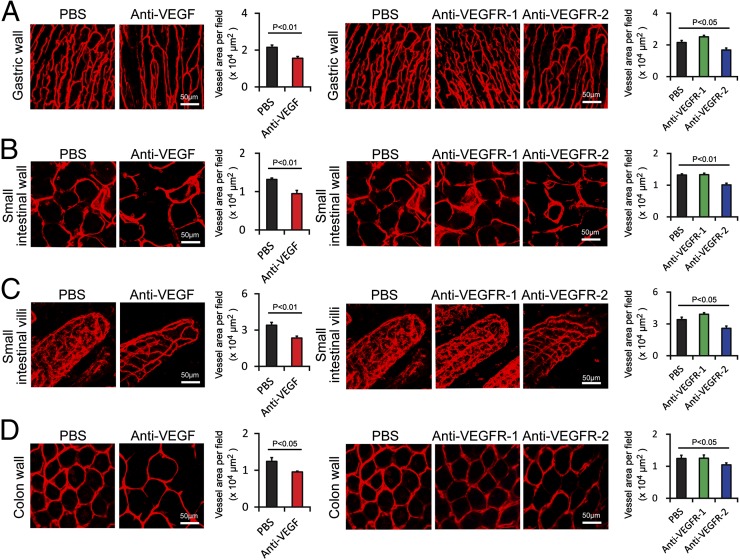
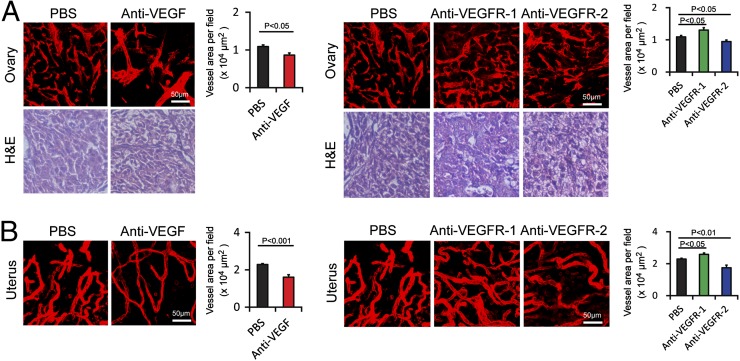
Examination of vasculatures in the gastric wall, small intestinal wall, and colon wall showed significant reduction of microvessel density in response to the VEGF blockade (Fig. 2 A, B, and D). A similar vascular regressive effect was also found in the VEGFR-2–treated groups. Intriguingly, microvessels in small intestinal villi showed marked decrease of vessel density in response to VEGF or VEGFR-2 blockade (Fig. 2C). However, systemic treatment with the VEGFR-1–specific blockade did not produce any obvious vascular changes (Fig. 2 A–D). In the ovary, systemic administration of VEGF and VEGFR-2 blockades produced significant effects on vascular regression whereas VEGFR-1 blockade did not show any vascular regressive effect. Surprisingly, treatment with the VEGFR-1 blockade increased vessel density in the ovarian tissue (Fig. 3A). There were no obvious structural changes upon anti-VEGF blockades as detected by hematoxylin/eosin histological staining. Similar to the ovary tissue, VEGF- and VEGFR-2–specific blockades significantly decreased vascular density in the uterus whereas VEGFR-1 increased vascular density in this tissue (Fig. 3B). These data demonstrate that the VEGF–VEGFR-2 signaling system is essentially required for maintenance of a subset of vasculatures in these tissues and organs.
Fig. 2.
Impact of anti-VEGF blockades on vasculature in gastrointestinal tracts. (A) CD31+ gastric wall microvessels (red). Vessel areas were quantified (20× magnification, n = 8 fields per group). (B) CD31+ small intestine wall (red). Vessel areas were quantified (20× magnification, n = 8 fields per group). (C) CD31+ small intestine villi microvessels (red). Vessel areas were quantified (20× magnification, n = fields 8 per group). (D) CD31+ colon wall microvessels in anti-VEGF or buffer-treated healthy mice were detected using CD31+ endothelial cell signals (red). Vessel areas were quantified (20× magnification, n = 8 fields per group). Data are presented as means ± SEM.
Fig. 3.
Impact of anti-VEGF blockades on vasculature in female reproductive system. (A) CD31+ ovary microvessels (red). H&E staining was used to reveal tissue structures. Vessel areas were quantified (20× magnification, n = 8 fields per group). (B) CD31+ uterus microvessels (red). Vessel areas were quantified (20× magnification, n = 8 per fields group). Data are presented as means ± SEM.
Vascular Changes in Kidney, Liver, Pancreas, and Other Tissues.
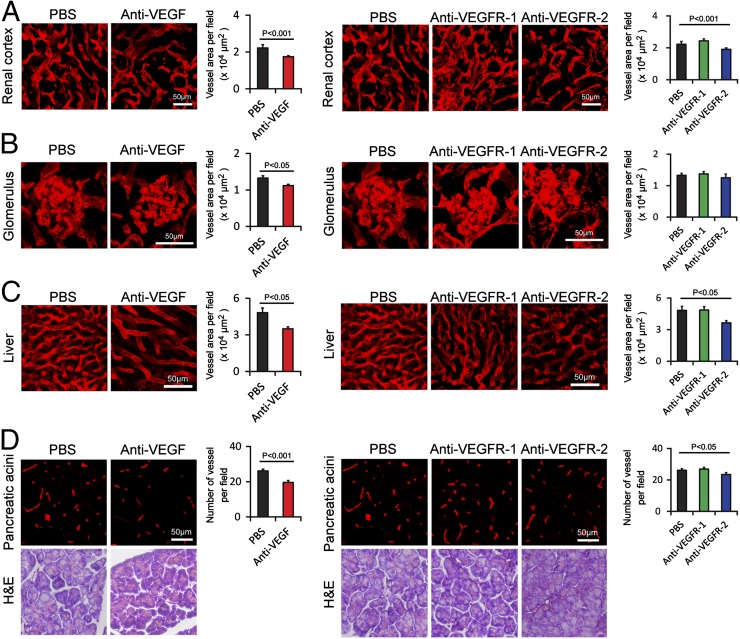
Among all analyzed tissues, renal cortex and glomeruli in the kidney showed significant reduction of vascular density in response to the VEGF-specific blockade (Fig. 4 A and B). The hepatic sinusoidal vasculature also responded to the VEGF-specific blockade, leading to a significant reduction of microvessel density (Fig. 4C). In the pancreatic acini area, the anti-VEGF treatment resulted in significant decrease of vessel numbers (Fig. 4D). Consistent with these findings, systemic delivery of the VEGFR-2 blockade produced a similar vascular regressive phenotype in these tissues whereas anti–VEGFR-1 blockade did not affect the vessel density (Fig. 4 A–D). We have also examined vasculatures in a number of other tissues including different regions of the brain, retina, thymus, myocardium, skeletal muscles, and bone marrow. In general, no significant changes were observed in these tissues and organs in response to the three VEGF blockades, with the exception of significant reduction of vascular density in thymus in response to VEGF and VEGFR-2 blockades (Figs. S1–S6).
Fig. 4.
Impact of anti-VEGF blockades on vasculature in kidney, liver, and pancreas. (A) CD31+ renal cortex microvessels (red). Vessel areas were quantified (20× magnification, n = 8 fields per group). (B) CD31+ glomerulus microvessels (red). Vessel areas were quantified (20× magnification, n = 8 fields per group). (C) CD31+ liver microvessels (red). Vessel areas were quantified (20× magnification, n = 8 fields per group). (D) Endomucin+ pancreatic acini microvessels were detected in paraffin-embedded tissues (red). H&E staining was used to reveal tissue structures. Vessel numbers were quantified (20× magnification, n = 8 fields per group). Data are presented as means ± SEM.
Reversible Vascular Density and Architecture Recovery.
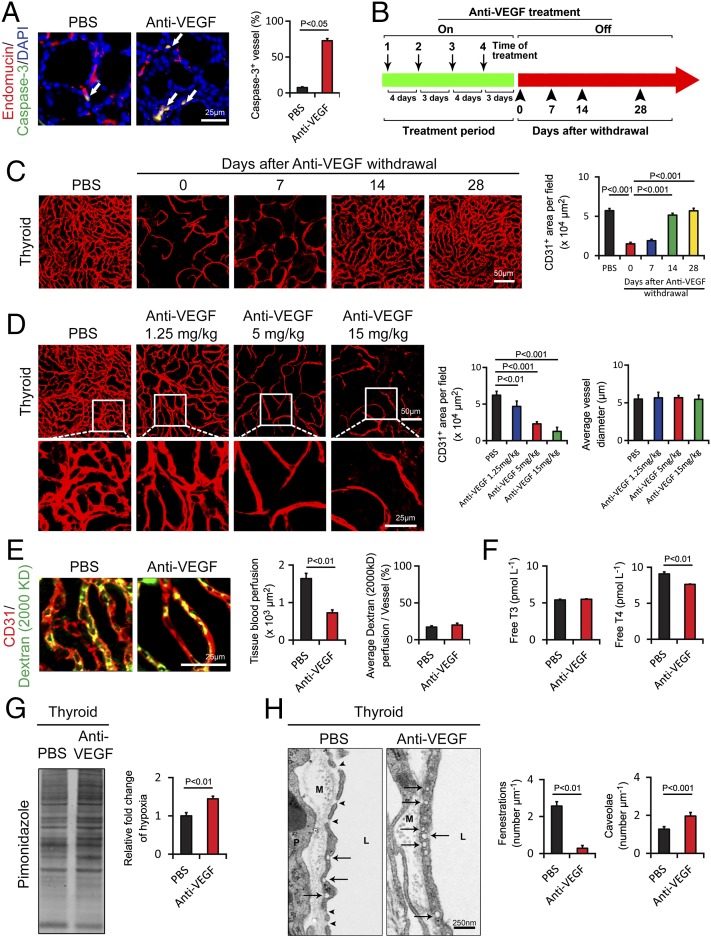
Consistent with anti-VEGF–induced vascular regression, a substantial number of endothelial cells in anti-VEGF–treated thyroid vessels underwent cellular apoptosis with expression of the activated caspase-3 in endomucin+ endothelial structures (Fig. 5A). In fact, a more than sevenfold increase of apoptotic endothelial cell percentage was detected in the anti-VEGF–treated group relative to the untreated control group (Fig. 5A). Despite the existence of a substantial number of apoptotic endothelial cells in anti-VEGF–treated samples, thyroid follicular and other nonendothelial cells rarely became apoptotic during the 2-wk treatment period. In concordance with vascular reduction, anti-VEGF–treated thyroid tissue exhibited significant tissue hypoxia (Fig. 5G). Vascular blockade also induced thyroid vessel regression in a dose-dependent manner without altering vessel diameters (Fig. 5D). To study the anti-VEGF–induced vascular rarefaction, we performed an “on–off” experiment in which anti-VEGF drugs were delivered to healthy mice for 2 wk, followed by analysis of the thyroid vasculature at different time points (Fig. 5B). Notably, anti-VEGF–induced thyroid vascular regression was nearly completely recovered after 2-wk cessation of VEGF blockade, and vascular density and architecture returned to the same levels seen in the untreated animals (Fig. 5C). However, we did not observe a “rebound” effect. These data demonstrate that anti-VEGF–induced vascular rarefaction is completely reversible after discontinuation of anti-VEGF treatment.
Fig. 5.
Endothelial cell apoptosis, cessation-induced vascular recovery, time course, tissue hypoxia, vescular fenestration, and thyroid functional alterations in response to anti-VEGF treatment. (A) At 2 wk after treatment with VEGF blockade, paraffin-embedded thyroid tissue sections were triple immunostained with an anti–caspase-3 antibody (green), an anti-endomucin antibody (red), and DAPI (blue). Arrows point to endothelial apoptotic cells. Caspase-3+ apoptotic endothelial cells were quantified (n = 8 per group). (B) Schematic diagram showing the treatment regimen and cessation of anti-VEGF therapy. On, on drug; Off, off drug. (C) Thyroid vascular alterations in response to VEGF blockade and withdrawal of treatment at various time points. CD31+microvessels in thyroid tissues of anti-VEGF–treated and nontreated groups were quantified (n = 8 fields per group). (D) Thyroid vascular alterations in response to various doses of VEGF blockade and quantification of vascular CD31+ density and the average of vascular diameter (n = 8 fields per group). (E) Vascular perfusions were double immunostained with CD31 (red) and a lysinated fluorescein-labeled dextran (2,000 kDa; green). Total tissue and individual vessel perfusion were quantified (n = 8 fields per group). (F) Serum levels of thyroid hormone free T3 and free T4 as measured using an ELISA method (n = 3 samples per group). (G) Quantification of tissue hypoxia in response to anti-VEGF treatment (n = 3 samples per group). (H) Thyroid vascular fenestrations in anti-VEGF–treated and nontreated tissue samples and numbers of fenestrae and caveolae were quantified (n = 8 samples per group). M, matrix; P, perivascular cell; L, lumen. Arrows point to caveolae, and arrowheads indicate fenestrae. Data are presented as means ± SEM.
Anti-VEGF–Induced Functional Impacts in Thyroid Gland.
Anti-VEGF–induced thyroid vessel regression promoted us to study the function impact of VEGF blockade. First, we measured thyroid tissue blood perfusion in anti-VEGF–treated and nontreated groups. In agreement with vascular density reduction, anti-VEGF–treated thyroid exhibited marked reduction of blood perfusion as measured using fluorescein-labeled 2,000-kDa dextran (Fig. 5E). However, blood perfusion in each individual vessel in anti-VEGF–treated and nontreated groups was not altered (Fig. 5E). Blood vessels in endocrine organs are known to contain fenestrated endothelium, and vascular fenestrations are crucial for maintenance of endocrine organ functions (28, 29). Furthermore, VEGF is the crucial factor for induction and maintenance of vascular fenestrations in endocrine organs. We next investigated the impact of anti-VEGF treatment on alteration of vascular fenestrations in thyroid gland. As expected, thyroid gland contained highly fenestrated microvasculatures with approximately more than 2 fenestrae per μm (Fig. 5H). Remarkably, VEGF blockade virtually completely suppressed the formation of endothelial fenestrations in thyroid vessels (Fig. 5H), suggesting that VEGF in thyroid acts as a homeostatic factor for maintenance of vascular fenestrations. Interestingly, concomitant with reduction of vascular fenestrations, the number of endothelial caveolae was significantly increased in anti-VEGF–treated thyroid tissues relative to the vehicle-treated group (Fig. 5H). To further study the functional impact of anti-VEGF treatment in modulation of thyroid gland functions, mice were treated with VEGF blockade for a prolonged period of 4 wk. Importantly, the circulating level of the predominant thyroid hormone free thyroxine (T4), but not the minimal isoform of free triiodothyronine (T3), was significantly decreased in the VEGF blockade-treated mice compared with that of the vehicle-treated group (Fig. 5F). These findings provide compelling evidence of the endocrine functional impairment of thyroid gland after prolonged treatment with VEGF blockade.
Discussion
Since the US Food and Drug Administration (FDA) approval of the first antiangiogenic drug, bevacizumab, for treatment of metastatic colorectal cancer in 2004 (2), this anti-VEGF drug has been widely used for treatment of various human cancers, including ovarian, breast, lung, glioblastoma, and renal cell carcinoma (RCC) (17–21, 30, 31). In general, addition of bevacizumab to the standard chemotherapy significantly improves beneficial effects in cancer patients although the bevacizumab-related overall survival benefits remain modest (32). Bevacizumab in combination with chemotherapy has become the first-line standard care for treatment of several cancer types, particularly for renal cell carcinoma, non-small cell lung carcinoma, colorectal cancer, and gastrointestinal stromal tumors (19–21, 33). Additionally, ramucirumab and aflibercept, as VEGFR-2–specific and VEGF trap, respectively, are under consideration or already approved for FDA approval for oncology use (34–36). In fact, several FDA-approved TKIs that block VEGFRs, such as sunitinib, sorafenib, and pazopanib, are also commonly used antiangiogenic drugs in the clinic for treatment of human patients with RCC and other cancer types (37–39). Clinical experiences with bevacizumab and other anti-VEGF agents show that these drugs produce a range of adverse effects commonly seen in cancer patients, including hypertension, renal vascular injury often manifested by proteinuria and thrombotic microangiopathy, gastrointestinal perforation, and congestive heart failure (40–42). The anti-VEGF agent-induced broad adverse effects demonstrate that these drugs have a broad impact on vasculatures in multiple healthy tissues and organs. Although this important issue has been known for a long time, there are limited data and knowledge on how these drugs affect healthy vasculatures in various tissues. In the present study, we chose to study the systemic impact of VEGF-specific inhibitors on mice to recapitulate the clinical situation.
Among all analyzed tissues and organs, the most affected vasculatures in response to mouse VEGF and VEGFR-2 blockades have been observed in endocrine organs, particularly in thyroid. It is known that anti-VEGF agents could trigger hypothyroidism (43), suggesting that they potentially target vasculatures in the thyroid gland in human patients. We have also observed marked vascular changes in pancreatic islets where insulin-producing β-cells are located. The clinical significance of the anti-VEGF–induced vascular changes in pancreatic islets remains unknown. Similarly, clinical relevance of the anti-VEGF–induced vascular changes in adrenal cortex needs to be established. Based on our findings, we can reasonably conclude that VEGF is essentially required for maintenance of vascular homeostasis in certain endocrine organs. In the established vasculature in endocrine organs, VEGF, together with several other vascular factors, may display two main functions: maintenance of endothelial cell survivals and vascular fenestrations. We further provide evidence showing that anti-VEGF–induced thyroid endothelial apoptosis as a potential mechanism of vascular regression, indicating that VEGF is an essential survival factor of vasculatures in endocrine glands. In the same line of this view, treatment with VEGF blockade almost completely blocked vascular fenestrations in thyroid vessels. These findings reconcile with the known vascular survival and permeability functions of VEGF (9, 14). Another interesting aspect of our study is that anti–VEGFR-2, but not anti–VEGFR-1, produced similar effects as VEGF blockade, demonstrating that VEGFR-2 is the primary functional receptor that transduces VEGF-mediated vascular homeostasis. Inversely, in some tissues, VEGFR-1 blockade produced a trend or significant effect to increase rather than to decrease vascular density in some tissues, suggesting that VEGFR-1 negatively regulates VEGF functions. In fact, such a negative function of VEGFR-1 in regulation of angiogenesis and homeostasis has been thoroughly discussed elsewhere (9).
Despite induction of endothelial apoptosis, cessation of VEGF blockade treatment resulted in a rapid and reversible recovery of vascular density and architecture of the thyroid vasculature, suggesting that regressed vessels can be regenerated. Although the detailed mechanism underlying vascular regeneration warrants further investigation, there was a similar regenerative mechanism of anti-VEGF–treated tumor vessels, in which angiogenic vessels grow along the basement sheet mainly made by collagen IV (44). Another interesting point is that withdrawal of VEGF blockade takes a relatively short time (approximate 2 wk) to fully recover the regressed vasculature despite the long half-life of the antibody. One possible explanation is that anti-VEGF treatment itself markedly increases VEGF expression levels in various tissues as seen in non–tumor-bearing mice (45). In supporting this view, anti-VEGF–treated tissues such as thyroid show an increased level of hypoxia, a known factor that markedly up-regulates VEGF expression levels (46, 47). High levels of VEGF rapidly neutralize the unbound free antibody molecules, resulting in defective neutralization of the antibody to VEGF. Within a relatively short period of anti-VEGF treatment, thyroid gland and perhaps other endocrine organs, exhibits extremely high capacity of compensatory functional recovery in response to vascular regression, leading to no obvious functional changes. However, prolonged treatment and persistent exposure to VEGF blockade resulted in low-level production of endocrine hormones such as thyroxine. Functional impairment of thyroid functions by chronic exposure to VEGF blockade could result from two vascular defects: vascular density reduction and elimination of vascular fenestrations.
Anti-VEGF–induced vascular changes in glomeruli may, in part, explain this type of side effect in human cancer patients (48, 49). Our data also show that VEGF-dependent vascular plasticity occurs in a tissue- and organ-specific manner. Of note, endocrine abnormalities seem to be uncommon during clinical practice of antiangiogenic drugs (50). Vasculatures in several other organs and tissues such as myocardium, brain, and skeletal muscles remain unaffected despite vascular exposure to the same dose of anti-VEGF drugs.
Taken together, our preclinical findings with systemic delivery of anti-VEGF–specific drugs in mice are in general concordance with clinically manifested adverse effects caused by these drugs in human cancer patients. Thus, our data provide a structural basis of broad targets in healthy tissues and also reveal the physiological functions of VEGF in maintenance of vascular homeostasis in various tissues and organs. Understanding the broad impacts of these anti-VEGF–specific drugs may help us to minimize or even to avoid side effects in future clinical practice.
Methods
All animal studies were approved by the Northern Stockholm Experimental Animal Ethical Committee (Stockholm, Sweden). Statistical analyses were performed using the standard two-tailed Student t test. Details are provided in SI Methods.
Supplementary Material
Acknowledgments
We thank Imclone for providing VEGFR-1– and VEGFR-2–specific neutralizing antibodies and Simcere Pharmaceuticals for providing VEGF blockade. We thank Dr. Kjell Hultenby for technical support in electron microscopy work. Y.C.´s laboratory is supported by research grants from the Swedish Research Council, the Swedish Cancer Foundation, the Karolinska Institute Foundation, the Karolinska Institute Distinguished Professor Award, the Torsten Söderbergs Foundation, Söderbergs Stiftelse, the Tianjin Natural Science Foundation (Center for Molecular Medicine-Tianjin Grant 09ZCZDSF04400) for international collaboration between Tianjin Medical University and Karolinska Institutet, ImClone Systems Inc./EliLilly, the European Union Integrated Project of Metoxia (Project 222741), and European Research Council Advanced Grant ANGIOFAT (Project 250021).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1301331110/-/DCSupplemental.
References
- 1.Folkman J. Tumor angiogenesis: Therapeutic implications. N Engl J Med. 1971;285(21):1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 2.Hurwitz H, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 3.Senger DR, et al. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219(4587):983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 4.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246(4935):1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 5.Coultas L, Chawengsaksophak K, Rossant J. Endothelial cells and VEGF in vascular development. Nature. 2005;438(7070):937–945. doi: 10.1038/nature04479. [DOI] [PubMed] [Google Scholar]
- 6.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407(6801):249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 7.Yancopoulos GD, et al. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407(6801):242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 8.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9(6):669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 9.Cao Y. Positive and negative modulation of angiogenesis by VEGFR1 ligands. Sci Signal. 2009;2(59):re1. doi: 10.1126/scisignal.259re1. [DOI] [PubMed] [Google Scholar]
- 10.Sato Y, et al. Properties of two VEGF receptors, Flt-1 and KDR, in signal transduction. Ann N Y Acad Sci. 2000;902:201–205, discussion 205–207. doi: 10.1111/j.1749-6632.2000.tb06314.x. [DOI] [PubMed] [Google Scholar]
- 11.Witmer AN, Dai J, Weich HA, Vrensen GF, Schlingemann RO. Expression of vascular endothelial growth factor receptors 1, 2, and 3 in quiescent endothelia. J Histochem Cytochem. 2002;50(6):767–777. doi: 10.1177/002215540205000603. [DOI] [PubMed] [Google Scholar]
- 12.LeCouter J, et al. Identification of an angiogenic mitogen selective for endocrine gland endothelium. Nature. 2001;412(6850):877–884. doi: 10.1038/35091000. [DOI] [PubMed] [Google Scholar]
- 13.Vittet D, Ciais D, Keramidas M, De Fraipont F, Feige JJ. Paracrine control of the adult adrenal cortex vasculature by vascular endothelial growth factor. Endocr Res. 2000;26(4):843–852. doi: 10.3109/07435800009048607. [DOI] [PubMed] [Google Scholar]
- 14.Eriksson A, et al. Small GTP-binding protein Rac is an essential mediator of vascular endothelial growth factor-induced endothelial fenestrations and vascular permeability. Circulation. 2003;107(11):1532–1538. doi: 10.1161/01.cir.0000055324.34758.32. [DOI] [PubMed] [Google Scholar]
- 15.Kamba T, et al. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol Heart Circ Physiol. 2006;290(2):H560–H576. doi: 10.1152/ajpheart.00133.2005. [DOI] [PubMed] [Google Scholar]
- 16.Ignoffo RJ. Overview of bevacizumab: A new cancer therapeutic strategy targeting vascular endothelial growth factor. Am J Health Syst Pharm. 2004;61(21) Suppl 5:S21–S26. doi: 10.1093/ajhp/61.suppl_5.S21. [DOI] [PubMed] [Google Scholar]
- 17.Miklos GL. Bevacizumab in neoadjuvant treatment for breast cancer. N Engl J Med. 2012 doi: 10.1056/NEJMc1202229. 366(17):1638, author reply 1639–1640. [DOI] [PubMed] [Google Scholar]
- 18.Mountzios G, Pentheroudakis G. Bevacizumab in ovarian cancer. N Engl J Med. 2012 doi: 10.1056/NEJMc1201044. 366(13):1257, author reply 1258. [DOI] [PubMed] [Google Scholar]
- 19.Sculier JP, Meert AP, Paesmans M. Bevacizumab for non-small-cell lung cancer. N Engl J Med. 2007;356(13):1373–1374, author reply 1374–1375. [PubMed] [Google Scholar]
- 20.Sharieff W. (2004) Bevacizumab in colorectal cancer. N Engl J Med 351(16):1690–1691; author reply 1690–1691. [PubMed]
- 21.Sonpavde G. Bevacizumab in renal-cell cancer. N Engl J Med. 2003;349(17):1674. doi: 10.1056/NEJM200310233491719. [DOI] [PubMed] [Google Scholar]
- 22.Yu Y, et al. A humanized anti-VEGF rabbit monoclonal antibody inhibits angiogenesis and blocks tumor growth in xenograft models. PLoS ONE. 2010;5(2):e9072. doi: 10.1371/journal.pone.0009072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xue Y, et al. Anti-VEGF agents confer survival advantages to tumor-bearing mice by improving cancer-associated systemic syndrome. Proc Natl Acad Sci USA. 2008;105(47):18513–18518. doi: 10.1073/pnas.0807967105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang D, et al. Antiangiogenic agents significantly improve survival in tumor-bearing mice by increasing tolerance to chemotherapy-induced toxicity. Proc Natl Acad Sci USA. 2011;108(10):4117–4122. doi: 10.1073/pnas.1016220108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao R, et al. VEGFR1-mediated pericyte ablation links VEGF and PlGF to cancer-associated retinopathy. Proc Natl Acad Sci USA. 2010;107(2):856–861. doi: 10.1073/pnas.0911661107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garon EB, et al. A randomized, double-blind, phase III study of docetaxel and ramucirumab versus docetaxel and placebo in the treatment of stage IV non-small-cell lung cancer after disease progression after 1 previous platinum-based therapy (REVEL): Treatment rationale and study design. Clin Lung Cancer. 2012;13(6):505–509. doi: 10.1016/j.cllc.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 27.Fan L, Iseki S. Immunohistochemical localization of vascular endothelial growth factor in the endocrine glands of the rat. Arch Histol Cytol. 1998;61(1):17–28. doi: 10.1679/aohc.61.17. [DOI] [PubMed] [Google Scholar]
- 28.Palade GE, Simionescu M, Simionescu N. Structural aspects of the permeability of the microvascular endothelium. Acta Physiol Scand Suppl. 1979;463:11–32. [PubMed] [Google Scholar]
- 29.Renkin EM. Relation of capillary morphology to transport of fluid and large molecules: a review. Acta Physiol Scand Suppl. 1979;463:81–91. [PubMed] [Google Scholar]
- 30.Wong ET, Brem S. Taming glioblastoma by targeting angiogenesis: 3 years later. J Clin Oncol. 2011;29(2):124–126. doi: 10.1200/JCO.2010.32.5282. [DOI] [PubMed] [Google Scholar]
- 31.Friedman HS, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 32.Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358(19):2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lièvre A, Landi B, Mitry E, Taïeb J. [Antiangiogenic agents and gastrointestinal cancers] Gastroenterol Clin Biol. 2008;32(5 Pt 1):504–520. doi: 10.1016/j.gcb.2008.01.042. [DOI] [PubMed] [Google Scholar]
- 34.Spratlin JL, Mulder KE, Mackey JR. Ramucirumab (IMC-1121B): A novel attack on angiogenesis. Future Oncol. 2010;6(7):1085–1094. doi: 10.2217/fon.10.75. [DOI] [PubMed] [Google Scholar]
- 35.Spratlin JL, et al. Phase I pharmacologic and biologic study of ramucirumab (IMC-1121B), a fully human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2. J Clin Oncol. 2010;28(5):780–787. doi: 10.1200/JCO.2009.23.7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramlau R, et al. Aflibercept and Docetaxel versus Docetaxel alone after platinum failure in patients with advanced or metastatic non-small-cell lung cancer: A randomized, controlled phase III trial. J Clin Oncol. 2012;30(29):3640–3647. doi: 10.1200/JCO.2012.42.6932. [DOI] [PubMed] [Google Scholar]
- 37.Motzer RJ, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356(2):115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 38.Escudier B, et al. TARGET Study Group Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356(2):125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 39.Bramwell VH. Pazopanib and the treatment palette for soft-tissue sarcoma. Lancet. 2012;379(9829):1854–1856. doi: 10.1016/S0140-6736(12)60739-9. [DOI] [PubMed] [Google Scholar]
- 40.des Guetz G, Uzzan B, Chouahnia K, Morère JF. Cardiovascular toxicity of anti-angiogenic drugs. Target Oncol. 2011;6(4):197–202. doi: 10.1007/s11523-011-0204-7. [DOI] [PubMed] [Google Scholar]
- 41.Randall LM, Monk BJ. Bevacizumab toxicities and their management in ovarian cancer. Gynecol Oncol. 2010;117(3):497–504. doi: 10.1016/j.ygyno.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tol J, et al. Gastrointestinal ulceration as a possible side effect of bevacizumab which may herald perforation. Invest New Drugs. 2008;26(4):393–397. doi: 10.1007/s10637-008-9125-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rini BI. Review: Thyroid function abnormalities in patients receiving VEGF-targeted therapy. Clin Adv Hematol Oncol. 2011;9(4):337–338. [PubMed] [Google Scholar]
- 44.Mancuso MR, et al. Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J Clin Invest. 2006;116(10):2610–2621. doi: 10.1172/JCI24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ebos JM, Lee CR, Christensen JG, Mutsaers AJ, Kerbel RS. Multiple circulating proangiogenic factors induced by sunitinib malate are tumor-independent and correlate with antitumor efficacy. Proc Natl Acad Sci USA. 2007;104(43):17069–17074. doi: 10.1073/pnas.0708148104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359(6398):843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 47.Makino Y, et al. Inhibitory PAS domain protein is a negative regulator of hypoxia-inducible gene expression. Nature. 2001;414(6863):550–554. doi: 10.1038/35107085. [DOI] [PubMed] [Google Scholar]
- 48.Launay-Vacher V, Deray G. Hypertension and proteinuria: A class-effect of antiangiogenic therapies. Anticancer Drugs. 2009;20(1):81–82. doi: 10.1097/CAD.0b013e3283161012. [DOI] [PubMed] [Google Scholar]
- 49.Zhu X, Wu S, Dahut WL, Parikh CR. Risks of proteinuria and hypertension with bevacizumab, an antibody against vascular endothelial growth factor: Systematic review and meta-analysis. Am J Kidney Dis. 2007;49(2):186–193. doi: 10.1053/j.ajkd.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 50.Perren TJ, et al. ICON7 Investigators A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365(26):2484–2496. doi: 10.1056/NEJMoa1103799. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.