Abstract
Individuals with autism spectrum disorders (ASDs) often show insensitivity to the human voice, a deficit that is thought to play a key role in communication deficits in this population. The social motivation theory of ASD predicts that impaired function of reward and emotional systems impedes children with ASD from actively engaging with speech. Here we explore this theory by investigating distributed brain systems underlying human voice perception in children with ASD. Using resting-state functional MRI data acquired from 20 children with ASD and 19 age- and intelligence quotient-matched typically developing children, we examined intrinsic functional connectivity of voice-selective bilateral posterior superior temporal sulcus (pSTS). Children with ASD showed a striking pattern of underconnectivity between left-hemisphere pSTS and distributed nodes of the dopaminergic reward pathway, including bilateral ventral tegmental areas and nucleus accumbens, left-hemisphere insula, orbitofrontal cortex, and ventromedial prefrontal cortex. Children with ASD also showed underconnectivity between right-hemisphere pSTS, a region known for processing speech prosody, and the orbitofrontal cortex and amygdala, brain regions critical for emotion-related associative learning. The degree of underconnectivity between voice-selective cortex and reward pathways predicted symptom severity for communication deficits in children with ASD. Our results suggest that weak connectivity of voice-selective cortex and brain structures involved in reward and emotion may impair the ability of children with ASD to experience speech as a pleasurable stimulus, thereby impacting language and social skill development in this population. Our study provides support for the social motivation theory of ASD.
Keywords: auditory cortex, nucleus accumbens
The human voice is a critical communication signal for children. Infants’ engagement by the acoustical features of speech (1) is thought to serve at least two critical developmental functions. First, attraction to the human voice guides early speech perception (2), which in turn underlies subsequent development of language skills (3). Second, speech provides critical emotional value to children (4) and promotes bonding between infants and their parents (5). For example, hearing the adult voice soothes infants during moments of distress (6), and it is thought that this form of vocally mediated comfort constitutes a pleasurable and reinforcing experience during the early stages of development (7). The rewarding and emotional nature of speech has also been documented in studies of older children. In stressful situations, children experience increased oxytocin release upon hearing their mother’s voice (4). Release of this hormone promotes affiliative behaviors and is closely linked with emotion (8) and reward processing (9).
Social impairments are a primary deficit in autism spectrum disorders (ASDs) (10). A common observation in individuals with ASD is a relative indifference to the human voice, a trait noted throughout Kanner’s initial report on autism (11). Kanner writes of one of his patients, “He did not register any change of expression when spoken to,” and of another, “he did not respond to being called or to any other words addressed to him” (11). In contrast to typically developing (TD) children, who are extremely engaged by (12), and sensitive to (13), human vocal stimuli, children with ASD are often oblivious to such stimuli (14, 15). Anecdotal (11) and retrospective (15) accounts, as well as experimental investigations, have shown that children with ASD do not automatically orient to vocal stimuli (16), nor do they show a preference for vocal, compared with nonvocal, sounds (17).
It is not known why children with ASD are often indifferent to human vocalizations. One possibility is that deficits associated with social motivation and cognition (18–20) cause indifference to human vocalizations in ASD. The social motivation theory of ASD posits that deficits in representing the reward value of social stimuli, including speech, impedes children with ASD from actively engaging with these stimuli and consequently impairs social skill development (18). An alternative possibility is that individuals with ASD have a sensory deficit in which abnormal processing of the acoustical features of sound precludes access to brain systems serving human vocalization and speech recognition (21).
Investigations into the neural basis of human voice processing with the use of functional MRI (fMRI) have begun to provide clues regarding the biological basis of speech perception in ASD. For example, adults with ASD fail to activate voice-selective regions of bilateral superior temporal cortex (22) that are reliably activated in neurotypical subjects (22, 23). Beyond this, little is known about brain regions underlying voice processing and their links with distributed systems involved in language, reward, and affective information processing. Critically, to date, no study has examined whether large-scale intrinsic functional connectivity of voice-selective superior temporal sulcus (STS) regions is altered in ASD. This is somewhat surprising given that aberrant brain connectivity is one of the most consistent findings in the autism neuroimaging literature (24, 25).
Functional connectivity MRI has recently emerged as a powerful method for the examination of intrinsic functional relationships across the human brain (26, 27). By identifying specific functional systems impaired in clinical populations, functional connectivity MRI can help constrain our knowledge of distributed circuits that underlie sensory, cognitive, and affective dysfunction in children with neurodevelopmental disorders such as ASDs (28, 29). Moreover, this method is particularly advantageous for studying clinical and developmental populations in that it is free from potential behavioral confounds associated with task-based fMRI studies (30).
Here we examine intrinsic functional circuitry of voice-selective temporal cortex in TD children and children with ASD to test competing models of social and communication impairments in ASD. Models that view ASD symptomatology as a deficit in social motivation and cognition would predict abnormal connectivity between the posterior STS (pSTS) and the reward circuit, including the ventral tegmental area (VTA), nucleus accumbens (NAc), orbitofrontal cortex (OFC) (31), and the amygdala (19, 20). In contrast, models based on sensory processing deficits in ASD (21) would predict that a key component of pSTS connectivity would include abnormal connectivity with primary auditory cortical regions, which are critical for acoustical processing of speech (32). We investigated voice-selective regions of left- and right-hemisphere pSTS (23) based on their putative roles in speech comprehension. Bilateral pSTS are critical for a number of speech and language-related processes (33) whereas right-hemisphere pSTS is also associated with the analysis of the emotional content of speech (34). Based on the social motivation theory of ASD (18), we hypothesized that children with ASD would show aberrant intrinsic connectivity of voice-selective pSTS throughout brain regions implicated in reward and emotion, including the OFC, NAc, insula, and amygdala (31). Such findings would support a role for motivational and affective factors, rather than low-level sensory abnormalities, in speech-related communication deficits in children with ASD.
Results
pSTS Functional Connectivity in TD Children and Children with ASD.
To understand the basic functional circuitry associated with human voice-selective areas in children, we first examined functional connectivity of the pSTS separately within the TD and ASD groups (Fig. 1, Left). Results indicate that the left-hemisphere pSTS has significant connectivity with superior temporal gyrus (STG) and STS bilaterally in TD children and children with ASD, with connectivity extending posteriorly into bilateral angular gyrus (AG) in the ASD group and anteriorly into mid-STS, STG, and planum temporale in the TD group. TD and ASD groups also showed significant connectivity with ventrolateral prefrontal cortex; however, in the TD group, this connectivity was limited primarily to left-hemisphere OFC, whereas, in the ASD group, connectivity was present bilaterally in pars triangularis (Brodmann area 45). TD children also showed significant connectivity with the basal ganglia, including the NAc, putamen and ventral caudate; however, there was no significant connectivity with the basal ganglia in children with ASD. Similarly, TD children showed significant connectivity between the left-hemisphere pSTS and visual cortical structures, including bilateral occipital pole and left-hemisphere lingual gyrus; however, the left-hemisphere lateral occipital cortex was the only occipital lobe structure that showed significant connectivity with the left-hemisphere pSTS in the ASD group.
Fig. 1.
Within-group functional connectivity results for left- and right-hemisphere voice-selective cortex. (Left) TD children and children with ASD showed significant connectivity between left-hemisphere pSTS and a distributed cortical network. The seed region used in this analysis was a 6-mm sphere centered at the Montreal Neurological Institute (MNI) coordinates [−63, −42, 9] (23). (Right) In contrast to TD children, right-hemisphere pSTS connectivity is sparse for children with ASD and is restricted primarily to superior temporal cortex. The seed used in this analysis was a 6-mm sphere centered at MNI coordinates [57, −31, 5] (23). Images are thresholded at P < 0.000001 for voxel height and an extent of 100 voxels. aMTG, anterior middle temporal gyrus; BA 45, Brodmann area 45 (pars triangularis); FG, fusiform gyrus; pSTG, posterior superior temporal gyrus; OP, occipital pole; PT, planum temporale; Thal, thalamus.
Next, we examined within-group functional connectivity for the right-hemisphere pSTS seed. Results show that TD children have extensive connections between right- hemisphere pSTS and several frontal, temporal, and parietal cortical regions; however, in children with ASD, connectivity was limited to a relatively small region of superior temporal cortex (Fig. 1, Right). Specifically, TD and ASD groups showed significant connectivity with right-hemisphere mid-STS; however, in the TD group, connectivity extended anteriorly along the STS and was also present in similar regions in left-hemisphere superior temporal cortex. TD individuals also showed significant connectivity in left-hemisphere OFC, bilateral ventromedial prefrontal cortex (vmPFC), AG, and subcortical structures, including right-hemisphere thalamus, caudate, and putamen.
Aberrant pSTS Connectivity in Children with ASD.
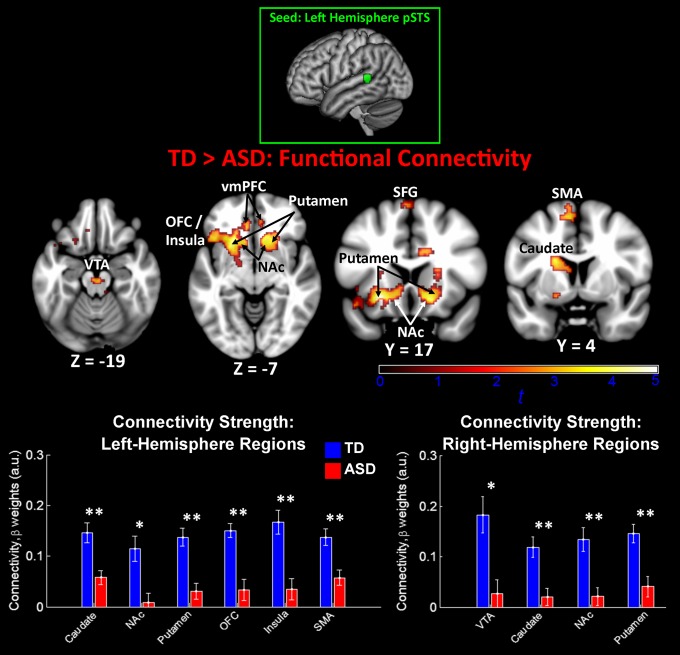
We then examined group differences in connectivity patterns between children with ASD and TD children for the left-hemisphere pSTS seed. Results for the TD>ASD contrast showed a specific and striking pattern of ASD-related underconnectivity between the left-hemisphere pSTS and distributed structures of the dopaminergic reward pathway (Fig. 2). ASD-related underconnectivity was evident in bilateral VTA of the brainstem, the NAc and putamen of the basal ganglia, vmPFC, as well as the left-hemisphere caudate, anterior insula, and OFC. One structure outside of the reward pathway, the left-hemisphere supplementary motor area, also showed ASD underconnectivity. Group differences in connectivity strength were driven by relatively strong positive connectivity in TD subjects and significantly reduced positive connectivity in the ASD subjects (Fig. 2, Lower). Importantly, ASD underconnectivity was not evident in any regions in parietal, occipital, or temporal cortex, including auditory cortical structures. No voxels in the brain showed significant differences for the ASD>TD contrast, indicating that there was no ASD hyperconnectivity between left-hemisphere pSTS and other brain structures.
Fig. 2.
Between-group functional connectivity results for left-hemisphere voice-selective cortex. Group differences for the TD>ASD contrast indicated ASD underconnectivity between left-hemisphere pSTS and structures of the reward network, including the VTA, nucleus accumbens (NAc), insula, and OFC. No voxels showed significant connectivity for the ASD>TD contrast. The seed used in this analysis was a 6-mm sphere centered in left-hemisphere pSTS at MNI coordinates [−63, −42, 9] (23). Images are thresholded at P < 0.01 for voxel height and an extent of 100 voxels. Mean connectivity differences between TD children and children with ASD are plotted in the bar graphs for six left-hemisphere and four right-hemisphere regions (error bars represent SEM). SFG, superior frontal gyrus; SMA, supplementary motor area.
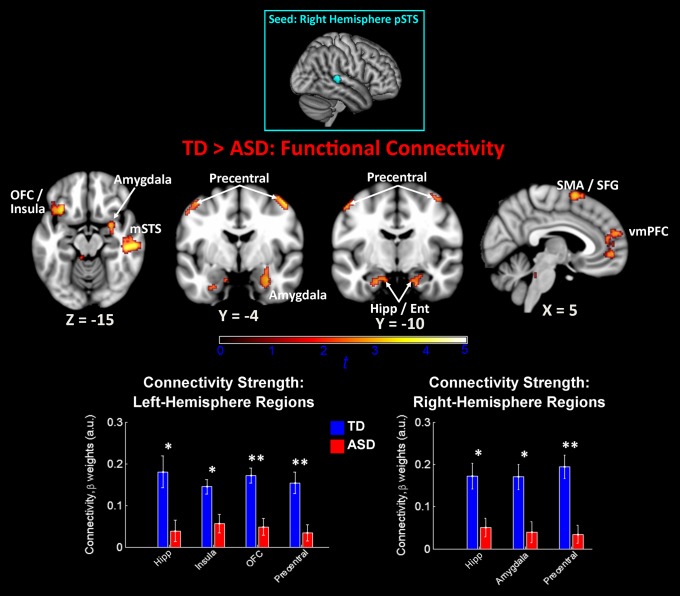
We then examined differences between TD and ASD groups in intrinsic connectivity patterns for voice-selective right-hemisphere pSTS regions. Results for the TD>ASD contrast from the first of these two right-hemisphere pSTS functional connectivity analyses showed no ASD underconnectivity with any cortical structures; however, results for the second pSTS seed showed widespread ASD underconnectivity across a range of cortical structures (Fig. 3). These structures included bilateral hippocampus, precentral and superior frontal gyri, left-hemisphere OFC, mid-STS, and middle frontal gyrus, and right-hemisphere amygdala, vmPFC, and crus I of the cerebellum. Again, group mean results showed that group differences in connectivity strength were driven by relatively strong positive connectivity in TD subjects and significantly reduced positive connectivity in ASD subjects (Fig. 3, Lower). Importantly, there was no ASD underconnectivity between either of the right-hemisphere pSTS seeds and the VTA or structures of the basal ganglia. Consistent with left-hemisphere pSTS connectivity results, no voxels in the brain showed significant differences for the ASD>TD contrast, indicating that there was no ASD hyperconnectivity between bilateral pSTS and other brain structures.
Fig. 3.
Between-group functional connectivity results for right-hemisphere voice-selective cortex. Group differences for the TD>ASD contrast indicated ASD underconnectivity between right-hemisphere pSTS and an array of cortical regions. No voxels showed significant connectivity for the ASD>TD contrast. The seed used in this analysis was a 6-mm sphere centered at MNI coordinates [57, −31, 5] (23). Images are thresholded at P < 0.01 for voxel height and an extent of 100 voxels. Mean connectivity differences between TD children and children with ASD are plotted in the bar graphs for four left-hemisphere and three right-hemisphere regions (error bars represent SEM). Ent, entorhinal cortex; Hipp, hippocampus; mSTS, mid-superior temporal sulcus; SFG, superior frontal gyrus; SMA, supplementary motor area.
Our next goal was to examine whether reward and affect-related ASD underconnectivity was specific to voice-selective auditory regions of pSTS or, alternatively, whether ASD underconnectivity was also evident between primary auditory cortex (PAC) and these downstream brain structures, thereby representing a more general auditory connectivity phenomenon. To examine this question, we performed two additional functional connectivity analyses in which the seed regions were bilateral PAC (35). Results from this analysis show that TD and ASD groups had comparable connectivity between PAC and all downstream brain structures identified in the pSTS connectivity analysis (SI Text). Independent-samples t tests performed on β-values from TD and ASD PAC connectivity analyses failed to reach statistical significance at the P < 0.01 level for all left-hemisphere (SI Text) and right-hemisphere (SI Text) connections, and only one connection was significant at the P < 0.05 level (right-hemisphere Te1.0 region to left-hemisphere precentral gyrus; P = 0.0498).
Between-Group Functional Connectivity Differences Examined with “Scrubbing” Procedures.
To investigate whether group differences in pSTS functional connectivity were influenced by group differences in subject movement (36), we used the scrubbing method on individual subjects’ resting state data (36) and repeated left- and right-hemisphere pSTS functional connectivity analyses. Consistent with the initially reported findings (Fig. 2), scrubbed results for the left-hemisphere pSTS seed show significantly reduced ASD connectivity in bilateral NAc and left-hemisphere OFC and anterior insula (SI Text). Connectivity with the VTA was also evident, albeit at a reduced threshold (P < 0.05, height). For the right-hemisphere pSTS seed, children with ASD showed significantly reduced connectivity in the right-hemisphere amygdala, bilateral hippocampus and precentral gyrus and left-hemisphere OFC, as before, and additional clusters in left-hemisphere amygdala and AG, bilateral temporal pole, and the cerebellum (SI Text).
pSTS Connectivity Is Related to Symptom Severity in Children with ASD.
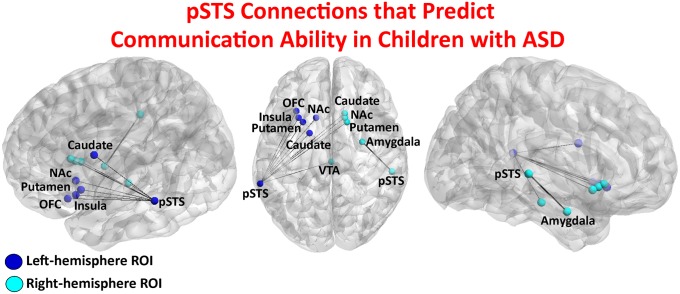
The final goal of this work was to examine whether connectivity strength between voice-selective pSTS and structures of the reward system and amygdala (Fig. 4) were predictive of ASD symptom severity on standardized measures of communication abilities. Results from binary logistic regression analysis showed that connectivity strength between the left-hemisphere pSTS and multiple regions of the reward pathway was predictive of communication subtest scores of the Autism Diagnostic Observation Schedule (ADOS; P = 0.008) and Autism Diagnostic Interview (ADI; P = 0.003).
Fig. 4.
Voice-selective pSTS connections entered into regression models for brain-behavior analyses. Functional connectivity between bilateral pSTS and the distributed reward circuit, including VTA, NAc, OFC, and anterior insula, as well as the amygdala, predicted communication subtests of the ADOS (R2 = 0.713, P = 0.008) and ADI (R2 = 0.740, P = 0.003) in children with ASD. Because of the narrow range of clinical symptom scores, logistic regression was performed, and scatter plots are not depicted for the ADOS and ADI measures.
Discussion
ASD has long been associated with abnormal processing of the human voice (11). Consistent with the social motivation theory of autism (18), we show that high-functioning, verbally fluent children with ASD (Table 1) have reduced intrinsic brain connectivity between voice-selective cortical regions and a distributed reward processing system that includes the VTA, NAc, anterior insula, vmPFC, and OFC (37), as well as the amygdala, a critical structure for processing emotional content in speech (38). Furthermore, the strength of functional connectivity between voice-selective cortex and reward centers in the brain predicts standardized scores of communication abilities in children with ASD. Children with ASD showed similar patterns of connectivity between bilateral PAC and superior temporal cortex as TD children, which is inconsistent with sensory-based models of human vocalization deficits in autism (21). Our results suggest that weak connectivity of voice-selective cortex and brain structures involved in reward and emotion may impair the ability of children with ASD to experience speech as a pleasurable stimulus, thereby impacting language and social skill development in this population.
Table 1.
Participant demographics
| Characteristic | ASD (n = 20) | TD (n = 19) | P value |
| Age | 9.96 ± 1.59 | 9.88 ± 1.61 | 0.88 |
| Sex, M/F | 16/4 | 15/4 | 0.94* |
| Full-scale IQ | 112.6 ± 17.8 | 112.2 ± 15.8 | 0.95 |
| ADOS social† | 8.2 ± 2.1 | — | — |
| ADOS communication† | 3.6 ± 1.5 | — | — |
| ADI-A social | 20.4 ± 5.4 | — | — |
| ADI-B communication | 15.9 ± 5.1 | — | — |
| ADI-C repetitive behaviors | 5.8 ± 2.5 | — | — |
| Word reading | 113.8 ± 12.3 | 109.8 ± 12.5 | 0.31 |
| Reading comprehension | 109.9 ± 14.9 | 104.1 ± 18.5 | 0.28 |
| Movement (RMS), mm | 0.33 (± 0.23) | 0.30 (± 0.24) | 0.70 |
ADI, Autism Diagnostic Interview; ADOS, Autism Diagnostic Observation Schedule.
χ2 test.
Score missing for one participant.
Social Motivation Theory and Reward Circuitry in ASD.
The etiology of the pronounced social deficits in ASD remains elusive, and several hypotheses have been proposed to explain these deficits (39–41). The social motivation theory states that impaired salience and reward value attributed to faces and vocal stimuli has a causal effect on social skill development in children with ASD (18). The reward circuit consists of a distributed set of brain regions that includes the midbrain VTA, NAc of the basal ganglia, anterior cingulate cortex, vmPFC, and the OFC (37), and activity in this pathway is known to modulate auditory cortical representations (42). Previous task-related fMRI studies have reported impaired function in these brain structures in individuals with ASD. For example, it has been shown that children with ASD exhibit reduced activation of the reward pathway, including the NAc and OFC, while viewing smiling faces (43). Moreover, reduced activation in reward regions has also been shown for nonsocial stimuli (44), supporting a more general reward-related impairment in ASD. Our findings provide support for the social motivation theory by showing diminished intrinsic connectivity between voice-selective cortex and most of the brain regions previously implicated in the social motivation theory, including the NAc, OFC, vmPFC, and amygdala (31). These results demonstrate that abnormal reward-related processes are not limited to visual social stimuli (43, 44) and that auditory voice-selective brain regions that are important for social information processing are also affected in ASD.
Critically, brain connectivity between voice-selective pSTS and brain structures implicated in reward and affective processes was predictive of the social communication scores of the ADOS and ADI. These findings suggest that aberrant brain connectivity associated with the reward pathway may be a primary mechanism underlying weakness in perceiving speech as a socially meaningful and rewarding stimulus in children with ASD (17, 45). Although the present results cannot provide information about the causal relationship between brain connectivity of voice-selective cortex and the ability to perceive speech as a rewarding and socially meaningful stimulus, we suggest that this model represents a parsimonious and plausible explanation for this auditory behavioral phenotype. Moreover, the significant relationship between impaired reward circuitry and social communication symptom severity are central predictions of the social motivation theory (18, 31).
Brain Circuitry Underlying Prosody and Emotional Information in ASD.
Germane to the study of speech processing in ASD, strong empirical evidence has accumulated from behavioral studies showing that individuals with ASD have pronounced deficits for extracting prosodic information from speech, which conveys emotional state information regarding the speaker through intonation and rhythm (46). Importantly, it has been shown that, in TD individuals, the processing of prosodic information is performed in right-hemisphere temporal cortex (34) and right-hemisphere amygdala (47). Connectivity results from the present study provide evidence regarding the neural basis for impaired prosodic speech processing in ASD. Specifically, our results show weak intrinsic coupling between right-hemisphere voice-selective cortex and the amygdala. We hypothesize that this disconnection may play a role in impeding access of auditory-based information, such as prosodic cues, to regions of the brain necessary for emotional learning and memory (48).
Our results also address more general hypotheses linking ASD with amygdala dysfunction. The amygdala has long received attention from ASD researchers because of its established role in social behavior (49), and abnormal amygdala function has been hypothesized to contribute to social deficits in ASD (20, 50). Specifically, it has been proposed that the amygdala is critical for identifying emotional information from complex visual stimuli such as mental state information that can be detected from the eye region of an individual (50). Consistent with previous findings in the visual domain (51), results from our study provide support for the hypothesized role of the amygdala in autism by showing abnormal functional connectivity of the amygdala in children with ASD. Critically, our findings extend this hypothesis by linking amygdala dysfunction with auditory-based social processing.
Implications for Models of Auditory and Speech Processing in Individuals with ASD.
Auditory perception in individuals with ASD is poorly understood and includes a number of paradoxical observations. For example, many children with ASD experience an increased sensitivity to the loudness of sounds (52), yet they often display insensitivity to the human voice, one of the most common of sounds in their environment (11, 45). A model for considering different stages of voice perception in TD adults was proposed by Belin et al. According to this model, speech is first subjected to a low-level acoustical analysis, followed by voice structural analysis, then, in parallel, vocal content, affect, and speaker recognition units are processed (53). This is a useful model for considering auditory and speech information processing in ASD given the variety of auditory deficits reported in this population. Because all levels of auditory processing described in the model of Belin et al. have been implicated in behavioral (11, 45, 54) and neurobiological (17, 22, 55, 56) investigations of ASD, it is plausible that a relatively early stage of this hierarchy is impaired in ASD, thereby negatively impacting all higher levels. Considering the present results linking voice-selective cortex with the reward system, we propose that weak connectivity between the voice structural analysis module in the model of Belin et al. and the reward system is specifically impaired in ASD, negatively impacting all higher-level speech related processes. In support of this hypothesis, we found no evidence for underconnectivity between PAC and reward and affective brain circuitry in children with ASD (SI Text), which suggests that a reward-related connectivity deficit does not impact the acoustical analysis module of this model. Beyond voice structural analysis, the pSTS has also been more broadly implicated in the processing of communicative intent (57), and, from this perspective, the present results may reflect a weakness in connectivity between brain structures that facilitate the recognition and extraction of communicative significance inherent to vocal stimuli and structures of the reward pathway.
Conclusion
We demonstrate that childhood ASD is associated with under-connectivity between voice-selective posterior temporal cortical regions and reward circuitry, providing important insights into the behavioral and clinical phenotype of abnormal speech and language processing observed in the disorder. Critically, aberrant brain connectivity was associated with the severity of social communicative deficits in children with ASD. Our findings shed light on the neurobiological bases of one of the core deficits in ASD by identifying key dysfunctional circuits associated with human voice processing. Taken together, our study provides support for the social motivation theory of ASD.
Materials and Methods
Participants.
The Stanford University Institutional Review Board approved the study protocol. Parental consent and the child's assent were obtained for all evaluation procedures, and children were paid for their participation in the study. Participants were recruited locally from schools and clinics near Stanford University. All children were required to have a full-scale intelligence quotient (IQ) >70, as measured by the Wechsler Abbreviated Scale of Intelligence (58). A group of 20 children who met ASD criteria on module 3 of the ADOS (59) or criteria for autism on the ADI–Revised (60) were matched for full-scale IQ, age, and sex with a group of 20 TD children (Table 1) using a previously described algorithm (29). Importantly, children in the ASD sample are considered “high-functioning” and had fluent language skills and above-average reading skills (Table 1). Nevertheless, these children are generally characterized as having communication impairments, especially in the area of reciprocal conversation. One control participant was excluded from the analysis as a result of issues related to data quality. As a result, the final group consisted of 20 children with ASD and 19 TD children. These data were used in recent publications from our group (29, 61) and are publicly available (http://fcon_1000.projects.nitrc.org/indi/abide/).
Data Acquisition and Preprocessing.
For the resting-state fMRI scan, participants were instructed to keep their eyes closed and remain still for the duration of a 6-min scan. Whole-brain functional images were acquired on a 3-T Signa scanner (GE Healthcare). Details are provided in SI Materials and Methods.
Region of Interest Selection.
Coordinates for the pSTS regions of interest (ROIs) were chosen based on a previous study that showed cortical regions selective for vocal stimuli compared with acoustical control conditions in neurotypical adults (23). Results from this study showed that left- and right-hemisphere pSTS are selective for the human voice compared with a number of control sounds, including environmental sounds, scrambled voices, and amplitude modulated noise. This previous study reported coordinates for the contrast of vocal stimuli minus control sounds in one left-hemisphere pSTS and two right-hemisphere pSTS regions. These peaks were used in the present study as seed regions for the functional connectivity analyses. Details are provided in SI Materials and Methods.
Functional Connectivity Analysis.
For each ROI, a resting-state time series was extracted by averaging the time series of all voxels within it. The resulting ROI time series was then used as a covariate of interest in a linear regression whole-brain analysis. A global time series, computed across all brain voxels, along with six motion parameters, were used as additional covariates to remove confounding effects of physiological noise and participant movement. The ASD and TD groups did not significantly differ in motion (P > 0.7) or have average rms movement >0.35 mm. To demonstrate the robustness of our findings against potential movement confounds, we performed additional supplementary analyses. We computed correlations between movement parameters and brain connectivity values and found that there was no significant correlation between mean brain connectivity values and rms of displacement for any of the ROIs examined. Between-group functional connectivity maps were calculated by using independent-samples t tests on individual subjects’ functional connectivity contrast images. Between-group maps were thresholded at P < 0.01 uncorrected for height and a voxel cluster extent of 100 (corresponding to P < 0.01 for height and P < 0.01 for extent). Although our analysis and interpretation focuses on between-group functional connectivity differences that directly compare children with ASD and TD children, for the sake of completeness, we have also presented within-group functional connectivity maps (Fig. 1), which were generated by using one-sample t tests of individual functional connectivity contrast images. Within-group functional connectivity maps were thresholded at P < 0.000001 uncorrected for height and 100 voxels for extent. Details are provided in SI Materials and Methods.
Functional Connectivity Analysis with Scrubbing Procedures.
To ensure that our findings are not severely confounded by participant motion, we performed additional analyses in which we applied the data-scrubbing method proposed by Power et al. (36). Details are provided in SI Materials and Methods.
Brain-Behavior Regression Analysis.
To investigate whether the degree of connectivity between pSTS ROIs and brain structures identified in the between-group analysis predicts communication symptom severity in ASD, we used binary logistic regression. We first calculated connectivity strength between ROIs identified in the functional connectivity analysis by identifying the voxel with peak group connectivity differences within reward-related structures and the amygdala (Table S1). The time series for these point-ROIs were extracted for each subject, and Pearson correlation coefficients for each subject were calculated for the 10 connections specified in Fig. 4. We then used binary logistic regression to model the relationship between the dependent variable, which were binarized scores on ADI and ADOS communications subscales, and the independent variables, which were Fisher-transformed Pearson correlation coefficients describing connectivity strength between pSTS and brain structures in the reward pathway and amygdala. The reason for performing a regression analysis with the use of binary rather than continuous ADI and ADOS values is that the distributions for these subtests in our sample are narrow (Table 1) and are better suited for a classification-based approach. Therefore, the ADI and ADOS analyses examined whether connectivity strength between pSTS and brain structures in the reward pathway and amygdala could predict group membership in the “more severe” or “less severe” ASD group based on binary scores on ADI and ADOS communications subscales. We used a median split to group subjects in either the more severe or less severe ASD groups. SPSS software (IBM) was used for all regression analyses.
Supplementary Material
Acknowledgments
The authors thank Tianwen Chen for assistance with data analysis, Carl Feinstein for helpful comments on previous drafts of this manuscript, and M. Barth, A. Khouzam, C. Young, C. Tenison, S. Santhanam, and the staff at the Lucas Center for Imaging for assistance with data collection. This work was supported by National Research Service Award F32DC010322 (to D.A.A.), National Institutes of Health (NIH) Career Development Award K01MH092288 (to L.Q.U.), NIH Grants DC011095 and MH084164, the Singer Foundation, and the Stanford Institute for Neuro-Innovation and Translational Neurosciences (to V.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1302982110/-/DCSupplemental.
References
- 1.Vouloumanos A, Werker JF. Listening to language at birth: Evidence for a bias for speech in neonates. Dev Sci. 2007;10(2):159–164. doi: 10.1111/j.1467-7687.2007.00549.x. [DOI] [PubMed] [Google Scholar]
- 2.Christophe A, Dupoux E, Bertoncini J, Mehler J. Do infants perceive word boundaries? An empirical study of the bootstrapping of lexical acquisition. J Acoust Soc Am. 1994;95(3):1570–1580. doi: 10.1121/1.408544. [DOI] [PubMed] [Google Scholar]
- 3.Kuhl PK, Conboy BT, Padden D, Nelson T, Pruitt J. Early speech perception and later language development: Implications for the “critical period”. Lang Learn Dev. 2005;1:237–264. [Google Scholar]
- 4.Seltzer LJ, Ziegler TE, Pollak SD. Social vocalizations can release oxytocin in humans. Proc Biol Sci. 2010;277:2661–2666. doi: 10.1098/rspb.2010.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeCasper AJ, Fifer WP. Of human bonding: Newborns prefer their mothers’ voices. Science. 1980;208(4448):1174–1176. doi: 10.1126/science.7375928. [DOI] [PubMed] [Google Scholar]
- 6.Thoman EB, Korner AF, Beasonwilliams L. Modification of responsiveness to maternal vocalization in neonate. Child Dev. 1977;48:563–569. [Google Scholar]
- 7.Lamb ME. Developing trust and perceived effectance in infancy. In: Lipsitt LP, editor. Advances in Infancy Research. Vol 1. Norwood, NJ: Ablex; 1981. pp. 101–127. [Google Scholar]
- 8.Baumgartner T, Heinrichs M, Vonlanthen A, Fischbacher U, Fehr E. Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron. 2008;58(4):639–650. doi: 10.1016/j.neuron.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Strathearn L, Fonagy P, Amico J, Montague PR. Adult attachment predicts maternal brain and oxytocin response to infant cues. Neuropsychopharmacology. 2009;34(13):2655–2666. doi: 10.1038/npp.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baio J. Prevalence of autism spectrum disorders–Autism and Developmental Disabilities Monitoring Network, 14 sites, United States, 2008. MMWR Surveill Summ. 2012;61:1–19. [PubMed] [Google Scholar]
- 11.Kanner L. Autistic disturbances of affective contact. Nerv Child. 1943;2:217–250. [PubMed] [Google Scholar]
- 12.Alegria J, Noirot E. Neonate orientation behaviour towards human voice. Int J Behav Dev. 1978;1:291–312. doi: 10.1016/0376-6357(83)90044-X. [DOI] [PubMed] [Google Scholar]
- 13.Eimas PD, Siqueland ER, Jusczyk P, Vigorito J. Speech perception in infants. Science. 1971;171(3968):303–306. doi: 10.1126/science.171.3968.303. [DOI] [PubMed] [Google Scholar]
- 14.Clancy H, McBride G. The autistic process and its treatment. J Child Psychol Psychiatry. 1969;10(4):233–244. doi: 10.1111/j.1469-7610.1969.tb02084.x. [DOI] [PubMed] [Google Scholar]
- 15.Ornitz EM, Guthrie D, Farley AH. The early development of autistic children. J Autism Child Schizophr. 1977;7(3):207–229. doi: 10.1007/BF01538999. [DOI] [PubMed] [Google Scholar]
- 16.Dawson G, et al. Early social attention impairments in autism: Social orienting, joint attention, and attention to distress. Dev Psychol. 2004;40(2):271–283. doi: 10.1037/0012-1649.40.2.271. [DOI] [PubMed] [Google Scholar]
- 17.Kuhl PK, Coffey-Corina S, Padden D, Dawson G. Links between social and linguistic processing of speech in preschool children with autism: Behavioral and electrophysiological measures. Dev Sci. 2005;8(1):F1–F12. doi: 10.1111/j.1467-7687.2004.00384.x. [DOI] [PubMed] [Google Scholar]
- 18.Dawson G, et al. Neural correlates of face and object recognition in young children with autism spectrum disorder, developmental delay, and typical development. Child Dev. 2002;73(3):700–717. doi: 10.1111/1467-8624.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pelphrey KA, Carter EJ. Brain mechanisms for social perception: lessons from autism and typical development. Ann N Y Acad Sci. 2008;1145:283–299. doi: 10.1196/annals.1416.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adolphs R, Sears L, Piven J. Abnormal processing of social information from faces in autism. J Cogn Neurosci. 2001;13(2):232–240. doi: 10.1162/089892901564289. [DOI] [PubMed] [Google Scholar]
- 21.Dinstein I, et al. Unreliable evoked responses in autism. Neuron. 2012;75(6):981–991. doi: 10.1016/j.neuron.2012.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gervais H, et al. Abnormal cortical voice processing in autism. Nat Neurosci. 2004;7(8):801–802. doi: 10.1038/nn1291. [DOI] [PubMed] [Google Scholar]
- 23.Belin P, Zatorre RJ, Lafaille P, Ahad P, Pike B. Voice-selective areas in human auditory cortex. Nature. 2000;403(6767):309–312. doi: 10.1038/35002078. [DOI] [PubMed] [Google Scholar]
- 24.Müller RA, et al. Underconnected, but how? A survey of functional connectivity MRI studies in autism spectrum disorders. Cereb Cortex. 2011;21(10):2233–2243. doi: 10.1093/cercor/bhq296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain. 2004;127(pt 8):1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- 26.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. 2003;100(1):253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 28.Menon V. Large-scale brain networks and psychopathology: A unifying triple network model. Trends Cogn Sci. 2011;15(10):483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Uddin LQ, et al. Salience network based classification and prediction of symptom severity in children with autism. JAMA Psychiatry. 2013 doi: 10.1001/jamapsychiatry.2013.104. 10.1001/jamapsychiatry.2013.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uddin LQ, Supekar K, Menon V. Typical and atypical development of functional human brain networks: Insights from resting-state FMRI. Front Syst Neurosci. 2010;4:21. doi: 10.3389/fnsys.2010.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chevallier C, Kohls G, Troiani V, Brodkin ES, Schultz RT. The social motivation theory of autism. Trends Cogn Sci. 2012;16(4):231–239. doi: 10.1016/j.tics.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kraus N, et al. Auditory neurophysiologic responses and discrimination deficits in children with learning problems. Science. 1996;273(5277):971–973. doi: 10.1126/science.273.5277.971. [DOI] [PubMed] [Google Scholar]
- 33.Vigneau M, et al. Meta-analyzing left hemisphere language areas: Phonology, semantics, and sentence processing. Neuroimage. 2006;30(4):1414–1432. doi: 10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 34.Buchanan TW, et al. Recognition of emotional prosody and verbal components of spoken language: An fMRI study. Brain Res Cogn Brain Res. 2000;9(3):227–238. doi: 10.1016/s0926-6410(99)00060-9. [DOI] [PubMed] [Google Scholar]
- 35.Morosan P, et al. Human primary auditory cortex: Cytoarchitectonic subdivisions and mapping into a spatial reference system. Neuroimage. 2001;13(4):684–701. doi: 10.1006/nimg.2000.0715. [DOI] [PubMed] [Google Scholar]
- 36.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haber SN, Knutson B. The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35(1):4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frühholz S, Grandjean D. Amygdala subregions differentially respond and rapidly adapt to threatening voices. Cortex. 2013;49(5):1394–1403. doi: 10.1016/j.cortex.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 39.Baron-Cohen S, Knickmeyer RC, Belmonte MK. Sex differences in the brain: Implications for explaining autism. Science. 2005;310(5749):819–823. doi: 10.1126/science.1115455. [DOI] [PubMed] [Google Scholar]
- 40.Kaiser MD, Pelphrey KA. Disrupted action perception in autism: Behavioral evidence, neuroendophenotypes, and diagnostic utility. Dev Cogn Neurosci. 2012;2(1):25–35. doi: 10.1016/j.dcn.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oberman LM, Ramachandran VS. The simulating social mind: The role of the mirror neuron system and simulation in the social and communicative deficits of autism spectrum disorders. Psychol Bull. 2007;133(2):310–327. doi: 10.1037/0033-2909.133.2.310. [DOI] [PubMed] [Google Scholar]
- 42.Bao S, Chan VT, Merzenich MM. Cortical remodelling induced by activity of ventral tegmental dopamine neurons. Nature. 2001;412(6842):79–83. doi: 10.1038/35083586. [DOI] [PubMed] [Google Scholar]
- 43.Scott-Van Zeeland AA, Dapretto M, Ghahremani DG, Poldrack RA, Bookheimer SY. Reward processing in autism. Autism Res. 2010;3(2):53–67. doi: 10.1002/aur.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dichter GS, Richey JA, Rittenberg AM, Sabatino A, Bodfish JW. Reward circuitry function in autism during face anticipation and outcomes. J Autism Dev Disord. 2012;42(2):147–160. doi: 10.1007/s10803-011-1221-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klin A. Young autistic children’s listening preferences in regard to speech: A possible characterization of the symptom of social withdrawal. J Autism Dev Disord. 1991;21(1):29–42. doi: 10.1007/BF02206995. [DOI] [PubMed] [Google Scholar]
- 46.Pronovost W, Wakstein MP, Wakstein DJ. A longitudinal study of the speech behavior and language comprehension of fourteen children diagnosed atypical or autistic. Except Child. 1966;33(1):19–26. doi: 10.1177/001440296603300104. [DOI] [PubMed] [Google Scholar]
- 47.Sander K, Scheich H. Auditory perception of laughing and crying activates human amygdala regardless of attentional state. Brain Res Cogn Brain Res. 2001;12(2):181–198. doi: 10.1016/s0926-6410(01)00045-3. [DOI] [PubMed] [Google Scholar]
- 48.Murty VP, Labar KS, Adcock RA. Threat of punishment motivates memory encoding via amygdala, not midbrain, interactions with the medial temporal lobe. J Neurosci. 2012;32(26):8969–8976. doi: 10.1523/JNEUROSCI.0094-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bonda E, Petrides M, Ostry D, Evans A. Specific involvement of human parietal systems and the amygdala in the perception of biological motion. J Neurosci. 1996;16(11):3737–3744. doi: 10.1523/JNEUROSCI.16-11-03737.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baron-Cohen S, et al. The amygdala theory of autism. Neurosci Biobehav Rev. 2000;24(3):355–364. doi: 10.1016/s0149-7634(00)00011-7. [DOI] [PubMed] [Google Scholar]
- 51.Rudie JD, et al. Reduced functional integration and segregation of distributed neural systems underlying social and emotional information processing in autism spectrum disorders. Cereb Cortex. 2012;22(5):1025–1037. doi: 10.1093/cercor/bhr171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khalfa S, et al. Increased perception of loudness in autism. Hear Res. 2004;198(1-2):87–92. doi: 10.1016/j.heares.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 53.Belin P, Bestelmeyer PE, Latinus M, Watson R. Understanding voice perception. Br J Psychol. 2011;102(4):711–725. doi: 10.1111/j.2044-8295.2011.02041.x. [DOI] [PubMed] [Google Scholar]
- 54.Paul R, Augustyn A, Klin A, Volkmar FR. Perception and production of prosody by speakers with autism spectrum disorders. J Autism Dev Disord. 2005;35(2):205–220. doi: 10.1007/s10803-004-1999-1. [DOI] [PubMed] [Google Scholar]
- 55.Russo N, Nicol T, Trommer B, Zecker S, Kraus N. Brainstem transcription of speech is disrupted in children with autism spectrum disorders. Dev Sci. 2009;12(4):557–567. doi: 10.1111/j.1467-7687.2008.00790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang AT, Lee SS, Sigman M, Dapretto M. Neural basis of irony comprehension in children with autism: The role of prosody and context. Brain. 2006;129(pt 4):932–943. doi: 10.1093/brain/awl032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shultz S, Vouloumanos A, Pelphrey K. The superior temporal sulcus differentiates communicative and noncommunicative auditory signals. J Cogn Neurosci. 2012;24(5):1224–1232. doi: 10.1162/jocn_a_00208. [DOI] [PubMed] [Google Scholar]
- 58.Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio: Harcourt; 1999. [Google Scholar]
- 59.Lord C, et al. The autism diagnostic observation schedule-generic: A standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30(3):205–223. [PubMed] [Google Scholar]
- 60.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 61.Lynch CJ, et al. Default mode network in childhood autism: Posteromedial cortex heterogeneity and relationship with social deficits. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.