Abstract
Metabotropic glutamate receptor 5 (mGluR5) plays important roles in modulating neural activity and plasticity and has been associated with several neuropathological disorders. Previous work has shown that genetic ablation or pharmacological inhibition of mGluR5 disrupts fear extinction and spatial reversal learning, suggesting that mGluR5 signaling is required for different forms of adaptive learning. Here, we tested whether ADX47273, a selective positive allosteric modulator (PAM) of mGluR5, can enhance adaptive learning in mice. We found that systemic administration of the ADX47273 enhanced reversal learning in the Morris Water Maze, an adaptive task. In addition, we found that ADX47273 had no effect on single-session and multi-session extinction, but administration of ADX47273 after a single retrieval trial enhanced subsequent fear extinction learning. Together these results demonstrate a role for mGluR5 signaling in adaptive learning, and suggest that mGluR5 PAMs represent a viable strategy for treatment of maladaptive learning and for improving behavioral flexibility.
The ability of animals to adapt their behaviors to a changing environment is essential for survival. Acquisition of new memories and behavioral flexibility is inherently linked to enable the organism to modify its response to novel information. To study the biological underpinnings of adaptive learning, a number of behavioral models have been established that have enabled us to better understand the circuits and molecular mechanisms involved. One of the most prominent models of adaptive learning is fear extinction in which a previously conditioned aversive memory can be suppressed or extinguished by repeated presentation of a nonaversive stimulus. Fear extinction has been extensively investigated primarily because it serves as a model to study the neural processes underlying behavioral exposure therapy, which is commonly used to address maladaptive memories such as those associated with post-traumatic stress disorder (PTSD). It has been proposed that biological agents that can facilitate fear extinction in animal models might also provide useful therapeutic agents for human PTSD (Graham et al. 2011; Myers et al. 2011). Agents targeting several different neurotransmitter receptor systems, including glutamate, have been demonstrated in animal models to have potential as adjunct treatment (Myers et al. 2011). Several of these agents target synaptic plasticity molecules, demonstrating a direct link between neural plasticity and adaptive learning. Another model of adaptive learning is spatial reversal, in which animals learn to form new search strategies for repositioned targets. Although executed in different contexts, emerging evidence suggests that fear extinction and spatial reversal learning require similar molecules and share similar synaptic mechanisms (Marsicano et al. 2002; Varvel and Lichtman 2002; Duffy et al. 2008; Xu et al. 2009; Luscher and Huber 2010; Park et al. 2012).
In recent studies we demonstrated that genetic ablation of the metabotropic glutamate receptor 5 (mGluR5) in mice disrupted fear extinction and spatial reversal learning, suggesting that signaling through this receptor is required for these adaptive behaviors (Xu et al. 2009). In support of an important role for these receptors in extinction learning, systemic administration or local microperfusion of mGluR5 antagonists into the medial prefrontal cortex inhibited recall of extinction memories (Fontanez-Nuin et al. 2010). These studies suggest that activation of mGluR5 is a requisite for the reversal learning and thus enhancing mGluR5 signaling may facilitate the extinction of fear memory and other previously acquired memories.
There are eight G-protein coupled metabotropic glutamate receptors in the mammalian genome and they are divided into three groups based on their sequence homology, signal transduction pathways, and pharmacological properties. mGluR5 is a member of the group 1 family of Gq protein-coupled glutamate receptors and modulates neural activity via linkage to various intracellular signaling cascades (Conn and Pin 1997). mGluR5 is expressed throughout the central nervous system (CNS), and has been demonstrated to be involved in an array of cellular functions, such as synaptic and cellular plasticity, which likely underlie their contribution to memory and cognition. Cellular signaling functions of mGluR5 are primarily mediated by the phospholipase C pathway, activation of which leads to production of lipid second messengers and cascades of protein phosphorylation (Conn and Pin 1997). mGluR5 has been proposed to be physically connected with NMDA receptors in the postsynaptic density, and activation of mGluR5 positively modulates NMDA receptor-mediated postsynaptic currents (Sepulveda-Orengo et al. 2013). The importance of mGluR5 signaling to normal brain function is reflected in its association to several neurological and neurodevelopmental disorders, including fragile X syndrome (Dolen and Bear 2008), schizophrenia (Conn et al. 2009), Parkinson’s related dyskinesia (Johnson et al. 2009), anxiety, depression, and addiction (Carroll 2008). The potential of mGluR5 as a therapeutic target for these diseases has led to a concerted effort to produce selective ligands for these receptors. Several studies have demonstrated that positive allosteric modulators (PAMs) of mGluR5 can enhance synaptic plasticity assayed in vitro (Ayala et al. 2009) and hippocampal dependent memory tasks (Balschun et al. 2006; Ayala et al. 2009; Fowler et al. 2013).
Here we tested whether administration of a selective mGluR5 PAM could enhance adaptive learning. PAMs represent a novel class of therapeutic agent that have advantages over orthosteric agonists because of their higher subtype specificity, achieved by targeting the nonconserved regions of different receptors, and also because of their use dependency as they only exert their influence on receptors after activation by the endogenous ligand. ADX47273 is a recently identified potent and selective mGluR5 PAM that has been demonstrated to increase mGluR5 function in both in vitro and in vivo assays (Liu et al. 2008; Ayala et al. 2009). Here we found that systemic administration of ADX47273 enhanced two distinct forms of adaptive learning: ADX47273 enhanced fear extinction learning when administered during the memory retrieval–reconsolidation phase. Separately, ADX47273 also improved reversal learning in the Morris Water Maze (MWM). We propose that mGluR5 PAMs are potentially a useful co-therapy for treatment of certain forms of anxiety disorders and for improving behavioral flexibility.
Results
mGluR5 PAM enhances reversal learning in the MWM
In prior work we had found that mGluR5 knockout mice are impaired in reversal learning in the MWM, suggesting that signaling through this receptor is crucial to this form of adaptive learning (Xu et al. 2009). We tested whether enhancing mGluR5 signaling with ADX47273 can affect reversal learning in the MWM. Wild-type mice (C57/bl6) (n = 25) randomly divided in two groups (n = 12 for vehicle group, n = 13 for drug group) underwent a training procedure consisting of 3 d (D1–D3) of visible platform training (three trials per day), followed by 7 d (D4–D10) of hidden platform training (three trials per day) and 4 d of reversal training (D11–D14) (Fig. 1A). As expected, in these tests when no drug was administered both groups performed equally well in the visible (two-way repeated-measures [RM] ANOVA, F(1,23) = 1.29, P > 0.05) (Fig. 1B) and hidden platform task (two-way RM ANOVA, F(1,23) = 0.01, P > 0.05) (Fig. 1C). Animals were then trained on D11 and D12 to find a hidden platform that was moved to the quadrant opposite its original position. This first reversal training set comprised of seven trials divided in 2 d (three trials on reversal 1 day 1 [R1D1] and four trials on R1D2). Thirty minutes before each trial, animals were administered either ADX47273 (15 mg/kg i.p.) or vehicle. Upon completion of trials on the first reversal training, we performed a second set of reversal training that also comprised of seven trials over 2 d (R2D1 and R2D2) by moving the platform position a further time. In all, each animal underwent 14 trials during the two sets of reversal training. Averaged escape latency for each trial is shown in Figure 1D. We found that the ADX47273 group performed better than the vehicle group, displaying significantly shorter escape latencies (two-way RM ANOVA, F(1,23) = 5.073, P < 0.05) (Fig. 1D). The enhanced performance was more pronounced in the first two trials each day, whereas by the final session on each training day the vehicle and ADX47273 group performed equally well (Fig. 1D). Analysis of path lengths also revealed a significant enhancement of reversal spatial learning by ADX47273 (data not shown), whereas no difference was detected in swimming speed between the two groups (Fig. 1E). Three probe tests were conducted on D11 (to test memory after hidden platform learning) and on D13 and D15 (to test memory after each round of reversal learning). These probe tests revealed no difference between the two groups, suggesting that ADX47273 has no effect on spatial long-term memory (LTM) once animals have acquired the tasks (data not shown). Together these results demonstrate that administration of mGluR5 PAMs can enhance distinct forms of adaptive spatial learning in mice.
Figure 1.
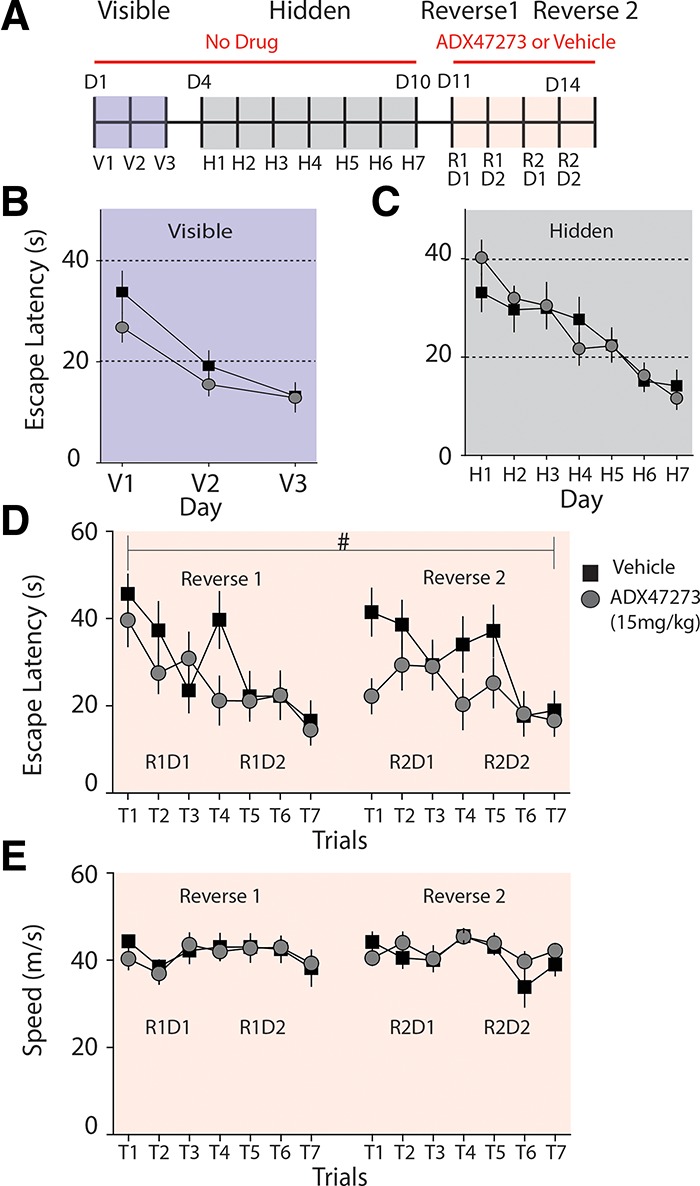
ADX47273 enhanced reversal learning in the MWM. (A) Schematic of experimental design for MWM. D1–D3 animals were trained with a visible platform. D4–D10 mice were subjected to a hidden platform test. For D11 and D12, the platform was moved to the opposite quadrant, and mice were trained in reversal learning for two consecutive days with a total of seven trials. Finally, the platform was again moved to the opposite quadrant and mice were allowed seven trials for 2 d to relearn the new location (D13 and D14). Twenty to 30 min prior to each reversal trial, animals were injected with ADX47272 or vehicle. Three probe tests were conducted on D11, D13, and D15 at the beginning of each day (data not shown). (B, C) Escape latency of the mice during each day of the visible platform (B) and hidden platform test (C). (D) Escape latency during the reversal test. Drug group, n = 13; vehicle group, n = 12. (#) Two-way RM ANOVA, P < 0.05. (E) Swim speeds during the reversal test.
ADX47273 does not enhance single-session fear extinction
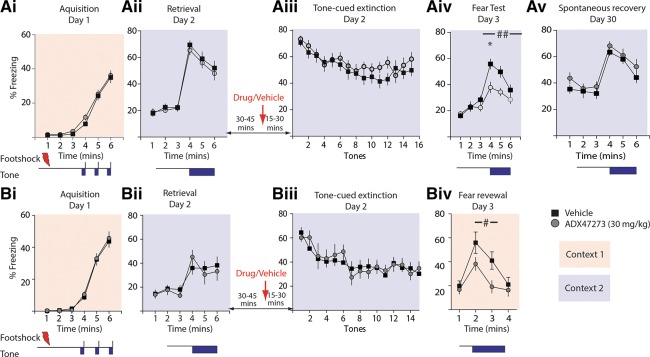
mGluR5 knockout mice have deficits in fear extinction, a form of adaptive learning that is critical to the appropriate response to fearful stimuli and which can go awry in anxiety disorder such as post-traumatic stress disorder (Rauch et al. 2006). General memory processes, including fear extinction, can be partitioned into three distinct phases: acquisition, consolidation, and retrieval, and the mGluR5 PAM ADX47273 might differentially affect these phases. First, to determine if ADX47273 has effects on the acquisition of fear extinction, we administered ADX47273 prior to single-session fear extinction training in a contextual fear extinction paradigm. Pilot experiment using the same dose of ADX47273 (15 mg/kg) as we had previously used in the MWM reversal failed to show effects when administered prior to extinction (data not shown). We therefore used a higher dose of ADX47273 in these experiments (30 mg/kg) that has previously been demonstrated to be the optimal concentration for alleviating deficits in an animal model of psychosis (Schlumberger et al. 2009). Animals were randomly divided into a drug group (n = 12) and a vehicle group (n = 12). On Day 1, animals of both groups received three footshocks during fear training (Fig. 2Ai). On Day 2, animals were administered either ADX47273 (30 mg/kg) or vehicle 30 min before being returned to the fear inducing conditioning chamber for within-session extinction in the presence of the drug or vehicle. Both vehicle and drug groups demonstrated fear extinction that was not different between the two groups (two-way RM ANOVA, F(1,22) = 0.1067, P > 0.05) (Fig. 2Aii). On Day 3, both groups were returned to the conditioning chamber for 6 min without drug administration to assess their extinction memory. There was no difference between the drug group and vehicle group in freezing (two-way RM ANOVA, F(1,22) = 0.18, P > 0.05) (Fig. 2Aiii), suggesting that PAM administration prior to a single day within-session extinction training has no effect on fear extinction.
Figure 2.
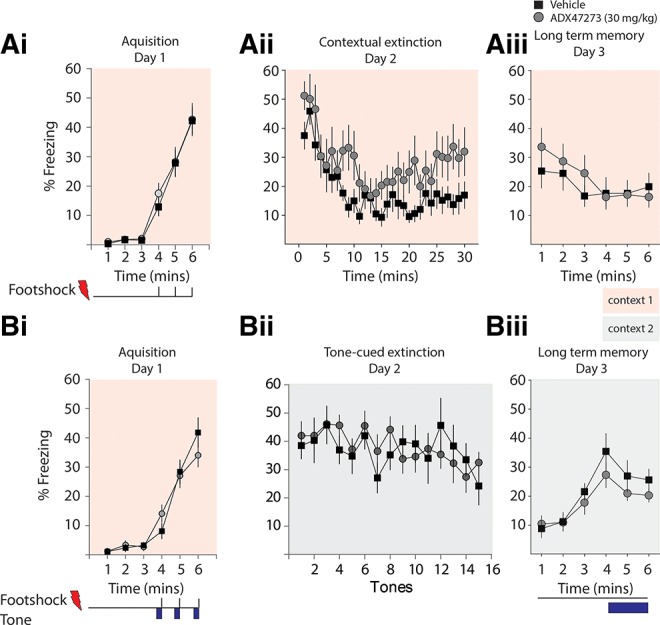
Prior administration of ADX47273 did not enhance single-session fear extinction. (Ai–Aiii) Freezing during contextual fear conditioning on Day 1 (Ai); 30-min contextual fear extinction on Day 2 (Aii); and 6 min of LTM test on Day 3 (Aiii). Drug group, n = 12; vehicle group, n = 12. (Bi–Biii) Freezing during tone cued fear extinction. Fear conditioning (three tone–shock pairings) on Day 1 (Bi); tone-cued fear extinction on Day 2 (15 tones of 1 min at 2-min intervals, shown are freezing during tone presentations) (Bii); and tone-cued LTM test on Day 3 (3-min tone after 3 min of acclimation) (Biii). Drug group, n = 8; vehicle group, n = 8.
We performed similar experiments (Fig. 2B) to test whether ADX47273 has effects on the extinction of tone-cued fear memory. On Day 1, animals (n = 16) were conditioned with three pairings of tones and co-terminating footshocks (Fig. 2Bi). On Day 2, animals were administered either ADX47273 (30 mg/kg) (n = 8) or vehicle (n = 8) 30 min prior to being introduced to a new context (context 2, extinction chamber) and presented with 15 1-min tones of 1-min interval without footshocks. Freezing percentage during each tone presentation is presented in Figure 2Bii. Evidently, the ADX47273 group did not demonstrate any difference in freezing behavior in the within-session fear extinction period (two-way RM ANOVA, F(1,14) = 0.038, P > 0.05) (Fig. 2Bii). On Day 3, both groups were returned to the extinction chamber and were tested for their fear extinction memory using a single 3-min tone presentation. Again there was no difference in the freezing of mice in either group (two-way RM ANOVA, F(1,14) = 1.79, P > 0.05) (Fig. 2Biii). Therefore, mGluR5 PAM administered prior to extinction training has no effect on single-session extinction of either tone-cued or contextual fear memory.
Multi-session extinction is not enhanced by co-administration of ADX47273
In the next experiment, we tested whether ADX47273 would be effective in a multi-trial contextual fear memory extinction paradigm. Mice were again divided into a drug group (n = 9) and a vehicle group (n = 8). On Day 1, animals were conditioned with three footshocks (Fig. 3Ai). On the next seven consecutive days, animals of both groups were returned to the conditioning chamber for 6 min each day without footshocks. Thirty minutes before this daily extinction training, animals were administered either ADX47273 (30 mg/kg i.p.) or vehicle. Freezing in both groups decreased during the multi-session extinction training; however, there was no difference observed in the freezing of mice in either group (two-way RM ANOVA, F(1,15) = 0.36, P > 0.05) (Fig. 3Aii).
Figure 3.
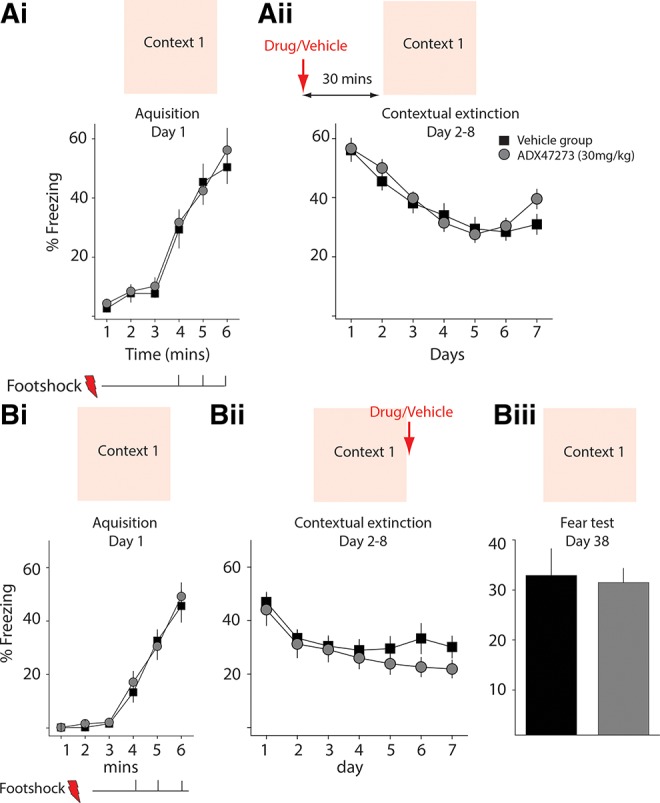
ADX47273 administered prior to or after multi-session extinction did not enhance fear extinction. (Ai,Aii) Effects of prior administration of ADX47273 on extinction. Freezing during contextual fear conditioning (three footshocks) on Day 1 (Ai) and contextual extinction training during seven consecutive days (Aii). ADX47273 or vehicle was administered 30 min prior to training. Drug group, n = 9; vehicle group, n = 8. (Bi–Biii) Effects of post-extinction administration of ADX47273. Contextual fear conditioning on Day 1 (Bi) and multi-session extinction training during seven subsequent consecutive days. ADX47273 or vehicle was administered immediately after training (Bii). (Biii) LTM test performed 1 mo after extinction. Drug group, n = 12; vehicle group, n = 11.
To test whether ADX47273 affected reconsolidation of fear memory, we changed the timing of ADX47273 administration to follow extinction training. Animals were again conditioned with three footshocks on Day 1 (Fig. 3Bi). From Day 2 to Day 8, we performed multi-session extinction experiments in which animals were briefly returned to the conditioning chamber for 6 min each day without footshocks. ADX47273 was administered immediately following each extinction session (Fig. 3Bii). Again we found that ADX47273-treated animals had similar freezing during the multi-session extinction training (two-way RM ANOVA, F(1,21) = 0.38, P > 0.05) (Fig. 3Bii), suggesting that ADX47273 did not affect reconsolidation of fear memory in this multi-session extinction paradigm. When animals were tested again (6-min exposure to the conditioning chamber) after 1 mo, the two groups showed similar levels of freezing again (t-test, t(21) = 0.24, P > 0.05) (Fig. 3Biii), indicating that long-term fear memory was not disrupted. Together, these results demonstrate that the mGluR5 PAM had no effect in a multi-session fear extinction paradigm when administered either before or after training in contextual fear extinction.
mGluR5 PAM during reactivation–reconsolidation window enhances extinction
Memories are stabilized after consolidation, but it has been proposed that memories might be vulnerable to disruption or editing when they are actively retrieved (Nader et al. 2000). Reactivating fear memory shortly before performing extinction training renders the memory labile. Intervention during this reconsolidation window can facilitate subsequent fear extinction in both rodents (Monfils et al. 2009; Clem and Huganir 2010) and humans (Kindt et al. 2009; Schiller et al. 2010). Based upon this, we reasoned that ADX47273 might be more effective in fear reduction when given during the retrieval–reconsolidation window. We first tested whether the insertion of a single retrieval trial by itself facilitated extinction in mice in our experiments. Mice were assigned into a retrieval group (n = 10) and a nonretrieval group (n = 10). On Day 1 of the experiment, animals were trained in a standard associative aversive task in which footshocks were paired with a tone (Fig. 4A). On Day 2, both groups were brought to in a novel context (extinction chamber) for a total of 6 min. A single tone presentation (180 sec) was given only to the retrieval group but not the nonretrieval group (Fig. 4B). A recent study using a reconsolidation-based protocol found that mice required more robust CS presentations to undergo fear extinction (Clem and Huganir 2010), and therefore in our experiments we elected to use a longer tone (180 sec) as a strong CS reminder to potentially produce a more robust behavioral extinction. Animals were immediately returned to their home cages after this single retrieval trial and 60 min later they were reintroduced to the extinction chamber where they underwent a single session, multi-trial extinction protocol. In order to match total time of tone exposure for the two groups, 12 tones (60 sec) were presented to the retrieval group and 15 tones were given to nonretrieval group (Fig. 4C). On Day 3, mice were returned to the extinction context where a single tone (180 sec) was presented (Fig. 4D). The two groups responded equally to the tone, suggesting there was no effect of the single retrieval trial by itself on extinction memory in this paradigm (two-way RM ANOVA, F(1,18) = 0.003, P > 0.05). On Day 4, mice were placed in the training context (context 1) to test for the renewal of conditioned fear after extinction training (Fig. 4E). To minimize contextual freezing, tone presentation was given 60 sec after introduction instead of 180 sec (Fig. 4E). Again we found no difference in the amount of freezing time between the retrieval group and nonretrieval group (two-way RM ANOVA, F(1,18) = 0.159, P > 0.05). Therefore, under our experimental conditions, a single retrieval trial did not enhance fear extinction in mice.
Figure 4.
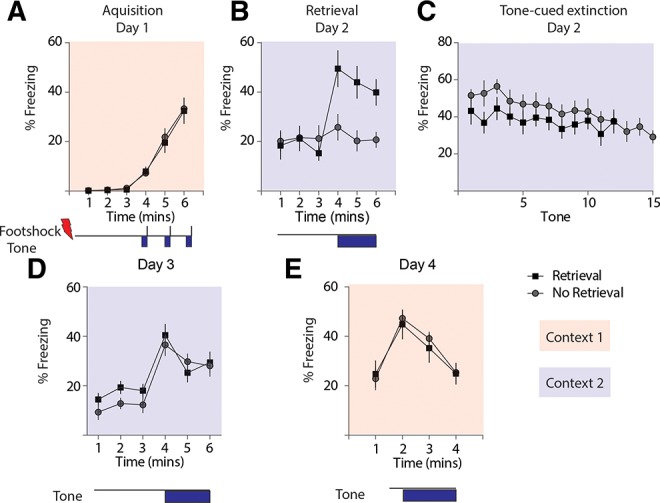
Single retrieval trial alone did not enhance fear extinction. (A) Freezing during tone-cued fear conditioning in Day 1. (B) Freezing in the extinction context. Single CS presentation (180 sec) was given only to the retrieval group but not to the nonretrieval group. (C) Within-session fear extinction after the single retrieval trial. The retrieval group received 12 1-min tones at 2-min intervals, and the nonretrieval group received 15 tones. (D) Fear extinction memory test for spontaneous recovery in the extinction context. Animals were given a single 3-min tone after 3 min of acclimation. (E) Fear renewal test conducted in the fear acquisition context. On Day 4, animals were returned to the fear acquisition chamber and received a single 3-min tone presentation. Retrieval group, n = 10; nonretrieval group, n = 10.
To test whether administration of ADX47273 during the retrieval–reconsolidation window had effects on fear extinction we repeated the training protocol just described. On Day 1, the two groups of animals (vehicle group, n = 20; ADX47273 group, n = 19) were conditioned with three tone–footshock pairings (Fig. 5Ai). On Day 2, the tone-cued memory was reactivated by a single tone presentation (180 sec) in a novel context (context 2, extinction chamber); both groups displayed similar freezing to the tone presentation (two-way RM ANOVA, F(1,37) = 0.41, P > 0.05) (Fig. 5Aii). Animals were then returned to the home cages after this single retrieval trial and 30–45 min later were administered ADX47274 (30 mg/mL, i.p.) or vehicle. Mice were allowed to remain in their home cages for an additional 15–30 min before being reintroduced to the extinction chamber where they underwent a single session extinction protocol (Fig. 5Aiii). ADX47274 did not enhance within-session extinction (two-way RM ANOVA, F(1,37) = 0.87, P > 0.05) and freezing in both groups declined at a similar rate over the extinction trials (two-way RM ANOVA F(14,518) = 0.84 for treatment–time interactions, P > 0.05) (Fig. 5Aiii). On Day 3, mice were returned to the extinction context and tested for their fear extinction memory. A single tone of 180 sec was presented. We found that the ADX47273-treated group froze to a much lesser degree to tone than vehicle-treated animals (two-way RM ANOVA, F(1,37) = 7.61, P < 0.01). There was no overall difference in basal levels of freezing prior to the tone presentation (two-way RM ANOVA, F(1,37) = 0.16, P > 0.05) (Fig. 5Avi). Therefore, ADX47273 facilitated fear extinction when administered shortly after retrieval. We tested a subgroup of these animals again after 30 d (without drug) for spontaneous fear recovery (n = 15 from ADX group, n = 16 from vehicle-treated group). Curiously, however, we found that the two groups froze equally before tone (two-way RM ANOVA, F(1,29) = 1.01, P > 0.05) and upon tone presentation (two-way RM ANOVA, F(1,29) = 1.09, P > 0.05) (Fig. 5Av). Thus, although mGluR5 PAM administration specifically during a retrieval–reconsolidation window enhances extinction memory 24 h after extinction, the treatment does not affect long-term spontaneous recovery of the fear response.
Figure 5.
ADX47273 administration coupled to a single retrieval trial enhances fear extinction. (Ai) Freezing during tone-cued fear conditioning in Day 1. (Aii) Tone-cued freezing upon a single CS presentation (180 sec) on Day 2 (retrieval). (Aiii) Within-session fear extinction after the single retrieval trial. The extinction protocol consisted of 15 1-min tones at 2-min intervals. (Aiv) Fear memory test in the extinction context on Day 4 (short-term spontaneous recovery). (Av) Fear memory test in the extinction context after 1 mo (long-term spontaneous recovery). (Ai–Aiv) Drug group, n = 19; vehicle group, n = 20. (Av) Drug group, n = 15; vehicle group, n = 16. (Bi–Biii) Freezing during tone-cued conditioning (Bi), retrieval trial (Bii), and extinction (Biii). (Biv) Renewal test conducted in the fear acquisition context (context 2,). Drug group, n = 7; vehicle group, n = 8. (#) Two-way RM ANOVA, P < 0.05, (##) P < 0.01, (*) post-hoc Bonferroni test, P < 0.05.
In a separate group of animals, we tested whether ADX47273 administered in conjunction with a single retrieval trial could affect fear renewal. After training, animals were exposed to a single retrieval trial and administered vehicle (n = 7) or ADX47273 (n = 8) and then underwent extinction as before. There was no difference between the two groups in fear acquisition in Day 1 (two-way RM ANOVA, F(1,13) = 0.14, P > 0.05) (Fig. 5Bi), and retrieval (two-way RM ANOVA, F(1,13) = 0.002, P > 0.05) (Fig. 5Bii) and extinction (two-way RM ANOVA, F(1,13) = 0.0196, P > 0.05) in Day 2 (Fig. 5Biii). On Day 3 after extinction, mice were placed into the training context (context 1) to test for the renewal of conditioned fear. The renewal test on Day 3 was 4 min in duration with a single tone presentation (180 sec) started at 60 sec (Fig. 5Biv). We found that the drug-treated group froze to a much lesser degree to tone than vehicle animals (two-way RM ANOVA, F(1,13) = 4.49, P = 0.05) (Fig. 5Biv). Taken together our results demonstrate that that ADX47273 administered during the retrieval–reconsolidation window suppresses both short-term spontaneous recovery and fear renewal.
ADX47273 enhances mGluR5-mediated long-term depression in the hippocampus
mGluR5 signaling has been demonstrated to play several roles in cellular plasticity and excitability. To determine the specificity of ADX47273, we examined how this mGluR5 PAM affected a well-studied form of hippocampal plasticity. Previously it has been demonstrated that paired-pulse low frequency stimulation (PP-LFS) induces a form of long-term depression (LTD) in the CA1 of the hippocampus that is independent of NMDA receptor activation, but dependent on mGluR5 activation (Kemp and Bashir 1997; Huber et al. 2001). We therefore made recordings of field excitatory postsynaptic potentials (fEPSPs) from hippocampal slices and examined PP-LFS LTD at Schaffer collateral-CA1 synapses. In slices from wild-type (mGluR5+/+) animals, PP-LFS induced a small but significant depression of the synaptic response. Thirty-five to 45 min after induction the fEPSP was significantly depressed compared to the baseline level (88.1 ± 5.96%, n = 7 slices from four mice) (Fig. 6A). In an interleaved experiment, PP-LFS-induced LTD was significantly enhanced when ADX47273 (10 μM) was present (68.4 ± 2.8%, n = 6 slices from four mice). Statistical analysis revealed a significant effect of ADX47273 (t-test, P < 0.05) (Fig. 6A). In recordings in slices from mGluR5 knockout mice (mGluR5−/−) PP-LFS did not produce an appreciable depression of the synaptic response (96.6 ± 7.17%, n = 7 slices from four mice) and, importantly, ADX47273 did not enhance LTD in mGluR5−/− slices (91.7 ± 2.75% n = 6 slices from four mice, P > 0.05, t-test) (Fig. 6B). This demonstrates both that mGluR5 is required for PP-LFS in the CA1 and that ADX47273 selectively enhances mGluR5 signaling.
Figure 6.
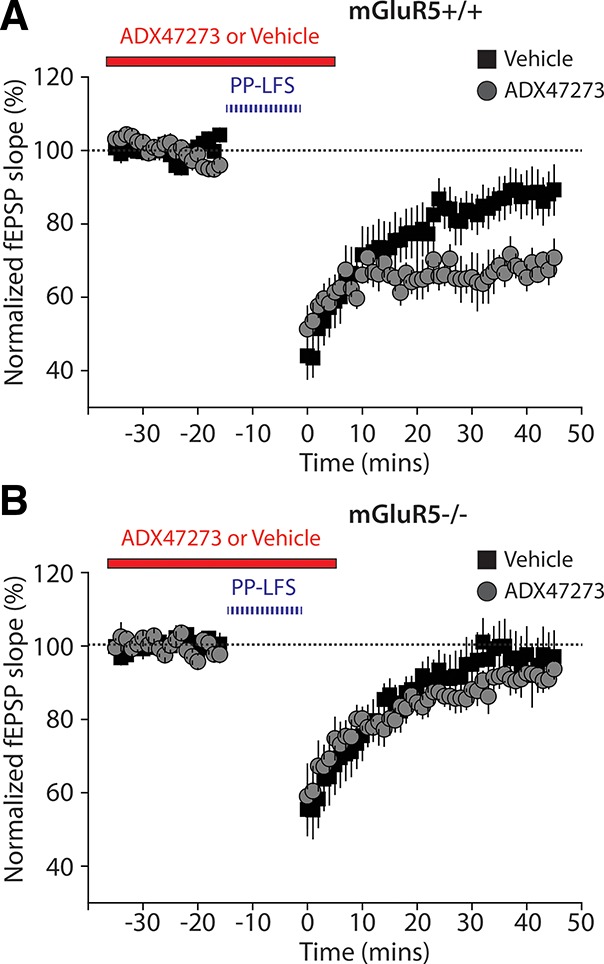
ADX47273 enhances PP-LFS induced LTD in the CA1 region of the hippocampus. (A) Normalized fEPSP slopes recorded in CA1 from slices from wild-type mice. Interleaved experiments were performed in the presence of vehicle or ADX47273. LTD was significantly enhanced in the presence of the mGluR5 PAM. The PP-LFS protocol consisted of 900 pairs of stimuli (40-msec inter-stimulus interval) delivered at 1 Hz for 15 min. (B) PP-LFS failed to induce LTD in slices taken from mGluR5−/− mice. ADX47273 had no effect on mGluR5−/− slices. PP-LFS protocol consists of 900 pairs of stimuli (40-msec inter-stimulus interval) delivered at 1 Hz for 15 min.
Discussion
Multiple mechanisms have been proposed to contribute to adaptive learning (Davis et al. 2006; Quirk et al. 2010). Genetic or pharmacological disruption of mGluR5 signaling causes deficits in fear extinction and reversal learning in the MWM, suggesting that mGluR5 activation is required for these two distinct forms of adaptive learning (Xu et al. 2009; Fontanez-Nuin et al. 2010). Here we demonstrate that enhancing mGluR5 signaling by administration of a selective mGluR5 PAM during a memory retrieval–reconsolidation window is particularly effective in facilitating adaptive learning that underlies fear extinction. Unexpectedly, ADX47273 had no effect when it was administered before or after extinction in a standard paradigm. One possibility to explain this is that the drug needs to be present during the consolidation or reconsolidation process and a single administration in the two standard paradigms is not effective during the memory consolidation process, which may occur many hours after the extinction training and after the drug has been metabolized (Schlumberger et al. 2009).
Previous in vitro studies have demonstrated that ADX47273 has no activity at any of the other mammalian mGluRs (Glu1-8) (Liu et al. 2008). In a previous study we demonstrated that ADX47273 can enhance DHPG-induced LTD in rat hippocampal slices (Ayala et al. 2009). To further demonstrate that ADX47273 has a selective effect on mGluR5 signaling, we examined PP-LFS induced LTD; an mGluR5-mediated form of plasticity in slices from wild-type mice LTD was enhanced by the mGluR5 PAM. But, as expected for a selective mGluR5 ligand, no effect was observed in slices from mGluR5−/− mice (Fig. 6). While further circuit analysis is required to demonstrate how mGluR5 signaling contributes to fear extinction, these results further confirm that ADX47273 is a useful tool for investigating the behavioral and cellular roles of mGluR5.
Memory processes are complex, but it is evident that memories are not formed as permanent structures but can be edited and modified in dynamic ways. Once information has been encoded and stored, it can be retrieved. Brief retrieval of recent memories triggers a reconsolidation process during which the memory becomes labile and susceptible to modifications (Nader et al. 2000; Sara 2000; Alberini 2005; Inda et al. 2011). This dynamic nature of the memory process suggests that intervention during periods of retrieval and reconsolidation can be used to alter maladaptive memory, and may represent a way to facilitate the extinction of unwanted memories such as traumatic fear. It has been reported that even administering a single retrieval trial shortly before fear extinction training can lead to persistent attenuation of fear memories in both rodents and human subjects (Monfils et al. 2009; Clem and Huganir 2010; Schiller et al. 2010). Exposure to a single retrieval trial with the subsequent ability to suppress renewal or spontaneous recovery of fear would have significant clinical implications for behavioral therapy regimes; however, these findings have not been universally accepted, with several recent studies failing to replicate the original findings (Chan et al. 2010; Costanzi et al. 2011; Ishii et al. 2012). In our control experiments we also examined whether an isolated retrieval trial before the extinction session could suppress spontaneous recovery (in the extinction context [Fig. 4D]) or renewal (in the fear context [Fig. 4E]). In each case we found no effect of the retrieval trial by itself but, interestingly, when ADX47273 was administered during the reactivation–reconsolidation window, reduced freezing was observed in subsequent tests for spontaneous recovery and renewal of fear (Fig. 5) suggesting that intervention with mGluR5 PAMs may be useful as an adjunct to increase the effectiveness of reconsolidation-based behavior therapies which are used to address maladaptive memory such as traumatic fear. Recently, the β-adrenergic receptor antagonist propranolol administered in combination with memory retrieval was found to have a similar effect (Przybyslawski et al. 1999; Brunet et al. 2008; Kindt et al. 2009; but also see Tollenaar et al. 2009; Muravieva and Alberini 2010). Given the clinical significance of reconsolidation-based behavioral therapy regimes for humans, finding adjuncts to facilitate the editing of memories will provide important tools for co-therapy. Our study provides evidence that mGluR5 PAMs may be one such drug class. It should be noted, however, that in these experiments we found that at a later timepoint of 1 mo post-extinction, normal spontaneous recovery was observed indicating that fear memory was not completely erased with our current experimental regime (Fig. 5Av). Future studies will be required to determine if more robust retrieval–extinction protocols with different drug regimens will produce more significant suppression of spontaneous recovery.
We also found that administration of ADX47273 enhanced reversal learning in the MWM. In this case mGluR5 PAM administered directly before the reversal training decreased the latency with which the animals relearned the position of the platform. This enhancement of adaptive learning in a very different context suggests that mGluR5 may have effects more generally on adaptive learning and behavioral flexibility. Recent studies using a different mGluR5 PAM, CDPPB, have demonstrated that enhancement of mGluR5 function increases extinction of conditioned place preference to cocaine (Gass and Olive 2009), or extinction of an operant self-administration task (Cleva et al. 2011). In this study, CDPPB enhanced extinction of cocaine self-administration when administered prior to, or after, each extinction session, suggesting that CDPPB may facilitate both the acquisition and consolidation of the extinction process (Cleva et al. 2011). In a second study by the same group, CDPPB did not affect extinction of methamphetamine-seeking behavior, suggesting that the effects of mGluR5 PAMs may not generalize to extinction of all drug-seeking behavior (Widholm et al. 2011). Differences in the efficacy of the drug in facilitating different types of learning behaviors may be due to the intrinsic properties of different types of memories, the properties of which might dictate the tolerance to behavioral and pharmacological modifications (Milner et al. 1998). Finally, in addition to extinction of fear and drug-related memory and spatial memories, mGluR5 PAMs have also been found to increase behavioral flexibility in animal models of schizophrenia (Stefani and Moghaddam 2010). Therefore, it appears that mGluR5 PAMs can enhance several forms of adaptive learning processes and there may be some shared general mechanisms between these different forms of memory.
The synaptic bases for adaptive learning mechanisms are not known; however, several cellular processes have been identified which correlate with fear extinction learning. Studies in the lateral amygdala have proposed that LTD and removal of surface AMPA receptors contribute to fear extinction (Lin et al. 2000, 2003; Clem and Huganir 2010; Kim et al. 2010). LTD in the amygdala is dependent on NMDA receptor activity and thus may be modulated by Group 1 mGluRs (Luscher and Huber 2010). Therefore, ADX47273 given during the retrieval–reconsolidation window may facilitate the removal of AMPA receptors from synapses in the lateral amygdala or hippocampus, both brain regions that are known to contribute to fear extinction memory. Another possibility is that ADX47273 may exert its actions in the infralimbic region of the medial prefrontal cortex, a region where mGluR5 activation has been proposed to contribute to fear extinction (Fontanez-Nuin et al. 2010; Sepulveda-Orengo et al. 2013). These possibilities are not mutually exclusive and it is possible that mGluR5 signaling in multiple brain regions contributes to fear extinction. Further studies are necessary to determine the neural circuits and the cellular basis for the involvement of mGluR5 in fear extinction.
Adaptive memory processes are required for many different forms of learning and maladaptive recruitment of these processes can contribute to diverse pathophysiologies. It is interesting to speculate that similar cellular processes are required for distinct forms of adaptive learning with an important role for mGluR5 signaling in each of these forms of memory. In sum, these results further demonstrate the involvement of mGluR5 signaling in adaptive learning, and suggest that mGluR5 PAMs may represent a viable strategy to improve the effectiveness of extinction-based behavioral therapy for anxiety disorders, the treatment of drug addiction, and for improving behavioral flexibility.
Materials and Methods
Animals
Animals used in all behavioral experiments were male C57/bl6 wild-type mice at the age of 2–4 months. Animals were either directly acquired from Jackson Laboratory or generated by breeding pairs established at Northwestern University. For electrophysiology experiments, mGluR5−/− mice and littermates (mGluR5+/+) were used (Xu et al. 2009). The mutant strain has been backcrossed to C57/bl6 wild-type mice for at least six generations. Animals were group housed in a room with 14-h light/10-h dark cycle. Food and water were provided ad libitum. All experiments were approved by the Institutional Animal Care and Use Committees at Northwestern University.
Materials
ADX47273 was custom synthesized as previously described (Ayala et al. 2009; Engers et al. 2009) and was dissolved in a vehicle consisting of 45% w/v 2-hydroxypropyl-β-cyclodextrin (Sigma-Aldrich) by sonication. Drug and vehicle were administrated intraperitoneally in all experiments.
Fear conditioning
Fear conditioning cages (30 cm L × 30 cm W × 25 cm H) were constructed of clear Plexiglas. The floors of the cages were equipped with stainless steel rods designed for shock delivery to mice. The chambers were cleaned with 70% ethanol before and after use. An automated video tracking system (Limelight) was used to monitor mice in the fear conditioning paradigm. Contextual fear conditioning training consisted of 3 min of baseline monitoring followed by three footshocks (0.7 mA, 1 sec) given at 1-min intervals. For tone-cued fear conditioning training, animals received three pairs of 20-sec tone (85 dB, 2900 Hz) co-terminated with 1-sec footshock (0.7 mA) given at 1-min intervals. Contextual fear extinction and fear memory tests were conducted in the same conditioning chamber and with the same visual cues. Tone-cued fear extinction and fear memory tests were conducted in a novel context that had a different cage floor (white plastic), altered shape (circular), changed size (25 cm in diameter), altered wall pattern, and a novel scent (Clidox) wiped on the walls. All experiments were conducted in sound-attenuated chambers.
Morris Water Maze
The Morris Water Maze experiments were conducted as previously described, with some modification (Xu et al. 2009). A water tank (120-cm diameter) was filled with water at room temperature. The water was made opaque with white nontoxic Crayola washable paint. A transparent platform (8 × 13 cm) was submerged 1 cm below the surface of opaque water. An automated video tracking system was used to record the swim path, velocity, and time taken to reach the platform (latency) or the time spent in each zone. Mice were first trained to find the visible platform for three consecutive days (three trials per day, Days 1–3). Mice that failed to find the platform within 60 sec were placed onto the platform. Mice were allowed to remain on the platform for 15 sec after each trial. Hidden platform training was conducted 1 d after the completion of the visible platform training. Mice were trained for 7 d (three trials per day, Days 4–10) to find the submerged platform at a fixed position (center of NE quadrant) with the aid of distal cues in the testing room. For reverse platform training the hidden platform was moved from the NE quadrant to the SW quadrant without changing the distal visual cues. Mice were trained to find this new platform location for 2 d (Days 11 and 12, three trails on Day 11 and four trials on Day 12). After this first round of reverse training, the platform was again moved to the opposite quadrant (NE) and mice were allowed seven trials for 2 d to relearn the new location (Days 13 and 14).
Electrophysiology
Animals were anaesthetized and rapid cardiac perfusion was performed with ice-cold sucrose artificial cerebrospinal fluid (ACSF) solution containing (in mM) 85 NaCl, 2.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 25 glucose, 75 sucrose, 0.5 CaCl2, and 4 MgCl2, equilibrated with 95% O2 and 5% CO2 before decapitation and removal of the brain. Transverse hippocampal slices were made from the ventral hippocampus and transferred to a holding chamber, initially at 26°C, for 1 h; during this time the slicing solution was exchanged for a normal ACSF solution containing (in mM) 125 NaCl, 2.4 KCl, 1.2 Na2PO4, 25 NaHCO3, 25 glucose, 1 CaCl2, and 2 MgCl2, and gradually allowed to return to room temperature. Individual slices were transferred to a recording chamber and visualized under DIC optics. For field recordings electrodes were fabricated from borosilicate glass at a resistance of 3–5 MΩ and filled with regular ACSF. EPSPs were evoked using a glass electrode placed in the stratum radiatum to stimulate Schaffer collateral inputs to CA1. Data were collected and analyzed using pClamp 9 software (Axon instruments). The PP-LFS LTD induction protocol consisted of 900 pairs of stimuli (40 msec inter-stimulus interval) delivered at 1 Hz for 15 min. Sampled data were analyzed offline using Clampfit 9.2. All fEPSP slopes were normalized to the average slope calculated during 20 min of baseline prior to PP-LFS.
Statistical analyses were conducted with GraphPad Prism. For the multiple trial experiments, two-way repeated-measures ANOVA (RM ANOVA) were conducted to assess the effects of both drug treatment and sessions/trials. Post-hoc Bonferroni pairwise comparisons were performed when significant effects were found by two-way ANOVA. Differences between two means were assessed with t-tests. Data are presented as mean ± SEM. Differences were considered significant when P < 0.05.
Acknowledgments
This work was supported by NIH/NINDS (R01NS058894 to A.C.), NIH/NIMH (R01MH099114 to A.C.), NIH/NIMH (K01MH094464 to J.X.), and a McKnight Foundation Memory and Cognitive Disorders Award (A.C.). We thank Dr. Jelena Radulovic for helpful comments.
References
- Alberini CM 2005. Mechanisms of memory stabilization: Are consolidation and reconsolidation similar or distinct processes? Trends Neurosci 28: 51–56 [DOI] [PubMed] [Google Scholar]
- Ayala JE, Chen Y, Banko JL, Sheffler DJ, Williams R, Telk AN, Watson NL, Xiang Z, Zhang Y, Jones PJ, et al. 2009. mGluR5 positive allosteric modulators facilitate both hippocampal LTP and LTD and enhance spatial learning. Neuropsychopharmacology 34: 2057–2071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balschun D, Zuschratter W, Wetzel W 2006. Allosteric enhancement of metabotropic glutamate receptor 5 function promotes spatial memory. Neuroscience 142: 691–702 [DOI] [PubMed] [Google Scholar]
- Brunet A, Orr SP, Tremblay J, Robertson K, Nader K, Pitman RK 2008. Effect of post-retrieval propranolol on psychophysiologic responding during subsequent script-driven traumatic imagery in post-traumatic stress disorder. J Psychiatr Res 42: 503–506 [DOI] [PubMed] [Google Scholar]
- Carroll FI 2008. Antagonists at metabotropic glutamate receptor subtype 5: Structure activity relationships and therapeutic potential for addiction. Ann N Y Acad Sci 1141: 221–232 [DOI] [PubMed] [Google Scholar]
- Chan WY, Leung HT, Westbrook RF, McNally GP 2010. Effects of recent exposure to a conditioned stimulus on extinction of Pavlovian fear conditioning. Learn Mem 17: 512–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clem RL, Huganir RL 2010. Calcium-permeable AMPA receptor dynamics mediate fear memory erasure. Science 330: 1108–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleva RM, Hicks MP, Gass JT, Wischerath KC, Plasters ET, Widholm JJ, Olive MF 2011. mGluR5 positive allosteric modulation enhances extinction learning following cocaine self-administration. Behav Neurosci 125: 10–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, Pin JP 1997. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol 37: 205–237 [DOI] [PubMed] [Google Scholar]
- Conn PJ, Lindsley CW, Jones CK 2009. Activation of metabotropic glutamate receptors as a novel approach for the treatment of schizophrenia. Trends Pharmacol Sci 30: 25–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzi M, Cannas S, Saraulli D, Rossi-Arnaud C, Cestari V 2011. Extinction after retrieval: Effects on the associative and nonassociative components of remote contextual fear memory. Learn Mem 18: 508–518 [DOI] [PubMed] [Google Scholar]
- Davis M, Myers KM, Chhatwal J, Ressler KJ 2006. Pharmacological treatments that facilitate extinction of fear: Relevance to psychotherapy. NeuroRx 3: 82–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolen G, Bear MF 2008. Role for metabotropic glutamate receptor 5 (mGluR5) in the pathogenesis of fragile X syndrome. J Physiol 586: 1503–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy S, Labrie V, Roder JC 2008. d-Serine augments NMDA-NR2B receptor-dependent hippocampal long-term depression and spatial reversal learning. Neuropsychopharmacology 33: 1004–1018 [DOI] [PubMed] [Google Scholar]
- Engers DW, Rodriguez AL, Williams R, Hammond AS, Venable D, Oluwatola O, Sulikowski GA, Conn PJ, Lindsley CW 2009. Synthesis, SAR and unanticipated pharmacological profiles of analogues of the mGluR5 ago-potentiator ADX-47273. ChemMedChem 4: 505–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontanez-Nuin DE, Santini E, Quirk GJ, Porter JT 2010. Memory for fear extinction requires mGluR5-mediated activation of infralimbic neurons. Cereb Cortex 21: 727–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler SW, Walker JM, Klakotskaia D, Will MJ, Serfozo P, Simonyi A, Schachtman TR 2013. Effects of a metabotropic glutamate receptor 5 positive allosteric modulator, CDPPB, on spatial learning task performance in rodents. Neurobiol Learn Mem 99: 27–31 [DOI] [PubMed] [Google Scholar]
- Gass JT, Olive MF 2009. Positive allosteric modulation of mGluR5 receptors facilitates extinction of a cocaine contextual memory. Biol Psychiatry 65: 717–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham BM, Langton JM, Richardson R 2011. Pharmacological enhancement of fear reduction: Preclinical models. Br J Pharmacol 164: 1230–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber KM, Roder JC, Bear MF 2001. Chemical induction of mGluR5- and protein synthesis-dependent long-term depression in hippocampal area CA1. J Neurophysiol 86: 321–325 [DOI] [PubMed] [Google Scholar]
- Inda MC, Muravieva EV, Alberini CM 2011. Memory retrieval and the passage of time: From reconsolidation and strengthening to extinction. J Neurosci 31: 1635–1643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii D, Matsuzawa D, Matsuda S, Tomizawa H, Sutoh C, Shimizu E 2012. No erasure effect of retrieval-extinction trial on fear memory in the hippocampus-independent and dependent paradigms. Neurosci Lett 523: 76–81 [DOI] [PubMed] [Google Scholar]
- Johnson KA, Conn PJ, Niswender CM 2009. Glutamate receptors as therapeutic targets for Parkinson’s disease. CNS Neurol Disord Drug Targets 8: 475–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp N, Bashir ZI 1997. NMDA receptor-dependent and -independent long-term depression in the CA1 region of the adult rat hippocampus in vitro. Neuropharmacology 36: 397–399 [DOI] [PubMed] [Google Scholar]
- Kim J, Song B, Hong I, Kim J, Lee J, Park S, Eom JY, Lee CJ, Lee S, Choi S 2010. Reactivation of fear memory renders consolidated amygdala synapses labile. J Neurosci 30: 9631–9640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindt M, Soeter M, Vervliet B 2009. Beyond extinction: Erasing human fear responses and preventing the return of fear. Nat Neurosci 12: 256–258 [DOI] [PubMed] [Google Scholar]
- Lin HC, Wang SJ, Luo MZ, Gean PW 2000. Activation of group II metabotropic glutamate receptors induces long-term depression of synaptic transmission in the rat amygdala. J Neurosci 20: 9017–9024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Lee CC, Gean PW 2003. Involvement of a calcineurin cascade in amygdala depotentiation and quenching of fear memory. Mol Pharmacol 63: 44–52 [DOI] [PubMed] [Google Scholar]
- Liu F, Grauer S, Kelley C, Navarra R, Graf R, Zhang G, Atkinson PJ, Popiolek M, Wantuch C, Khawaja X, et al. 2008. ADX47273 [S-(4-fluoro-phenyl)-{3-[3-(4-fluoro-phenyl)-[1,2,4]-oxadiazol-5-yl]-piper idin-1-yl}-methanone]: A novel metabotropic glutamate receptor 5-selective positive allosteric modulator with preclinical antipsychotic-like and procognitive activities. J Pharmacol Exp Ther 327: 827–839 [DOI] [PubMed] [Google Scholar]
- Luscher C, Huber KM 2010. Group 1 mGluR-dependent synaptic long-term depression: Mechanisms and implications for circuitry and disease. Neuron 65: 445–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgansberger W, et al. 2002. The endogenous cannabinoid system controls extinction of aversive memories. Nature 418: 530–534 [DOI] [PubMed] [Google Scholar]
- Milner B, Squire LR, Kandel ER 1998. Cognitive neuroscience and the study of memory. Neuron 20: 445–468 [DOI] [PubMed] [Google Scholar]
- Monfils MH, Cowansage KK, Klann E, LeDoux JE 2009. Extinction–reconsolidation boundaries: Key to persistent attenuation of fear memories. Science 324: 951–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muravieva EV, Alberini CM 2010. Limited efficacy of propranolol on the reconsolidation of fear memories. Learn Mem 17: 306–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Carlezon WA Jr, Davis M 2011. Glutamate receptors in extinction and extinction-based therapies for psychiatric illness. Neuropsychopharmacology 36: 910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader K, Schafe GE, LeDoux JE 2000. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature 406: 722–726 [DOI] [PubMed] [Google Scholar]
- Park S, Lee S, Kim J, Choi S 2012. Ex vivo depotentiation of conditioning-induced potentiation at thalamic input synapses onto the lateral amygdala requires GluN2B-containing NMDA receptors. Neurosci Lett 530: 121–126 [DOI] [PubMed] [Google Scholar]
- Przybyslawski J, Roullet P, Sara SJ 1999. Attenuation of emotional and nonemotional memories after their reactivation: Role of β adrenergic receptors. J Neurosci 19: 6623–6628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Pare D, Richardson R, Herry C, Monfils MH, Schiller D, Vicentic A 2010. Erasing fear memories with extinction training. J Neurosci 30: 14993–14997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Phelps EA 2006. Neurocircuitry models of posttraumatic stress disorder and extinction: Human neuroimaging research—past, present, and future. Biol Psychiatry 60: 376–382 [DOI] [PubMed] [Google Scholar]
- Sara SJ 2000. Retrieval and reconsolidation: Toward a neurobiology of remembering. Learn Mem 7: 73–84 [DOI] [PubMed] [Google Scholar]
- Schiller D, Monfils MH, Raio CM, Johnson DC, Ledoux JE, Phelps EA 2010. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature 463: 49–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlumberger C, Pietraszek M, Gravius A, Klein KU, Greco S, More L, Danysz W 2009. Comparison of the mGlu(5) receptor positive allosteric modulator ADX47273 and the mGlu(2/3) receptor agonist LY354740 in tests for antipsychotic-like activity. Eur J Pharmacol 623: 73–83 [DOI] [PubMed] [Google Scholar]
- Sepulveda-Orengo MT, Lopez AV, Soler-Cedeno O, Porter JT 2013. Fear extinction induces mGluR5-mediated synaptic and intrinsic plasticity in infralimbic neurons. J Neurosci 33: 7184–7193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani MR, Moghaddam B 2010. Activation of type 5 metabotropic glutamate receptors attenuates deficits in cognitive flexibility induced by NMDA receptor blockade. Eur J Pharmacol 639: 26–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollenaar MS, Elzinga BM, Spinhoven P, Everaerd W 2009. Psychophysiological responding to emotional memories in healthy young men after cortisol and propranolol administration. Psychopharmacology 203: 793–803 [DOI] [PubMed] [Google Scholar]
- Varvel SA, Lichtman AH 2002. Evaluation of CB1 receptor knockout mice in the Morris water maze. J Pharmacol Exp Ther 301: 915–924 [DOI] [PubMed] [Google Scholar]
- Widholm JJ, Gass JT, Cleva RM, Olive MF 2011. The mGluR5 positive allosteric modulator CDPPB does not alter extinction or contextual reinstatement of methamphetamine-seeking behavior in rats. J Addict Res Ther S1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Zhu Y, Contractor A, Heinemann SF 2009. mGluR5 has a critical role in inhibitory learning. J Neurosci 29: 3676–3684 [DOI] [PMC free article] [PubMed] [Google Scholar]