Abstract
Background
Process-based ecophysiological crop models are pivotal in assessing responses of crop productivity and designing strategies of adaptation to climate change. Most existing crop models generally over-estimate the effect of elevated atmospheric [CO2], despite decades of experimental research on crop growth response to [CO2].
Analysis
A review of the literature indicates that the quantitative relationships for a number of traits, once expressed as a function of internal plant nitrogen status, are altered little by the elevated [CO2]. A model incorporating these nitrogen-based functional relationships and mechanisms simulated photosynthetic acclimation to elevated [CO2], thereby reducing the chance of over-estimating crop response to [CO2]. Robust crop models to have small parameterization requirements and yet generate phenotypic plasticity under changing environmental conditions need to capture the carbon–nitrogen interactions during crop growth.
Conclusions
The performance of the improved models depends little on the type of the experimental facilities used to obtain data for parameterization, and allows accurate projections of the impact of elevated [CO2] and other climatic variables on crop productivity.
Keywords: Acclimation to elevated CO2, climate change, crop models, impact assessment, model improvement, nitrogen
INTRODUCTION
World agriculture faces daunting challenges to meet growing demands for food, energy and other agricultural products. Crop production is, however, strongly affected by climate change. Lobell et al. (2011) showed that climate trends since 1980 were large enough in many countries to offset a significant proportion of the potential increases in average crop yields due to technological advances, CO2 fertilization and other factors. By 2100, potentially, atmospheric [CO2] will rise to 1000 µmol mol−1, temperature will rise by 2–4 °C or more, precipitation will become more variable, and episodes of extreme weather will become more frequent and intense and last longer (IPCC, 2007). It is important to assess whether there will be sufficient food and energy production under future climate conditions. Such an assessment can also assist in developing adaptation strategies that improve the resilience of crop systems to stresses induced by climate change.
Numerous studies have assessed the impact of climate change on productivity of major crops. Whilst simple regression analysis can detect a non-linear response of crop yields to warm climate (Schlenker and Roberts, 2009; Lobell et al., 2011), process-based crop simulation models (hereafter ‘crop models’) combined with climate scenario models are considered necessary to assess the impact of climate change on crop production (Porter et al., 1995; Hulme et al., 1999; White et al., 2004; Challinor et al., 2009; Semenov and Halford, 2009; Soussana et al., 2010). Early assessments at the global level (e.g. Rosenzweig and Parry, 1994) often used simple crop models based on empirical experimental data to define impacts of elevated [CO2] and other factors on crop processes. More mechanistic ecophysiological models, although not necessarily defined originally for climate change impact assessment, are increasingly used (e.g. Tubiello and Ewert, 2002).
Research on mechanistic crop models, according to Tardieu (2010), has a history of approx. 50 years since the earliest models such as developed by de Wit (1959). These models predict crop productivities based on quantitative functional relationships for underlying processes (photosynthesis, respiration, transpiration, assimilate partitioning, etc.) and their response to environmental variables. Thus, they are believed to be suitable for projecting the impact of future climate scenarios on crop productivity at various (field, regional, national, global) scales (Challinor et al., 2009).
Although crop models have continuously been refined (Weiss, 2003; Priesack and Gayler, 2009), knowledge gaps limit the ability of current crop models to reflect responses to global change factors (White et al., 2004). Many reports emphasize the need to review critically and improve crop models for assessments of climate change impacts (e.g. Lawlor and Mitchell, 1991; Tubiello et al., 2007a; Ziska and Bunce, 2007; Challinor et al., 2009; Soussana et al., 2010). Rötter et al. (2011) indicated that many of the current models used for estimating potential impacts of climate change do not incorporate the latest knowledge about how crops respond to changing climates and management practices. Yet, reports dedicated towards how to improve crop models for climate impact assessment are rare.
The objective of this paper is to outline how models for assessing the impact of elevated [CO2] can be improved. The current status in using crop models for assessing the impact of elevated [CO2] is briefly reviewed. I then analyse whether there are differences in quantitative functional relationships for a number of traits between plants grown under the elevated CO2 and those grown under ambient conditions. Key issues for modelling crop responses to elevated [CO2] will be discussed.
PERFORMANCE OF EXISTING CROP MODELS
Models for climate change impact assessment need to capture responses of crop growth to all major environmental variables. Much research has focused on crop responses to elevated atmospheric [CO2] (Long et al., 2004), using various experimental facilities [such as growth chamber, temperature-gradient tunnel, open-top chamber and free-air CO2 enrichment (FACE) technology]. As a result, the impact of elevated [CO2] has been a constant focus in modelling. One of the first studies that assessed climate impacts on crop production was conducted by Rosenzweig and Parry (1994). The physiological effects of [CO2] on crop growth were considered to be mediated through increased rates of net photosynthesis and reduced stomatal openings as reported from early experimental results in enclosure chambers. The ratio of photosynthetic rates at 555 µmol mol−1 to that at 330 µmol mol−1 CO2 for soybean (Glycine max), wheat (Triticum aestivum), rice (Oryza sativa) and maize (Zea mays) were 1·21, 1·17, 1·17 and 1·06, respectively. Stomatal resistance was assumed to increase from 34·4 to 49·7 s m−1 in C3 crops and from 55·8 to 87·4 s m−1 in C4 crops with [CO2] increase from 330 to 555 µmol mol−1. More recently, Tubiello and Fischer (2007) even used the ultra-simple model AEZ to simulate crop response to elevated [CO2], i.e. as a multiplier of the harvest yield obtained under current [CO2]. The multiplier was derived from experiments under controlled conditions, which indicated a 25 % increase in yield of C3 crops (such as wheat, rice and soybean), and a 10 % increase in the yield of C4 crops (such as maize and sugarcane) for a doubling of the current atmospheric [CO2]. Empirical approaches to include impacts of elevated [CO2] have also been used in other crop models, e.g. CERES (Tubiello et al., 1999).
Tubiello et al. (2007a) reported that some models used in impact assessment have not been evaluated against FACE data. Where this has been carried out, Tubiello and Ewert (2002) found that five widely used crop models reproduced well the effects of elevated [CO2] on wheat in the Maricopa FACE experiment of Kimball et al. (1995). However, this statement was challenged by Long et al. (2005, 2006) and Ainsworth et al. (2008a), who re-analysed modelled CO2 enhancement ratio compared with experimental response ratio (Fig. 1). They concluded that current crop models over-estimated the CO2 fertilization effect both under well-watered conditions and under drought-stress conditions. CO2 enhancement ratios from similar experimental set-ups were also over-predicted by other models, e.g. APSIM (Asseng et al., 2004) and SPASS (Biernath et al., 2011).
Fig. 1.
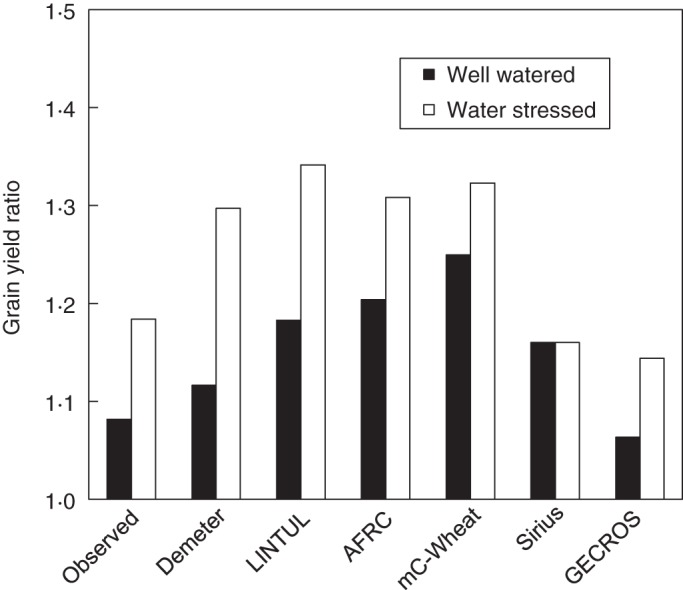
Observed stimulation of grain yields of well-watered and water-stressed wheat crops grown under ambient CO2 (370 µmol mol−1) and elevated CO2 (550 µmol mol−1) for the free-air CO2 enrichment (FACE) experiment conducted in Maricopa, Arizona, USA (see Kimball et al., 1995). The stimulation ratio was expressed as the average ratio of grain yield under elevated CO2 to the yield under ambient CO2 for the two growing seasons 1992–1993 and 1993–1994. The modelled stimulation ratio from five crop models (Demeter, LINTUL, AFRC, mC-wheat, Sirius) previously evaluated by Tubiello and Ewert (2002) and that from model GECROS evaluated by Yin and Struik (2010) were also given. The observed stimulation effects was not over-predicted by GECROS. Other models tended to over-estimate the effects, largely leading Long et al. (2006), Ainsworth et al. (2008a) and Leakey et al. (2009) to conclude that model parameterization based on chamber experiments is inappropriate to project crop response to elevated CO2 under field conditions. Reproduced from Yin and Struik (2010) with permission.
The observed CO2 enhancement ratio on yield and other plant traits is generally lower in FACE than in enclosed chambers (e.g. de Graaff et al., 2006; Ainsworth et al., 2008a). In my opinion, this may be explained by the difference in the scale of experiments (typically plots of >300 m2 in FACE vs. <4 m2 in enclosure studies). Because of reduced chance of mutual shading, leaves in enclosures tend to receive higher light; under high-light conditions photosynthesis is more likely limited by Rubisco capacity than by electron transport. Rubsico-limited photosynthetic rates are stimulated more by elevated [CO2] than electron transport-limited rates (Kirschbaum, 2011). The increased photosynthetic rates can result in higher leaf area that will further lead to more light interception and biomass production. However, there have also been doubts about whether the lower enhancement values in FACE reflect a flaw of the FACE technology. For example, in the experiment of Kimball et al. (1995) control plots lacked the blowers that were installed in the elevated [CO2] plots. Such a difference may have resulted in a relatively lower measured response to elevated [CO2] than if both treatments were similarly equipped (Kimball, 2013). Furthermore, Holtum and Winter (2003) and Bunce (2012) showed that frequent fluctuations in [CO2], as commonly occurred in FACE, may diminish the response of leaf photosynthesis to elevated [CO2]. One can predict from the convex nature of the photosynthetic [CO2] response curve that fluctuations in [CO2] can lead the FACE systems to an underestimation of steady-state photosynthetic rates at projected future [CO2] concentrations. If fluctuations are highly irregular and variable, steady-state photosynthesis may not be achieved and the impact becomes complicated to predict quantitatively. Ainsworth and Long (2004), however, argued that [CO2] fluctuations seem an unlikely explanation of the lower stimulation in the FACE experiments. First, there was no evidence for the difference in photosynthetic electron transport rate between constant and fluctuating elevated [CO2] in wheat for oscillations of a half-cycle of 30 s or less, which would include most of the fluctuations observed in the FACE systems (Hendrey et al., 1997). Second, fluctuations in [CO2] are also observed in open-top chambers, albeit to a lesser extent.
Long et al. (2004, 2005, 2006) and Ainsworth et al. (2008a) therefore expressed concern that there may be some quantitative differences in how crops respond to elevated [CO2] in FACE and chamber experiments. They suggested that controlled chamber environments were not the best experimental facilities to parameterize crop models for estimating CO2 response ratio of crop yield, as FACE has revealed factors in field conditions that were not identified by chamber experiments (e.g. increased herbivory). The need for larger-scale FACE experiments to determine how inter- and intraspecific variations in crop yield are affected by [CO2] in combination with other aspects of climate change was collectively proposed by a large group of scientists (Ainsworth et al., 2008b). I will examine this controversial issue later.
There have been debates (e.g. Tubiello et al., 2007b) on the statement of Long et al. (2005, 2006) and Ainsworth et al. (2008a) about quantitative differences in crop response to elevated [CO2] between FACE and chamber experiments. Ziska and Bunce (2007) analysed a large set of compiled data and found little evidence that relative increases of crop yield in response to future [CO2] obtained using a number of enclosure methodologies are quantitatively different from those with FACE results for rice, wheat and soybean. They suggested that instead of focusing on methodological disparities per se, improved projection of the impact of future climate could be achieved by better characterization of other biotic/abiotic uncertainties associated with projected changes in [CO2] and incorporation of these uncertainties into crop models. For example, they highlighted the result of Matsui et al. (1997) that at air temperatures above 30 °C, the percentage of filled rice spikelets under elevated [CO2] was lower than that under ambient [CO2], a trend that is opposite to the positive interaction between [CO2] and temperature on leaf photosynthetic rates (Long, 1991). Such a response of spikelet fertility could be explained, at least in part, by the reduction in transpirational cooling, higher panicle temperature and thus increased pollen sterility under elevated [CO2] conditions.
Obviously, the arguments of Ziska and Bunce (2007) were proposed from different perspectives from those of Long et al. (2005, 2006) and Ainsworth et al. (2008a). According to Long et al. (2006) and Ainsworth et al. (2008a), most existing crop models are unable to accurately predict the impact of elevated [CO2] on crop growth and yield, especially considering the interaction of [CO2] with other climatic factors, unless substantial calibrations of model parameter values are made. One possibility is that major crop models were developed mainly from ambient [CO2] conditions; when used to assess the impact of climate change, only some parameters were modified from elevated-[CO2] experiments (Soussana et al., 2010). This raises a question whether elevated [CO2] alters quantitative functional relationships for plant growth used in crop models.
DOES ELEVATED [CO2] CHANGE FUNCTIONAL RELATIONSHIPS OF PLANT GROWTH?
This question will be discussed by analysing several physiological processes or traits.
Leaf photosynthesis
Crop models quantify processes related to crop carbon (C) or biomass accumulation in order to predict final seed yield. Many crop models use a simple approach relating daily biomass increase as a function of daily intercepted solar radiation multiplied by radiation use efficiency (RUE) (e.g. Asseng et al., 2004; Ko et al., 2010; also see review of White et al., 2011). Other models quantify specifically crop photosynthesis and respiration. To calculate photosynthesis, often either an empirical light response equation (e.g. Matthews et al., 1997; Tubiello et al., 1999) or a mechanistic biochemical model (Grant, 2001; Rodriguez et al., 2001) is first used to estimate leaf photosynthesis rate, which is then scaled up to the canopy level.
It has been shown theoretically (van Oijen et al., 2004) and experimentally (e.g. van Oijen et al., 1999; Sakai et al., 2006) that RUE varies with [CO2] concentration. Any RUE-based crop models need to adjust RUE empirically to varying [CO2] (e.g. Asseng et al., 2004; Ko et al., 2010). In the crop models where leaf photosynthesis is modelled using an empirical light-response equation, an underlying parameter – photosynthetic rate under saturated light (Pmax) – was modified to depend on the [CO2] concentration (Matthews et al., 1997; Tubiello et al. 1999), whereas the other underlying parameter – initial light-use efficiency (ɛ) – was assumed to vary (Matthews et al., 1997) or not to vary (Tubiello et al., 1999) with the [CO2] concentration. Experimental measurements (e.g. Ehleringer and Björkman, 1977) have long shown that under the ambient O2 conditions, ɛ does vary with [CO2] in C3 plants. Even the parameters Vcmax (maximum carboxylation rate of Rubsico), Jmax (maximum electron transport rate under saturated lights) and TPU (potential rate of triose phosphate utilization) of a biochemical leaf-photosynthesis model proposed by Farquhar et al. (1980) and extended by Sharkey (1985) were found to decrease with increasing growth [CO2] concentrations in cotton (Gossypium hirsutum) plants (Harley et al., 1992). All these indicate that elevated [CO2] will result in changes in parameter values or functional relationships of these traits used in crop models.
Harley et al. (1992), however, also showed that photosynthetic parameters Vcmax, Jmax and TPU correlate linearly with leaf nitrogen (N) content, and the relationships between these parameters and leaf N varied little with growth [CO2] in cotton (Fig. 2). Similar linear relationships between Vcmax or Jmax and leaf N across contrasting [CO2] were widely reported, for example in ryegrass (Lolium perenne) (Nijs et al., 1995) and in rice (Nakano et al., 1997), or across diverse species (Ellsworth et al., 2004). A small deviation of the Vcmax–leaf N relationship under elevated [CO2] from that under the ambient [CO2] (Fig. 2) could be explained by elevated [CO2]-induced decrease in the investment of leaf N in Rubisco (Sage et al., 1989; Leakey et al., 2009); however, the significance of the deviation was only marginal (Harley et al., 1992). This indicates that the aforementioned changes in these parameters with [CO2] were predominantly due to elevated [CO2]-induced decrease in leaf N. An eventual decline in leaf N after plants grow for some period of time under elevated [CO2] is commonly observed (e.g. Wong, 1990; Conroy and Hocking, 1993; Luo et al., 1998; Dijkstra et al., 1999; van Oijen et al., 1999; Kim et al., 2003; Sakai et al., 2006; Zhu et al., 2009). The linear relationships between Vcmax (or Jmax, TPU) and leaf N assume that leaf photosynthetic N is linearly related to total N, which may generally be the case (e.g. Hikosaka and Terashima, 1995). Macro-scale photosynthesis parameters (e.g. RUE, Pmax, ɛ), however, even being related to leaf N, are still affected by [CO2]. Moreover, their relationship with N is often non-linear. For example, Sakai et al. (2006) showed the quadratic relationship between RUE and N in rice, with higher RUE under elevated than ambient [CO2] conditions. Similarly, a hyperbolic relationship between Pmax and N was shown by Hirose et al. (1997) for Abutilon theophrasti, with higher Pmax under elevated [CO2] conditions.
Fig. 2.
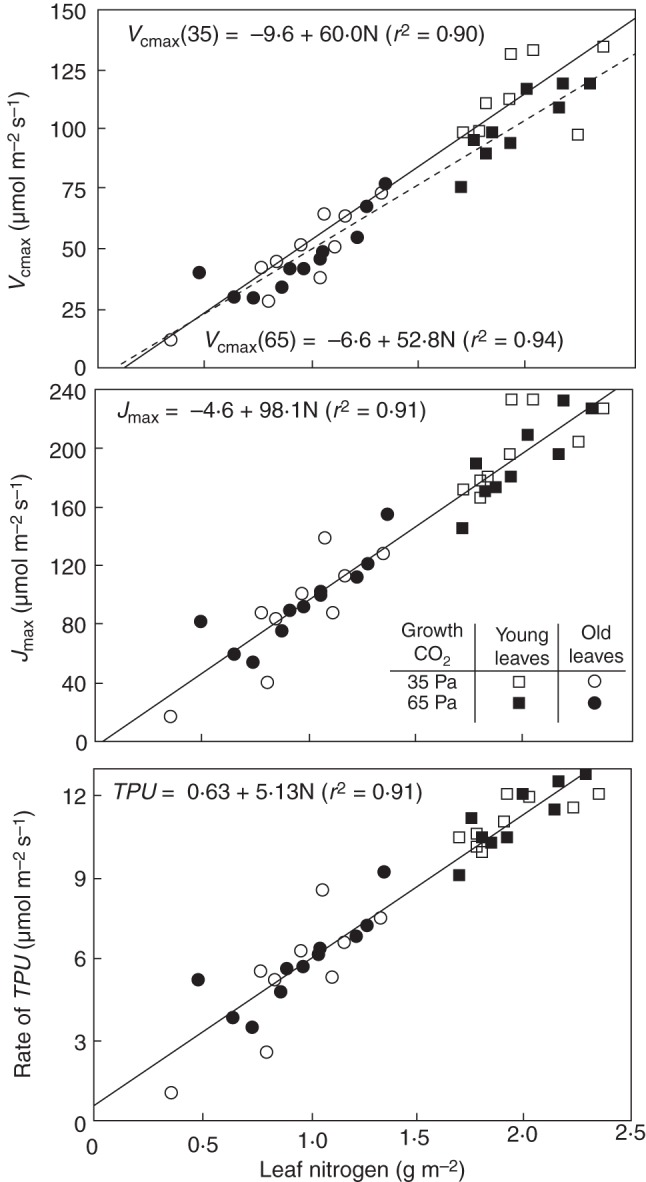
Estimates of photosynthetic parameters Vcmax, Jmax and TPU plotted as a function of leaf N in cotton plants. Squares, estimates obtained from recently fully expanded leaves; circles, from leaves up to 18 d after full expansion. For Vcmax, independent linear regressions were obtained for leaves of plants grown in 35 (open symbols) and 65 Pa CO2 (filled symbols); regressions for Jmax and TPU data are based on combined 35 and 65 Pa data (reproduced from Harley et al., 1992) with permission.
This also exemplifies an often asked question in crop modelling – how deep, but not deeper, should one go to obtain stable values of a set of parameters in order to model a process in response to environmental variables? A common view of crop modellers is that parameterization of the biochemical model for different crops is difficult and time consuming (Tubiello and Ewert, 2002; Biernath et al., 2011). Given the availability of a wealth of information for the key enzyme constants (see review of Yin and Struik, 2009), which are believed to be conservative among C3 or C4 species, the task of parameterization can focus on a few key parameters, estimated from readily available data (e.g. Medlyn et al., 2002) or measurements (e.g. Yin et al., 2009). Yin and Struik (2009) showed that an empirical leaf-photosynthesis model, as used by Matthews et al. (1997), incorrectly predicts the interaction of temperature and elevated [CO2] on parameters Pmax or ɛ. This problem can be overcome by expressing Pmax and ɛ based on formulae of the Farquhar et al. (1980) biochemical model, as done by Mitchell et al. (1995) and van Oijen et al. (2004).
Leaf photosynthesis rate and stomatal conductance (gs) are closely coupled (Wong et al., 1979), and gs has a profound effect on energy balance and leaf temperature, and hence on water use. An additional advantage of using the biochemical model is that once the model is coupled with an equation for gs, the coupled model reliably predicts the response of gs to environmental variables, including [CO2] (Ball et al., 1987; Leuning, 1995; Yin and Struik, 2009), and performs better than an empirical multiplicative gs model (Li et al., 2012). A similar argument may apply to modelling mesophyll conductance in response to [CO2] and light levels (Yin et al., 2009).
Leaf respiration
Besides photosynthesis, respiration has been examined extensively to determine whether it is altered by elevated [CO2]. Review reports based on meta-analysis of multiple experiments reveal an overall effect of CO2 on leaf respiration. Such analyses made by Poorter et al. (1992; based on published results for 47 species) and by Wang and Curtis (2002; 33 species) revealed that leaf respiration per unit leaf area (Ra) was slightly increased for plants grown at high [CO2], whereas a small decrease was found when respiration was expressed on leaf weight basis (Rw, also known as ‘specific respiration rate’). Moreover, Wang and Curtis (2002) showed that the longer were plants exposed to elevated [CO2], the smaller was the increase in Ra and the greater was the reduction in Rw by elevated [CO2]. Most studies used for the meta-analysis did not measure leaf N content. Wullschleger et al. (1992) showed that leaf Rw differed among plants grown in three [CO2] treatments, and the relationship of Rw values, despite a large scatter within each [CO2] treatment, with leaf N concentration was shared by plants grown under three [CO2] values (Fig. 3). This means that leaf respiration, if expressed on leaf N basis, will be affected little by [CO2]. Ryan (1991) showed a similar linear relationship between Rw and N concentration for a wide variety of species and plant tissues. This relationship can explain the above-mentioned results of meta-analysis of Poorter et al. (1992) and Wang and Curtis (2002), as elevated [CO2] leads to a reduced leaf N concentration (Wong, 1990; Conroy and Hocking, 1993) and an increased leaf mass/area ratio (Lawlor and Mitchell, 1991; Poorter et al., 2009). Cannell and Thornley (2000) noted that maintenance respiration is generically related to total N content, rather than to biomass, because maintenance costs increase with tissue protein content and overall metabolic activity (Barnes and Hole, 1978). When maintenance is related to crop N content (which indirectly represents active protein content), there is little need for empirical correction of the maintenance coefficients for different growing organs or developmental stages (Cannell and Thornley, 2000), whereas such a correction is essential when maintenance respiration is related to biomass (Penning de Vries et al., 1989; Matthews et al., 1997).
Fig. 3.
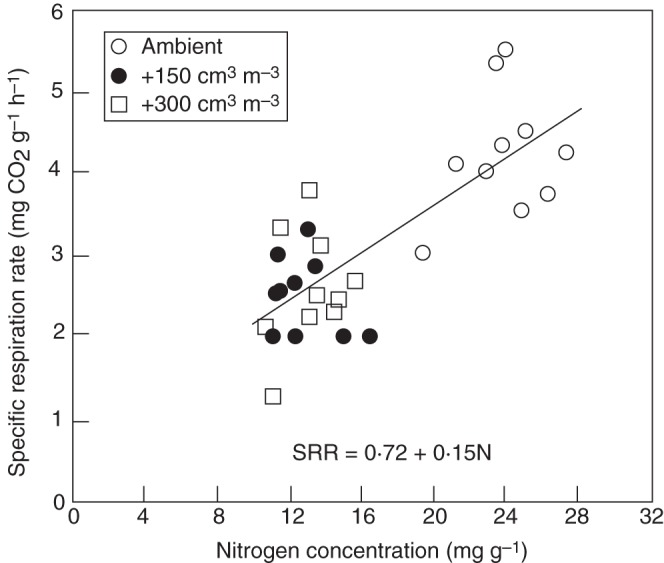
Leaf respiration rate on a weight basis (or: specific respiration rate – SRR) as a function of leaf nitrogen concentration for 22-d-old yellow-poplar leaves of different ages from sapling grown at each of three CO2 treatments (ambient, and two elevated [CO2], increased over ambient by 150 and 300 cm3 m−3, respectively). Reproduced from Wullschleger et al. (1992) with permission.
Canopy leaf area index
Not only can leaf photosynthesis or respiration be affected by elevated [CO2] but so too can canopy-scale traits such as leaf area index (LAI). As LAI determines the proportion of incoming radiation that is intercepted by a canopy, an accurate quantification of LAI is important for modelling crop response to elevated [CO2] (Ewert, 2004).
LAI varies significantly with [CO2] (e.g. Dijkstra et al., 1999; Kim et al., 2003; Sakai et al., 2006) and, generally, elevated [CO2] results in slightly higher LAI (see the review of Ainsworth and Long, 2004). Most models assume that this effect on LAI is indirect, via increased photosynthesis and leaf mass, which means a higher LAI under elevated [CO2]. But for the same measurement times, the increased LAI under elevated [CO2] was not always observed, and LAI could sometimes be even lower (Miglietta et al., 1998; Dijkstra et al., 1999; Kim et al., 2003; Sakai et al., 2006). Leaves usually become thicker under elevated [CO2] (Thomas and Harvey, 1983), which limits an increase of LAI. These results suggest that LAI is not determined only by C supply. Experimental data have shown that LAI is in fact highly related to the amount of leaf N in the canopy (Yin et al., 2003). Sakai et al. (2006) showed that the ratio of LAI under elevated to ambient [CO2] increases linearly with the ratio of canopy leaf N under elevated to ambient [CO2]. By plotting LAI against canopy leaf-N using the data of Kim et al. (2003) for rice, linear and logarithmic relations can be obtained for the early tillering phase and for the phase from panicle initiation to maturity, respectively (Fig. 4). Data points that did not follow either linear or logarithmic trends were those measured in the intermediate phase. These relationships coincide with the theoretical analysis of Yin et al. (2003) using a generic equation for LAI in relation to canopy leaf N. Note that in neither phase did elevated [CO2] alter the relationships (Fig. 4). Limited data points for wheat (e.g. Dijkstra et al., 1999; van Oijen et al., 1999) showed a similar pattern for the relationship. These suggest that the functional relationship for the canopy-size LAI, once being related to canopy-N status, is not altered by a change in [CO2]. The LAI–N relationship also generates a robust method for predicting leaf senescence in the canopy (Yin et al., 2000).
Fig. 4.
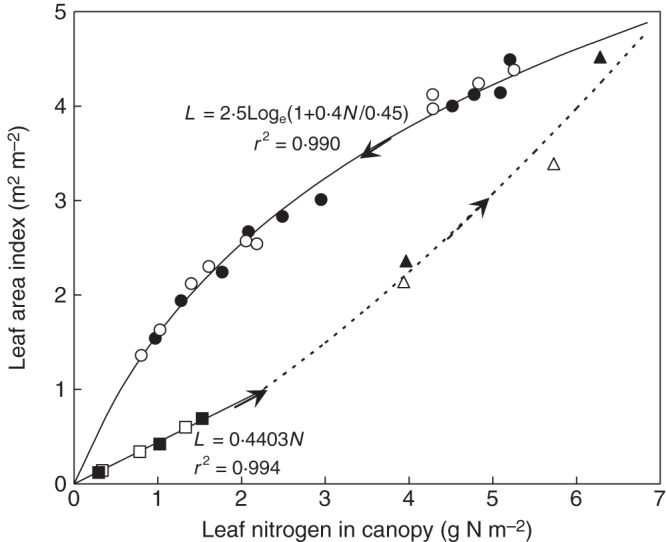
Canopy leaf area index (L) plotted against canopy leaf-nitrogen (N) for rice plants grown under ambient (open symbols) and elevated (i.e. 200 µmol mol−1 above ambient, filled symbols) CO2 levels in a FACE experiment of Kim et al. (2003) with three nitrogen supply levels, for the early tillering phases if L < 1 (squares), for the late phase from panicle initiation to maturation (circles), and for the intermediate phase (triangles). The solid line and curve represent a linear equation and a logarithmic equation, fitted to the combined data of the two CO2 levels, for the first phase and the third phase, respectively. The dotted curve was drawn from the expected transitional trajectory from the first to the third phase. Arrows indicate the temporal direction of L or N in each of the three phases.
Crop sink size
Leaf photosynthesis, respiration and LAI are traits related to the source of net photosynthetic assimilates for crop production. Often crop yields can be limited by sink size, and some crop models simulate a number of ‘sink’-related traits, such as the number of grains per unit area, in addition to the traits of ‘source’ activity. Crop sink size responds to elevated [CO2] (Lawlor and Mitchell, 1991; Mitchell et al., 1993).
It has been frequently observed that grain number increases with N accumulation in crops (see review of Makino, 2011). Horie et al. (1997) found a close relationship between the number of spikelets per m2 in rice and the amount of N accumulated through to the early reproductive stage (i.e. the critical period for forming spikelets), using data collected across locations of widely varied climate and edaphic conditions (Fig. 5A). Analysing data from FACE experiments (e.g. Kim et al., 2001), Kobayashi et al. (2006) found a similar relationship for the fertile spikelets per m2 and N accumulation through to the panicle initiation stage, which held across [CO2] and N supply rate (Fig. 5A). The difference in the two relationships could be due to the genetic difference in average grain weight between cultivars used: 22·6 mg for ‘Koshihihari’ (Horie et al., 1997) vs. 24·7 mg for ‘Akitakomachi’ (Kim et al., 2001). If the spikelet number is multiplied by average grain weight to fully represent the sink size, the difference in the relationships of Horie et al. (1997) and of Kobayashi et al. (2006) virtually disappears (Fig. 5B). The plots in Fig. 5 support that the empirical relationship for sink size, once being related to crop N status, was unaltered by elevated [CO2]. Limited data points from van Oijen et al. (1999) suggested that the conclusion also holds for wheat.
FIG. 5.
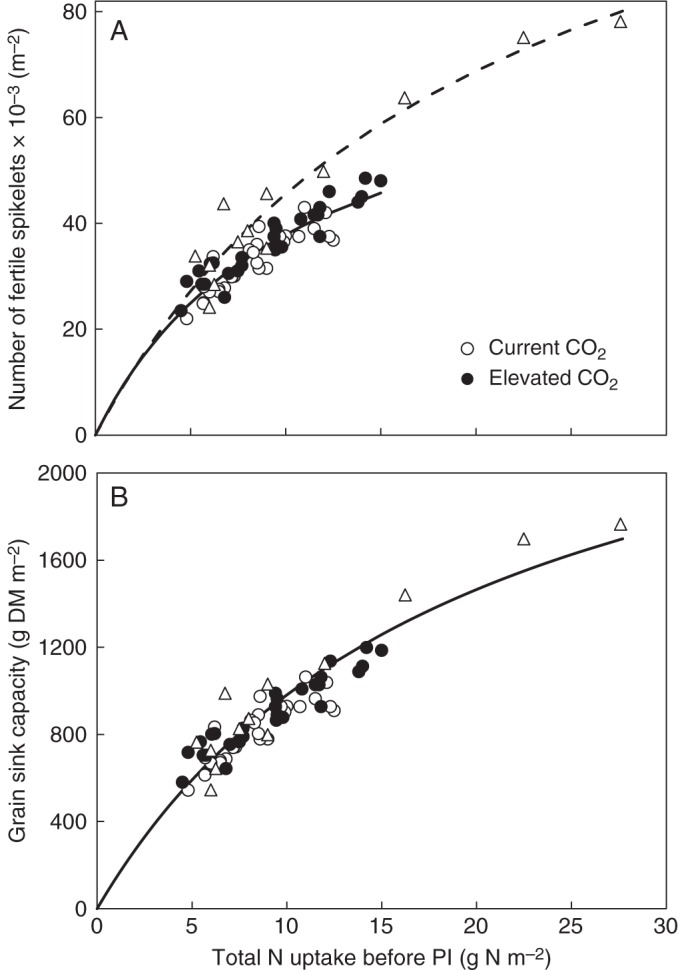
(A) Relationships shown by Kobayashi et al. (2006) between the number of fertile spikelets at harvest and nitrogen (N) accumulated through to panicle initiation (PI), in which observations in a rice FACE experiment for current CO2 and elevated are shown (as indicated in key), with the curve fitted to the FACE data (solid line) and the relationship (dashed line with triangles) reported by Horie et al. (1997). (B) Data re-analysed by correcting for the difference in average grain weight between the variety used in the FACE and that used by Horie et al. (1997) in such a way that the y-axis is represented as the full sink capacity i.e. number of fertile spikelets × average single grain dry-mass (DM) weight.
BALANCED QUANTIFICATION OF CARBON–NITROGEN INTERACTIONS TO MODEL PHOTOSYNTHEIC ACCLIMATION TO ELEVATED [CO2]
The above analyses indicate that elevated [CO2] changes little the functional relationships of plant growth if these relationships are expressed as a function of plant N status. This is in line with the early modelling concept of functional balance based on C–N interactions (e.g. Brouwer, 1962) and with early approaches of Thornley (e.g. Thornley, 1998) for grass modelling, although most of the classical work did not consider the effect of [CO2]. Therefore, it is necessary to couple above-ground C assimilation with below-ground N uptake in crop models if the models are able to predict crop yields under any [CO2] without much calibration needed. This echoes the suggestion of Stitt and Krapp (1999) that interpretation of experiments in elevated [CO2] requires monitoring the N status of the plants.
Based mainly on the concepts of the C–N interaction during crop growth, the crop model GECROS was developed (Yin and van Laar, 2005; see Supplementary Data). Compared with most existing crop models, GECROS has a more complex structure but requires fewer input parameters, because many of those used in more empirical models can be considered to be emergent properties of the mechanisms modelled by GECROS on the interactions of physiological processes.
Using data from a FACE experiment of Kimball et al. (1995) for wheat – the same data set that Tubiello et al. (1999), Tubiello and Ewert (2002), Long et al. (2006) and Ainsworth et al. (2008a) used to evaluate other models – Yin and Struik (2010) evaluated GECROS (v2·0) in estimating the impact of stimulation of yield by elevated [CO2]. The analysis did not consider the experimental flaw as pointed out by Kimball (2013). Like most previous models, GECROS correctly showed that the ‘CO2-fertilization’ effect was larger under drought than non-stress conditions (Fig. 1), although underlying physiological mechanisms simulated differ among the models. However, whereas earlier crop models tended to over-estimate yield responses to elevated [CO2], GECROS did not (Fig. 1) when standard parameters for instantaneous leaf photosynthesis and other growth processes of wheat were used. GECROS also simulated well absolute yields and total biomass across the treatments (Yin and Struik, 2010).
Yin and Struik (2010) attributed the good performance of GECROS without over-estimation to its ability in capturing the photosynthetic acclimation to elevated [CO2]. This acclimation refers to an observation that initial enhancement of photosynthesis by elevated [CO2] cannot be sustained over a longer term (e.g. Xu et al., 1994; Vandermeiren et al., 2002; Sakai et al., 2006), which has been considered as a general rule although the extent of acclimation depends on species (Sage et al., 1989; Bowes, 1993). Photosynthetic acclimation is probably not due to acclimation of gs to elevated [CO2] (Leakey et al., 2006) but can be explained by mechanisms at various biological scales (e.g. Stitt and Krapp, 1999; Long et al., 2004). Under elevated [CO2] conditions, the enhancement in N uptake may not keep pace with that of C gain (Kim et al., 2003). As a result, N dilution may occur as a result of increased C accumulation in plant materials (Skinner et al., 1999), and N and organic-N concentration of plants and leaves under elevated [CO2] may eventually be lower, irrespective of N availability (Wong, 1990; Conroy and Hocking, 1993; Pleijel and Uddling, 2012). One direct consequence of this effect is the previously discussed decrease of N content and photosynthesis at the leaf level, and an indirect consequence is faster senescence and reduced LAI at the canopy level for later stages, as observed experimentally (e.g. Miglietta et al., 1998; Kim et al., 2003). This effect of acclimation at both leaf and canopy levels is most evident under limited N conditions (e.g. Mitchell et al., 1993; Stitt and Krapp, 1999). Photosynthetic acclimation could reduce the positive effect of elevated [CO2] on crop yield by 50 % (Schapendonk et al., 2000). To simulate this acclimation effect, a crop model has to appropriately quantify the C and N interaction. GECROS captures this acclimation at both leaf and canopy levels. At the leaf level, the photosynthetic advantage of plants grown under elevated [CO2] will decrease with developmental stage as a result of a stronger decreasing leaf N content, compared with those under ambient [CO2]. At the canopy level, there is an additional contributory mechanism: C-determined LAI is initially higher and leaves will soon senesce faster under elevated than ambient [CO2] for a given canopy N content. As a result, LAI may eventually become smaller, compared with the canopy at ambient [CO2] (Yin and Struik, 2010).
The low measured stimulation of elevated [CO2] in the FACE experiments could be due to the aforementioned artefacts of the FACE system (Holtum and Winter, 2003; Bunce, 2012; Kimball, 2013). The GECROS-based analysis, however, suggests that the low stimulation of elevated [CO2] for wheat in the FACE system was at least partly due to photosynthetic acclimation at both leaf and canopy level. Most existing crop models are unable to simulate the photosynthetic acclimation to elevated [CO2], therefore tending to overestimate the stimulation of elevated [CO2], especially when model parameters for [CO2] response are calibrated from enclosure studies.
The evaluation of GECROS by Yin and Struik (2010) demonstrated that a robust crop model requiring minimum inputs does translate input information at the single-organ level over a short timescale (e.g. photosynthetic rates on single leaves in μmol m−2 s−1) to crop performance in a continuously changing field environment. Lenz (2007) and Lenz-Wiedemann et al. (2010) also showed good performance of GECROS in representing the growth of various crops and the interplay of water, C and N fluxes under field conditions. The separate evaluation of the model by Yin and Struik (2010) and Lenz-Wiedemann et al. (2010) confirms a well-recognized principle (e.g. Penning de Vries et al., 1989) that a good model should be able to extrapolate the results from one to another experiment. Thus, to accurately predict CO2 response of crop yield, one should not place emphasis on the type of experimental facilities to obtain data for parameterization, but on how the model is structured and how it is parameterized from available data. With a robust crop model, there is no need to seek for untested hypotheses (e.g. increased herbivory in FACE fields as compared with growth enclosures) for the failure to predict the CO2 response ratio of crop yield using data of controlled enclosure environments. Similarly, the reliance on a large-scale FACE experimentation, as emphasized by Ainsworth et al. (2008b), could be considerably reduced. This latter assertion is especially true if the FACE facilities, due to the impact of [CO2] fluctuation (Holtum and Winter, 2003; Bunce, 2012), cannot exactly mimic the real [CO2] environment of future climate.
CONCLUDING REMARKS
Both statistical modelling (e.g. Schlenker and Roberts, 2009; Lobell et al., 2011) and ecophysiological modelling approaches are currently used to assess the impact of climate change on crop production. Compared with statistical modelling, ecophysiological modelling research should play a greater role in facilitating the design of genetic and agronomic strategies of adaptation to climate change (Challinor et al., 2009; Semenov and Halford, 2009). Algorithms in current ecophysiological crop models to simulate [CO2] effects are, however, often empirical. Long et al. (2005, 2006) and Ainsworth et al. (2008a) indicated that when parameterized from controlled chamber environments, current crop models tend to overestimate the observed response of crop yield to elevated [CO2] in the FACE experiments. They further argued that controlled chamber environments were not the best experimental facilities for estimating CO2 response ratio of crop yield, as FACE has revealed factors in field conditions that were not identified by chamber experiments (e.g. increased herbivory). However, the lower than expected response to elevated [CO2] could actually be explained by: (1) the different size between enclosure and FACE experiments (see earlier discussions), (2) the impact of the flaw in FACE technology, e.g. the ‘blower artefact’ (Kimball, 2013) and more [CO2] fluctuation in FACE experiments (Holtum and Winter, 2003; Bunce, 2012) and (3) the inability of most crop models to accommodate photosynthetic acclimation to elevated [CO2]. Although further studies are needed to quantify the relative importance of the three explanations, the present paper discussed how to improve crop models to deal with the acclimation given that this acclimation is recognized as a general rule.
My analysis suggests that elevated [CO2] alters quantitative functional relationships only little if they are expressed as a function of plant N status (Figs 2–5), suggesting that fundamental expressions of plant performance can be captured in relatively simple models. This viewpoint has been supported by the success in modelling root–shoot partitioning using functional balance theory (Brouwer, 1962) and in modelling stomatal and mesophyll conductance using phenomenological equations (e.g. Ball et al., 1987; Leuning, 1995; Yin et al., 2009; Li et al., 2012). The analysis also showed that photosynthetic acclimation to elevated [CO2] can be an emerging property of an appropriate quantification of interactions between C- and N-related processes. Crop models with such functionalities for C–N interactions have a sophisticated structure, but require few empirical input parameters – an important property of the models when used to extrapolate both in time and in space for assessment of climate change impact (Lenz-Wiedemann et al., 2010). Their performance in projecting the impact of elevated [CO2] will depend little on the type of experimental facilities used to obtain data for parameterization.
Such a robust crop model is a basis for developing a general framework that models critical physiological processes and traits in response to all climatic factors, including extreme events (Rötter et al., 2011). It is also the basis to further improve the models based on new physiological understandings. Notably, my viewpoint stresses the need to model C–N relationships. However, photosynthesis and respiration should be related to active protein, whereas significant amounts of plant N can accumulate as nitrate (Cárdenas-Navarro et al., 1999). [CO2] enrichment was recently found to inhibit shoot nitrate assimilation into protein compounds, and this inhibition might alter the partitioning between leaf nitrate and protein and be responsible for photosynthetic acclimation to elevated [CO2] (Bloom et al., 2010; Pleijel and Uddling, 2012). This phenomenon might also be relevant to the differences in the [CO2] response between controlled-environment chambers and FACE experiments where crops may have different access to nitrate relative to ammonium as an N source. Once the mechanisms for nitrate-assimilation inhibition are elucidated, an approach that models individual pools for inorganic and organic N may enhance mechanistic prediction of elevated [CO2] effects on crop productivity.
In this paper I have discussed only generic aspects of crop responses to elevated [CO2], especially those related to photosynthesis and respiration. Other processes also respond to [CO2]. For example, early flowering was often associated with a higher canopy temperature induced by elevated [CO2] (Craufurd and Wheeler, 2009). Direct phenological (Springer and Ward, 2007) and morphogenetic (Thomas and Harvey, 1983) responses to elevated [CO2] are species-specific and need to be addressed differentially when developing models for specific crops. Finally, crop models should be combined with soil and pest models in order to account for indirect impacts on crops resulting from climate-induced changes in soil moisture and nutrient availability and in weed, insect and disease pressures (Soussana et al., 2010; Hatfield et al., 2011).
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of a detailed summary of the GECROS crop model.
ADDITIONAL NOTES IN RESPONSE TO THE COMMENTS OF KIMBALL (2013)
Following the online publication of this paper ahead of print, Kimball (2013) has noted several important points related to FACE experimentation and model projection of crop responses to elevated [CO2]. In particular, he points out the flaw (i.e. the ‘blower artefact’) of the FACE experiment reported in Kimball et al. (1995), conducted in 1992–93 and 1993–94.
It would have been more timely if this particular comment had been published earlier following publications such as Ainsworth et al. (2008a), who commented on the over-estimation of yield response to elevated [CO2] by existing crop models, based on data from the experiments of Kimball et al. (1995). I thank Dr Kimball for pointing out this experimental flaw explicitly, and as a result I have already made some small adjustments to several parts of this paper. To further respond to his comments, I put forth additional notes below.
The higher [CO2] response ratio from the 1995–96 and 1996–97 FACE experiments (see Kimball, 2013) cannot be entirely attributed to the removal of the experimental artefact that existed in the 1992–93 and 1993–94 experiments. Besides the possible difference in climates between the years, actual nitrogen fertilizer application was appreciably higher in the 1995–96 and 1996–97 experiments (350 kg ha−1) than in the 1992–93 and 1993–94 experiments (260–276 kg ha−1; see Ko et al., 2010). It is well known that plants generally respond more to elevated [CO2] in high- than in low-nitrogen environments (e.g. de Graaff et al., 2006; also shown by the 1995–96 and 1996–97 experiments).
The water stress treatment was not repeated in the 1995–96 and 1996–97 experiments. Although the 1992–93 and 1993–94 experiments had a technical flaw, the ratio in the relative [CO2] response between the two water treatments or the responses to water stress under either [CO2] environment should still be quite representative of the real values. Such information would be very useful to test crop models in reflecting underlying biological mechanisms of [CO2] × drought interactions, e.g. elevated [CO2] reduces the loss due to higher photorespiration under drought (Long et al., 2004). Relative to most other models, GECROS still predicts these ratios well (Fig. 6). Of course, it is necessary to use a wider range of FACE data to critically parameterize and evaluate the models, given the wide range of variation in reported crop responses to elevated [CO2] (Ainsworth et al., 2008a).
Fig. 6.
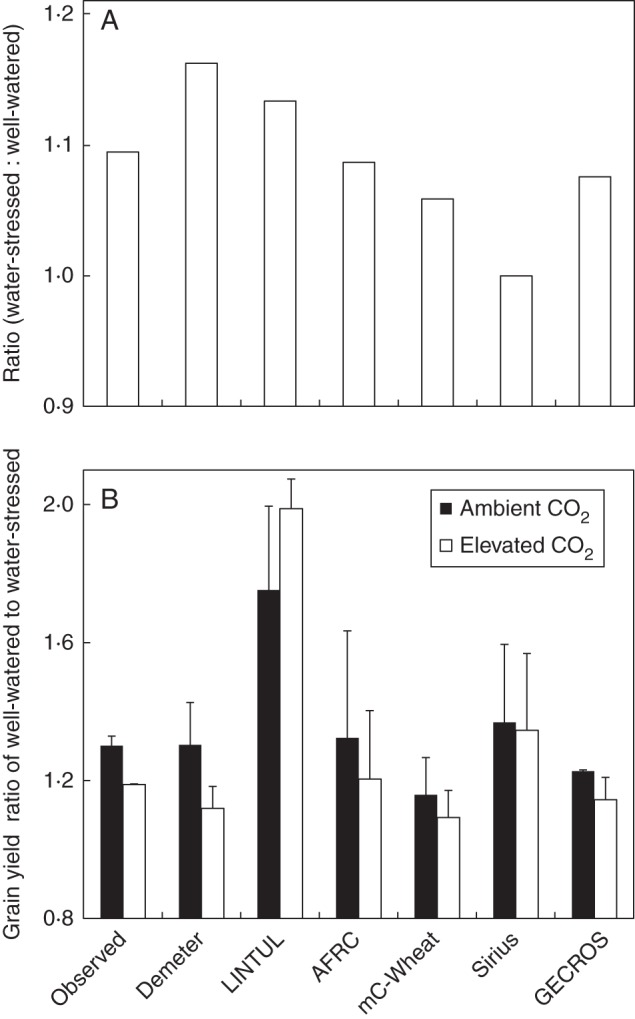
(A) The ratio in stimulation of grain yield by elevated [CO2] (relative to ambient [CO2]) in the water-stressed treatment to that in the well-watered treatment for a wheat crop grown in the experiment reported by Kimball et al. (1995). This ratio was calculated from data as shown in Fig. 1. (B) The ratio of grain yield in the well-watered treatment to that in the water-stressed treatment under two different [CO2] environmental conditions for the same crop. For further details see Fig. 1. The error bars represent standard errors for variation between two growing seasons.
Kimball (2013) also discusses genotype-specific responses, and mentions a few Chinese FACE experiments that have reported a high response ratio to elevated [CO2] in hybrid rice cultivars (see references therein). However, no inbred rice cultivar was used as a control in these FACE experiments. So, this higher value may simply add to the inconsistency already known in the literature on crop response to [CO2], and not necessarily reflect an intrinsically higher response of hybrid rice. As mentioned in the Concluding Remarks above, my paper addresses only generic aspects of crop response to CO2. Crop- (or genotype-) specific responses need to be addressed differentially for specific crops or genotypes.
Kimball (2013) emphasizes respective advantages of FACE and non-FACE in climate change research. However, my current paper never really questions the merits of these experimental facilities and their value in supporting modelling. In addition, Kimball (2013) stresses the importance of both relative and absolute responses to elevated [CO2]. This is again not very different from the discussions in this current paper, where I also indicate model performance in simulating absolute values for yield and biomass, although not in the format of a figure. In addition, models should also be evaluated in terms of intermediate physiological variables (such as time course of LAI and canopy transpiration), rather than only end-of-season yield traits.
Supplementary Material
ACKNOWLEDGEMENTS
Prof. P. C. Struik and several anonymous reviewers are acknowledged for their comments on earlier versions of the manuscript. I thank Prof. Ken Giller for checking the English text.
LITERATURE CITED
- Ainsworth EA, Long SP. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytologist. 2004;165:351–372. doi: 10.1111/j.1469-8137.2004.01224.x. [DOI] [PubMed] [Google Scholar]
- Ainsworth EA, Leakey ADB, Ort DR, Long SP. FACE-ing the facts: inconsistencies and interdependence among field, chamber and modelling [CO2] impacts on crop yield and food supply. New Phytologist. 2008a;179:5–9. doi: 10.1111/j.1469-8137.2008.02500.x. [DOI] [PubMed] [Google Scholar]
- Ainsworth EA, Beier C, Calfapietra C, et al. Next generation of elevated [CO2] experiments with crops: a critical investment for feeding the future world. Plant, Cell and Environment. 2008b;31:1317–1324. doi: 10.1111/j.1365-3040.2008.01841.x. [DOI] [PubMed] [Google Scholar]
- Asseng S, Jamieson PD, Kimball B, et al. Simulated wheat growth affected by rising temperature, increased water deficit and elevated atmospheric CO2. Field Crops Research. 2004;85:85–102. [Google Scholar]
- Ball JT, Woodrow IE, Berry JA. A model predicting stomatal conductance and its contribution to the control of photosynthesis under different environmental conditions. In: Biggens J, editor. Progress in photosynthesis research. Dordrecht: Martinus-Nijhoff; 1987. pp. 21–224. [Google Scholar]
- Barnes A, Hole C.C. A theoretical basis of growth and maintenance respiration. Annals of Botany. 1978;42:1217–1221. [Google Scholar]
- Biernath C, Gayler S, Bittner S, et al. Evaluating the ability of four crop models to predict different environmental impacts on spring wheat grown in open-top chambers. European Journal of Agronomy. 2011;35:71–82. [Google Scholar]
- Bloom AJ, Burger M, Asensio JSR, Cousins AB. Carbon dioxide enrichment inhibits nitrate assimilation in wheat and Arabidopsis. Science. 2010;328:899–903. doi: 10.1126/science.1186440. [DOI] [PubMed] [Google Scholar]
- Bowes G. Facing the inevitable: plants and increasing atmospheric CO2. Annual Review of Plant Physiology and Plant Molecular Biology. 1993;44:309–332. [Google Scholar]
- Brouwer R. Nutritive influences on the distribution of dry matter in the plant. Netherlands Journal of Agricultural Science. 1962;10:399–408. [Google Scholar]
- Bunce JA. Responses of cotton and wheat photosynthesis and growth to cyclic variation in carbon dioxide concentration. Photosynthetica. 2012;50 495–400. [Google Scholar]
- Cannell MGR, Thornley JHM. Modelling the components of plant respiration: some guiding principles. Annals of Botany. 2000;85:45–54. [Google Scholar]
- Cárdenas-Navarro R, Adamowicz S, Robin P. Nitrate accumulation in plants: a role for water. Journal of Experimental Botany. 1999;50:613–624. [Google Scholar]
- Challinor AJ, Ewert F, Arnold S, Simelton E, Franser E. Crops and climate change: progress, trends, and challenges in simulating impacts and informing adaptation. Journal of Experimental Botany. 2009;60:2775–2789. doi: 10.1093/jxb/erp062. [DOI] [PubMed] [Google Scholar]
- Conroy J, Hocking P. Nitrogen nutrition of C3 plants at elevated atmospheric CO2 concentrations. Physiologia Plantarum. 1993;89:570–576. [Google Scholar]
- Craufurd PQ, Wheeler TR. Climate change and the flowering time of annual crops. Journal of Experimental Botany. 2009;60:2529–2539. doi: 10.1093/jxb/erp196. [DOI] [PubMed] [Google Scholar]
- Dijkstra P, Schapendonk AHCM, Groenwold K, Jansen M, Van de Geijn S. Seasonal changes in the response of winter wheat to elevated atmospheric CO2 concentration grown in open-top chambers and field tracking enclosures. Global Change Biology. 1999;5:563–576. [Google Scholar]
- Ehleringer J, Björkman O. Quantum yields for CO2 uptake in C3 and C4 plants. Dependence on temperature, CO2, and O2 concentration. Plant Physiology. 1977;59:86–90. doi: 10.1104/pp.59.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellsworth DS, Reich PB, Naumburg ES, Koch GW, Kubiske ME, Smith SD. Photosynthesis, carboxylation and leaf nitrogen responses of 16 species to elevated pCO2 across four free-air CO2 enrichment experiments in forest, grassland and desert. Global Change Biology. 2004;10:2121–2138. [Google Scholar]
- Ewert F. Modelling plant responses to elevated CO2: how important is leaf area index? Annals of Botany. 2004;93:619–627. doi: 10.1093/aob/mch101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar GD, von Caemmerer S, Berry JA. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta. 1980;149:78–90. doi: 10.1007/BF00386231. [DOI] [PubMed] [Google Scholar]
- de Graaff M-E, van Groenigen K-J, Six J, Hungate B, van Kessel C. Interactions between plant growth and soil nutrient cycling under elevated CO2: a meta-analysis. Global Change Biology. 2006;12:2077–2091. [Google Scholar]
- Grant RF. A review of the Canadian ecosystem model ecosys. In: Shaffer M, editor. Modeling carbon and nitrogen dynamics for soil management. Boca Raton, FL: CRC Press; 2001. pp. 173–264. [Google Scholar]
- Harley PC, Thomas RB, Reynolds JF, Strain BR. Modelling photosynthesis of cotton grown in elevated CO2. Plant, Cell and Environment. 1992;15:271–282. [Google Scholar]
- Hatfield JL, Boote KJ, Kimball BA, et al. Climate change impacts on agriculture: implications for crop production. Agronomy Journal. 2011;103:351–370. [Google Scholar]
- Hendrey GR, Long SP, McKee IF, Baker NR. Can photosynthesis respond to short-term fluctuations in atmospheric carbon dioxide? Photosynthesis Research. 1997;51:179–184. [Google Scholar]
- Hikosaka K, Terashima I. A model of the acclimation of photosynthesis in the leaves of C3 plants to sun and shade with respect to nitrogen use. Plant, Cell and Environment. 1995;18:605–618. [Google Scholar]
- Hirose T, Ackerly DD, Traw MB, Ramseier D, Bazzaz FA. CO2 elevation, canopy photosynthesis, and optimal leaf area index. Ecology. 1997;78:2339–2350. [Google Scholar]
- Horie T, Ohnishi M, Angus JF, Lewin LG, Tsukaguchi T, Matano T. Physiological characteristics of high-yielding rice inferred from cross-location experiments. Field Crops Research. 1997;52:55–57. [Google Scholar]
- Hulme M, Barrow EM, Arnell NW, Harrison PA, Johns TC, Downing TE. Relative impacts of human-induced climate change and natural climate variability. Nature. 1999;397:688–691. [Google Scholar]
- Holtum JAM, Winter K. Photosynthetic CO2 uptake in seedlings of two tropical tree species exposed to oscillating elevated concentrations of CO2. Planta. 2003;218:152–158. doi: 10.1007/s00425-003-1089-1. [DOI] [PubMed] [Google Scholar]
- IPCC. Contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change. Cambridge: Cambridge University Press; 2007. [Google Scholar]
- Kim HY, Lieffering M, Miura S, Kobayashi K, Okada M. Growth and nitrogen uptake of CO2-enriched rice under field conditions. New Phytologist. 2001;150:223–229. [Google Scholar]
- Kim HY, Lieffering M, Kobayashi K, Okada M, Miura S. Seasonal changes in the effects of elevated CO2 on rice at three levels of nitrogen supply: a free air CO2 enrichment (FACE) experiment. Global Change Biology. 2003;9:826–837. [Google Scholar]
- Kimball BA. Comment on ‘Improving ecophysiological simulation models to predict the impact of elevated atmospheric CO2 concentration on crop productivity’ by X. Yin. Annals of Botany. 2013;112:477–478. doi: 10.1093/aob/mct130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball BA, Pinter PJ, Garcia RL, et al. Productivity and water use of wheat under free-air CO2 enrichment. Global Change Biology. 1995;1:429–442. [Google Scholar]
- Kirschbaum MUF. Does enhanced photosynthesis enhance growth? Lessons learned from CO2 enrichment studies. Plant Physiology. 2011;155:117–124. doi: 10.1104/pp.110.166819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko J, Ahuja L, Kimball B, et al. Simulation of free air CO2 enriched wheat growth and interactions with water, nitrogen, and temperature. Agricultural and Forest Meteorology. 2010;150:1331–1346. [Google Scholar]
- Kobayashi K, Okada M, Kim HY, Lieffering M, Miura S, Hasegawa T. Paddy rice responses to free-air [CO2] enrichment. Ecological Studies 187. In: Nösberger J, Long SP, Norby RJ, Stitt M, Hendrey GR, Brum H, editors. Managed ecosystems and CO2: case studies, processes, and perspectives. Berlin: Springer; 2006. pp. 87–104. [Google Scholar]
- Lawlor DW, Mitchell AC. The effects of increased CO2 on crop photosynthesis and productivity: a review of field studies. Plant, Cell and Environment. 1991;14:807–818. [Google Scholar]
- Leakey ADB, Bernacchi CJ, Ort DR, Long SP. Long-term growth of soybean at elevated [CO2] does not cause acclimation of stomatal conductance under fully open-air conditions. Plant, Cell and Environment. 2006;29:1794–1800. doi: 10.1111/j.1365-3040.2006.01556.x. [DOI] [PubMed] [Google Scholar]
- Leakey ADB, Ainsworth EA, Bernacchi CJ, Rogers A, Long SP, Ort DR. Elevated CO2 effects on plant carbon, nitrogen, and water relations: six important lessons from FACE. Journal of Experimental Botany. 2009;60:2859–2876. doi: 10.1093/jxb/erp096. [DOI] [PubMed] [Google Scholar]
- Lenz VIS. A process-based crop growth model for assessing Global Change effects on biomass production and water demand – A component of the integrative Global Change decision support system DANUBIA. PhD thesis: University of Cologne; 2007. [Google Scholar]
- Lenz-Wiedemann VIS, Klar CW, Schneider K. Development and test of a crop growth model for application within a Global Change decision support system. Ecological Modelling. 2010;221:314–329. [Google Scholar]
- Leuning R. A critical appraisal of a combined stomatal–photosynthesis model for C3 plants. Plant, Cell and Environment. 1995;18:339–355. [Google Scholar]
- Li G, Lin L, Dong Y, et al. Testing two models for the estimation of leaf stomatal conductance in four greenhouse crops: cucumber, chrysanthemum, tulip and lilium. Agricultural and Forest Meteorology. 2012;165:92–103. [Google Scholar]
- Lobell DB, Schlenker W, Costa-Roberts J. Climate trends and global crop production since 1980. Science. 2011;333:616–620. doi: 10.1126/science.1204531. [DOI] [PubMed] [Google Scholar]
- Long SP. Modification of the response of photosynthetic productivity to rising temperature by atmospheric CO2 concentrations: has its importance been underestimated? Plant, Cell and Environment. 1991;14:729–739. [Google Scholar]
- Long SP, Ainsworth EA, Rogers A, Ort DR. Rising atmospheric carbon dioxide: plants FACE the future. Annual Review of Plant Biology. 2004;55:591–628. doi: 10.1146/annurev.arplant.55.031903.141610. [DOI] [PubMed] [Google Scholar]
- Long SP, Ainsworth EA, Leakey ADB, Morgan PB. Global food insecurity. Treatment of major food crops with elevated carbon dioxide or ozone under large-scale fully open-air conditions suggests recent models may have overestimated future yields. Philosophical Transactions of the Royal Society B: Biological sciences. 2005;360 doi: 10.1098/rstb.2005.1749. 2011–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long SP, Ainsworth EA, Leakey ADB, Nösberger J, Ort DR. Food for thought: lower-than-expected crop yield stimulation with rising CO2 concentrations. Science. 2006;312:1918–1921. doi: 10.1126/science.1114722. [DOI] [PubMed] [Google Scholar]
- Luo Y, Sims DA, Griffin KL. Nonlinearity of photosynthetic responses to growth in rising atmospheric CO2: an experimental and modelling study. Global Change Biology. 1998;4:173–183. [Google Scholar]
- Makino A. Photosynthesis, grain yield, and nitrogen utilization in rice and wheat. Plant Physiology. 2011;155:125–129. doi: 10.1104/pp.110.165076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T, Namuco OS, Ziska LH, Horie T. Effects of high temperature and CO2 concentration on spikelet sterility in indica rice. Field Crops Research. 1997;51:213–219. [Google Scholar]
- Matthews RB, Kropff MJ, Horie T, Bachelet D. Simulating the impact of climate change on rice production in Asia and evaluating options for adaptation. Agricultural Systems. 1997;54:399–425. [Google Scholar]
- Medlyn BE, Dreyer E, Ellsworth D, et al. Temperature response of parameters of a biochemically based model of photosynthesis. II. A review of experimental data. Plant, Cell and Environment. 2002;25:1167–1179. [Google Scholar]
- Miglietta F, Magliulo V, Bindi M, et al. Free air CO2 enrichment of patato (Solanum tuberosum L.): development, growth and yield. Global Change Biology. 1998;4:163–172. [Google Scholar]
- Mitchell RAC, Mitchell VJ, Driscoll SP, Franklin J, Lawlor DW. Effects of increased CO2 concentration and temperature on growth and yield of winter wheat at two levels of nitrogen application. Plant, Cell and Environment. 1993;16:521–529. [Google Scholar]
- Mitchell RAC, Lawlor DW, Mitchell VJ, Gibbard CI, White EM, Porter JR. Effects of elevated CO2 and increased temperature on winter-wheat – test of ARCWHEAT1 simulation model. Plant, Cell and Environment. 1995;18:736–748. [Google Scholar]
- Nakano H, Makino A, Mae T. The effect of elevated partial pressure of CO2 on the relationship between photosynthetic capacity and N content in rice leaves. Plant Physiology. 1997;115:191–198. doi: 10.1104/pp.115.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijs I, Behaeghe T, Impens I. Leaf nitrogen content as a predictor of photosynthetic capacity in ambient and global change conditions. Journal of Biogeography. 1995;22:177–183. [Google Scholar]
- van Oijen M, Schapendonk AHCM, Jansen MJH, Pot CS, Maciorowski R. Do open-top chambers overestimate the effects of rising CO2 on plants? An analysis using spring wheat. Global Change Biology. 1999;5:411–421. [Google Scholar]
- van Oijen M, Dreccer MF, Firsching K-H, Schnieders BJ. Simple equations for dynamic models of the effects of CO2 and O3 on light use efficiency and growth of crops. Ecological Modelling. 2004;179:39–60. [Google Scholar]
- Penning de Vries FWT, Jansen DM, ten Berge HFM, Bakema A. Simulation of ecophysiological processes of growth in several annual crops. Los Banos: IRRI, and Wageningen: Pudoc; 1989. [Google Scholar]
- Pleijel H, Uddling J. Yield vs. quality trade-offs for wheat in response to carbon dioxide and ozone. Global Change Biology. 2012;18:596–605. doi: 10.1111/j.1365-2486.2011.02489.x. [DOI] [PubMed] [Google Scholar]
- Poorter H, Gifford RM, Kriedemann PE, Wong SC. A quantitative analysis of dark respiration and carbon content as factors in the growth response of plants to elevated CO2. Australian Journal of Plant Physiology. 1992;40:501–513. [Google Scholar]
- Poorter H, Niinemets Ü, Poorter L, Wright IJ, Villar R. Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. New Phytologist. 2009;182:565–588. doi: 10.1111/j.1469-8137.2009.02830.x. [DOI] [PubMed] [Google Scholar]
- Porter JR, Leigh RA, Semenov MA, Miglietta F. Modelling the effects of climatic change and genetic modification on nitrogen use by wheat. European Journal of Agronomy. 1995;4:419–429. [Google Scholar]
- Priesack E, Gayler S. Agricultural crop models: concepts of resource acquisition and assimilate partitioning. In: Lüttge U, et al., editors. Progress in botany 70. Berlin: Springer; 2009. pp. 195–222. [Google Scholar]
- Rodriguez D, Ewert F, Goudriaan J, Manderscheid R, Burkart S, Weigel HJ. Modelling the response of wheat canopy assimilation to atmospheric CO2 concentrations. New Phytologist. 2001;150:337–346. [Google Scholar]
- Rosenzweig C, Parry ML. Potential impact of climate change on world food supply. Nature. 1994;367:133–138. [Google Scholar]
- Rötter RP, Carter TR, Olesen JE, Porter JR. Crop-climate models need an overhaul. Nature Climate Change. 2011;1:175–177. [Google Scholar]
- Ryan MG. Effects of climate change on plant respiration. Ecological Applications. 1991;1:157–167. doi: 10.2307/1941808. [DOI] [PubMed] [Google Scholar]
- Sage RF, Sharkey TD, Seemann JR. Acclimation of photosynthesis to elevated CO2 in five C3 species. Plant Physiology. 1989;89:590–596. doi: 10.1104/pp.89.2.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai H, Hasegawa T, Kobayashi K. Enhancement of rice canopy carbon gain by elevated CO2 is sensitive to growth stage and leaf nitrogen concentration. New Phytologist. 2006;170:321–332. doi: 10.1111/j.1469-8137.2006.01688.x. [DOI] [PubMed] [Google Scholar]
- Schapendonk AHCM, van Oijen M, Dijkstra P, Pot SC, Jordi WJRM, Stoopen GM. Effects of elevated CO2 concentration on photosynthetic acclimation and productivity of two potato cultivars grown in open-top chambers. Australian Journal of Plant Physiology. 2000;27:1119–1130. [Google Scholar]
- Schlenker W, Roberts MJ. Nonlinear temperature effects indicate severe damage to U.S. crop yields under climate change. Proceedings of the National Academy of Sciences of the USA. 2009;106:15594–15598. doi: 10.1073/pnas.0906865106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenov MA, Halford NG. Identifying target traits and molecular mechanisms for wheat breeding under a changing climate. Journal of Experimental Botany. 2009;60:2791–2804. doi: 10.1093/jxb/erp164. [DOI] [PubMed] [Google Scholar]
- Sharkey TD. Photosynthesis in intact leaves of C3 plants: physics, physiology and rate limitations. The Botanical Review. 1985;51:53–105. [Google Scholar]
- Skinner RH, Morgan JA, Hanson JD. Carbon and nitrogen reserve remobilization following defoliation: nitrogen and elevated CO2 effects. Crop Science. 1999;39:1749–1756. [Google Scholar]
- Soussana JF, Graux A-I, Tubiello FN. Improving the use of modelling for projections of climate change impacts on crops and pastures. Journal of Experimental Botany. 2010;61:2217–2228. doi: 10.1093/jxb/erq100. [DOI] [PubMed] [Google Scholar]
- Springer C, Ward J. Flowering time and elevated atmospheric CO2. New Phytologist. 2007;178:63–67. doi: 10.1111/j.1469-8137.2007.02196.x. [DOI] [PubMed] [Google Scholar]
- Stitt M, Krapp A. The interaction between elevated carbon dioxide and nitrogen nutrition: the physiological and molecular background. Plant, Cell and Environment. 1999;22:583–621. [Google Scholar]
- Tardieu F. Why work and discuss the basic principles of plant modelling 50 years after the first plant models. Journal of Experimental Botany. 2010;61:2039–2041. doi: 10.1093/jxb/erq135. [DOI] [PubMed] [Google Scholar]
- Thomas JF, Harvey CN. Leaf anatomy of four species grown under long-term continuous CO2 enrichment. Botanical Gazette. 1983;144:303–309. [Google Scholar]
- Thornley JHM. Modelling shoot:root relations: the only way forward? Annals of Botany. 1998;81:165–171. [Google Scholar]
- Tubiello FN, Ewert F. Simulating the effects of elevated CO2 on crops: approaches and applications for climate change. European Journal of Agronomy. 2002;18:57–74. [Google Scholar]
- Tubiello FN, Fischer G. Reducing climate change impacts on agriculture: global and regional effects of mitigation, 2000–2080. Technological Foresting & Social Change. 2007;74:1030–1056. [Google Scholar]
- Tubiello FN, Rosenzweig C, Kimball BA, et al. Testing CERES-wheat with Free-air Carbon dioxide Enrichment (FACE) experiment data: CO2 and water interactions. Agronomy Journal. 1999;91:247–255. [Google Scholar]
- Tubiello FN, Soussana J-F, Howden SM. Crop and pasture response to climate change. Proceedings of the National Academy of Sciences of the USA. 2007a;104:19686–19690. doi: 10.1073/pnas.0701728104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubiello FN, Amthor JS, Boote KJ, et al. Crop response to elevated CO2 and world food supply: a comment on “Food for Thought …” by Long et al., Science 312:1918–1921, 2006. European Journal of Agronomy. 2007b;26:215–223. [Google Scholar]
- Vandermeiren K, Black C, Lawson T, Casanova MA, Ojanperä K. Photosynthetic and stomatal responses of potatoes grown under elevated CO2 and/or O3 – results from the European CHIP-programme. European Journal of Agronomy. 2002;17:337–352. [Google Scholar]
- Wang X, Curtis P. A meta-analytical test of elevated CO2 effects on plant respiration. Plant Ecology. 2002;161:251–261. [Google Scholar]
- Weiss A. Introduction. Agronomy Journal. 2003;95:1–3. [Google Scholar]
- White JW, McMaster GS, Edmeades GO. Genomics and crop response to global change: what have we learned? Field Crops Research. 2004;90:165–169. [Google Scholar]
- White JW, Hoogenboom G, Kimball BA, Wall GW. Methodologies for simulating impacts of climate change on crop production. Field Crops Research. 2011;124:357–368. [Google Scholar]
- de Wit CT. Potential photosynthesis of crop surfaces. Netherland Journal of Agricultural Sciences. 1959;7:141–149. [Google Scholar]
- Wong S-C. Elevated atmospheric partial pressure of CO2 and plant growth. II Non-structural carbohydrate content in cotton plants and its effect on growth parameters. Photosynthesis Research. 1990;23:171–180. doi: 10.1007/BF00035008. [DOI] [PubMed] [Google Scholar]
- Wong SC, Cowan IR, Farquhar GD. Stomatal conductance correlates with photosynthetic capacity. Nature. 1979;282:424–426. [Google Scholar]
- Wullschleger SD, Norby RJ, Gunderson CA. Growth and maintenance respiration in leaves of Liriodendron tulipifera L. exposed to long-term carbon dioxide enrichment in the field. New Phytologist. 1992;121:515–523. [Google Scholar]
- Xu D-Q, Gifford RM, Chow WS. Photosynthetic acclimation in pea and soybean to high atmospheric CO2 partial pressure. Plant Physiology. 1994;106:661–671. doi: 10.1104/pp.106.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X, van Laar HH. Crop systems dynamics: an ecophysiological simulation model for genotype-by-environment interactions. Wageningen: Wageningen Academic Publishers; 2005. [Google Scholar]
- Yin X, Struik PC. C3 and C4 photosynthesis models: an overview from the perspective of crop modelling. NJAS-Wageningen Journal of Life Sciences. 2009;57:27–38. [Google Scholar]
- Yin X, Struik PC. Modelling the crop: from system dynamics to systems biology. Journal of Experimental Botany. 2010;61:2171–2183. doi: 10.1093/jxb/erp375. [DOI] [PubMed] [Google Scholar]
- Yin X, Schapendonk AHCM, Kropff MJ, van Oijen M, Bindraban PS. A generic equation for nitrogen-limited leaf area index and its application in crop growth models for predicting leaf senescence. Annals of Botany. 2000;85:579–585. [Google Scholar]
- Yin X, Lantinga EA, Schapendonk AHCM, Zhong X. Some quantitative relationships between leaf area index and canopy nitrogen content and distribution. Annals of Botany. 2003;91:893–903. doi: 10.1093/aob/mcg096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X, Struik PC, Romero P, Harbinson J, Evers JB, van der Putten PEL, Vos J. Using combined measurements of gas exchange and chlorophyll fluorescence to estimate parameters of a biochemical C3 photosynthesis model: a critical appraisal and a new integrated approach applied to leaves in a wheat (Triticum aestivum) canopy. Plant, Cell and Environment. 2009;32:448–464. doi: 10.1111/j.1365-3040.2009.01934.x. [DOI] [PubMed] [Google Scholar]
- Zhu C, Zhu J, Zeng Q, et al. Elevated CO2 accelerates flag-leaf senescence in wheat due to ear photosynthesis which causes greater ear nitrogen sink capacity and ear carbon sink limitation. Functional Plant Biology. 2009;36:291–299. doi: 10.1071/FP08269. [DOI] [PubMed] [Google Scholar]
- Ziska LH, Bunce JA. Predicting the impact of changing CO2 on crop yields: some thoughts on food. New Phytologist. 2007;175:607–618. doi: 10.1111/j.1469-8137.2007.02180.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


