Abstract
Background and Aims
Gynodioecy (coexistence of females and hermaphrodites) is a sexual system that occurs in numerous flowering plant lineages. Thus, understanding the features that affect its maintenance has wide importance. Models predict that females must have a seed fitness advantage over hermaphrodites, and this may be achieved via seed quality or quantity. Females in a population of Fragaria vesca subsp. bracteata, a long-lived gynodioecious perennial, do not demonstrate a seed quantity advantage, so this study explored whether females produced better quality seed via maternal sex effects or avoidance of inbreeding depression (IBD).
Methods
Families of selfed and outcrossed seed were created using hermaphrodite mothers and families of outcrossed seed were created using female mothers. The effects of these pollination treatments were assessed under benign conditions early in life and under varied conditions later in life. To test for an effect of maternal sex, fitness components and traits associated with acclimation to variable environments of progeny of outbred hermaphrodites and females were compared. To test for expression of IBD, fitness parameters between inbred and outbred progeny of hermaphrodites were compared.
Key Results
Offspring of females were more likely to germinate in benign conditions and survive in harsh resource environments than outbred progeny of hermaphrodites. IBD was low across most life stages, and both the effect of maternal sex on progeny quality and the expression of IBD depended on both maternal family and resource condition of the progeny.
Conclusions
The effect of maternal sex and IBD on progeny quality depended on resource conditions, maternal lineage and progeny life stage. In conjunction with known lack of differences in seed quantity, the quality advantages and IBD observed here are still unlikely to be sufficient for maintenance of gynodioecy under nuclear inheritance of male sterility.
Keywords: Fragaria vesca subsp. bracteata, gynodioecy, inbreeding depression, strawberry, family-level variation, environmental variation, maternal sex
INTRODUCTION
The evolutionary pathway from hermaphroditism (combined-sex individuals) to dioecy (separate-sexed individuals) often involves gynodioecy as an intermediate step (Charlesworth and Charlesworth, 1978; Ross, 1978; Barrett, 2002). Gynodioecy, a dimorphic sexual system where hermaphrodites coexist with females, occurs in approx. 7 % of angiosperms (Richards, 1997). Females result from male sterility mutations in either the nuclear or mitochondrial genome, and in the latter case nuclear genes that restore pollen fertility may also be present (Lewis, 1941).
Under any of these sex-determining systems, an advantage is required to maintain females because they are only able to pass on genes via ovules, and thus are reproductively disadvantaged relative to hermaphrodites who can contribute to the next generation though both ovules and pollen. Under nuclear inheritance of male sterility, females need a 2-fold reproductive advantage for maintenance among hermaphrodites (Lewis, 1941; Charlesworth and Charlesworth, 1978). In contrast, when sex is determined by mitochondrial genes [i.e. cytoplasmic male sterility (CMS)] alone, the advantage required is far less because male sterility genes are maternally inherited (Lewis, 1941; Lloyd, 1974). Finally, under cytoplasmic–nuclear control, female frequencies could become cyclical, with low female frequencies occurring when the cost of restoration (e.g. via nuclear restorers) is very low, and higher frequencies occurring under times of high cost (Bailey and Delph, 2007).
Females can offset reproductive disadvantage by producing more or better quality progeny than hermaphrodites (Darwin, 1877). In particular, females can reallocate resources not spent on pollen to produce more seeds or higher quality seeds. In some gynodioecious species, females produce more seeds than hermaphrodites (Shykoff et al., 2003; Chang, 2006; Spigler and Ashman, 2012), but females in other species, including Silene acaulis, do not demonstrate a seed quantity advantage (Delph and Carroll, 2001). In such cases, female maternal advantage may arise through other mechanisms, such as seed quality. Seed quality disparities could arise through the following mechanisms: differences in maternal provisioning, genetic factors (i.e. maternal sex effects) and/or avoidance of inbreeding depression (IBD). First, seeds of females could be larger or contain more nutrients. However, Delph and colleagues (1999) tested this in S. acaulis (Caryophyllaceae) and found that seeds of females were not better provisioned than seeds of hermaphrodites, suggesting a genetic component to advantage. Secondly, females could carry a cytotype that increases progeny fitness relative to hermaphrodites, or there could be negative pleiotropic effects of nuclear restorers that affect progeny of hermaphrodites (Delph and Mutikainen, 2003). Such maternal sex advantages are expected to be expressed early in life (Stevens, 1988; Wolfe and Shmida, 1997). For example, in Sidalcea oregana subsp. spicata, a female maternal advantage was shown for germination in the greenhouse and juvenile growth rate in the field, but not during reproductive stages (Ashman, 1992). Many empirical studies on female maternal sex advantage do not examine progeny quality beyond juvenile stages, thus vital information regarding the influence of maternal sex could be gained from longer studies on progeny quality. Finally, as obligate outcrossers, females can avoid IBD and produce more fit offspring than those of selfing hermaphrodites. Progeny produced by selfing can exhibit reduced vigour due to the loss of heterozygosity or via expression of recessive deleterious alleles (Charlesworth and Willis, 2009). The effect of self-fertilization on progeny fitness, however, may vary among hermaphrodites in mixed-mating populations as some maternal lineages may have purged their deleterious alleles to a greater extent than others (Lande and Schemske, 1985; Dudash et al., 1997). Moreover, IBD can vary with environmental conditions as well as life stages (Husband and Schemske, 1996; Armbruster and Reed, 2005). For example, Wolfe (1993) indicated that the magnitude of IBD in Hydrophyllum appendiculatum increased in late life stages (reproduction, adult size) and under more stressful environments, particularly competition.
While a number of studies consider how sex-differential fecundity (reviewed in Shykoff et al.. 2003) and IBD (Sakai et al., 1989; Molina-Freaner and Jain, 1993; Kohn and Biardi, 1995; Delph and Lloyd, 1996; Mutikainen and Delph, 1998; Chang, 2007) may influence the maintenance of females, far fewer consider both effects simultaneously (however, see Jolls and Chenier, 1989; Ashman, 1992; Maki, 1993; Sakai et al., 1997; Thompson and Tarayre, 2000; Koelewijn and Van Damme, 2004), and we found only one study that tested the influence of resource level on the magnitude of these effects (Norman et al., 1995). However, a full characterization of how maternal sex effects and IBD may contribute to the maintenance of gynodioecy must include multiple life history traits, a variety of maternal genotypes, as well as varied environmental conditions (McCauley and Bailey, 2009).
Gynodioecious species closely related to those that exhibit sub-dioecy and dioecy are a vital tool in the study of sexual system evolution (Charlesworth and Charlesworth, 1978; Ross, 1978; Barrett, 1992; Ehlers and Bataillon, 2007; Goldberg et al., 2010), and Fragaria vesca subsp. bracteata is key to the understanding of these transitions in the strawberry clade. Not only is it the putative maternal contributor to both sub-dioecious F. virginiana and dioecious F. chiloensis (Njuguna et al., 2013), but it is reported to share the sex-determining mechanism with these species (Ahmadi and Bringhurst, 1991). Li and co-workers (2012) found that females in F. v. subsp. bracteata lack a seed production advantage over hermaphrodites, and that hermaphrodites are highly selfing in the field. Thus, in this study, we sought to extend prior knowledge by assessing seed quality differences between the sex morphs. Specifically, we answer the following questions. (1) Is there an overall effect of maternal sex on progeny quality or does it depend upon life stage, ecological context and maternal genotype? (2) Does maternal sex influence the ability of progeny to respond to variable environments? (3) Do progeny of self-pollinated hermaphrodites exhibit IBD, and does the degree of IBD vary among life stages, environmental conditions and/or maternal genotype? (4) Does IBD result in reduced ability to respond to variable environments?
METHODS
Study system
Fragaria vesca subsp. bracteata (Rosaceae) is a long-lived herbaceous perennial that occurs in woodlands of western North America (Staudt, 1999). Habitats in the Pacific Northwest are characterized by low light [mean ± s.e.; range, 35 ± 9 %; 13–61 % full photosynthetically active radiation (PAR) in the summer], and dry (27 ± 4%; 16–39 % soil moisture) but nutrient-rich soils (P, 41·4 ± 14·0 ppm; K, 242·4 ± 46·9 ppm; and N, 0·34 ± 0·50 %; T.-L. Ashman, unpubl. res.).
Sex is determined by a nuclear gene with the allele for male sterility (femaleness) dominant to that for hermaphroditism (Ahmadi and Bringhurst, 1991). Both hermaphrodites and females can reproduce clonally through plantlets on stolons and sexually via seeds (Staudt, 1999; Schulze et al., 2012). Populations vary in female frequency (mean 19 ± 3 % female; range 0–46 %; T.-L. Ashman, unpubl. res.). This species is pollinated by insects, and hermaphrodites are self-compatible (Staudt, 1999). In the study population, hermaphrodites have a mixed-mating system with a mean selfing rate of 0·76 (range 0·43–1·1), and produced 30 % more seeds than females because they produced more flowers per plant under greenhouse conditions (Li et al., 2012).
Plant material and pollination treatments
Plants were originally collected from a population on Mary's Peak, OR, USA (N44°29′18·4′′, W123°32′14·7′′) and were maintained in the greenhouse. Twenty-two hermaphrodites and 25 females known to be genetically distinct based on simple sequence repeat (SSR) markers (Li et al., 2012) were selected as maternal plants. Flowers on individual hermaphrodite plants were either self-pollinated (HS) or outcross-pollinated (HO), whereas those on females were outcrossed (FO). Hermaphrodites were emasculated prior to pollination and, for each treatment, pollen was applied to receptive stigmas of 2–3 flowers per plant with a toothpick. Pollen was collected from multiple genotypes for outcross pollinations, but each recipient was pollinated with only one random donor. Mature and aborted seeds were enumerated on ripe fruit, and seeds were collected for each maternal plant cross type.
Progeny growth conditions
To assess progeny vigour, we planted seeds from 18 hermaphrodite and 19 female parents (only those that produced ≥24 mature seeds/pollination treatment were used). Twenty-four seeds/maternal genotype × pollination treatment (n = 1320) were randomly planted in 288-well trays filled with Sunshine® #3 Germination Mix and placed in a growth chamber at 20/15.5 °C and 14/10 h light, day/night. Seeds were watered, trays were rotated, and germination was scored daily for 1 month.
To determine whether maternal sex effects or expression of IBD were context dependent, we transplanted 12 progeny from each family into one of three different levels of soil fertility (nutrient/water) crossed by two levels of sunlight. Two seedlings from each family experienced each condition (n = 660). Three levels of soil resources were achieved by varying the soil composition and the watering/fertilization regime in 280 mL pots as follows: (1) high soil resources, 3:1 mix of Fafard #4 soil to sand; (2) medium soil resources, 1:1 soil to sand; and (3) low soil resources, 1:3 soil to sand. Plants were randomly placed in the greenhouse and fertilized as follows: 55 ± 1 mg (mean ± s.e.) of 13:13:13 N:P:K (Nutricote® Total Controlled Release Fertilizer with Micronutrients Type 100) in the medium resource condition and 110 ± 3 mg in the high resource condition. The low resource treatment did not receive any additional fertilizer beyond that provided from the soil medium. Plants were watered as needed to achieve the desired treatment level, and soil moisture was confirmed in 2–3 pots per treatment combination 24 h post-watering on a bi-monthly basis. The percentage soil moisture was 35 ± 2 % in the high treatment, 23 ± 0·1 % in the medium treatment and 20 ± 0·1 % in the low treatment. Two levels of sunlight were achieved with shade cloth: (1) shaded plants were individually covered with a 15·2 × 15·2 cm square of 90 % shade cloth (DeWitt®) fastened to a wire frame (these plants received approx. 16 ± 2 % full sunlight); and (2) non-shaded plants had wire frames but no shade cloth, and received full sunlight. All plants were arranged randomly on greenhouse benches, and growth conditions were between 13 and 33 °C.
Plants received an 8 week winter treatment in a growth chamber (4 °C and darkness). They were then returned to the greenhouse where they experienced 24 °C and 10 h daylength, and the shade treatments were reinstated. For the next 6 weeks, the plants in the low soil resource treatment received 10 ppm, medium received 25 ppm and high received 50 ppm of 10:30:20 N:P:K fertilizer (Peter's Professional® Bloom Booster), and soil moisture was maintained as above.
Plant traits and analyses
Early life traits
We determined the proportion of seed formation per flower as the number of mature seeds divided by total ovules, and the proportion of germination as the number of germinated seedlings divided by the total number of seeds planted. For each family, we harvested the seedlings that were not transplanted (total n = 567; range of n per family, 3–12), dried them overnight at 40 °C, and weighed them. We calculated average seedling biomass per maternal genotype by cross type as the total biomass divided by seedling number.
Early life analyses of IBD and maternal sex effects
We used a generalized linear mixed model (PROC GLIMMIX) with a binary distribution to determine whether the probability of seed formation or germination varied between progeny of selfed and outcrossed hermaphrodites (pollination treatment). Maternal genotype and the genotype × pollination treatment were random factors, and their significance was determined using the COVTEST GLM option. Mean seedling biomass per maternal genotype cross type was natural log-transformed to improve normality, and was analysed using a general linear model (PROC GLM) with pollination treatment as a fixed effect, maternal genotype and maternal genotype × pollination treatment as random effects (Johnston and Schoen, 1994) and mean number of days since germination as a covariate. A significant pollination effect indicates IBD or outbreeding depression, whereas a significant pollination × genotype effect indicates that the effect of inbreeding varies among maternal genotypes.
To determine whether maternal sex influenced the probability of seed formation and germination, we used a generalized mixed linear model with maternal genotype nested within maternal sex. Mean seedling biomass was analysed using analysis of covariance (ANCOVA; PROC GLM), with maternal sex as a fixed effect and mean days since germination as a covariate. All analyses were conducted with SAS v. 9.3 (SAS Institute, 2008).
Late life traits
After 2 months of exposure to varied environmental treatments, we recorded investment in asexual reproduction and adult vegetative size. Stolons and plantlets (hereafter asexual biomass) were harvested, dried, and weighed to the nearest 0·1 mg to estimate asexual reproduction per plant. We used the product of the width of the central leaflet on the largest trifoliate leaf and the total number of leaves as an estimate of adult vegetative size following Ashman (2005). To determine if inbreeding influences traits associated with acclimation to light availability, we measured petiole length and leaf specific mass (Witkowski and Lamont, 1991; Huber, 1996). We calculated leaf specific mass (dry biomass per area) for each plant by drying a circular punch (6·0 mm diameter) from the middle leaflet on the largest leaf, and weighing it to the nearest 0·01 mg (Cahn C-31 Microbalance). The length of the largest petiole was measured to the nearest 0·1 mm using an electronic caliper. In addition, survival was scored on a bi-weekly basis throughout the experiment (8 months).
Analysis of maternal sex effects on late life traits
We analysed natural log-transformed asexual biomass, adult vegetative size, petiole length and leaf specific mass with mixed general linear models (PROC GLM), with maternal sex, shade, soil resources and all interactions as fixed effects, and maternal genotype (nested within sex) and all of its interactions as random effects. The majority of plants survived in the high resource condition (<3·6 % death); therefore, the probability of survival in low and medium resource treatments combined was analysed with generalized mixed linear model (PROC GLIMMIX), with maternal genotype (nested within sex) as a random effect.
Analysis of IBD in late life traits
To determine whether the effect of inbreeding depended on environmental conditions, asexual biomass, adult vegetative size, petiole length and leaf specific mass were analysed with general linear models (PROC GLM), with pollination treatment, shade and soil resources, and all interactions as fixed effects. Maternal genotype and its interactions were random effects. Data were natural log-transformed following Johnston and Schoen (1994). Transformations for asexual biomass, however, did not achieve normality, and although analysis of variance (ANOVA) is robust to deviation in normality (Zar, 1974), the results must be viewed with caution. Probability of survival was analysed using a generalized mixed linear model (PROC GLIMMIX) with a binary distribution, with maternal genotype as a random factor. Survival was evaluated in the low and medium resource treatments only, since mortality was negligible in the high resource treatment.
Calculation of stage-specific and cumulative IBD
For each maternal genotype, we calculated IBD according to the formula δ = 1 – (ωs/ωo) if ωs < ωo and δ = (ωo/ωs) – 1 if ωs > ωo at each stage (probability of seed formation, probability of germination, asexual biomass and probability of survival) where ωs and ωo are family mean fitness of self-pollinated and outcross progeny, respectively (Lande and Schemske, 1985; Ågren and Schemske, 1993; Husband and Schemske, 1996). To test whether IBD varied with life stage, stage-specific IBD was analysed using a general linear model (PROC GLM), with stage as a fixed effect and maternal genotype as a random effect. Relative cumulative fitness was calculated according to the above equations using the product of the fitness values of each trait for each maternal family. Most plants did not produce flowers (sexual reproduction), and thus asexual biomass is included in the cumulative fitness calculation as it reflects asexual reproduction, which may be particularly important to population growth for this clonally reproducing species (Schulze et al., 2012).
RESULTS
Effect of maternal sex and resource context in early life
Progeny of females exhibited a higher probability of germination in benign conditions and survival in stressful conditions, but, for most cases, the maternal genotype more strongly influenced the traits of progeny. Specifically, in early life traits, females and outcrossed hermaphrodites were equally as likely to produce viable seeds (57 ± 3 % vs. 59 ± 3 %; F1,44 = 0·08, P = 0·78; Fig. 1), but, within a sex, certain mothers had a higher probability of producing fertile seed than others (range 18–91 %; Z = 4·47, P < 0·001). A significant effect of maternal sex on seed germination was found, as offspring of females were significantly more likely to germinate than those of outcrossed hermaphrodites, although the difference was small (97 ± 0·8 % vs. 93 ± 1 %; F1, 47·6 = 4·11; P < 0·05; Fig. 1). Maternal genotype also affected the progeny germination rate (range: HO, 83–100 %; FO, 71–100 %; Z = 1·97, P < 0·03). Seedling biomass did not differ however between hermaphrodite and female mothers (HO, 6·1 ± 0·3; FO, 6·4 ± 0·3 mg; F1, 32 = 0·46; P = 0·50).
Fig. 1.

Mean ± s.e. proportion of seed formation, germination and survival of progeny from outcrossed females (FO) and hermaphrodites (HO) for Fragaria vesca subsp. bracteata in the greenhouse.
Effect of maternal sex and resource context in later life
In the later life stages, asexual biomass and vegetative size of progeny were affected by soil resources and shade conditions regardless of maternal sex (Table 1). For example, plants receiving full sun were 24 % larger on average than plants experiencing shade (P = 0·02, Table 1). Resource conditions influenced the magnitude of the maternal sex effect in both traits (vegetative size, Sex × S, F2,35 = 6·7, P = 0·01; asexual biomass, Sex × S × SR, F2,70 = 3·27, P = 0·04). Specifically, for vegetative size, under shade conditions progeny of females were on average larger than those of outcrossed hermaphrodites, but no significant differences were found under full sun (shade, 186·6 ± 9·3 vs. 156·7 ± 8·5; full sun, 216·8 ± 13·8 vs. 231·9 ± 14·9). Although all plants responded to shade via petiole lengthening and decreased leaf thickness, maternal sex did not affect this response (Table 1).
Table 1.
Summary of F-values for mixed-model analyses of variance on asexual biomass, vegetative size, petiole length and leaf specific mass, of progeny of females and outcrossed hermaphrodites from Fragaria vesca subsp. bracteata grown in the greenhouse
| Source of variation | d.f. | Asexual biomass | Vegetative size | Petiole length | Leaf specific mass |
|---|---|---|---|---|---|
| Sex | 1 | 0·76 | 1·20 | 1·83 | 1·14 |
| S | 1 | 80·99*** | 6·02* | 279·85*** | 5·8* |
| SR | 1 | 418·4*** | 297·16*** | 146·75*** | 2·88† |
| S × SR | 2 | 34·5*** | 12·99*** | 4·71* | 0·91 |
| Sex × S | 2 | 1·33 | 6·7* | 2·03 | 1·60 |
| Sex × SR | 2 | 1·05 | 0·60 | 0·17 | 1·10 |
| Sex × S × SR | 2 | 3·27* | 0·39 | 1·68 | 1·13 |
| G(Sex) | 35 | 2·58*** | 2·91*** | 2·18** | 0·69 |
| G(Sex) × S | 35 | 1·22 | 1·13 | 0·52 | 0·70 |
| G(Sex) × SR | 70 | 1·46* | 1·12 | 1·00 | 0·74 |
| G(Sex) × S × SR | 68–70 | 0·64 | 1·00 | 0·80 | 0·79 |
| Error | 187–213 | . | . | . | . |
Maternal sex (Sex), shade (S) and soil resource (SR) are fixed effects, and maternal genotype (G) is a random effect.
† 0·05 < P < 0·07; *P < 0·05; **P < 0·01; ***P < 0·001.
Most (90 %) of the progeny mortality occurred after exposure to the simulated winter conditions. Progeny of outcrossed hermaphrodites were less likely to survive than those of females (80 ± 3 % vs. 69 ± 4 %; F1,40·51 = 4·64, P < 0·04; Fig. 1), but maternal genotype did not influence survivorship (Z = –0·45, P = 0·65). A greater proportion of death occurred in the low soil resource condition, but shade did not affect offspring survival (F1,48·07 = 30·86, P < 0·0001; F1,39·7 = 0·21, P = 0·65, respectively).
Inbreeding depression in early life
The type of pollination did not influence probability of seed formation or any other early life trait of progeny of hermaphrodites. Self-pollination was as equally likely to result in viable seeds as outcross pollination (59 ± 3 % vs. 59 ± 4 %; F1,19 = 0·04, P = 0·83), but some genotypes displayed IBD while others displayed outbreeding depression (i.e. the difference in probability of seed production between outcrossed and selfed ranged from –58 % to 83 %; Z = 2·95, P < 0·003). Seeds of self-pollinated hermaphrodites were equally likely to germinate as those that were outcrossed (93 ± 1 % vs. 92 ± 1 %; F1,18·55 = 0·00, P = 0·96) across all maternal genotypes (range, 65–100 %; mother by pollination type, Z = 1·67, P = 0·09). Lastly, mean seedling biomass was similar for outcrossed and inbred progeny (6·1 ± 0·3 vs. 5·1 ± 0·3 mg; F1,31 = 2·80, P = 0·10).
Effect of inbreeding depression and resource context in later life
All late life traits were affected by soil resources or shade, or both (Table 2). For instance, shade reduced vegetative size by 27 %, plants in the low resource condition were 70 % smaller than plants in the high treatment (Table 2) and the combination reduced vegetative size by 76 %. Furthermore, the effect of pollination treatment was strongly dependent on soil resources and maternal genotype (e.g. for vegetative size, asexual biomass and leaf specific mass, P × SR × G: all P < 0·001; Table 2). Likewise, petiole length and leaf specific mass, measures of physiological response to environmental conditions, were affected by all resource treatments (e.g. for petiole length: S, SR, S × SR: all P < 0·05; Table 2). There was a strong effect of maternal genotype on these traits as well (G, P < 0·0001; Table 2). The magnitude of the effect of pollination treatment on leaf specific mass was influenced by both soil conditions and maternal lineage (P × SR × G; P < 0·0001). Inbred progeny produced 8 % shorter petioles than outbred progeny under shaded conditions (P × S; P < 0·05; Table 2, Fig. 2). Inbreeding affected both asexual biomass and vegetative size, but the response was highly dependent upon maternal genotype (P × G; P < 0·01; Table 2). For example, some maternal families demonstrated IBD and others demonstrated outbreeding depression for vegetative size (Fig. 3). Progeny of self-pollinated hermaphrodites were less likely to survive than progeny of outcrossed hermaphrodites (58 ± 4 % vs. 69 ± 4 %; F1,17·81 = 4·28, P = 0·053). Maternal genotype by pollination type did not influence survivorship (range, 25–88 %; Z = 0·43, P = 0·67). Soil resources, but not shade, affected offspring survival (F1,17·22 = 56·28, P < 0·0001; F1,16·19 = 0·06, P = 0·81, respectively).
Table 2.
Summary of F-values for mixed-model analyses of variance on asexual biomass, vegetative size, petiole length and leaf specific mass of progeny of self- and outcrossed hermaphrodites of Fragaria vesca subsp. bracteata grown in the greenhouse
| Source of variation | d.f. | Asexual biomass | Vegetative size | Petiole length | Leaf specific mass |
|---|---|---|---|---|---|
| P | 1 | 2·24 | 1·19 | 2·88 | 1·93 |
| G | 17 | 6·29*** | 6·89*** | 3·97*** | 52·61*** |
| S | 1 | 53·84*** | 22·14** | 214·68** | 5·19* |
| SR | 2 | 412·44*** | 161·85*** | 143·28*** | 3·24† |
| G × SR | 34 | 1·53 | 1·96** | 1·10 | 43·09*** |
| G × S | 17 | 2·01 | 0·98 | 0·56 | 0·69 |
| P × S | 1 | 1·01 | 0·58 | 4·5* | 1·49 |
| P × SR | 2 | 0·19 | 0·01 | 1·16 | 1·87 |
| P × G | 17 | 3·35*** | 2·3** | 1·39 | 53·25*** |
| S × SR | 2 | 21·3*** | 5·57** | 3·97* | 0·71 |
| P × S × SR | 2 | 1·60 | 0·51 | 1·41 | 0·99 |
| P × S × G | 34 | 1·17 | 1·26 | 0·65 | 0·66 |
| P × SR × G | 34 | 1·98** | 1·98** | 0·55 | 48·98*** |
| S × SR × G | 34 | 1·04 | 1·44 | 0·79 | 0·69 |
| P × S × SR × G | 26–33 | 1·01 | 1·36 | 0·94 | 0·83 |
| Error | 175–207 |
Pollination (P), shade (S) and soil resources (SR) are fixed effects, and maternal genotype (G) is a random effect.
† 0·05 < P < 0·07; *P < 0·05; **P < 0·01; ***P < 0·0001.
Fig. 2.
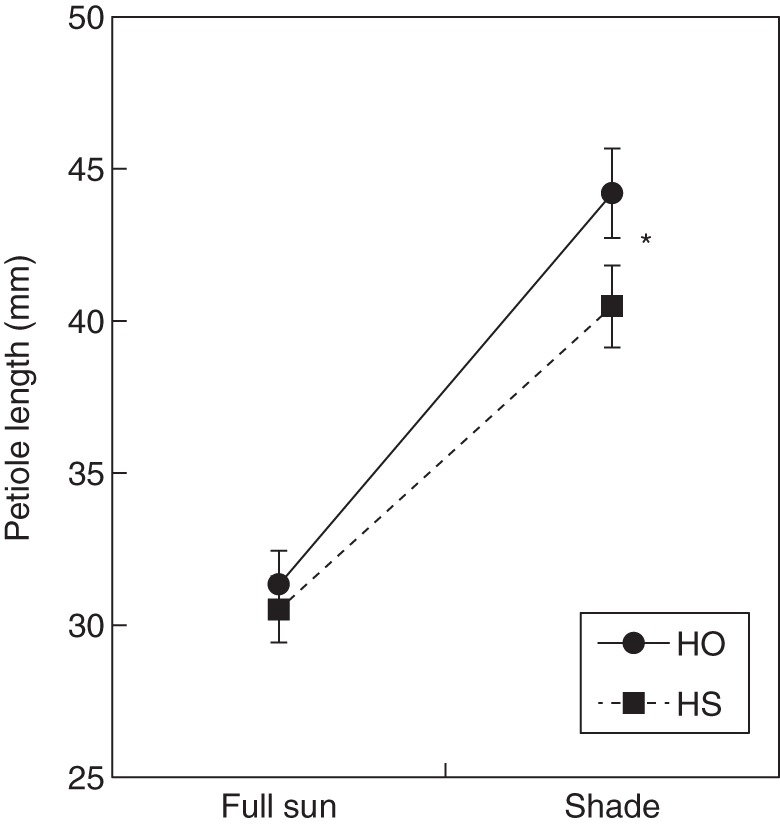
Mean ± s.e. petiole length of progeny from outcrossed (HO) and self-pollinated hermaphrodites (HS) of Fragaria vesca subsp. bracteata grown under full sun or shaded conditions in the greenhouse.
Fig. 3.
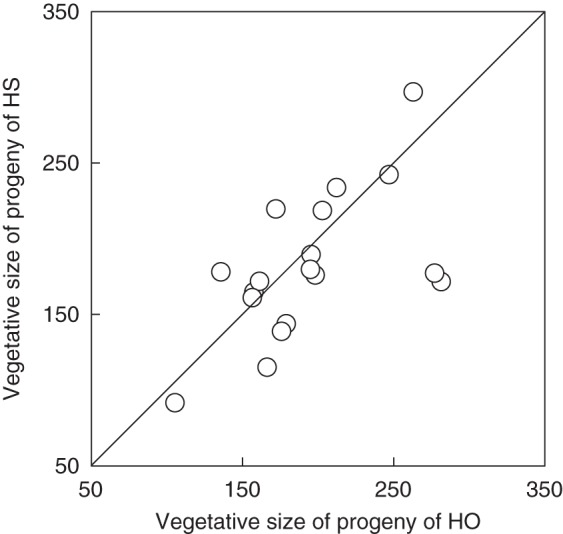
Maternal genotype mean ± s.e. vegetative size of progeny of outcrossed (HO) and self-pollinated hermaphrodites (HS) of Fragaria vesca subsp. bracteata grown in the greenhouse. Maternal families above the line expressed outbreeding depression, and those below the line expressed inbreeding depression.
Effect of life stage on IBD and cumulative IBD
The magnitude of IBD varied with life history trait (F3,51 = 4·20, P < 0·01, Fig. 4). Inbreeding depression increased with life stage, from outbreeding depression at seed formation, to negligible IBD for germination, to significant IBD for asexual biomass and adult survival. Cumulative IBD was low (0·11 ± 0·10; Fig. 4) but varied extensively among maternal genotypes (Fig. 5). If we exclude seed formation from the calculation of cumulative IBD, as it may be influenced by maternal environment, cumulative IBD is slightly higher (0·21 ± 0·06).
Fig. 4.
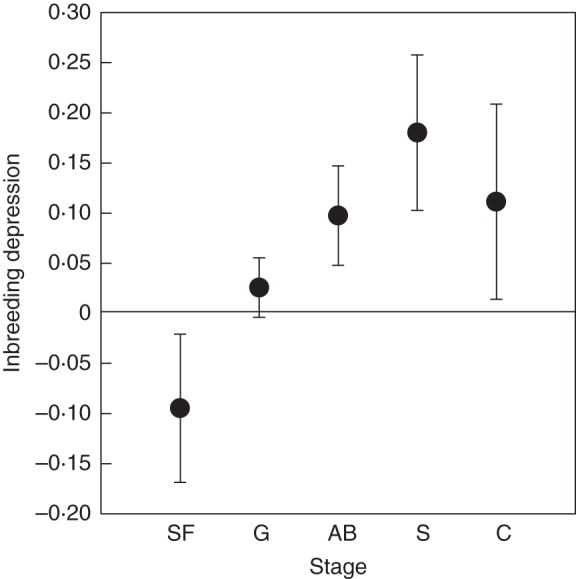
Stage-specific and cumulative inbreeding depression (mean ± s.e.) for Fragaria vesca subsp. bracteata grown in the greenhouse. Stages shown are parental seed formation (SF), progeny germination (G), asexual biomass (AB), survival (S) and cumulative inbreeding depression (C).
Fig. 5.
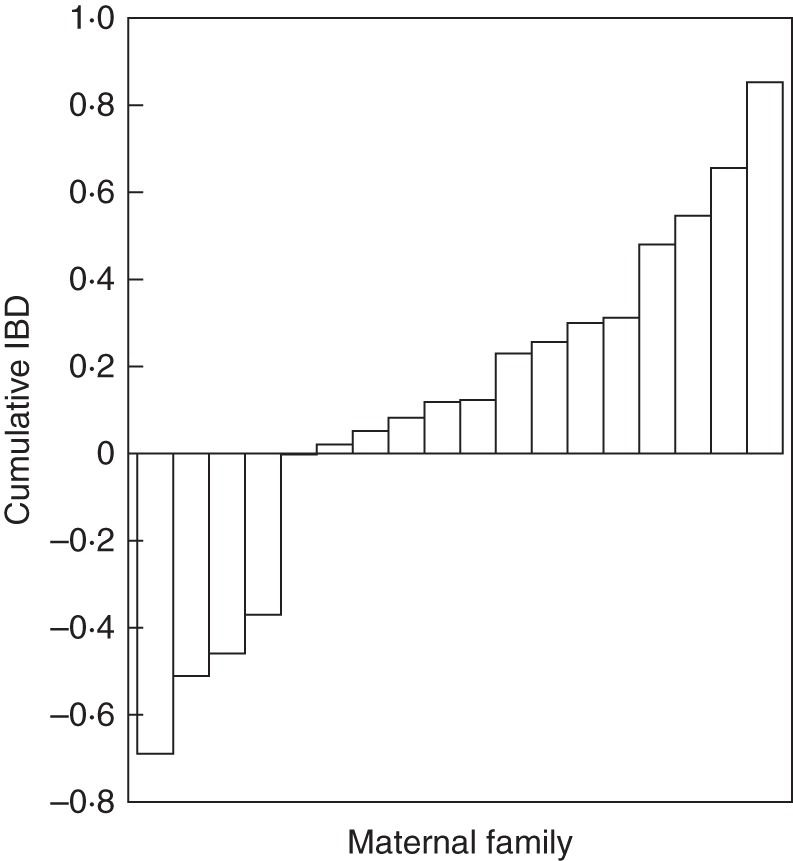
Cumulative inbreeding depression values for 18 hermaphrodite maternal genotypes of Fragaria vesca subsp. bracteata.
DISCUSSION
In F. v. subsp. bracteata we found maternal sex advantages at two crucial life stages, germination and survival, but not in a number of other traits, although maternal family-level variation was large. Expression of trait-specific IBD was highly variable among environmental contexts and maternal families, and overall cumulative IBD was moderate to low. Taken together, neither the estimated degree of IBD nor the maternal sex effects measured here appear sufficient to generate a quality advantage high enough to maintain females under theoretical models with assumptions of nuclear dominant inheritance of male sterility. Below, we discuss these results in greater detail.
Maternal sex effects
It has long been hypothesized that females, able to reallocate resources not used for pollen production, could produce higher quality progeny (Darwin, 1877; Lewis, 1941). Here, we found a maternal sex advantage in an early and important life history trait, probability of germination (Fig. 1), and this coincides with findings in studies which also controlled for outcross pollination status (Ashman, 1992; Wolfe and Shmida, 1997; but see Eckhart, 1992; Koelewijn and Van Damme, 2005). Li and colleagues (2012) found that hermaphrodites produce more seeds than females as a consequence of increased flower production. However, we found that seeds of hermaphrodites are less likely to germinate than those of females, suggesting a trade-off between seed production and germination success. The difference in progeny seed germination between the sexes was small (4 %) but statistically significant. One needs to consider, however, that these small germination differences could be exacerbated under field conditions. Although maternal sex did not affect most other traits, we found that progeny of females exhibited a greater adult survival rate compared with those of outcrossed hermaphrodites under stressful environmental conditions (Fig. 1). Most studies find that maternal sex influences seedling survival (Shykoff, 1988; Delph and Mutikainen, 2003), but here we show that survival to later life stages is also influenced, which could be very important in a long-lived plants such as this one.
Variation of IBD among life stages
Expression of IBD increased throughout the life cycle in F. v. subsp. bracteata (Fig. 1), consistent with a review by Husband and Schemske (1996) which revealed that primarily self-pollinating species demonstrated increasing levels of IBD with life stage. Several factors could contribute to the variation in IBD across life stages. First, pleiotropic effects of mutations associated with IBD that are expressed early in life may go undetected, but have cumulative negative effects later in life as gene products become involved in a wider variety of functions (Husband and Schemske, 1996). Secondly, the increase in IBD could be attributable to the fact that the early life history traits were examined in benign resource conditions, whereas later life traits were studied in harsher resource environments. Unfortunately, we cannot differentiate between these two hypotheses with our current data. Further, we must acknowledge that had we measured sexual reproductive traits or survival to later life stages, we may have documented even higher levels of IBD late in life. Indeed, expression of IBD in F. v. subsp. bracteata depended not only on life history trait (Fig. 4), but also on soil resources/shade condition (Fig. 2, Table 2) and maternal family (Fig. 5, Table 2).
Variation of IBD among environmental conditions and maternal genotypes
Although Keller and Waller (2002) argue that generalizations regarding IBD and habitat stress should be avoided, approx. 75 % of species reviewed by Armbruster and Reed (2005) demonstrated an increase in IBD in response to increased levels of environmental stress. This pattern is hypothesized to result from inbred individuals being unable to withstand stress as well as outcrossed (more heterozygous) progeny (Dudash, 1990; Roff, 1997). In F. v. subsp. bracteata, expression of IBD was affected by resource condition and maternal genotype for vegetative size and asexual biomass (Table 2). Thus, particular families exhibited an increase in IBD in response to more stressful nutrient/water conditions, while others did not. Our results join others in highlighting the fact that environmental variation can interact with maternal genotype to create complex patterns of the expression of IBD. Pray et al. (1994) found that in the beetle, Tribolium castaneum, IBD was expressed by particular maternal lineages under one environmental condition, but not in another. Similarly, Norman et al. (1995) found IBD to depend upon maternal lineage and environmental condition in Schiedea lydgatei. Interestingly, we also revealed that environmental context may affect IBD because inbred and outbred plants are differentially plastic. Specifically, inbred progeny were less able to respond to shade through petiole elongation than outbred progeny (Fig. 2). Under shaded conditions of forest edges this may put them at a competitive disadvantage.
Although the interactions between environmental resource condition and maternal genotype are very important for expression of IBD in F. v. subsp. bracteata, maternal genotype alone strongly affected IBD (Fig. 5; Table 2). Family variation in selfing history (Li et al., 2012) could be the cause of this variation (Lande and Schemske, 1985). In fact, the majority of studies have found variation in IBD among familial lines (Sakai et al., 1989; Dudash, 1990; Ågren and Schemske, 1993; Dudash et al., 1997; Mutikainen and Delph, 1998; Culley et al., 1999; Waller et al., 2008; reviewed in Armbruster and Reed, 2005).
Cumulative IBD
Based on the traits we measured, cumulative IBD is low to average. Since IBD is expected to decline in populations that are predominantly selfing (Lande and Schemske, 1985; Husband and Schemske, 1996), the low levels of IBD here are consistent with the high selfing rate (approx. 75 %) among hermaphrodites in this population (Li et al., 2012). However, the wide variation among families may also reflect the fact that hermaphrodites can be the progeny of females and thus the product of outcrossing. The cumulative IBD value estimated with seed formation allows for comparison with the data reviewed by Husband and Schemske (1996) and reveals that our value (0·11) is low but within the range for highly selfing species (mean, 0·23; range, –0·15 to 0·51; Husband and Schemske, 1996). This suggests that despite our resource treatments, the greenhouse was still a rather benign environment, an idea substantiated by the low overall mortality. Alternatively, exclusion of sexual reproduction and survival to later life stages might have contributed to an underestimate of cumulative IBD.
Maintenance of gynodioecy
If the sex determination system of this species is nuclear (Ahmadi and Bringhurst, 1991), then the female advantage necessary for maintenance within a gynodioecious population must be 2-fold (Lewis, 1941). In this study, progeny of females have a higher probability of germinating and surviving, but together these quality advantages do not appear sufficient for female maintenance. We also found that IBD in this population is minimal and depends on life history trait, maternal lineage and environment. In order for nuclear male sterility to spread, the inequality d > (1 – k)/2 must be satisfied (Charlesworth and Charlesworth, 1987) where d is IBD and k is the differential in seed fertility between females and hermaphrodites. Li et al. (2012) found k = –0·35, and that IBD for this population would have to be approx. 0·87, which is much higher than values we observed in this experiment. The inequality is clearly not satisfied with d = 0·11 (0·21; without seed formation). There are at least three factors that could account for this lack of fit. First, our estimate of cumulative IBD may be an underestimate as it did not include sexual reproduction or adult survival in this long-lived perennial. Moreover, our estimate of overall cumulative IBD treats the test environments as equally frequent, and if low resource environments are more common in the wild than rich ones, then IBD would be higher. Secondly, the standard theory (e.g. Charlesworth and Charlesworth, 1987) may not include all relevant factors. For instance, spatial variation and external factors may affect model parameters and thus predicted sex ratios (McCauley and Bailey, 2009). Thirdly, if the genetics of sex determination do not fit the published framework (Ahmadi and Bringhurst, 1991), then the current model may be inappropriate. Thus, while our work demonstrated the value of simultaneous evaluation of multiple factors, it also reveals that there is still additional research to be conducted before we can draw final conclusions regarding the mechanisms by which females are maintained in F. v. subsp. bracteata.
ACKNOWLEDGEMENTS
We would like to thank B. Chaun, K. Dehart, D. Jackson, B. McTeague, G. Meindl, K. Schuller and E. York for assistance in the greenhouse, R. Spigler and G. Arceo-Gómez for guidance on statistical analyses, and the Ashman lab members and anonymous reviewers for comments or discussion that improved the manuscript. This work was supported by the NSF [DEB 1020523 to T-L.A. and Graduate Research Fellowship to M.H.K.] and a Science Education Grant from the Howard Hughes Medical Institute to the Department of Biological Sciences [52006957 to R.M.D.].
LITERATURE CITED
- Ågren J, Schemske DW. Outcrossing rate and inbreeding depression in two annual monoecious herbs, Begonia hirsuta and B. semiovata. Evolution. 1993;47:125–135. doi: 10.1111/j.1558-5646.1993.tb01204.x. [DOI] [PubMed] [Google Scholar]
- Ahmadi H, Bringhurst RS. Genetics of sex expression in Fragaria species. American Journal of Botany. 1991;78:504–514. [Google Scholar]
- Armbruster P, Reed D. Inbreeding depression in benign and stressful environments. Heredity. 2005;95:235–242. doi: 10.1038/sj.hdy.6800721. [DOI] [PubMed] [Google Scholar]
- Ashman T-L. The relative importance of inbreeding and maternal sex in determining progeny fitness in Sidalcea oregana subsp. spicata, a gynodioecious plant. Evolution. 1992;46:1862–1874. doi: 10.1111/j.1558-5646.1992.tb01174.x. [DOI] [PubMed] [Google Scholar]
- Ashman T-L. The limits on sexual dimorphism in vegetative traits in a gynodioecious plant. American Naturalist. 2005;166:S5–S16. doi: 10.1086/444598. [DOI] [PubMed] [Google Scholar]
- Bailey MF, Delph LF. A field guide to models of sex-ratio evolution in gynodioecious species. Oikos. 2007;116:1609–1617. [Google Scholar]
- Barrett SCH. Gender variation and the evolution of dioecy in Wurmbea dioica (Liliaceae) Journal of Evolutionary Biology. 1992;5:423–444. [Google Scholar]
- Barrett SCH. The evolution of plant sexual diversity. Nature Reviews Genetics. 2002;3:274–284. doi: 10.1038/nrg776. [DOI] [PubMed] [Google Scholar]
- Chang SM. Female compensation through the quantity and quality of progeny in a gynodioecious plant, Geranium maculatum (Geraniaceae) American Journal of Botany. 2006;93:263–270. doi: 10.3732/ajb.93.2.263. [DOI] [PubMed] [Google Scholar]
- Chang SM. Gender-specific inbreeding depression in a gynodioecious plant Geranium maculatum (Geraniaceae) American Journal of Botany. 2007;94:1193–2004. doi: 10.3732/ajb.94.7.1193. [DOI] [PubMed] [Google Scholar]
- Charlesworth B, Charlesworth D. A model for the evolution of dioecy and gynodioecy. American Naturalist. 1978;112:975–997. [Google Scholar]
- Charlesworth D, Charlesworth B. Inbreeding depression and its evolutionary consequences. Annual Review of Ecology and Systematics. 1987;18:237–268. [Google Scholar]
- Charlesworth D, Willis J. The genetics of inbreeding depression. Nature Reviews Genetics. 2009;10:783–796. doi: 10.1038/nrg2664. [DOI] [PubMed] [Google Scholar]
- Culley TM, Weller SG, Sakai AK, Rankin AE. Inbreeding depression and selfing rates in a self-compatible, hermaphroditic species, Schiedea membranacea (Caryophyllaceae) American Journal of Botany. 1999;86:980–987. [PubMed] [Google Scholar]
- Darwin C. The different forms of flowers on plants of the same species. Chicago: The University of Chicago Press; 1877. [Google Scholar]
- Delph LF, Carroll SB. Factors affecting relative seed fitness and female frequency in a gynodioecious species. Silene acaulis. Evolutionary Ecology Research. 2001;3:487–505. [Google Scholar]
- Delph LF, Lloyd DG. Inbreeding depression in the gynodioecious shrub Hebe subalpina (Scrophulariaceae) New Zealand Journal of Botany. 1996;34:241–247. [Google Scholar]
- Delph LF, Mutikainen P. Testing why the sex of the maternal parent affects seedling survival in a gynodioecious species. Evolution. 2003;57:231–239. doi: 10.1111/j.0014-3820.2003.tb00258.x. [DOI] [PubMed] [Google Scholar]
- Delph LF, Bailey MF, Marr DL. Seed provisioning in gynodioecious Silene acaulis (Caryophyllaceae) American Journal of Botany. 1999;86:140–144. [PubMed] [Google Scholar]
- Dudash MR. Relative fitness of selfed and outcrossed progeny in a self-compatible, protandrous species, Sabatia angularis L. (Gentianaceae): a comparison in three environments. Evolution. 1990;44:1129–1139. doi: 10.1111/j.1558-5646.1990.tb05220.x. [DOI] [PubMed] [Google Scholar]
- Dudash MR, Carr DE, Fenster CB. Five generations of enforced selfing and outcrossing in Mimulus guttatus: inbreeding depression variation at the population and family level. Evolution. 1997;51:54–65. doi: 10.1111/j.1558-5646.1997.tb02388.x. [DOI] [PubMed] [Google Scholar]
- Eckhart VM. Resource compensation and the evolution of gynodioecy in Phacelia linearis (Hydrophyllaceae) Evolution. 1992;46:1313–1328. doi: 10.1111/j.1558-5646.1992.tb01126.x. [DOI] [PubMed] [Google Scholar]
- Ehlers BK, Bataillon T. ‘Inconstant males’ and the maintenance of labile sex expression in subdioecious plants. New Phytologist. 2007;174:194–211. doi: 10.1111/j.1469-8137.2007.01975.x. [DOI] [PubMed] [Google Scholar]
- Goldberg MT, Spigler RB, Ashman TL. Comparative genetic mapping points to different sex chromosomes in sibling species of wild strawberry (Fragaria) Genetics. 2010;186:1425–1433. doi: 10.1534/genetics.110.122911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber H. Plasticity of internodes and petioles in prostrate and erect Potentilla species. Functional Ecology. 1996;10:401–409. [Google Scholar]
- Husband BC, Schemske DW. Evolution of the magnitude and timing of inbreeding depression in plants. Evolution. 1996;50:54–70. doi: 10.1111/j.1558-5646.1996.tb04472.x. [DOI] [PubMed] [Google Scholar]
- Johnston MO, Schoen DJ. On the measurement of inbreeding depression. Evolution. 1994;48:1735–1741. doi: 10.1111/j.1558-5646.1994.tb02209.x. [DOI] [PubMed] [Google Scholar]
- Jolls CL, Chenier TC. Gynodioecy in Silene vulgaris (Caryophyllaceae): progeny success, experimental design, and maternal effects. American Journal of Botany. 1989;76:1360–1367. [Google Scholar]
- Keller LF, Waller DM. Inbreeding effects in wild populations. Trends in Ecology and Evolution. 2002;17:230–241. [Google Scholar]
- Koelewijn HP, Van Damme JMM. Effects of seed size, inbreeding and maternal sex on offspring fitness in gynodioecious Plantago coronopus. Journal of Ecology. 2005;93:373–383. [Google Scholar]
- Kohn JH, Biardi JE. Outcrossing rates and inferred levels of inbreeding depression in gynodioecious Cucurbita foetidissima (Cucurbitaceae) Heredity. 1995;75:77–83. [Google Scholar]
- Lande R, Schemske DW. The evolution of self-fertilization and inbreeding depression in plants. I. Genetic models. Evolution. 1985;39:24–40. doi: 10.1111/j.1558-5646.1985.tb04077.x. [DOI] [PubMed] [Google Scholar]
- Lewis D. Male sterility in natural populations of hermaphrodite plants. The equilibrium between females and hermaphrodites with different types of inheritance. New Phytologist. 1941;40:56–63. [Google Scholar]
- Li J, Koski MH, Ashman T-L. Functional characterization of gynodioecy in Fragaria vesca subsp. bracteata (Rosaceae) Annals of Botany. 2012;109:545–552. doi: 10.1093/aob/mcr279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd DG. Theoretical sex ratios of dioecious and gynodioecious angiosperms. Heredity. 1974;32:11–34. [Google Scholar]
- Maki M. Outcrossing and fecundity advantage of females in gynodioecious Chionographis japonica var. kurohimensis (Liliaceae) American Journal of Botany. 1993;80:629–634. [Google Scholar]
- McCauley DE, Bailey MF. Recent advances in the study of gynodioecy: the interface of theory and emiricism. Annals of Botany. 2009;104:611–620. doi: 10.1093/aob/mcp141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Freaner F, Jain SK. Inbreeding effects in a gynodioecious population of the colonizing species Trifolium hirtum All. Evolution. 1993;47:1472–1479. doi: 10.1111/j.1558-5646.1993.tb02169.x. [DOI] [PubMed] [Google Scholar]
- Mutikainen P, Delph LF. Inbreeding depression in gynodioecious Lobelia siphilitica: among-family differences override between-morph differences. Evolution. 1998;52:1572–1582. doi: 10.1111/j.1558-5646.1998.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Njuguna W, Liston A, Cronn R, Ashman T-L, Bassil N. Insights into phylogeny, sex function and age of Fragaria based on whole chloroplast genome sequencing. Molecular Phylogenetics and Evolution. 2013;66:17–29. doi: 10.1016/j.ympev.2012.08.026. [DOI] [PubMed] [Google Scholar]
- Norman JK, Sakai AK, Weller SG, Dawson TE. Inbreeding depression in morphological and physiological traits of Schiedea lydgatei (Caryophyllaceae) in two environments. Evolution. 1995;49:297–306. doi: 10.1111/j.1558-5646.1995.tb02242.x. [DOI] [PubMed] [Google Scholar]
- Pray LA, Schwartz JM, Goodnight CJ, Stevens L. Environmental dependency of inbreeding depression: implications for conservation biology. Conservation Biology. 1994;8:562–568. [Google Scholar]
- Richards AJ. Plant breeding systems. London: Chapman & Hall; 1997. [Google Scholar]
- Roff DA. Evolutionary quantitative genetics. New York: Chapman & Hall; 1997. [Google Scholar]
- Ross M. The evolution of gynodioecy and subdioecy. Evolution. 1978;32:174–188. doi: 10.1111/j.1558-5646.1978.tb01107.x. [DOI] [PubMed] [Google Scholar]
- Sakai AK, Karoly K, Weller SG. Inbreeding depression in Schiedea globosa and S. salicaria (Caryophyllaceae), subdioecious and gynodioecious Hawaiian species. American Journal of Botany. 1989;76:437–444. [Google Scholar]
- Sakai AK, Weller SG, Chen M-L, Chou S-Y, Tasanont C. Evolution of gynodioecy and maintenance of females: the role of inbreeding depression, outcrossing rates, and resource allocation in Schiedea adamantis (Caryophyllaceae) Evolution. 1997;51:724–736. doi: 10.1111/j.1558-5646.1997.tb03656.x. [DOI] [PubMed] [Google Scholar]
- SAS Institute. SAS version 9.3. Cary: NC; 2008. [Google Scholar]
- Schulze J, Rufener R, Erhardt A, Stoll P. The relative importance of sexual and clonal reproduction for population growth in the perennial herb Fragaria vesca. Population Ecology. 2012;54:1–12. [Google Scholar]
- Shykoff JA. Maintenance of gynodioecy in Silene acaulis (Caryophyllaceae): stage-specific fecundity and viability selection. American Journal of Botany. 1988;75:844–850. [Google Scholar]
- Shykoff JA, Kolokotronis SO, Collin CL, López-Villavicencio M. Effects of male sterility on reproductive traits in gynodioecious plants: a meta-analysis. Oecologia. 2003;135:1–9. doi: 10.1007/s00442-002-1133-z. [DOI] [PubMed] [Google Scholar]
- Spigler RB, Ashman TL. Gynodioecy to dioecy: are we there yet? Annals of Botany. 2012;109:531–543. doi: 10.1093/aob/mcr170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudt G. Systematics and geographic distribution of the American strawberry species: Taxonomic studies in the genus Fragaria (Rosaceae: Potentilleae) Berkeley: University of California Press; 1999. [Google Scholar]
- Stevens D. On the gynodioecious polymorphism in Saxifraga granulata L. (Saxifragaceae) Biological Journal of the Linnean Society. 1988;35:15–28. [Google Scholar]
- Thompson JD, Tarayre M. Exploring the genetic basis and proximate cause of female fertility advantage in gynodioecious Thymus vulgaris. Evolution. 2007;54:1510–1520. doi: 10.1111/j.0014-3820.2000.tb00697.x. [DOI] [PubMed] [Google Scholar]
- Waller DM, Dole J, Bersch AJ. Effects of stress and phenotypic variation on inbreeding depression in Brassica rapa. Evolution. 2008;62:917–931. doi: 10.1111/j.1558-5646.2008.00325.x. [DOI] [PubMed] [Google Scholar]
- Witkowski E, Lamont BB. Leaf specific mass confounds leaf density and thickness. Oecologia. 1991;88:486–493. doi: 10.1007/BF00317710. [DOI] [PubMed] [Google Scholar]
- Wolfe LM. Inbreeding depression in Hydrophyllum appendiculatum: role of maternal effects, crowding, and parental mating history. Evolution. 1993;47:374–386. doi: 10.1111/j.1558-5646.1993.tb02100.x. [DOI] [PubMed] [Google Scholar]
- Wolfe LM, Shmida A. The ecology of sex expression in a gynodioecious Israeli desert shrub (Ochradenus baccatus) Ecology. 1997;78:101–110. [Google Scholar]
- Zar J. Biostatistical analysis. Englewood Cliffs, NJ: Prentice-Hall, Inc; 1974. [Google Scholar]


