Abstract
This study describes the contribution of de novo glucose synthesis by the kidney to blood glucose homeostasis in rats. The net glucose release by the kidney in vivo was measured by an isotope-dilution method, which calculated the extent of dilution of injected [14C]glucose by glucose newly synthesized in the kidney. The extent of dilution was determined from the difference between the decrease of the actual blood glucose concentration and that of the radioactivity of [14C]glucose, after injecting [14C]glucose into functionally hepatectomized rats.
The results indicate that the net glucose release by the kidney in vivo in normal fed rats was 0.75±0.13 mg/dl per min, and that its contribution to blood glucose was 25.9±5.0%. When unilateral nephrectomy was performed, under the same conditions, renal net glucose release was one-half of that in rats with two intact kidneys, which indicates the quantitative accuracy of the isotope-dilution method employed in this study.
In rats starved for 24 h, the renal net glucose release increased to 0.99±0.08 mg/dl per min. Diabetic rats showed a remarkably higher renal net glucose of 2.28±0.33 mg/dl per min, which was 360% of the normal level. Treatment of diabetic rats with insulin, restored the renal net glucose release to the normal level. In acidotic rats, renal net glucose release was as great as 1.03±0.15 mg/dl per min, which suggests that the acid-base balance participates in control of renal glucose output. Measurements every 6 h throughout the day showed that glucose was supplied from the kidney at a constant rate without any circadian rhythm.
These data suggest that renal gluconeogenesis is of physiological importance in the maintenance of homeostasis of blood glucose.
Full text
PDF
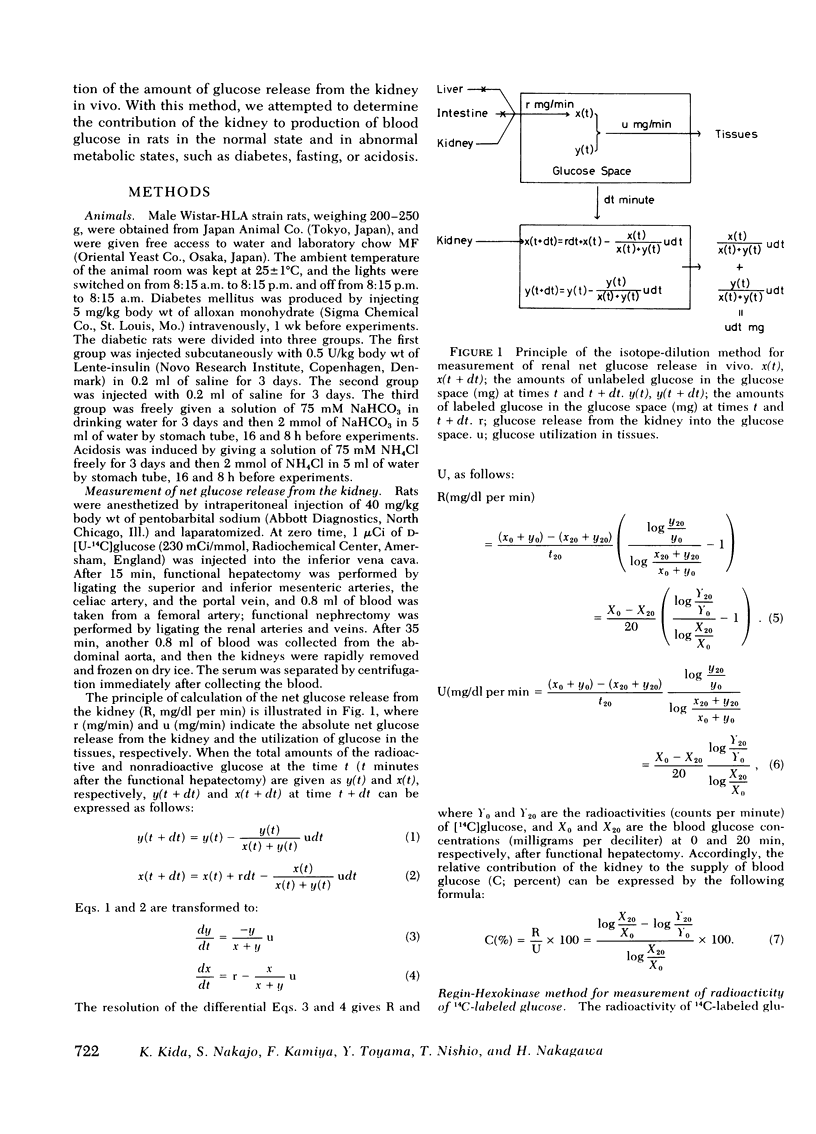




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archer L. T., Benjamin B., Lane M. M., Hinshaw L. B. Renal gluconeogenesis and increased glucose utilization in shock. Am J Physiol. 1976 Sep;231(3):872–879. doi: 10.1152/ajplegacy.1976.231.3.872. [DOI] [PubMed] [Google Scholar]
- BOLLMAN J. L., GRINDLAY J. H. Measurement of renal gluconeogenesis. Am J Physiol. 1952 Jul;170(1):38–44. doi: 10.1152/ajplegacy.1952.170.1.38. [DOI] [PubMed] [Google Scholar]
- Bahlmann J., Ochwadt B., Schröder E. Uber den Glucose- und Lactat-Stoffwechsel von isolierten Hndenieren. Pflugers Arch Gesamte Physiol Menschen Tiere. 1965 Oct 26;286(3):207–219. [PubMed] [Google Scholar]
- Benoy M. P., Elliott K. A. The metabolism of lactic and pyruvic acids in normal and tumour tissues: Synthesis of carbohydrate. Biochem J. 1937 Aug;31(8):1268–1275. doi: 10.1042/bj0311268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill P. C., Belloni F. L., Churchill M. C. Net renal glucose release in the rat. Am J Physiol. 1973 Sep;225(3):528–531. doi: 10.1152/ajplegacy.1973.225.3.528. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN M. S., HENRY W. L., HUDDLESTUN B., LEVINE R. Action of insulin on transfer of sugars across cell barriers; common chemical configuration of substances responsive to action of the hormone. Am J Physiol. 1953 May;173(2):207–211. doi: 10.1152/ajplegacy.1953.173.2.207. [DOI] [PubMed] [Google Scholar]
- Goldman H. Oxytocin modulation of blood flow in the unanesthetized male rat. Am J Physiol. 1968 Apr;214(4):860–862. doi: 10.1152/ajplegacy.1968.214.4.860. [DOI] [PubMed] [Google Scholar]
- Goodman A. D., Fuisz R. E., Cahill G. F., Jr Renal gluconeogenesis in acidosis, alkalosis, and potassium deficiency: its possible role in regulation of renal ammonia production. J Clin Invest. 1966 Apr;45(4):612–619. doi: 10.1172/JCI105375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUGGETT A. S., NIXON D. A. Use of glucose oxidase, peroxidase, and O-dianisidine in determination of blood and urinary glucose. Lancet. 1957 Aug 24;273(6991):368–370. doi: 10.1016/s0140-6736(57)92595-3. [DOI] [PubMed] [Google Scholar]
- ITZHAKI R. F., GILL D. M. A MICRO-BIURET METHOD FOR ESTIMATING PROTEINS. Anal Biochem. 1964 Dec;9:401–410. doi: 10.1016/0003-2697(64)90200-3. [DOI] [PubMed] [Google Scholar]
- KREBS H. A., BENNETT D. A., DE GASQUET P., GASQUET P., GASCOYNE T., YOSHIDA T. Renal gluconeogenesis. The effect of diet on the gluconeogenic capacity of rat-kidney-cortex slices. Biochem J. 1963 Jan;86:22–27. doi: 10.1042/bj0860022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCANN W. P., JUDE J. R. The synthesis of glucose by the kidney. Bull Johns Hopkins Hosp. 1958 Aug;103(2):77–93. [PubMed] [Google Scholar]
- Nagai K., Suda M., Yamagishi O., Toyama Y., Nakagawa H. Studies on the circadian rhythm of phosphoenolpyruvate carboxykinase. III. Circadian rhythm in the kidney. J Biochem. 1975 Jun;77(6):1249–1254. [PubMed] [Google Scholar]
- Nakagawa H., Nagai K. Cold adaptation. I. Effect of cold-exposure on gluconeogenesis. J Biochem. 1971 May;69(5):923–934. doi: 10.1093/oxfordjournals.jbchem.a129543. [DOI] [PubMed] [Google Scholar]
- Nishiitsutsuji-Uwo J. M., Ross B. D., Krebs H. A. Metabolic activities of the isolated perfused rat kidney. Biochem J. 1967 Jun;103(3):852–862. doi: 10.1042/bj1030852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen O. E., Felig P., Morgan A. P., Wahren J., Cahill G. F., Jr Liver and kidney metabolism during prolonged starvation. J Clin Invest. 1969 Mar;48(3):574–583. doi: 10.1172/JCI106016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REINECKE R. M. Renal arteriovenous changes in lactic acid and sugar in the eviscerated rat. Am J Physiol. 1955 Aug;182(2):243–246. doi: 10.1152/ajplegacy.1955.182.2.243. [DOI] [PubMed] [Google Scholar]
- Smith O. K., Long C. N. Renal gluconeogenesis in eviscerated diabetic rats. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1618–1622. doi: 10.1073/pnas.68.7.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner A. L., Goodman A. D., Treble D. H. Effect of metabolic acidosis on renal gluconeogenesis in vivo. Am J Physiol. 1968 Jul;215(1):211–217. doi: 10.1152/ajplegacy.1968.215.1.211. [DOI] [PubMed] [Google Scholar]
- Suda M., Nagai K., Nakagawa H. Studies on the circadian rhythm of phosphoenolpyruvate carboxykinase activity in rats. I. Mechanism of circadian increase in liver enzyme with special reference to hormonal and dietary effects. J Biochem. 1973 Apr;73(4):727–738. doi: 10.1093/oxfordjournals.jbchem.a130135. [DOI] [PubMed] [Google Scholar]



