Abstract
Background and Aims
Aluminium is toxic in acid soils because the soluble Al3+ inhibits root growth. A mechanism of Al3+ tolerance discovered in many plant species involves the release of organic anions from root apices. The Al3+-activated release of citrate from the root apices of Al3+-tolerant genotypes of barley is controlled by a MATE gene named HvAACT1 that encodes a citrate transport protein located on the plasma membrane. The aim of this study was to investigate whether expressing HvAACT1 with a constitutive promoter in barley and wheat can increase citrate efflux and Al3+ tolerance of these important cereal species.
Methods HvAACT1
was over-expressed in wheat (Triticum aestivum) and barley (Hordeum vulgare) using the maize ubiquitin promoter. Root apices of transgenic and control lines were analysed for HvAACT1 expression and organic acid efflux. The Al3+ tolerance of transgenic and control lines was assessed in both hydroponic solution and acid soil.
Key Results and Conclusions
Increased HvAACT1 expression in both cereal species was associated with increased citrate efflux from root apices and enhanced Al3+ tolerance, thus demonstrating that biotechnology can complement traditional breeding practices to increase the Al3+ tolerance of important crop plants.
Keywords: Acid soil, aluminium, resistance, tolerance, wheat, transgenic, barley, Hordeum vulgare, Triticum aestivum
INTRODUCTION
Approximately 30 % of total arable lands are acidic (von Uexkull and Mutert, 1995; Ma and Ryan, 2010). The two main geographical regions of acid soils are the humid northern temperate zone and the humid tropics (von Uexkull and Mutert, 1995). Acid soils limit crop yields due to nutrient deficiencies and mineral toxicities. Non-adapted plants grown on acid soils typically have smaller root systems because high concentrations of soluble aluminium (Al3+) inhibit root elongation (Munns, 1965). Al3+ can begin to inhibit root growth of wheat (Triticum aestivum) within minutes or hours in simple hydroponic solutions (Ryan et al., 1992). Longer exposures result in thickened roots, damaged root caps and lesions in the epidermal and cortical tissues near the apices (Foy, 1984). Al3+ absorbed by plants can rapidly trigger other stress responses such as oxidative stress and callose production (Sivaguru et al., 2000; Yamamoto et al., 2001; Horst et al., 2010) as well as interfering with cytosolic Ca2+ homeostasis, cell wall metabolism and nutrient uptake (Pineros and Tester, 1993; Gassmann and Schroeder, 1994).
Variation in Al3+ tolerance occurs within many crop species including maize (Zea mays), wheat, barley (Hordeum vulgare), rice (Oryza sativa) and sorghum (Sorghum bicolor) (Foy, 1988; Furlani et al., 1987; Foy et al., 1993; Koyama et al., 2003; Kochian et al., 2004; Magalhaes et al., 2007; Ryan et al., 2011). This variation provides opportunities for breeders to develop new cultivars better suited to acid soils. Barley is among the most Al3+-sensitive cereal crops and, although genotypic variation exists within this species, Al3+-tolerant genotypes are more sensitive than Al3+-tolerant wheat plants. A seedling-based screen of diverse barley genotypes identified the cultivars Kearney and ‘Golden Promise’ as being Al3+ sensitive and ‘Dayton’ and several Japanese cultivars as being among the most tolerant (Moroni et al., 2010). Hexaploid wheat has a greater variation in Al3+ tolerance than barley, with differences in root growth that can vary by >10-fold in short-term growth assays or in field screens (Rengel and Jurkic, 1992; Bona et al., 1993; Ryan et al., 1995; Pinto-Carnide and Guedes-Pinto, 1999; Garvin and Carver, 2003; Tang et al., 2003; Raman et al., 2008; Dai et al., 2009).
Al3+ tolerance mechanisms in wheat and barley rely on the release of organic anions from root apices (Ma et al., 2001; Ryan et al., 2001; Delhaize et al., 2007). Malate and citrate are the major organic anions released from wheat and barley, respectively (Ma et al., 2001; Ryan et al., 2001). Once these anions are released from root cells they bind the Al3+ and exclude it from the sensitive root apices. Efflux is largely restricted to the root apices and in most cases is activated by exposure to Al3+.
The Al3+ tolerance genes controlling organic anion efflux from roots have been isolated from several crop species (Zhou et al., 2011; Delhaize et al., 2012a). The pattern that has emerged to date indicates that genes controlling malate efflux belong to the ALMT (aluminium-activated malate transporter) family of genes and genes controlling citrate efflux belong to the MATE (multidrug and toxic compound extrusion) family. The first Al3+ tolerance gene cloned from wheat, TaALMT1, was identified by transcript analysis (Sasaki et al., 2004), and the first MATE genes involved in Al3+ tolerance were identified in sorghum (SbMATE) (Magalhaes et al., 2007) and barley (HvAACT1) by map-based cloning (Furukawa et al., 2007). Both HvAACT1 and TaALMT1 are constitutively expressed in roots, and Al3+-tolerant cultivars show significantly greater expression in the root apices than sensitive cultivars (Sasaki et al., 2004; Furukawa et al., 2007). In contrast, Al3+ induces SbMATE expression in sorghum, with tolerant genotypes attaining a higher expression level than sensitive genotypes (Magalhaes et al., 2007).
Raising the productivity of marginal land will be essential for increasing the food requirement of a growing population. Land management practices such as liming and the use of Al3+-tolerant germplasm are complementary strategies for managing acid soils. Transgenic strategies have the potential to enable crops normally sensitive to acid soils such as barley to be grown effectively on a wide range of acid soils (Ryan et al., 2011). A diverse range of genes have been expressed in plants to enhance their Al3+ tolerance. In view of the importance of organic anion efflux in tolerance mechanisms, most of these studies have targeted genes involved in organic anion synthesis and organic anion transport. For example, the wheat TaALMT1 gene enhanced the Al3+ tolerance of barley, wheat and Arabidopsis thaliana (Delhaize et al., 2004; Pereira et al., 2010; Ryan et al., 2011). Expression of TaALMT1 in barley was found to confer Al3+-activated malate efflux and a significant increase in Al3+ tolerance (Delhaize et al., 2004). The Al3+ tolerance of some transgenic wheat lines in both hydroponic and soil experiments was similar to levels measured in naturally Al3+-tolerant wheat lines such as ET8 (Pereira et al., 2010). MATE transporters have also been transformed into model species such as tobacco and arabidopsis to increase Al3+ tolerance (Furukawa et al., 2007; Magalhaes et al., 2007), but have not been extensively assessed in crop species such as barley (Fujii et al., 2012).
The aim of this study was to investigate whether expressing HvAACT1 with a constitutive promoter in barley and wheat can increase citrate efflux and Al3+ tolerance of these important cereal species.
MATERIALS AND METHODS
Genotypes
‘Golden Promise’, an Al3+-sensitive cultivar of barley, Hordeum vulgare, was transformed with HvAACT1. The Al3+-tolerant cultivar ‘Dayton’ was used as a control for assessing citrate efflux and Al3+ tolerance. The two cultivars of wheat (Triticum aestivum) transformed with the HvAACT1 gene were the Al3+-sensitive cultivar ‘Bob White 26’ (‘BW26’) and the Al3+-tolerant cultivar ‘Fielder’. The Al3+-tolerant wheat cultivar ‘Carazinho’ was included as a positive control in several experiments because it possesses both Al3+-activated malate efflux and constitutive citrate efflux from root apices (Ryan et al., 2009).
Barley transformation
HvAACT1 cDNA was obtained from JianFeng Ma (Okayama University, Japan) and cloned into pWBvec8 (Wang et al., 1998) which uses the maize ubiquitin promoter to drive transgene expression. The plasmid was introduced into Agrobacterium (strain AGL-1) by electroporation. The Agrobacterium was then used to transform barley (‘Golden Promise’) as described by Tingay et al. (1997). Genomic DNA was isolated from the 119 primary transgenic (T0) plants, and polymerase chain reaction (PCR) was used to confirm the presence of the transgene. The primers used (forward: 5′-TGCAGCATCTATTCATATGC-3′; reverse: 5′-AGAGGTAGAGCCCCGTCGT-3′) did not amplify the endogenous HvAACT1 gene of barley because the forward primer targets the maize ubiquitin intron included in the construct. Primary T0 and segregating T1 lines were grown for several days in hydroponics and tested for citrate efflux and Al3+ tolerance. A sub-set of these lines was chosen to generate T2 seed for further analysis. The transgenic plants (either homozygous or hemizygous) and null plants within these families were identified by PCR or by an agar-based antibiotic resistance screen using leaf segments (Wang et al., 2007).
Wheat transformation
Two wheat cultivars were transformed with HvAACT1 using the biolistic method as described by Pereira et al. (2010). HvAACT1 cDNA was ligated into the pWubi_tml vector where expression of the transgene is driven by the maize ubiquitin promoter (Wang et al., 1998). Embryos were co-bombarded with the pCMneoSTLS2 vector which contained a geneticin (G418) resistance gene to enable selection of transgenic plants. A total of 56 T0 wheat plants generated from ‘BW26’ and one line from ‘Fielder’ were used to generate T1 seed (segregating generation). Citrate efflux was measured from 10–15 plants in each of the 57 T1 wheat families (data not shown). Lines with the highest citrate efflux were used to generate T2 seed. Putative homozygous T2 lines and null lines were identified by PCR. A single ‘Fielder’ line named Ta(Fielder):T1_8 was also grown for T2 seed, but no null lines could be identified from this family so the parental line ‘Fielder’ was used as a control.
Quantitative real-time PCR
Root apices (5 mm) were collected and the total RNA was extracted using an RNeasy Mini kit (Qiagen). First-strand cDNA was obtained by reverse transcription using an Invitrogen kit with an oligo(dT) primer. The forward and reverse primers targeting HvAACT1 were 5′-AGCAGCCAAGACCTTGAGAA-3′ and 5′-GCCGAAAAGATCAGGAACAC-3′, respectively. The reference gene used for expression analysis in barley was actin, and the primers were 5′-GACTCTGGTGATGGTGTCAGC-3′ (forward) and 5′-GGCTGGAAGAGGACCTCAGG-3′ (reverse). The reference gene used for expression analysis in wheat was glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and the primers were 5′-TCAGACTCCTCCTTGATAGC-3′ (forward) and 5′-GTTGAGGGTTTGATGACCAC-3′ (reverse). The parental barley line ‘Golden Promise’ is an Al3+-sensitive cultivar and therefore shows low expression of HvAACT1, and wild-type wheat does not express HvAACT1.
Assaying citrate efflux and malate efflux from root apices
Seedlings were grown for 4 d in the nutrient solution described below (without added AlCl3). Root apices (5 mm) were excised from seedlings and washed for 20 min on a platform shaker (60 rpm) in sealed glass vials with 1 mL of control solution (0·2 mm CaCl2, pH 4·3). The control solution was then discarded and 1 mL of treatment solution (with or without AlCl3) was added. The vials containing the root tips were shaken (60 rpm) for 2 h. The enzyme assay used to determine citrate concentration is described by Wang et al. (2007). Briefly, the samples were dried on a rotary vacuum drier and resuspended in 114 µL of assay solution consisting of 100 mm Tris, pH 8·0, 0·25 µL of malate dehydrogenase (Sigma-Aldrich Cat. no. M-7383), 0·25 µL of lactate dehydrogenase (Roche Cat. no. 107042) and 14 µL of NADH (Sigma-Aldrich Cat. no. N8129) which was prepared by dissolving 16 mg of NADH and 15 mg of NaHCO3 in 2 mL of water. NADH consumption, read as the decrease in A340, was monitored after the reaction was initiated with 1 µL of citrate lyase (Sigma-Aldrich Cat. no. C0897) prepared by dissolving 5 mg of citrate lyase in 100 µL of water. The initial citrate content in each sample was calculated from a standard curve. Malate concentration was measured with an enzyme assay as described previously (Ryan et al., 1995).
Measurements of Al3+ tolerance in hydroponic culture
Seed were sterilized with 20 % bleach for 15 min, rinsed thoroughly and germinated in the dark for 2 d at 4° C and then 2 d at 28 °C. Root length of the seedlings was measured and they were placed in an aerated nutrient solution containing 500 µm KNO3, 500 µm CaCl2, 500 µm NH4NO3, 150 µm MgSO4, 10 µm KH2PO4, 2 µm Fe:EDTA, 11 µm H3BO3, 2 µm MnCl2, 0·35 µm ZnCl2 and 0·2 µm CuCl2. For barley, Al3+ tolerance was estimated by measuring net root length after 4 d in 0, 1, 2 and 4 µm AlCl3. For wheat, the concentrations were 0, 4 and 8 µm AlCl3 for the ‘BW26’-derived lines and 0, 4 and 30 µm AlCl3 for the ‘Fielder’-derived line. Relative root length (RRL) was estimated as: (net root growth in Al3+ treatment/net root growth in control solution) × 100 %.
Measurements of Al3+ tolerance in soil
An acidic red ferrosol soil was obtained from the Robertson region of New South Wales, Australia (34°35′S, 150°36′E). The pH of a 0·01 m CaCl2 extract was 4·33 and the exchangeable Al3+ was 21 % of the total cations in solution. A proportion of the soil was treated by adding 5 g of lime kg−1 dry soil, which raised the pH to 5·18 and reduced the exchangeable Al3+ to <1 % of the total cations in solution. The soil moisture was maintained at 90 % field capacity (333 mL kg−1 dry soil). Seeds were surface-sterilized and germinated as described above. For each line, seedlings with similar root lengths were planted into pots (two seedling per pot) that contained 1·3 kg of either acid or limed soil. After 6 d, the seedlings were harvested and the fresh shoot weight, fresh root weight and length of the two longest roots were measured. The lengths of the two longest roots were combined and the RRL was calculated as the value in acid soil relative to that in limed soil. The root system from each plant was scanned and analysed with WinRHIZO™ system to determine total root length. Errors associated with RRL (SERRL) were calculated as described below except x and y represent the mean net root length in the limed soil and the mean net root length in the acid soil. Relative root and shoot weight for each plant was calculated as the total dry weight of the roots or shoots in the acid soil compared with the limed soil.
Statistical analyses
Experiments performed in this study often compared a parameter, such as root length, in a treatment (e.g. Al3+ concentration or acid soil), with a control condition (e.g. no Al3+ or limed soil). This was done to account for the inherent differences in root growth that occur in control conditions. For instance, root growth of ‘Dayton’ plants was consistently shorter than that of ‘Bob White 26’ and the other lines when grown in the absence of Al3+. Since each measurement of root length has a mean and standard error associated with it, the ratio of the means to estimate the relative change has a new accumulated standard error. For instance, RRL is calculated as the ratio of root length in acid soil to that in limed soil. Therefore, RRL = x/y where x and y represent the mean net root length in the treatment and control conditions, respectively. The standard error for this ratio (SERRL) was estimated by eqn (1):
| (1) |
where SEx is the standard error of the Al3+ treatment and SEy is the standard error of the control.
Determining whether two RRL values are different from one another is problematic if there is no a priori reason to pair the original plants in the treatment and control conditions. To compare relative data (see Figs 2–4), we adapted an approach based on overlapping confidence limits (CLs) described by Payton et al. (2003). In brief, Payton et al. (2003) showed that the 84 % CL of means sampled from a normally distributed population will overlap 95 % of the time (if the standard errors are similar sizes). As the two standard errors become less homogenous (ratio ≥2), the size of the CL increases to maintain the same probability of overlap. The present study used this principle to compare T2 transgenic and null lines. Initially, the values of SERRL were calculated for the quotients [eqn (1)] and the 84 % CLs were calculated from the Z distribution by multiplying the standard error by 1·33. If the CLs did not overlap, the means were considered significantly different from one another at P < 0·05.
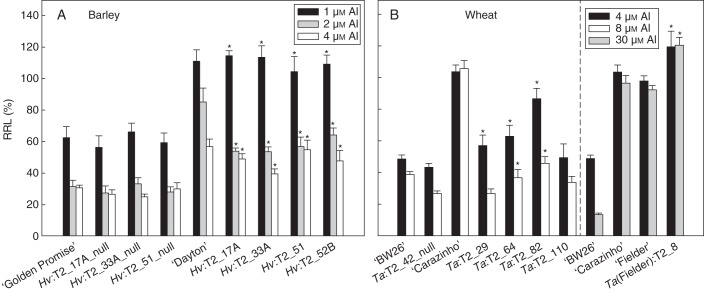
Fig. 2.
Al3+ tolerance of transgenic T2 barley and wheat lines in hydroponic culture. Net root growth was measured on seedlings after 4 d in nutrient solution containing different concentrations of AlCl3. (A) Relative root growth of transgenic barley lines, null lines, ‘Golden Promise’ and ‘Dayton’. Data show the means and standard error calculated from eqn (1) (n = 7). Asterisks above transgenic lines Hv:T2_17A, Hv:T2_33A and Hv:T2_51 indicate significant differences from their respective null lines at each Al3+ concentration (P < 0·05). Asterisks above line Hv:T2_52B indicate significant differences from the null lines. (B) Relative root growth of transgenic and control wheat lines including ‘Bob White 26’ (‘BW26’), the Al3+-sensitive parental cultivar of most transgenic lines, ‘Fielder’, the Al3+-tolerant parent wheat cultivar for one transgenic line, and ‘Carazinho’, an Al3+-tolerant cultivar. The ‘BW26’-derived lines (left) were tested at 4 and 8 µm AlCl3, while the ‘Fielder’-derived line (right) was tested at 4 and 30 µm AlCl3. Data show the mean and standard error calculated from eqn (1) (n = 7). Asterisks above the ‘BW26’-derived transgenic lines Ta:T2_29, Ta:T2_64 and Ta:T2_82 indicate significant differences from the null line at each Al3+ concentration (P0·05). Asterisks above the ‘Fielder’-derived transgenic line Ta(Fielder):T2_8 indicate significant differences from the ‘Fielder’ parental line at each Al3+ concentration (P < 0·05).
Fig. 3.
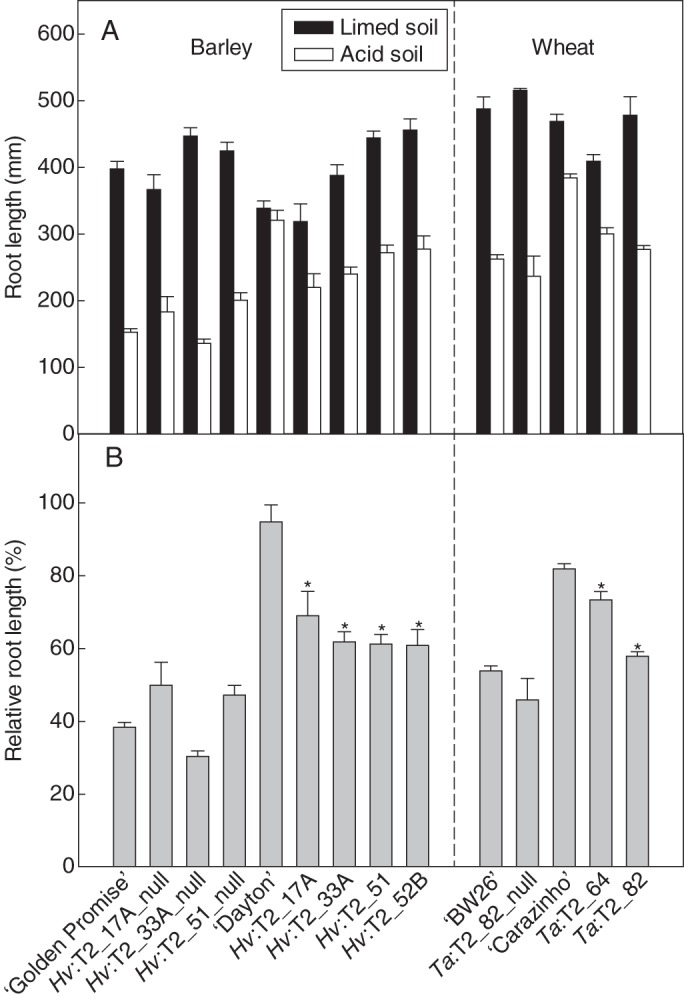
Relative root length of transgenic T2 barley and wheat lines grown in soil. (A) Combined length of the two longest roots from barley and wheat plants grown in acid and limed soils for 6 d. Data show the mean and standard error (n = 6). (B) Relative root length (RRL) of barley and wheat lines. RRL is calculated as the length of the two longest roots in acid soil expressed as a percentage of the two longest roots in limed soil. Transgenic lines, null lines and other control lines are indicated as for Fig. 2. Data show the mean of RRL and the accumulated standard error (n = 6). For the barley results, asterisks above transgenic lines Hv:T2_17A, Hv:T2_33A and Hv:T2_51 indicate significant differences from their respective null lines at each Al3+ concentration (P < 0·05). Asterisks above line Hv:T2_52B indicate significant differences from all the null lines. For the wheat results, asterisks above the transgenic lines Ta:T2_64 and Ta:T2_82 indicate significant differences from the null line Ta:T2_82_null (P < 0·05).
Fig. 4.

Representative photographs of transgenic and control barley plants harvested from soil experiments. Plants were grown for 6 d in an acid soil and the same soil amended with lime as indicated. (A) ‘Golden Promise’, the Al3+-sensitive parental cultivar; (B) ‘Dayton’, an Al3+-tolerant cultivar; (C) Hv:T2_33A_null is a null (non-transgenic) segregant line; (D) Hv:T2_33A is a T2 transgenic line over-expressing HvAACT1. Note that Hv:T2_33A_null and Hv:T2_33A are sister lines. The scale bar represents 10 cm.
RESULTS
Characterization of transgenic barley lines
Barley (‘Golden Promise’) was transformed with the HvAACT1 gene with an efficiency of approx. 30 %. Four transgenic T2 lines (Hv:T2_17A, Hv:T2_33A, Hv:T2_51 and Hv:T2_52B) and three null lines (Hv:T2_17A_null, Hv:T2_33A_null and Hv:T2_51_null) were selected for further detailed analysis. Transgenic and null lines with the same number are sister lines (e.g. Hv:T2_17A_null is a null segregant line of transgenic line Hv:T2_17A). The segregation ratio of the HvAACT1 transgene was assessed in the T1 lines by PCR. Plants of Hv_T1_51 segregated in a 3:1 ratio (transgenic to non-transgenic) and therefore this line was likely to contain a single transgenic insertion (χ2 tested at P < 0·05). The other lines segregated with ratios that differed significantly from 3:1, indicating that it was likely that more than one insertion of the transgene had occurred at unlinked loci.
Expression of HvAACT1 in these lines was compared with the wild-type parental line and with ‘Dayton’, an Al3+-tolerant barley, which expresses the endogenous HvAACT1 gene. Line Hv:T2_52B showed the highest expression among the transgenic lines, with nearly 3-fold greater expression than ‘Dayton’ (Fig. 1). Lines Hv:T2_17A and Hv:T2_33A had similar HvAACT1 expression levels to ‘Dayton’, whereas expression in Hv:T1_51 was about 30 % of that in ‘Dayton’. As expected, no expression was detected in the null lines or in ‘Golden Promise’.
Fig. 1.
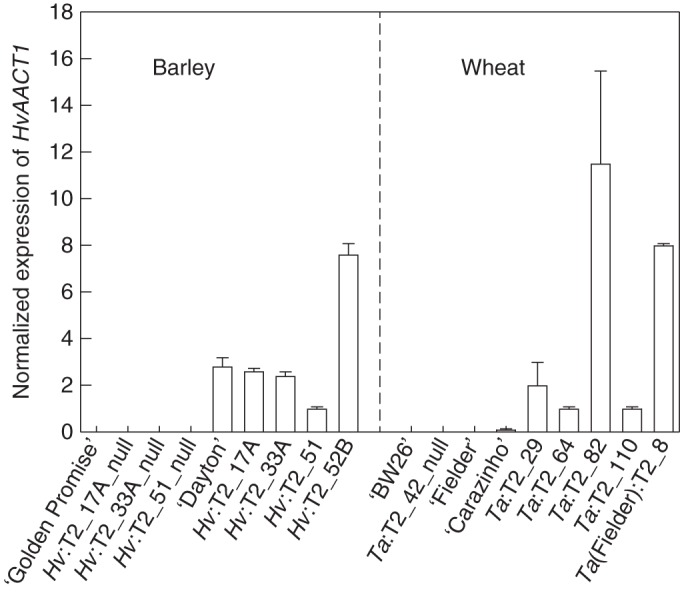
HvAACT1 expression in transgenic T2 barley and wheat lines. Data show the mean of the expression level normalized to actin (barley) and GAPDH (wheat), and the standard error from two biological replicates each with two technical replicates. Left: ‘Golden Promise’ (GP) is the Al3+-sensitive parental barley cultivar for the transgenic barley lines and ‘Dayton’ is an Al3+-tolerant barley cultivar. Right: ‘Bob White 26’ (‘BW26’) is the Al3+-sensitive parental wheat cultivar of most transgenic lines and ‘Fielder’ is the Al3+-tolerant parent wheat cultivar for one transgenic line. ‘Carazinho’ is an Al3+-tolerant wheat cultivar.
Organic anion efflux from excised root apices was measured in the presence of 50 µm Al3+ because HvAACT1 is activated by Al3+ (Fujii et al., 2012). Citrate efflux from each of the T2 transgenic lines was 3- to 4-fold greater than that in the null control lines and about half the efflux measured from ‘Dayton’. The Al3+-activated malate efflux was low and similar for all transgenic lines, as well as ‘Golden Promise’ (Table 1).
Table 1.
Organic anion efflux from wild-type and transgenic barley and wheat lines expressing HvAACT1*
| Lines | Citrate efflux (pmol per apex h−1) | Malate efflux (nmol per apex h−1) |
|---|---|---|
| Barley | ||
| ‘Golden Promise’ | 3·8 ± 0·2 | 0·3 ± 0·0 |
| ‘Dayton’ | 33·2 ± 6·4 | 0·3 ± 0·1 |
| Hv:T2_17A _null | 1·4 ± 0·6 | 0·2 ± 0·1 |
| Hv:T2_33A_null | 3·8 ± 1·1 | 0·2 ± 0·0 |
| Hv:T2_51_null | 1·7 ± 0·1 | 0·3 ± 0·1 |
| Hv:T2_17A | 14·9 ± 1·4 | 0·3 ± 0·0 |
| Hv:T2_33A | 9·7 ± 0·4 | 0·3 ± 0·1 |
| Hv:T2_51 | 14·7 ± 1·6 | 0·3 ± 0·1 |
| Hv:52B | 21·3 ± 4·4 | 0·3 ± 0·0 |
| Wheat | ||
| ‘BW26’ | 6·8 ± 1·2 | 0·1 ± 0·0 |
| Ta:T2_82_null | 6·8 ± 1·4 | 0·2 ± 0·0 |
| ‘Carazinho’ | 95·5 ± 4·9 | 1·0 ± 0·2 |
| Ta:T2_29 | 8·6 ± 2·5 | 0·1 ± 0·0 |
| Ta:T2_64 | 14·4 ± 2·8 | 0·2 ± 0·1 |
| Ta:T2_82 | 26·6 ± 3·9 | 0·1 ± 0·1 |
| Ta:T2_110 | 7·9 ± 0·5 | 0·2 ± 0·0 |
| ‘Fielder’ | 11·7 ± 2·7 | 1·8 ± 0·2 |
| Ta(Fielder):T2_8 | 53·6 ± 3·5 | 1·5 ± 0·6 |
* Citrate and malate efflux from excised root apices of transgenic T2 lines, null lines and wild-type controls. Efflux was measured in the presence of 50 µm AlCl3. Data show the mean and standard error (n = 4).
‘Golden Promise’ is the Al3+-sensitive parental barley cultivar of the transgenic lines and ‘Dayton’ is an Al3+-tolerant barley cultivar. ‘Bob White 26’ (‘BW26’) is the Al3+-sensitive parental wheat cultivar of most transgenic lines and ‘Fielder’ is the Al3+-tolerant parent wheat cultivar for one transgenic line as indicated. ‘Carazinho’ is an Al3+-tolerant wheat used as another control.
The Al3+ tolerance was first evaluated by measuring root growth in hydroponic solutions containing 0, 1, 2 and 4 µm Al3+. In the absence of Al3+, the length of the longest root was similar for all lines (55–65 mm; data not shown). The RRL in all four transgenic lines at 1 µm Al3+ was approx. 110 %, which was similar to that of ‘Dayton’ (Fig. 2A). In contrast, RRL in ‘Golden Promise’ and the three null lines was <60 %. Similar differences were detected at the higher Al3+ concentrations, with the transgenic lines consistently showing a 2-fold greater RRL than the null lines. A statistical analysis based on overlapping CLs indicated that the transgenic lines Hv:T2_17A, Hv:T2_33A and Hv:T2_51 were significantly different from their respective nulls at all Al3+ concentrations. Transgenic line Hv:T2_52B was also significantly different from these nulls.
The Al3+ tolerance of the transgenic and control lines was also compared by measuring root length after 6 d growth in an acid soil with and without added lime (Fig. 3A). Representative photographs of these plants at the end of the experiment are shown in Fig. 4. The RRL was calculated as the combined length of the two longest roots in the acid soil compared with that in the limed soil. In the four transgenic lines, RRL was approx. 60 %, which was significantly greater (P < 0·05) than that of their respective null sister lines and ‘Golden Promise’, all of which showed an RRL of between 30 and 50 % (Fig. 3B). ‘Dayton’ was the most tolerant genotype in this experiment because RRL was 100 %. However, the size of the rhizosheath (soil loosely clinging to the root) of all lines was visibly smaller in the acid soil compared with the limed soil (Fig. 4), indicating that root hair growth was inhibited in the acid soil (Delhaize et al., 2012b). This indicates that over-expression of HvAACT1 increased the Al3+ tolerance of the roots more than the root hairs, even though a constitutive promoter was used. This could be related to the fact that root hairs are tip-growing cells and the HvAACT1 proteins may not be able to function well at the tip of these growing cells.
Total root length of ‘Dayton’ grown in acid soil was similar to total root length of plants grown in the limed soil (Fig. 5A). All other lines had shorter roots in the acid soil but differences in relative total root length (RTRL) were detected among some of the lines (Fig. 5B). For instance, RTRL in transgenic lines Hv:T2_17A and Hv:T2_33A was greater than in their respective null sister lines (P0·05), whereas Hv:T2_51 did not differ from its null sister line (Fig. 5B). Line Hv:T2_52B also did not differ from the other null lines. Root weight of these lines was also compared in the acid and the limed soil. Relative root weight (acid/limed) in the transgenic lines was 1·5- to 2-fold greater than the null lines (Table 2). Line Hv:T2_17A had the greatest relative root weight of the transgenic lines and was similar to ‘Dayton’ (Table 2). In contrast, no consistent differences were detected in shoot weight or relative shoot weight between transgenic and null lines (Table 2).
Fig. 5.
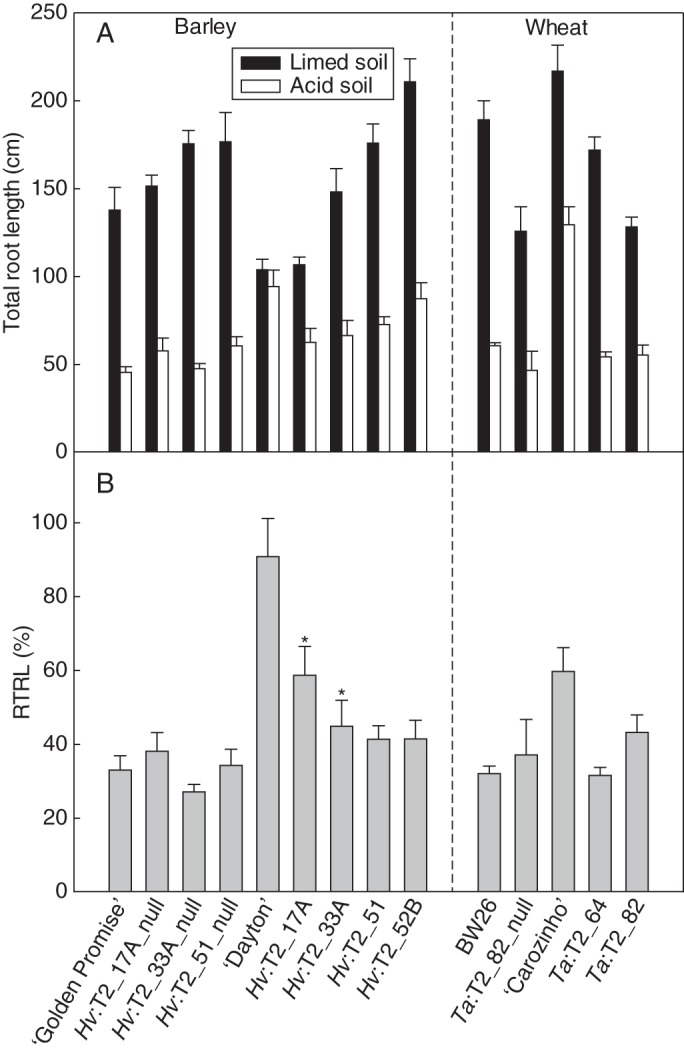
Total root length of transgenic T2 barley and wheat lines grown in soil. (A) Total root length after 6 d growth in acid and limed soils. (B) Relative total root length (RTRL) is expressed as a percentage of the total root length in acid soil relative to limed soil. The data show the mean of RTRL and the standard error (n = 6). Lines are described in Fig. 3. For the barley results, asterisks above transgenic lines Hv:T2_17A and Hv:T2_33A indicate significant differences from their respective null lines (P < 0·05).
Table 2.
Shoot and root fresh weights of transgenic barley and wheat lines compared with control lines grown in acid and limed soils*
| Lines | Shoot weight limed soil (g) | Shoot weight acid soil (g) | Relative shoot weight (%) | Root weight limed soil (g) | Root weight acid soil (g) | Relative root weight (%) |
|---|---|---|---|---|---|---|
| Barley | ||||||
| ‘Golden Promise’ | 0·217 ± 0·017 | 0·170 ± 0·005 | 78·4 ± 6·6 | 0·186 ± 0·018 | 0·111 ± 0·010 | 59·4 ± 7·9 |
| ‘Dayton’ | 0·188 ± 0·005 | 0·159 ± 0·017 | 84·4 ± 9·4 | 0·148 ± 0·005 | 0·187 ± 0·019 | 126·6 ± 13·7 |
| Hv:T2_17A _null | 0·222 ± 0·012 | 0·146 ± 0·011 | 65·9 ± 6·0 | 0·176 ± 0·012 | 0·139 ± 0·014 | 78·7 ± 9·7 |
| Hv:T2_33A_null | 0·242 ± 0·009 | 0·171 ± 0·007 | 70·8 ± 4·0 | 0·206 ± 0·01 | 0·118 ± 0·007 | 57·5 ± 4·4 |
| Hv:T2_51_null | 0·247 ± 0·009 | 0·173 ± 0·006 | 70·3 ± 3·5 | 0·203 ± 0·014 | 0·148 ± 0·011 | 72·8 ± 7·6 |
| Hv:T2_17A | 0·167 ± 0·014 | 0·134 ± 0·015 | 80·2 ± 11·2 | 0·115 ± 0·01 | 0·153 ± 020 | 132·9 ± 20·7 |
| Hv:T2_33A | 0·204 ± 0·015 | 0·120 ± 0·011 | 58·9 ± 6·8 | 0·169 ± 0·018 | 0·160 ± 0·021 | 95·1 ± 15·9 |
| Hv:T2_51 | 0·252 ± 0·014 | 0·152 ± 0·013 | 60·4 ± 6·1 | 0·157 ± 0·012 | 0·177 ± 0·01 | 112·4 ± 10·4 |
| Hv:T2_52B | 0·257 ± 0·011 | 0·173 ± 0·013 | 67·2 ± 6·0 | 0·224 ± 0·018 | 0·215 ± 0·021 | 96·3 ± 12·3 |
| Wheat | ||||||
| ‘BW26’ | 0·236 ± 0·010 | 0·181 ± 0·004 | 77 ± 3·6 | 0·254 ± 0·018 | 0·177 ± 0·005 | 70 ± 5·4 |
| ‘Carazinho’ | 0·219 ± 0·010 | 0·167 ± 0·009 | 76 ± 5·3 | 0·245 ± 0·015 | 0·201 ± 0·012 | 82 ± 7·0 |
| Ta:T2_42_null | 0·170 ± 0·013 | 0·112 ± 0·010 | 66 ± 7·5 | 0·196 ± 0·018 | 0·113 ± 0·011 | 57 ± 7·9 |
| Ta:T2_29 | 0·186 ± 0·004 | 0·130 ± 0·013 | 70 ± 7·1 | 0·215 ± 0·011 | 0·142 ± 0·013 | 66 ± 6·8 |
| Ta:T2_64 | 0·141 ± 0·012 | 0·098 ± 0·014 | 69 ± 11·5 | 0·148 ± 0·013 | 0·109 ± 0·022 | 74 ± 16·3 |
| Ta:T2_82 | 0·158 ± 0·02 | 0·111 ± 0·005 | 70 ± 9·3 | 0·186 ± 0·021 | 0·147 ± 0·006 | 79 ± 9·5 |
* Shoot and root fresh weight of plants grown in acid soil and limed soil for 6 d. Data show the mean of shoot and root fresh weight (g) and standard error (n = 6), and the mean of relative shoot and root weight and standard error (n = 6).
Plant lines are the same as described for Table 1.
Characterization of transgenic wheat lines
The barley HvAACT1 gene was expressed in wheat cultivars ‘Bob White 26’ (‘BW26’, Al3+ sensitive) and ‘Fielder’ (Al3+ tolerant). Experiments were performed on four T2 transgenic lines (Ta:T2_29, Ta:T2_64, Ta:T2_82 and Ta:T2_110) and two null lines (Ta:T2_42_null and Ta:T2_82_null) generated from ‘BW26’. Only a single transgenic T2 line named Ta(Fielder):T2_8 was generated in the ‘Fielder’ cultivar, but no null sister line could be isolated from this family, indicating that it contains multiple inserts.
HvAACT1 expression was detected in the root apices of the T2 transgenic lines, with Ta:T2_82 and Ta(Fielder):T2_8 showing the highest levels (Fig. 1). No expression was detected in one null line tested, or in the wild-type wheat cultivars ‘BW26’, ‘Fielder’ or ‘Carazinho’.
Citrate and malate efflux was measured from excised root apices of the transgenic lines and controls in the presence of 50 µm Al3+. ‘Carazinho’ was included since it has citrate efflux which is controlled by a different gene, the endogenous TaMATE1B gene in wheat (Ryan et al., 2009; Tovkach et al., 2013). Citrate efflux from three transgenic lines [Ta(Fielder):T2_8, Ta:T2_64 and Ta:T2_82] was significantly greater than from the null and their respective wild-type cultivars, ‘Fielder’ and ‘BW26’ (Table 1). The two remaining T2 lines (Ta:T2_29 and Ta:T2_110) did not differ from the controls in these experiments. No malate efflux was detected from ‘BW26’ or any of the ‘BW26’-derived transgenic lines. Malate efflux was detected from the Al3+-tolerant cultivars ‘Carazinho’ and ‘Fielder’ and the transgenic line generated from ‘Fielder’, Ta(Fielder):T2_8. This is consistent with the known mechanism of Al3+ tolerance in wheat encoded by TaALMT1.
The Al3+ tolerance of the transgenic wheat lines was first evaluated by measuring RRL in hydroponic solutions containing increasing concentrations of Al3+ (Fig. 2B). For the ‘BW26’-derived lines, the concentrations were 0, 4 and 8 µm Al3+ and for the ‘Fielder’-derived line the concentrations were 0, 4 and 30 µm Al3+. A higher range of concentrations was used for ‘Fielder’ because it possesses an Al3+-tolerant allele of the TaALMT1 gene whereas ‘BW26’ possesses an Al3+-sensitive allele (see below). Two of the ‘BW26’-derived lines, Ta:T2_64 and Ta:T2_82, showed greater RRL than the null line and ‘BW26’ at both 2 and 4 µm Al3+ (Fig. 2B). Of the remaining two lines, Ta:T2_29 showed a greater RRL than the null at 4 µm only, and Ta:T2_110 was not significantly different from the null line at either concentration (Fig. 2B). These results are generally consistent with the measured citrate efflux from these lines. For the single ‘Fielder’-derived line, RRL was approx. 120 % in both 8 and 30 µm Al3+, which was significantly greater than in the wild-type ‘Fielder’ and ‘Carazinho’ in these short-term experiments. The inclusion of ‘BW26’ in this experiment demonstrates its greater sensitivity to Al3+ compared with ‘Fielder’ and ‘Carazinho’.
The Al3+ tolerance of a selection of these wheat lines was examined in an acid soil and in the same soil amended with lime. This experiment included the two ‘BW26’-derived transgenic lines (Ta:T2_64 and Ta:T2_82), a null line (Ta:T2_82_null), and wild-type cultivars ‘BW26’ and ‘Carazinho’. The ‘Fielder’-derived transgenic line was not included because there was insufficient seed. Relative shoot and root weights of the transgenic lines were not consistently different from those of the null lines and ‘BW26’ (Table 2). However the transgenic lines had longer roots in acid soil (Fig. 3), with RRL being significantly larger in the two transgenic lines than in the null line (Fig. 3B). ‘Carazinho’ had the greatest RRL at >80 % (Fig. 3B). ‘Carazinho’ showed a significantly greater RTRL than the other lines (Fig. 5B), but no differences were detected between the transgenic and null lines.
DISCUSSION
We have demonstrated that expression of HvAACT1 in barley and wheat with a constitutive promoter enhances Al3+-activated citrate efflux from roots and confers increased Al3+ tolerance. In these short-term experiments, effects on shoot growth were not apparent and would probably appear over a longer period as changes in root growth and function affect water and nutrient uptake (Zhao et al., 2003). The results were generally similar in the hydroponic experiments and soil trials, which reinforces the value of short-term hydroponic screens for assessing lines. In the hydroponic experiments, root growth of transgenic barley lines and ‘Dayton’ was stimulated in 1 µm Al3+. This response has been observed previously in other species and is interpreted as low concentrations of Al3+ alleviating pH toxicity by reducing the concentration of the more toxic H+ on, or adjacent to, the charged membrane surface of the root cells (Kinraide et al., 1992; Kinraide, 1993). Although the transgenic barley lines were significantly more Al3+ tolerant than null lines, they did not reach the level of tolerance displayed by the tolerant wild-type cultivar ‘Dayton’. Furthermore, HvAACT1 does not appear to be as effective for increasing Al3+ tolerance in transgenic barley as the wheat tolerance gene TaALMT1 (Delhaize et al., 2004). Barley plants expressing TaALMT1 are tolerant of 20 µm Al3+ (RRL >80 %) (Delhaize et al., 2004) whereas in the present study, plants expressing HvAACT1 are inhibited by 4 µm Al3+ in a hydroponic solution of similar composition. This indicates that Al3+ tolerance is determined not only by the effectiveness of organic acid in chelating Al3+, but also by the amount of organic acid released from root apices. In transgenic barley lines expressing TaALMT1 or HvAACT1, malate efflux was about 50 times greater than citrate efflux. For instance, in transgenic barley plants over-expressing TaALMT1, malate efflux was up to 1000 pmol per apex per hour (Delhaize et al., 2004), while in barley plants over-expressing HvAACT1 citrate efflux was about 20 pmol per apex per hour. Nevertheless, HvAACT1 expression does increase tolerance and provides opportunities for further enhancing the Al3+ tolerance of barley and wheat, as discussed later.
Furukawa et al. (2007) reported a positive relationship between HvAACT1 expression level and citrate efflux among wild-type genotypes of barley. In the current study, HvAACT1 expression in one of the transgenic barley lines (Hv:T2_52B) was almost 3-fold greater than in ‘Dayton’ despite showing less citrate efflux and a lower relative tolerance. Several technical and biological factors could explain the poor correlation between expression and citrate efflux when comparing wild-type plants with transgenic plants. First, gene expression is not necessarily directly correlated with the protein content so expression levels of the gene might not reflect expression of the phenotype. More importantly, the constitutive promoter in the transgenic plants might obscure the correlation detected among the wild-type plants because the pattern of expression in roots of the transgenic plants may differ from the pattern occurring naturally. For instance, HvAACT1 expression in wild-type barley is driven by its native promoter which confers relatively high expression in the epidermal cells compared with the other cells of the apex (Fujii et al., 2012). A correlation between relative HvAACT1 expression in these specific cells and citrate efflux from roots could be obscured in transgenic plants expressing HvAACT1 with a constitutive promoter where expression occurs in all cells. Not all root cells may contribute to the measured citrate efflux due to other anatomical, regulatory or metabolic restraints. It also remains possible that other factors such as the rate of citrate synthesis within the cells or the genetic background of barley influences the capacity to release citrate from roots.
Fujii et al. (2012) also expressed HvAACT1 in ‘Golden Promise’, but the aims of that study were quite different from those of the present work. Fujii et al. (2012) primarily investigated the allelic variation in HvAACT1 promoters and used native promoters derived from different genotypes to drive HvAACT1 expression. They analysed T1 lines, not T2 lines, and compared citrate efflux and Al3+ tolerance with wild-type ‘Golden Promise’ but not with null segregant lines or Al3+-tolerant wild-type cultivars. Furthermore, they measured citrate efflux from the whole root system of young seedlings instead of the root apex, which is more relevant for Al3+ tolerance. This could explain why they reported smaller increases in citrate efflux from their T1 lines compared with controls (2- to 4-fold) than we measured from T2 lines in the present study (4- to 10-fold). Nevertheless, Fujii et al. (2012) showed that HvAACT1 expression in transgenic barley lines using the native promoter from the Al3+-tolerant Murasakimochi cultivar could increase Al3+ tolerance above that measured in the parental cultivar ‘Golden Promise’.
Two of the transgenic wheat lines generated in this study with the highest expression levels, Hv(Fielder):T2_8 and Hv:T2_82, also exhibited the greatest citrate efflux and Al3+ tolerance in both hydroponic culture and acid soil. The ‘Fielder’-derived line showed citrate efflux in addition to malate efflux (controlled by the endogenous TaALMT1 gene) and was more tolerant of Al3+ than wild-type ‘Fielder’ in hydroponic culture. This suggests that citrate efflux and malate efflux may confer additive levels of Al3+ tolerance. However, since only a single transgenic line was characterized, this conclusion needs to be confirmed with additional lines and soil experiments. None of the transgenic barley lines or ‘BW26’-derived wheat lines showed Al3+-activated malate efflux, which is consistent with HvAACT1 specifically facilitating citrate efflux from these roots.
The efflux of organic anions from root cells involves two main processes: synthesis of organic anions and their transport across the plasma membrane. Ryan et al. (2001) argued that the transport step was more likely to be rate limiting since organic anions such as malate and citrate are common cellular components with high turnover rates. None of the transgenic barley lines expressing HvAACT1 showed greater Al3+ tolerance than the tolerant wild-type cultivar ‘Dayton’. It is possible that as the capacity to transport citrate out of the cell is increased, other processes begin to limit efflux. Consistent with this idea are the findings from other studies which show that enhanced expression of genes involved in organic anion synthesis can also increase Al3+ tolerance (see Ryan et al., 2011). For instance, expressing genes encoding organic acid synthesis in tobacco (Nicotiana tabacum) (de la Fuente et al., 1997; Han et al., 2009), alfalfa (Medicago sativa) (Barone et al., 2008) and arabidopsis (Koyama et al., 1999; Koyama et al., 2000) is reported to increase citrate efflux and Al3+ tolerance. Although other studies have not observed these same responses (Delhaize et al., 2001, 2003), it remains plausible that transgenic strategies which combine enhanced organic anion synthesis and transport will provide an additional benefit if organic anion supply begins to limit efflux. Alternative transgenic strategies which have successfully increased Al3+ tolerance to some degree have targeted genes involved in membrane lipid composition, stress responses and general metabolism (Ezaki et al., 2000; Basu et al., 2001; da Silva et al., 2006; Ryan et al., 2007; Trejo-Tellez et al., 2010). Future studies will explore whether combining natural tolerance traits in barley and wheat with transgenic strategies is effective for increasing Al3+ tolerance.
In conclusion, this study has demonstrated that genetically modifying barley and wheat to express the barley gene HvAACT1 gene can increase their Al3+ tolerance in hydroponic experiments and short-term soil trials. This transgene could be introgressed into naturally Al3+-tolerant genotypes of wheat and barley to assess whether the effects of the endogenous genes and transgenes are additive. To date, TaALMT1 confers the most effective level of Al3+ tolerance in transgenic barley (Delhaize et al., 2004) and wheat (Pereira et al., 2010), and combining it with HvAACT1 may further enhance the tolerance of these species. Further studies need to test the effectiveness of these transgenes in increasing grain yield as established for barley expressing TaALMT1 (Delhaize et al., 2009). Ultimately, field trials are needed to establish whether these strategies can increase production on acid soils above what is currently possible with natural germplasm.
ACKNOWLEDGEMENTS
We thank JianFeng Ma (Okayama University, Japan) for the use of HvAACT1 cDNA, and Terese Richardson (CSIRO, Australia) for assistance with wheat and barley transformation. This work was supported by Grains Research and Development Corporation of Australia.
LITERATURE CITED
- Barone P, Rosellini D, LaFayette P, Bouton J, Veronesi F, Parrott W. Bacterial citrate synthase expression and soil aluminum tolerance in transgenic alfalfa. Plant Cell Reports. 2008;27:893–901. doi: 10.1007/s00299-008-0517-x. [DOI] [PubMed] [Google Scholar]
- Basu U, Good AG, Taylor GJ. Transgenic Brassica napus plants overexpressing aluminium-induced mitochondrial manganese superoxide dismutase cDNA are resistant to aluminium. Plant, Cell and Environment. 2001;24:1269–1278. [Google Scholar]
- Bona L, Wright RJ, Baligar VC, Matuz J. Screening wheat and other small grains for acid soil tolerance. Landscape and Urban Planning. 1993;27:175–178. [Google Scholar]
- Dai SF, Yan ZH, Liu DC, Zhang LQ, Wei YM, Zheng YL. Evaluation on Chinese bread wheat landraces for low pH and aluminum tolerance using hydroponic screening. Agricultural Sciences in China. 2009;8:285–292. [Google Scholar]
- Delhaize E, Hebb DM, Ryan PR. Expression of a Pseudomonas aeruginosa citrate synthase gene in tobacco is not associated with either enhanced citrate accumulation or efflux. Plant Physiology. 2001;125:2059–2067. doi: 10.1104/pp.125.4.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhaize E, Ryan PR, Hocking PJ, Richardson AE. Effects of altered citrate synthase and isocitrate dehydrogenase expression on internal citrate concentrations and citrate efflux from tobacco (Nicotiana tabacum L.) roots. Plant and Soil. 2003;248:137–144. [Google Scholar]
- Delhaize E, Ryan PR, Hebb DM, Yamamoto Y, Sasaki T, Matsumoto H. Engineering high-level aluminum tolerance in barley with the ALMT1 gene. Proceedings of the National Academy of Sciences, USA. 2004;101:15249–15254. doi: 10.1073/pnas.0406258101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhaize E, Gruber BD, Ryan PR. The roles of organic anion permeases in aluminium resistance and mineral nutrition. FEBS Letters. 2007;581:2255–2262. doi: 10.1016/j.febslet.2007.03.057. [DOI] [PubMed] [Google Scholar]
- Delhaize E, Taylor P, Hocking PJ, Simpson RJ, Ryan PR, Richardson AE. Transgenic barley (Hordeum vulgare L.) expressing the wheat aluminium resistance gene (TaALMT1) shows enhanced phosphorus nutrition and grain production when grown on an acid soil. Plant Biotechnology Journal. 2009;7:391–400. doi: 10.1111/j.1467-7652.2009.00403.x. [DOI] [PubMed] [Google Scholar]
- Delhaize E, Ma JF, Ryan PR. Transcriptional regulation of aluminium tolerance genes. Trends in Plant Science. 2012a;17:341–348. doi: 10.1016/j.tplants.2012.02.008. [DOI] [PubMed] [Google Scholar]
- Delhaize E, James RA, Ryan PR. Aluminium tolerance of root hairs underlines the genotypic differences in rhizosheath size in wheat (Triticum aestivum) grown on acid soil. New Phytologist. 2012b;195:609–619. doi: 10.1111/j.1469-8137.2012.04183.x. [DOI] [PubMed] [Google Scholar]
- Ezaki B, Gardner RC, Ezaki Y, Matsumoto H. Expression of aluminum-induced genes in transgenic Arabidopsis plants can ameliorate aluminum stress and/or oxidative stress. Plant Physiology. 2000;122:657–665. doi: 10.1104/pp.122.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente JM, RamirezRodriguez V, CabreraPonce JL, HerreraEstrella L. Aluminum tolerance in transgenic plants by alteration of citrate synthesis. Science. 1997;276:1566–1568. doi: 10.1126/science.276.5318.1566. [DOI] [PubMed] [Google Scholar]
- Foy CD. Physiological effects of hydrogen, aluminum, and manganese toxicities in acid soil. In: Adams F, editor. Soil acidity and liming. Madison, WI: American Society of Agronomy, Inc; 1984. pp. 57–92. [Google Scholar]
- Foy CD. Plant adaptation to acid, aluminum-toxic soils. Communications in Soil Science and Plant Analysis. 1988;19:959–987. [Google Scholar]
- Foy CD, Duncan RR, Waskom RM, Miller DR. Tolerance of sorghum genotypes to an acid, aluminum toxic tatum subsoil. Journal of Plant Nutrition. 1993;16:97–127. [Google Scholar]
- Fujii M, Yokosho K, Yamaji N, et al. Acquisition of aluminium tolerance by modification of a single gene in barley. Nature Communications. 2012;3:713. doi: 10.1038/ncomms1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlani PR, Bastos CR, Borgonovi RA, Schaffert RE. Differential responses of sorghum genotypes for tolerance to aluminum in nutrient solutions. Pesquisa Agropecuaria Brasileira. 1987;22:323–330. [Google Scholar]
- Furukawa J, Yamaji N, Wang H, et al. An aluminum-activated citrate transporter in barley. Plant and Cell Physiology. 2007;48:1081–1091. doi: 10.1093/pcp/pcm091. [DOI] [PubMed] [Google Scholar]
- Garvin DF, Carver BF. Role of the genotype in tolerance to acidity and aluminum toxicity. In: Rengel Z, editor. Handbook of soil acidity. New York: Marcel Dekker; 2003. pp. 387–406. [Google Scholar]
- Gassmann W, Schroeder JI. Inwardly-rectifying K+ channels in roots hairs of wheat – a mechanism for aluminum-sensitive low-affinity K+ uptake amd membrane potential control. Plant Physiology. 1994;105:1399–1408. doi: 10.1104/pp.105.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YY, Zhang WZ, Zhang BL, Zhang SS, Wang W, Ming F. One novel mitochondrial citrate synthase from Oryza sativa L. can enhance aluminum tolerance in transgenic tobacco. Molecular Biotechnology. 2009;42:299–305. doi: 10.1007/s12033-009-9162-z. [DOI] [PubMed] [Google Scholar]
- Horst WJ, Wang YX, Eticha D. The role of the root apoplast in aluminium-induced inhibition of root elongation and in aluminium resistance of plants: a review. Annals of Botany. 2010;106:185–197. doi: 10.1093/aob/mcq053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinraide TB, Ryan PR, Kochian LV. Interactive effects of Al3+, H+, and other cations on root elongation considered in terms of cell-surface electrical potential. Plant Physiology. 1992;99:1461–1468. doi: 10.1104/pp.99.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinraide TB. Aluminum enhancement of plant-growth in acid rooting media – a case of reciprocal alleviation of toxicity by two toxic cations. Physiologia Plantarum. 1993;88:619–625. doi: 10.1111/j.1399-3054.1993.tb01380.x. [DOI] [PubMed] [Google Scholar]
- Kochian LV, Hoekenga OA, Pineros MA. How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Annual Review of Plant Biology. 2004;55:459–493. doi: 10.1146/annurev.arplant.55.031903.141655. [DOI] [PubMed] [Google Scholar]
- Koyama H, Takita E, Kawamura A, Hara T, Shibata D. Overexpression of mitochondrial citrate synthase gene improves the growth of carrot cells in Al-phosphate medium. Plant and Cell Physiology. 1999;40:482–488. doi: 10.1093/oxfordjournals.pcp.a029568. [DOI] [PubMed] [Google Scholar]
- Koyama H, Kawamura A, Kihara T, Hara T, Takita E, Shibata D. Overexpression of mitochondrial citrate synthase in Arabidopsis thaliana improved growth on a phosphorus-limited soil. Plant and Cell Physiology. 2000;41:1030–1037. doi: 10.1093/pcp/pcd029. [DOI] [PubMed] [Google Scholar]
- Koyama H, Ikka T, Kobayashi Y, Hasegawa M. Comparison of aluminum-tolerance and other stress factors associated with acid soil between Arabidopsis accessions. Plant and Cell Physiology. 2003;44:S164–S164. [Google Scholar]
- Ma JF, Ryan PR. Understanding how plants cope with acid soils. Functional Plant Biology. 2010;37:iii–vi. [Google Scholar]
- Ma JF, Ryan PR, Delhaize E. Aluminium tolerance in plants and the complexing role of organic acids. Trends in Plant Science. 2001;6:273–278. doi: 10.1016/s1360-1385(01)01961-6. [DOI] [PubMed] [Google Scholar]
- Magalhaes JV, Liu J, Guimaraes CT, et al. A gene in the multidrug and toxic compound extrusion (MATE) family confers aluminum tolerance in sorghum. Nature Genetics. 2007;39:1156–1161. doi: 10.1038/ng2074. [DOI] [PubMed] [Google Scholar]
- Moroni JS, Sato K, Scott BJ, et al. Novel barley (Hordeum vulgare L.) germplasm resistant to acidic soil. Crop and Pasture Science. 2010;61:540–553. [Google Scholar]
- Munns DN. Soil acidity and growth of a legume. II. Reactions of aluminium and phosphate in solution and effects of aluminium, phosphate, calcium, and pH on Medicago sativa L. and Trifolium subterraneum L. in solution culture. Australian Journal of Agricultural Research. 1965;16:743–755. [Google Scholar]
- Payton ME, Greenstone MH, Schenker N. Overlapping confidence intervals or standard error intervals: what do they mean in terms of statistical significance? Journal of Insect Science. 2003;3:34. doi: 10.1093/jis/3.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira JF, Zhou GF, Delhaize E, Richardson T, Zhou MX, Ryan PR. Engineering greater aluminium resistance in wheat by over-expressing TaALMT1. Annals of Botany. 2010;106:205–214. doi: 10.1093/aob/mcq058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineros M, Tester M. Plasma-membrane Ca2+ channels in roots of higher roots and their role in aluminum toxicity. Plant and Soil. 1993;156:119–122. [Google Scholar]
- Pinto-Carnide O, Guedes-Pinto H. Aluminum tolerance variability in rye and wheat Portuguese germplasm. Genetic Resources and Crop Evolution. 1999;46:81–85. [Google Scholar]
- Raman H, Ryan PR, Raman R, et al. Analysis of TaALMT1 traces the transmission of aluminum resistance in cultivated common wheat (Triticum aestivum L.) Theoretical and Applied Genetics. 2008;116:343–354. doi: 10.1007/s00122-007-0672-4. [DOI] [PubMed] [Google Scholar]
- Rengel Z, Jurkic V. Genotypic differences in wheat Al-tolerance. Euphytica. 1992;62:111–117. [Google Scholar]
- Ryan PR, Shaff JE, Kochian LV. Aluminum toxicity in roots – correlation among ionic currents, ion fluxes, and root elongation in aluminum-sensitive and aluminum-tolerant wheat cultivars. Plant Physiology. 1992;99:1193–1200. doi: 10.1104/pp.99.3.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan PR, Delhaize E, Randall PJ. Characterization of Al-stimulated efflux of malate from the apices of Al-tolerant wheat roots. Planta. 1995;196:103–110. [Google Scholar]
- Ryan PR, Delhaize E, Jones DL. Function and mechanism of organic anion exudation from plant roots. Annual Review of Plant Physiology and Plant Molecular Biology. 2001;52:527–560. doi: 10.1146/annurev.arplant.52.1.527. [DOI] [PubMed] [Google Scholar]
- Ryan PR, Liu Q, Sperling P, Dong B, Franke S, Delhaize E. A higher plant Δ8 sphingolipid desaturase with a preference for (Z)-isomer formation confers aluminum tolerance to yeast and plants. Plant Physiology. 2007;144:1968–1977. doi: 10.1104/pp.107.100446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan PR, Raman H, Gupta S, Horst WJ, Delhaize E. A second mechanism for aluminum resistance in wheat relies on the constitutive efflux of citrate from roots. Plant Physiology. 2009;149:340–351. doi: 10.1104/pp.108.129155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan PR, Tyerman SD, Sasaki T, et al. The identification of aluminium-resistance genes provides opportunities for enhancing crop production on acid soils. Journal of Experimental Botany. 2011;62:9–20. doi: 10.1093/jxb/erq272. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Yamamoto Y, Ezaki B, et al. A wheat gene encoding an aluminum-activated malate transporter. Plant Journal. 2004;37:645–653. doi: 10.1111/j.1365-313x.2003.01991.x. [DOI] [PubMed] [Google Scholar]
- da Silva ALS, Sperling P, Horst W, et al. A possible role of sphingolipids in the aluminium resistance of yeast and maize. Journal of Plant Physiology. 2006;163:26–38. doi: 10.1016/j.jplph.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Sivaguru M, Fujiwara T, Samaj J, et al. Aluminum-induced 1 → 3-beta-d-glucan inhibits cell-to-cell trafficking of molecules through plasmodesmata. A new mechanism of aluminum toxicity in plants. Plant Physiology. 2000;124:991–1005. doi: 10.1104/pp.124.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C, Nuruzzaman M, Rengel Z. Screening wheat genotypes for tolerance of soil acidity. Australian Journal of Agricultural Research. 2003;54:445–452. [Google Scholar]
- Tingay S, McElroy D, Kalla R, et al. Agrobacterium tumefaciens-mediated barley transformation. The Plant Journal. 1997;11:1369–1376. [Google Scholar]
- Tovkach A, Ryan PR, Richardson AE, et al. Transposon-mediated alteration of TaMATE1B expression in wheat confers constitutive citrate efflux from root apices. Plant Physiology. 2013;161:880–892. doi: 10.1104/pp.112.207142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejo-Tellez LI, Stenzel R, Gomez-Merino FC, Schmitt JM. Transgenic tobacco plants overexpressing pyruvate phosphate dikinase increase exudation of organic acids and decrease accumulation of aluminum in the roots. Plant and Soil. 2010;326:187–198. [Google Scholar]
- von Uexkull HR, Mutert E. Golbal extent, development and economic-impact of acid soils. Plant and Soil. 1995;171:1–15. [Google Scholar]
- Wang JP, Raman H, Zhou MX, et al. High-resolution mapping of the Alp locus and identification of a candidate gene HvMATE controlling aluminium tolerance in barley (Hordeum vulgare L.) Theoretical and Applied Genetics. 2007;115:265–276. doi: 10.1007/s00122-007-0562-9. [DOI] [PubMed] [Google Scholar]
- Wang MB, Li ZY, Matthews PR, Upadhyaya NM. Improved vectors for Agrobacterium tumefaciens-mediated transformation of monocot plants. In: Drew RA, editor. International Symposium on Biotechnology of Tropical and Subtropical Species Part 2. Vol. 461. 1998. pp. 401–408. ISHS Acta Horticulturae. [Google Scholar]
- Yamamoto Y, Kobayashi Y, Matsumoto H. Lipid peroxidation is an early symptom triggered by aluminum, but not the primary cause of elongation inhibition in pea roots. Plant Physiology. 2001;125:199–208. doi: 10.1104/pp.125.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ZQ, Ma JF, Sato K, Takeda K. Differential Al resistance and citrate secretion in barley (Hordeum vulgare L.) Planta. 2003;217:794–800. doi: 10.1007/s00425-003-1043-2. [DOI] [PubMed] [Google Scholar]
- Zhou GF, Delhaize E, Zhou MX, Ryan PR. Biotechnological solutions for enhancing the aluminium resistance of crop plants. In: Shanker A, Venkateswarlu B, editors. Abiotic stress in plants – mechanisms and adaptations. 2011. InTech. http://dx.doi.org/10.5772/25187 . [Google Scholar]