Abstract
Objective
Functional magnetic resonance imaging (fMRI) provides an opportunity to study the relationship between cerebral reorganization and functional recovery after stroke. The authors set out to demonstrate the feasibility of using fMRI to investigate mechanisms of recovery in individual patients presenting with severe motor impairment.
Methods
fMRI was performed during passive movement at both affected and unaffected wrists separately in 2 patients with pure motor stroke. Six scanning sessions were performed in each patient over the first 4 months after stroke. Seven control subjects were also studied, 1 of them over 6 sessions. The authors examined for longitudinal changes in cerebral responses to proprioceptive afferent input that correlated with motor recovery.
Results
In control subjects, passive movement of either wrist led to relative increases in brain activation in the contralateral sensorimotor cortex and supplementary motor area, the bilateral inferior parietal cortex and secondary somatosensory areas, and the ipsilateral cerebellum. Increases in brain activation correlating with motor recovery were observed in both the ipsilesional primary sensory and primary motor cortex in 1 patient with good motor recovery but not in another patient with poor recovery. No longitudinal changes were seen in the control subject.
Conclusions
These 2 case reports demonstrate that functionally relevant changes in cerebral organization can be identified in individual patients.
Keywords: fMRI, Stroke, Recovery, Plasticity
Functional imaging techniques such as positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) allow measurement of task-related brain activity in humans with excellent spatial resolution. Functional imaging experiments have demonstrated longitudinal changes in the organization of the cerebral motor output system that are related to recovery of motor function after stroke.1-3 In some cases, these recovery-related changes have been seen in individual patients.3 These studies require that the subjects have recovered enough motor skills to be able to perform the experimental task (e.g., hand grip, finger tapping), and thus patients who have lost the ability to move the hand altogether are unable to participate. These patients can be studied using alternative paradigms, such as passive rather than active limb movement, which provide an opportunity to probe the sensorimotor system in an entirely different way. In general, passive movement studies have demonstrated increases in brain responses to proprioceptive input with time after stroke, in regions such as the ipsilesional sensorimotor cortex, as well as the bilateral premotor cortex, supplementary motor area, and inferior parietal cortex.4-6 Studies employing active motor paradigms, on the other hand, have tended to show reductions in brain activation with time/recovery in secondary motor areas.1-3 The changes over time in active motor studies have been interpreted as representing increased efficiency within the motor system, as might be seen during motor skill learning.7 Longitudinal changes in the response to passive movement tasks might represent increased processing of sensory information relevant to motor output. The passive movement studies analyzed data grouped from several patients and did not distinguish time- or recovery-related changes in individual patients. Group studies are important and can contribute toward an understanding of the general principles of cerebral reorganization after stroke but are less informative about whether it underlies functional recovery in individual patients. If functional imaging techniques are ever to be used as surrogate markers of recovery, or as ways of predicting outcome, then intersubject variability in responses needs to be investigated. We set out to investigate the feasibility of studying the relationship between changes in cerebral organization and changes in motor performance in individual patients. Using fMRI, we measured passive movement–induced brain activation patterns over the first 4 months of recovery in 2 stroke patients with very different recovery profiles and investigated how these patterns in each of these patients changed in relation to motor recovery.
METHODS
Subjects
We selected 2 patients admitted to the Acute Brain Injury Unit at the National Hospital for Neurology and Neurosurgery within 7 days of one another, with complete paresis of the affected hand at 48 h after onset of symptoms. Further inclusion criteria were also applied to both patients: 1) first stroke ever, 2) sufficient language and cognitive ability to take part in the study, 3) absence of sensory deficit at bedside testing, and 4) no carotid artery stenosis ≥ 70%. Seven right-handed control subjects were also recruited. Full written consent was obtained from all subjects in accordance with the Declaration of Helsinki. The study was approved by the Joint Ethics Committee of the Institute of Neurology, UCL and National Hospital for Neurology and Neurosurgery, UCL Hospitals NHS Trust, London.
Behavioral Evaluation
In addition to full neurological examination, both patients were evaluated with the following outcome measures pertaining to motor function of the affected upper limb at each scanning session: 1) Action Research Arm Test (ARAT), 2) Motricity Index for upper limb, 3) grip strength (GRIP), and 4) nine-hole peg test (NHPT). GRIP represents the maximum hand grip strength of the affected hand divided by the maximum grip strength of the unaffected hand expressed as a percentage. NHPT was performed by measuring the time to place 9 pegs with each hand. If patients failed to place all 9 pegs within 60 s, the number of pegs successfully placed was recorded. Scores were recorded as pegs per second for each hand (averaged over 3 trials). The score for the affected hand was divided by the score for the unaffected hand and expressed as a percentage.
To obtain a single representative score for each patient at each scanning session, a principal component analysis was performed on the 4 sets of longitudinal scores as previously described.3
Experimental Paradigm
Subjects were studied using fMRI during passive wrist flexion-extension. To obtain the greatest signal change, the range of movements was determined as the largest that could be tolerated by both patients (± 45° either side of horizontal). The movements were performed at 1 Hz. The subject’s hands were fixed into a hinged wrist splint. An experimenter in the scanner room moved the subject’s wrist by means of a horizontal rod attached to the wrist splint. Left and right sides were studied in separate 20-s blocks, alternating with 20-s rest blocks. In total, six 20-s blocks of passive movement were performed on each side during a single scanning session. Subjects had their eyes covered throughout. Both patients were studied over 6 scanning sessions. One control subject was also studied longitudinally over 6 sessions to examine for nonspecific session effects. The remaining 6 control subjects were scanned on 1 occasion only. The 1st scan for each patient was performed at 10 to 15 days poststroke and thereafter at approximately 3, 4, 5, 6, and 16 weeks poststroke. The sessions were similarly spaced for 1 control subject undergoing a longitudinal study.
Data Acquisition
A Siemens VISION system (Siemens, Erlangen, Germany), operating at 2 T, was used to acquire both T1-weighted anatomical images (1 × 1 × 1.5 mm voxels) and T2*-weighted MRI transverse echoplanar images (EPI) (64 × 64.3 × 3 mm2 pixels, TE = 40 ms) with blood oxygenation level dependent (BOLD) contrast. The site of cerebral infarction was determined from the T1-weighted anatomical images. Each echoplanar image comprised forty-eight 1.8-mm thick contiguous axial slices taken every 3 mm, positioned to cover the whole cerebrum, with an effective repetition time (TR) of 3.65 s/vol. The first 6 volumes were discarded to allow for T1 equilibration effects.
Image Analysis
Imaging data were analyzed using Statistical Parametric Mapping (SPM2, Wellcome Department of Imaging Neuroscience, http://www.fil.ion.ucl.ac.uk/spm/) implemented in Matlab6 (The Mathworks Inc., Natick, MA).8,9 All volumes were realigned to the 1st volume to correct for interscan movement. To remove unwanted movement-related variance without removing variance attributable to the motor task, realigned images were processed using the “unwarp” toolbox in SPM2,10 which is predicated on the assumption that susceptibility-by-movement interaction is responsible for a sizeable part of residual movement-related variance. Given the observed variance (after realignment) and the realignment parameters, estimates of how deformations changed with subject movement were made, which were subsequently used to minimize movement-related variance.
To correct for their different acquisition times, the signal measured in each slice was shifted relative to the acquisition of the middle slice using sinc interpolation in time. The resulting volumes were then normalized to a standard EPI template based on the Montreal Neurological Institute (MNI) reference brain in Talairach space11 and resampled to 3 × 3 × 3 mm voxels. All normalized images were then smoothed with an isotropic 8-mm full-width half-maximum Gaussian kernel to account for intersubject differences and allow valid statistical inference according to Gaussian random field theory.12 The time series in each voxel were high-pass filtered at 1/128 Hz to remove low-frequency confounds and scaled to a grand mean of 100 over voxels and scans within each session. Images from the patient with a left-sided lesion (patient 2) were flipped about the midsagittal plane, to allow direct comparison between patients.
Each condition (left wrist movement, right wrist movement, rest) was modeled separately as a boxcar function convolved with a canonical synthetic hemodynamic response function, and used in a general linear model. Control group effects were determined using a multisubject fixed effects model. For the longitudinal analysis, a single-subject multisession fixed effects model was employed, as used previously.3 Voxels significant for the contrast left (or right) passive wrist movement compared to rest were determined for each session. Further analyses were performed as follows:
The main effects of wrist movement were determined for the control group (averaging across subjects), and for patient 1 and patient 2 separately (averaging across sessions), from the respective multisession fixed effects models.
Longitudinal analyses were performed for patient 1, patient 2, and for the single control subject undergoing multiple studies, separately. Contrasts were weighted with the mean corrected overall recovery scores (derived for each patient by principal component analysis) to identify voxels in which there is a linear correlation (either positive or negative) between the recovery score and the size of activation across sessions (the “task by recovery” interaction). The “task by session” interaction was determined for the longitudinal control subject to examine for nonspecific session effects.
To directly compare the correlations in patient 1 and patient 2, data from both patients were entered into a single multisession fixed effects model. Contrasts were weighted to identify voxels in which the correlation between magnitude of task-related activation and recovery scores was significantly different in one patient compared to the other.
All results were displayed as statistical parametric maps of the t statistic (SPM{t}) corrected for multiple comparisons across whole brain (P < 0.05). The correlation coefficient for each significant voxel was also calculated for each patient for descriptive purposes. Anatomical identification was performed by superimposing the maxima of activation foci both on the MNI brain and on the normalized structural images of each subject and labeling with the aid of the atlas of Duvernoy.13 The probability of the location of each significant voxel was determined from the cytoarchitectonic maps at www.bic.mni.mcgill.ca/cytoarchitectonics/.
RESULTS
Clinical Data
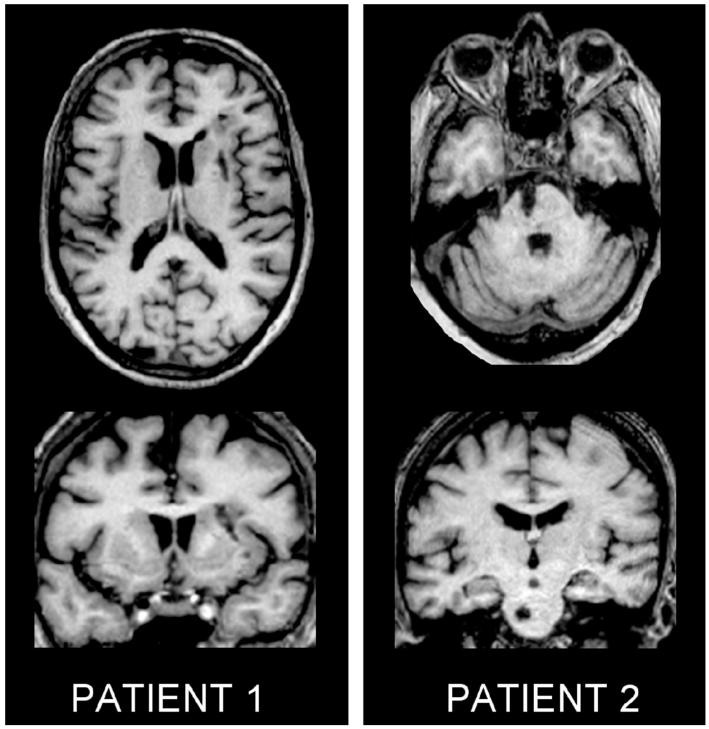
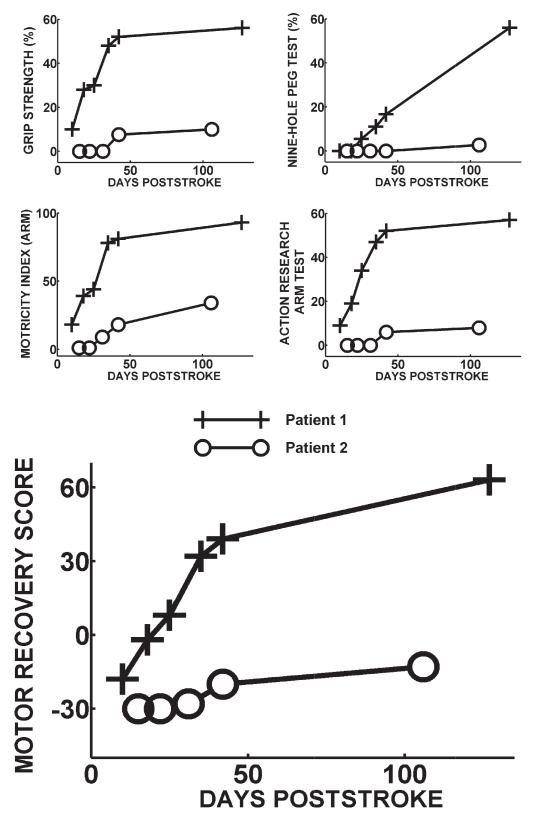
Patient 1 (71 years old) suffered a right striatocapsular infarction. Patient 2 (72 years old) suffered a left pontine infarction (Figure 1). Both patients received physiotherapy as in patients appropriate for their own needs. Outcome scores improved more for patient 1 who became independent by 4 months, than for patient 2 who remained wheelchair bound with poor arm function (Figure 2). Muscle tone was normal throughout the study in both patients. Seven control subjects were studied (age, 44–70 years; mean, 59.6 years).
Figure 1.
Axial (top) and coronal (bottom) T1-weighted MRI scans at the level of maximum infarct volume for each patient performed at the time of final fMRI.
Figure 2.
Motor recovery scores are plotted against days poststroke for patient 1 (+) and patient 2 (o). Grip strength represents the maximum hand grip strength for the affected hand divided by the maximum grip strength or the unaffected hand expressed as a percentage. Nine-hole peg test represents pegs/second for the affected hand divided by the pegs/second for the unaffected hand expressed as a percentage. The Motor Recovery Score (lower graph) represents the 1st principal component of the 4 measures used. This accounts for 90.6 percentage of the variance across all recovery scores.
Imaging Results
The 50 days poststroke scans for patient 2 were excluded from the analysis because of excessive movement-related artifact.
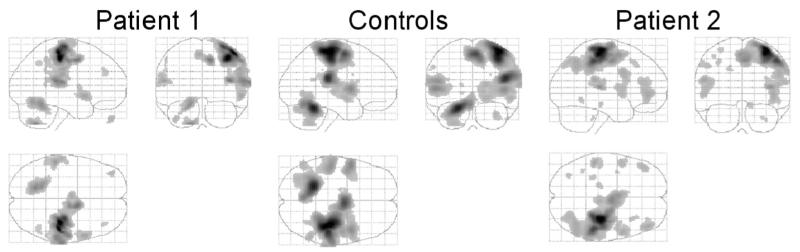
The main effects of passive movement compared to rest for controls (left hand), patient 1 (affected left hand), and patient 2 (affected right hand, results flipped about midsagittal line for comparison) are given in Table 1 and illustrated in Figure 3.
Table 1.
Brain Activation during Passive Movement
| Region | Control Group |
Patient 1 |
Patient 2 |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Talairach Coordinates in MNI Space |
Talairach Coordinates in MNI Space |
Talairach Coordinates in MNI Space |
|||||||||||||
| Side | X | y | z | Z Value | Side | X | y | z | Z Value | Side | X | y | z | Z Value | |
| Primary sensory cortex | R | 38 | −28 | 62 | >8.0 | R | 38 | −26 | 52 | >8.0 | R | 34 | −24 | 64 | >8.0 |
| R | 40 | −32 | 62 | >8.0 | R | 46 | −28 | 60 | >8.0 | R | 32 | −28 | 60 | >8.0 | |
| Primary motor cortex | R | 48 | −6 | 54 | 6.11 | — | — | — | — | — | — | — | — | — | — |
| Supplementary motor area | R | 4 | −4 | 58 | >8.0 | R | 12 | 0 | 48 | >8.0 | R | 4 | −8 | 50 | 7.24 |
| — | — | — | — | — | R | 12 | −12 | 48 | >8.0 | — | — | — | — | — | |
| Parietal operculum (S II) | R | 48 | −28 | 22 | >8.0 | — | — | — | — | — | R | 46 | −26 | 12 | 6.88 |
| L | −50 | −24 | 16 | 6.73 | L | −56 | −32 | 16 | >8.0 | L | −52 | −26 | 20 | 5.46 | |
| Inferior frontal gyrus (pars opercularis) | R | 46 | 6 | 4 | 7.13 | R | 60 | 12 | −4 | 6.81 | R | 54 | 6 | 32 | 5.14 |
| L | −54 | 6 | 6 | 7.51 | — | — | — | — | — | ||||||
| Dorsal prefrontal cortex | — | — | — | — | — | — | — | — | — | — | R | 44 | 44 | 0 | 5.23 |
| Thalamus | R | 18 | −14 | 12 | 4.96 | — | — | — | — | — | — | — | — | — | — |
| Cerebellum | L | −20 | −48 | −24 | >8.0 | L | −16 | −54 | −20 | >8.0 | — | — | — | — | — |
| L | −24 | −54 | −48 | 4.73 | L | −16 | −60 | −50 | >8.0 | — | — | — | — | — | |
Regions in which brain activation was detected during passive movement of the left wrist (controls) or affected wrist (patients). Images for patient 2 were flipped about the midsagittal line, for comparison with patient 1. Voxels are significant at P < 0.05, corrected for multiple comparisons across whole brain volume. MNI = Montreal Neurological Institute; R = right; L = left.
Figure 3.
The main effects of hand grip compared to rest for controls, patient 1, and patient 2. The results for controls and patient 1 are for passive movement of the left wrist. The results for patient 2 are for passive movement of the right wrist flipped about the midsagittal line for comparison. Results are shown as maximum intensity projections. The brain is shown from the right, top, and back. All voxels are significant at P < 0.001 (uncorrected) for the purposes of illustration.
No session effects (task-by-session interactions) were seen for the control subject studied longitudinally. Furthermore, no recovery-related effects (task-by-recovery interactions) were observed for passive movement of the unaffected wrist for either patient. For patient 1, a positive correlation was observed between size of activation and recovery scores in both ipsilesional M1 and S1 (Table 2). For patient 2, there was a positive correlation between size of activation and recovery scores in the contralesional inferior parietal cortex (x = −64, y = −22, z = 26, r2 = 0.86, P = 0.05). There were no negative correlations.
Table 2.
Differences in Recovery-Related Increases in Brain Activation between Patients
| Talairach Coordinates in MNI Space |
Patient 1 Correlation Analysis |
Patient 2 Correlation Analysis |
|||||
|---|---|---|---|---|---|---|---|
| Region | Side | X | y | z | Z Value | r 2 | r 2 |
| (A) Primary motor cortex | I | 44 | −20 | 54 | 6.64 | 0.9 | 0.01 |
| (B) Primary motor cortex | I | 40 | −24 | 46 | 5.74 | 0.92 | 0.08 |
| (C) Primary sensory cortex | I | 48 | −22 | 46 | 6.45 | 0.92 | 0.001 |
| (D) Primary sensory cortex | I | 48 | −26 | 54 | 4.87 | 0.77 | 0.02 |
MNI = Montreal Neurological Institute; I = ipsilesional.
Comparison between these correlation analyses revealed significant differences in the positive correlation slopes between the 2 patients in ipsilesional S1 and M1 (Table 2, Figure 4). The positive correlation in the inferior parietal cortex for patient 2, however, was not statistically different to changes seen in the same voxel in patient 1.
Figure 4.
Brain regions in which the correlation between magnitude of activation and recovery scores is significantly different for patient 1 compared to patient 2. Task-related signal change is plotted against motor recovery score for patient 1 (+, solid line = significant correlation) and patient 2 (○, dotted line = nonsignificant correlation) for voxels A to D (see Table 2 for coordinates and results of correlation analyses). Results for control group (passive movement of left wrist compared to rest) for the same voxel are plotted on the left-hand side of the graph for comparison. Significant voxels are overlaid onto axial slices of the T1-weighted structural image from patient 1.
DISCUSSION
We have measured changes in the magnitude of brain activation in response to proprioceptive input from the affected upper limb over the first 4 months after stroke in 2 patients with very different recovery profiles. We observed increases in ipsilesional primary motor (M1) and sensory (S1) cortex activation over sessions that correlated with improvements in upper limb motor function. These changes were significantly greater in a patient with good recovery compared to a patient with poorer recovery. Our results are unlikely to be due to changes in sensory perception, as neither patient had sensory deficits. Somatosensory-evoked potentials were not performed, but the magnitude of ipsilesional S1 activation at initial scanning was comparable to that seen in controls, suggesting preserved proprioceptive input in both patients. In addition, recovery-related increases in activation were seen in the inferior parietal cortex in the patient with poorer recovery, but we were unable to demonstrate that these were significantly different from changes seen in patient 1.
Time-related increases in passive movement–induced cerebral activation after stroke have previously been reported in both the sensorimotor cortex and inferior parietal cortex.5,6 The relevance of these results to functional recovery is not clear because analyses were performed on groups of patients as a whole, irrespective of their clinical outcomes. To advance our understanding of the principles of functionally relevant cerebral reorganization after stroke, it is crucial that changes in physiological measures (e.g., BOLD signal) are related to changes in behavioral performance in individuals as well as groups of patients. Detailed longitudinal studies of individual cases with fMRI are not common, and our purpose in reporting these 2 cases is to illustrate the feasibility of this approach, even in patients with initially severe impairment. Consequently, we have been able to identify changes in cerebral reorganization that might be clinically relevant in these individuals. The results suggest that this longitudinal approach has the potential to be useful in understanding the impact of therapeutic interventions over the whole period of rehabilitation.
It is important to acknowledge that our patients had lesions in different parts of the motor system and that the exact anatomy of the damage will have a major impact on subsequent patterns of recovery. This has been demonstrated empirically with respect to the corticospinal tract.14 These anatomical differences are likely to be the main reason for different recovery patterns in our 2 patients. It is interesting to speculate on the basis of what we know about the organization of fibers in the internal capsule14 that the important elements of the corticospinal system are less damaged in patient 1 than in patient 2. The ascending sensory pathways are likely to be intact in both patients because both have preserved sensory function and passive movement results in S1 activation to a similar extent in patients and controls. Thus, sensory input reaches S1 in both patients, which is important, as the acquisition of new motor skills relies in part on input from the sensory to the motor cortex.15 However, sensorimotor cortex reorganization only occurs in the patient in whom the M1 efferent projections are likely to be intact. We speculate therefore that reorganization in the ipsilesional sensorimotor cortex might facilitate motor recovery only if M1 still has a useful role to play in motor function, as is likely for patient 1 but probably less so for patient 2.
From our study and from those of others,4-6,16 it appears that in control subjects, proprioceptive input does increase neuronal activity in the inferior parietal cortex bilaterally. Reorganization in the contralesional inferior parietal cortex may be the “best option” for patient 2 given the anatomical constraints of his damaged system, but this reorganization has less direct influence over motor output and consequently motor recovery.
Thus, recovery-related increases seen in the ipsilesional sensorimotor cortex were associated with good recovery, but those in the inferior parietal cortex were less functionally relevant. The way the surviving brain regions are organized into a functional system is crucial for recovery, as it may well determine not only the ability of the damaged central nervous system to generate a motor output but also the ability of a therapeutic intervention, for example, sensory training, to induce activity-driven change in important brain regions. Functional anatomy depends not only on the anatomy of the damage but will also change as a result of prior treatment and experience.17 Structural imaging, however, cannot convey the functional organization of these remaining brain regions in the way that functional imaging can. Our previous longitudinal fMRI study of the motor output system after stroke indicates that each patient’s residual functional anatomy changes with time.3 The current case reports differ in that they deal with cerebral responses to a sensory stimulus with afferent input to both M1 and S1,18 which may be more relevant for understanding how certain treatments interact with the damaged central nervous system after stroke.
Clearly our 2 single case reports do not generalize to all stroke patients, but they do demonstrate that detailed correlations between physiological and behavioral data might reveal potentially important information about the recovery process in individual patients after stroke.
ACKNOWLEDGMENTS
NSW and RSJF are supported by the Wellcome Trust. NSW was previously supported by the Stroke Association during part of this work. MMB’s Chair in Stroke Medicine is supported by the Reta Lila Weston Trust for Medical Research. AJT holds the Garfield Weston Chair of Clinical Neurology and Neurological Rehabilitation. We thank Will Penny (Wellcome Department of Imaging Neuroscience) for help and advice concerning statistical analysis and Peter Aston (Wellcome Department of Imaging Neuroscience) for the building of the wrist splints. We also thank the staff of the Acute Brain Injury Unit and Neurorehabilitation Unit at the National Hospital for Neurology and Neurosurgery, Queen Square, London, for their assistance.
REFERENCES
- 1.Marshall RS, Perera GM, Lazar RM, Krakauer JW, Constantine RC, DeLaPaz RL. Evolution of cortical activation during recovery from corticospinal tract infarction. Stroke. 2000;31:656–61. doi: 10.1161/01.str.31.3.656. [DOI] [PubMed] [Google Scholar]
- 2.Calautti C, Leroy F, Guincestre JY, Marie RM, Baron JC. Sequential activation brain mapping after subcortical stroke: changes in hemispheric balance and recovery. Neuroreport. 2001;12:3883–6. doi: 10.1097/00001756-200112210-00005. [DOI] [PubMed] [Google Scholar]
- 3.Ward NS, Brown MM, Thompson AJ, Frackowiak RSJ. Neural correlates of motor recovery after stroke: a longitudinal fMRI study. Brain. 2003;126:2476–96. doi: 10.1093/brain/awg245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelles G, Spiekramann G, Jueptner M, et al. Evolution of functional reorganization in hemiplegic stroke: a serial positron emission tomographic activation study. Ann Neurol. 1999;46:901–9. doi: 10.1002/1531-8249(199912)46:6<901::aid-ana13>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 5.Loubinoux I, Carel C, Pariente J, et al. Correlation between cerebral reorganization and motor recovery after subcortical infarcts. Neuroimage. 2003;20:2166–80. doi: 10.1016/j.neuroimage.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 6.Tombari D, Loubinoux I, Pariente J, et al. A longitudinal fMRI study: in recovering and then in clinically stable sub-cortical stroke patients. Neuroimage. 2004;23:827–39. doi: 10.1016/j.neuroimage.2004.07.058. [DOI] [PubMed] [Google Scholar]
- 7.Hikosaka O, Nakamura K, Sakai K, Nakahara H. Central mechanisms of motor skill learning. Curr Opin Neurobiol. 2002;12:217–22. doi: 10.1016/s0959-4388(02)00307-0. [DOI] [PubMed] [Google Scholar]
- 8.Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- 9.Worsley KJ, Friston KJ. Analysis of fMRI time-series revisited—again. Neuroimage. 1995;2:173–81. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- 10.Andersson JL, Hutton C, Ashburner J, Turner R, Friston K. Modeling geometric deformations in EPI time series. Neuroimage. 2001;13:903–19. doi: 10.1006/nimg.2001.0746. [DOI] [PubMed] [Google Scholar]
- 11.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thieme; Stuttgart: 1998. [Google Scholar]
- 12.Friston KJ, Ashburner L, Poline JB, Frith CD, Frackowiak RSJ. Spatial registration and normalization of images. Hum Brain Mapp. 1995;2:165–89. [Google Scholar]
- 13.Duvernoy HM. The human brain: surface, blood supply, and three-dimensional anatomy. Springer-Verlag; New York: 1991. [Google Scholar]
- 14.Fries W, Danek K, Scheidtmann K, Hamburger C. Motor recovery following capsular stroke. Role of descending pathways from multiple motor areas. Brain. 1993;116:369–82. doi: 10.1093/brain/116.2.369. [DOI] [PubMed] [Google Scholar]
- 15.Pavlides C, Miyashita E, Asanuma H. Projection from the sensory to the motor cortex is important in learning motor skills in the monkey. J Neurophysiol. 1993;70:733–41. doi: 10.1152/jn.1993.70.2.733. [DOI] [PubMed] [Google Scholar]
- 16.Weiller C, Juptner M, Fellows S, et al. Brain representation of active and passive movements. Neuroimage. 1996;4:105–10. doi: 10.1006/nimg.1996.0034. [DOI] [PubMed] [Google Scholar]
- 17.Schallert T, Leasure JL, Kolb B. Experience-associated structural events, subependymal cellular proliferative activity, and functional recovery after injury to the central nervous system. J Cereb Blood Flow Metab. 2000;20:1513–28. doi: 10.1097/00004647-200011000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Fourment A, Chennevelle JM, Belhaj-Saif A, Maton B. Responses of motor cortical cells to short trains of vibration. Exp Brain Res. 1996;111:208–14. doi: 10.1007/BF00227298. [DOI] [PubMed] [Google Scholar]