Abstract
The purpose of the present study was to investigate the effect of intra-articular injections of autologous conditioned serum on human hip osteoarthritis and to test whether a potential treatment effect might be increased by additional injections of steroids and the recombinant interleukin-1 receptor antagonist protein anakinra. We compared the effects of autologous conditioned serum 46 hip osteoarthritis patients), autologous conditioned serum+cortisone (56 patients), and autologous conditioned serum+cortisone+recombinant interleukin-1 receptor antagonist protein (17 patients) in a retrospective clinical study by means of the Visual Analogue Scale for pain (pre- vs posttreatment). Over 14 months, treatment resulted in a large, statistically significant improvement for patients in all three groups, independent of the severity of osteoarthritis. Neither cortisone nor cortisone+recombinant interleukin-1 receptor antagonist protein increased the beneficial treatment effect over and above the effect of autologous conditioned serum alone. Autologous conditioned serum successfully reduces pain in hip osteoarthritis. In severe hip osteoarthritis, the sole application of autologous conditioned serum can be even more beneficial than the combination of autologous conditioned serum with steroids.
Key words: autologous conditioned serum, recombinant IL-1 receptor antagonist protein, hip osteoarthritis
Introduction
Osteoarthritis (OA) is one of the most common chronic conditions, particularly in elderly populations. Leading as it does to pain and loss of function, OA drastically reduces patients’ ability to work and their Quality of Life.1 Hip OA is the second most frequent type of OA affecting large joints and prevalence estimates vary from 3-11% of adults.2
Pharmacological therapies for OA used to be restricted to the symptomatic use of analgesics, non-steroidal anti-inflammatory drugs (NSAIDs), and intra-articular injections of steroids or hyaluronic acid (HA).1 To date, disease-modifying effects of steroids have only been demonstrated in animal and in in vitro studies, but not yet clinically in human in vivo studies.3 The clinical use of steroids in hip OA, therefore, remains controversial: cortisone injections are said to be short-acting, prone to potential adverse effects, and the level of evidence regarding their disease-modifying efficacy is low.4,5 Nor has the initial hope for a causal clinical effect of intra-articular HA injections been fulfilled. Most studies investigating the efficacy of HA injections in human hip OA are methodologically flawed. When compared to placebo control groups, HA does not significantly improve outcome, and little is known about long-term effects.5,6 Thus, there is still a need for a non-invasive, disease-modifying therapy of OA in general, and hip OA in particular.7,8
In OA, the destruction of hyaline cartilage constitutes the central pathological mechanism causing various mechanical and biological dysfunctions within the joint. Of the cytokines identified in osteoarthritic joints, interleukin-1 (IL-1) appears to be of particular importance. Accordingly, the IL-1 receptor antagonist protein (IRAP), a naturally occurring inhibitor of IL-1, has been reported to limit the intra-articular damage associated with IL-1.1,9 In animal models, many researchers have succeeded in positively modifying the osteoarthritic disease process by effectively antagonising IL-1.1,10,11
Autologous conditioned serum (ACS) is an example of a presumably disease-modifying treatment for OA based on antagonising the intra-articular effects of IL-1.12 Currently, Orthokin® (Orthogen, Düsseldorf, Germany) and Onoccomed® (Plasmaconcept AG, Bonn, Germany) are the only two commercially available ACS products. Both Orthokin® and Onoccomed® are prepared from venous whole blood incubated in the presence of glass spheres to initiate monocyte activation. The resulting conditioned serum contains elevated levels of various anti-inflammatory cytokines, such as IRAP, IL-4 and IL-10.1,12,13 After initial scepticism and the positive outcome of an animal model,14,15 recent prospective randomized controlled double-blind trials have provided first evidence in human samples demonstrating that Orthokin® is more effective than placebo and/or HA for the treatment of knee OA.1,16
One problem associated with ACS, however, is that it contains a combination of cytokines and growth factors, and their respective contributions to the clinical effects remain to be unraveled.7,12 Some authors, therefore, suggested antagonising selected cytokines, such as IL-1, individually in order to study their individual and unique contribution to the disease modification process. For example, the recombinant IRAP (rIRAP) anakinra is already in use for the evidence-based treatment of rheumatoid arthritis (RA).17,18 In addition, rIRAP has proved its efficacy as a treatment option in an animal OA model; first results in human knee OA and in patients with erosive hand OA,7,19,20 as well as in patients with acute anterior cruciate ligament tear, have also been promising.9 However, not all of the OA results are fully convincing: the presumably low concentration of rIRAP, due to its short half-life, has been discussed as one potential explanation for the fact that it sometimes fails to be an effective clinical treatment in human OA, as the concentration of (r)IRAP seems crucial for a clinically relevant treatment effect.7,21 Published studies agree that the concentration of IRAP must greatly exceed the concentration of IL-1 for it to effectively block the available IL-1 receptors and hence the effects triggered by IL-1.7,17 One possible way to increase the concentration of (r)IRAP (and thus presumably the antagonism of IL-1) would be the combined application of different IL-1 antagonists, including those which on their own might be ineffective clinically or less effective.
To the best of our knowledge, the combined effect of an intra-articular application of ACS (Orthokin® or Onoccomed®) and rIRAP or cortisone has not yet been investigated, either in animals or humans.
Aim of the study
Since the few previous human ACS studies were restricted to knee OA, we conducted the first clinical study to retrospectively investigate the effect of intra-articular ACS injections on hip OA. If ACS proved effective, we would then examine whether the treatment effect might be enhanced by the additional intraarticular application of cortisone and rIRAP.
Materials and Methods
Study design and population
Data collection and treatment took place at an orthopedic center. In order to be able to assess the overall efficacy of ACS, and that of cortisone and rIRAP over and above ACS, we conducted a retrospective, clinical, non-blinded and non-randomized intervention study with a modified dismantling design.22
Inclusion criteria were age over 30 years and clinical evidence of hip OA as judged by the treating physician and defined both by the presence of typical symptoms, such as pain and disability, and by radiographic evidence of OA, i.e. Kellgren/Lawrenc (K/L) hip grade 2-4.23 Patients with active infection, clinically relevant hematologic or abnormal clinical chemistry values, bone cancer, metastasis or tumorlike lesions in immediate proximity to the treated hip, and poor general health were excluded. All investigations were conducted in conformity with the ethical standards laid down in the 1975 Declaration of Helsinki (as revised in 2008), the ethics review board of the Centre for Molecular Orthopaedics, Düsseldorf, Germany, approved the study and its subsequent publication. Participation was voluntary and informed consent for participation in the study was obtained.
A total of 119 patients (150 hips) were assigned to one of the three treatment groups based on the treating physician’s clinical judgement. There was no placebo control group, such as saline injection. The treating physician also performed the interventions on his respective patients. All patients completed the Visual Analogue Scale (VAS) for pain,24 which ranges from 0 (no pain) to 10 (most severe pain), before the first injection was administered (pre-treatment VAS) and again 14.35±0.60 months after the last injection (post-treatment VAS). No patient dropped out or underwent surgery while enrolled in the study.
Interventions
To produce ACS, 50 mL of whole blood were taken from each patient using a special syringe with increased internal surface area (Orthogen, Düsseldorf, Germany); glass beads in the syringes increase the non-pyrogenic surface area and induce the dose-dependent production of IRAP (among others) by white blood cells in whole blood incubated at 37°C. After incubation, the blood-filled syringes were centrifuged, the serum supernatant was filtered (0.22 mm; Millipore, Carrigtwohill, Co. Cork, Ireland) and aliquoted into 6-8.2 mL portions. The aliquots were stored at -20°C until use.
The rIRAP was generated by diluting commercially available anakinra (Kineret®) and aliquoting it into portions of 0.2 mg each. Triamcinolone (Triam 10 Lichtenstein®) was used as a cortisone derivative. The following 3 groups of patients were studied:
autologous conditioned serum (ACS): in 46 patients (62 hips), a total of 5.94±0.03 injections of 2 mL of ACS each were administered;
ACS+cortisone (ACS+C): 56 patients (71 hips) received 5.70±0.08 injections of 2 mL ACS, and 1.94±0.15 injections of 10mg of Triamcinolone each;
ACS+cortisone+rIRAP (ACS+C+rIRAP): 17 patients (17 hips) were given 5.88±0.19 injections of 2 mL ACS, 2.88±0.32 injections of 10 mg of Triamcinolone, and 3.53±0.26 injections of 0.2 mg rIRAP each.
Statistical analysis
The Statistical Package for the Social Sciences (SPSS) 17.0 was used for data analysis. Means and standard errors of the mean are reported as M±SE.
Group differences regarding continuous variables were assessed by one-way between-subjects analyses of variance (ANOVAs), with Bonferroni-corrected pairwise post hoc comparisons; χ2 (Fisher’s exact) tests were used for dichotomous variables. The association between VAS scores and K/L hip grade (2-3: mild vs 4: severe OA) was tested by biserial correlation coefficients (rb).Pearson’s correlation coefficient (r) was computed to relate pretreatment to post-treatment VAS values. Differences between pre- and post-treatment VAS scores were tested with one-way withinsubjects ANOVAs for each group separately.
In order to evaluate potentially differential treatment effects despite the lack of randomization, 3 safeguards were applied. First, success rates (responders) were compared, as indicated by VAS pain reduction of at least 20%,25 using Fisher’s exact tests. Second, the interaction between intervention and the change in VAS score over time was analyzed in a mixed-design ANOVA. Third, an analysis of covariance (ANCOVA) was conducted with pretreatment VAS values as a covariate, thus allowing us to examine the treatment effect adjusting for the (potentially unequal) pretreatment VAS values. In all tests, P<0.05 was considered statistically significant.
As the computation of P values alone is not fully informative, particularly when samples are very small or large, Cohen’s method was adopted to additionally determine the power (1-β) and effect sizes,26 using the freely available G*Power 3 program.27 Following Cohen’s criteria for the interpretation of effect sizes, in AN(C)OVAs ɳ2≥0.14 indicates a large effect, ɳ2≥0.06 a moderate one, and ɳ2≥0.01 a small effect. In Fisher’s exact tests, the measure of effect size is ͩ, with ͩ≥0.50 a large, ͩ≥0.30 a moderate, and ͩ≥0.10 a small effect. The correlation coefficients r and rb are themselves measures of effect size, with rb≥0.50 a large, rb≥0.30 a moderate, and rb≥0.10 a small effect. With regard to power, 1-β>0.80 is considered large.
Results
Patients’ characteristics
There was no significant difference between groups with regard to age, gender, OA localization or severity, or average total number of ACS injections administered (all P values >0.05). Significant differences were, however, observed in the average number of cortisone injections, the percentage of patients with a hip prosthesis, and in the pre- and posttreatment VAS scores.
Patients in the ACS+C+rIRAP group were administered more cortisone injections (2.88±0.32) than those in the ACS+C group (1.94±0.15), but the difference was only moderate (P=0.01, ɳ2=0.08). The percentage of patients with a hip prosthesis was significantly larger in the two groups receiving additional cortisone injections (ACS+C 31.0%, ACS+C+rIRAP 47.1%), than in the ACS group (6.5%), but this difference was not large (P<0.001, ω=0.34). The pre-treatment and post-treatment VAS scores of ACS patients (5.50±0.30 vs 2.58±0.26) were significantly lower than for the ACS+C patients (pre-treatment 6.97±0.22, P<0.001, ɳ2=0.09; post-treatment 3.91±0.31, P<0.01, ɳ2=0.07). The effect sizes in both cases were moderate (Table 1).
Table 1.
Demographics and interventional characteristics in the Autologous Conditioned Serum (ACS), ACS+Cortisone (ACS+C), and ACS+C+recombinant IL-1 receptor antagonist protein (ACS+C+rIRAP) groups.
| Characteristic | Total (n=150) |
ACS (n=62) |
ACS+C (n=71) |
ACS+C+rIRAP (n=17) |
P | Effect size | power (1-β) |
Post hoc |
|---|---|---|---|---|---|---|---|---|
| Age | 62.08 ±0.71 | 61.52 ±0.91 | 63.04=1=1.14 | 60.12 ±2.28 | 0.37 | 0.01 (ɳ2) | 0.18 | - |
| Gender (female) | 77 (51.3%) | 30 (48.4%) | 41 (57.7%) | 6 (35.3%) | 0.21 | 0.14 (ω) | 0.31 | - |
| Hip OA localization (left) | 67 (44.7%) | 30 (48.4%) | 31 (43.7%) | 6 (35.3%) | 0.62 | 0.08 (ω) | 0.13 | - |
| Hip prosthesis (yes) | 34 (22.7%) | 4 (6.5%) | 22 (31.0%) | 8 (47.1%) | <0.001 | 0.34 (ω) | 0.97 | - |
| Severe OA (K/L hip grade: 4) | 54 (36.0%) | 16 (25.8%) | 30 (42.3%) | 8 (47.1%) | 0.09 | 0.18 (ω) | 0.49 | - |
| ACS injections | 5.82±0.05 | 5.94±0.03 | 5.70±0.08 | 5.88±0.19 | 0.06 | 0.04 (ɳ2) | 0.59 | - |
| Cortisone injections | - | - | 1.94±0.15 | 2.88±0.32 | <0.01 | 0.08 (ɳ2) | 0.78 | ACS+C<ACS+C+rIRAP |
| rlRAP injections | - | - | - | 3.53±0.26 | - | - | - | |
| Pre-treatment VAS | 6.27±0.18 | 5.50±0.30 | 6.97±0.22 | 6.22±0.60 | <0.001 | 0.09 (ɳ2) | 0.93 | ACS <ACS+C |
| Post-treatment VAS | 3.25±0.20 | 2.58±0.26 | 3.91±0.31 | 2.90±0.53 | <0.01 | 0.07 (ɳ2) | 0.85 | ACS <ACS+C |
N, number of hips; OA, osteoarthritis; VAS, Visual Analogue Scale (0=no pain, 10=most severe pain). The figures represent either M±SE or absolute frequencies (%). For continuous variables, P values were calculated by one-way between-subjects analyses of variance with the effect size ɳ2 (≥0.14= large, ≥0.06=moderate, ≥0.01=small) and Bonferroni-corrected post hoc tests. For dichotomous variables, P values were calculated by Fisher’s exact tests with the effect size ω(≥0.50=large, ≥0.30=moderate, ≥0.10=small). Power ≥0.80=large.
Overall treatment effects
Pre- and post-treatment VAS values were positively correlated (r=0.36, P<0.001). Pretreatment VAS scores correlated with K/L hip grade (rb=0.19, P=0.01), whereas the correlation between post-treatment score and K/L grade failed to attain significance (rb=0.11, P=0.09, 1-β=0.38). The differences between pre- and post-treatment VAS values were highly significant in all groups (all P<0.001), with very large effects (all ɳ2’s well over 0.14), i.e. all three interventions effectively reduced pain (Figure 1).
Figure 1.
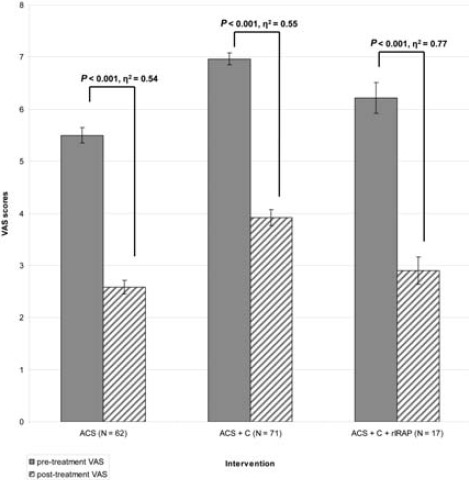
Mean pre- and post-treatment Visual Analogue Scale-scores in the Autologous Conditioned Serum (ACS), ACS+Cortisone (ACS+C), and ACS+Cortisone+recombinant IL-1 receptor antagonist protein (ACS+C+rIRAP) groups. N, number of hips; VAS, Visual Analogue Scale (0=no pain, 10=most severe pain). Error bars indicate standard errors of mean (SE). P values were calculated by one-way within-subjects analyses of variance (ANOVAs) with the effect size ?2 (?0.14=large, ?0.06=moderate, ?0.01=small).
The same pattern of results emerged when the analyses where conducted separately for patients with mild (n=96 hips) and severe OA (n=54 hips), indicating that the effects reported above were valid independently of OA severity (Figure 2).
Figure 2.
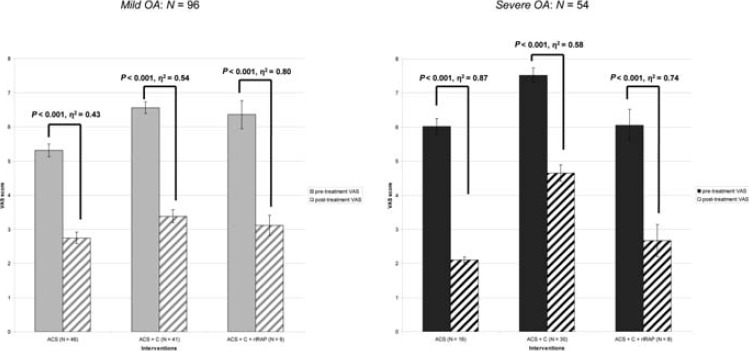
Mean pre- and post-treatment Visual Analogue Scale scores in the Autologous Conditioned Serum (ACS), ACS+Cortisone (ACS+C), and ACS+Cortisone+recombinant IL-1 receptor antagonist protein (ACS+C+rIRAP) groups as a function of the Kellgren/Lawrence (K/L) hip grade. OA, osteoarthritis; Mild=K/L hip grade 2-3; Severe=K/L hip grade 4; N=number of hips; N=number of hips; VAS=Visual Analogue Scale (0=no pain, 10=most severe pain). Error bars indicate standard errors of mean (SE). P values were calculated by one-way within-subjects analyses of variance (ANOVAs) with the effect size ɳ2 (≥0.14=large, ≥0.06=moderate, ≥0.01=small).
Differential treatment effects
We tested for differential treatment effects by comparing success rates (percentage of responders) across treatment groups. Overall, patients reported pain reduction in 119 of 150 hips (79.3%). There was no significant difference in success rates, which varied between 73.2% (ACS+C) and 94.1% (ACS+C+rIRAP), across interventions (P=0.14, ω=0.17, 1- β=0.45). Separate analyses for patients with mild OA (overall success rate 77.1%, range 73.2-100%) and severe OA (overall 83.3%, range 73.3-100%) showed similar results, i.e. high overall pain reduction with no significant differences between interventions (Table 2).
Table 2.
Success rates in the Autologous Conditioned Serum (ACS), ACS+Cortisone (ACS+C), and ACS+C+recombinant IL-1 receptor antagonist protein (ACS+C+rIRAP) groups.
| Total | Mild OA | Severe OA | ||||
|---|---|---|---|---|---|---|
| Overall | N=150 | 119 (79.3%) | N=96 | 74 (77.1%) | N=54 | 45 (83.3%) |
| ACS | N=62 | 51 (82.3%) | N=46 | 35 (76.1%) | N=16 | 16 (100%) |
| ACS+C | N=71 | 52 (73.2%) | N=41 | 30 (73.2%) | N=30 | 22 (73.3%) |
| ACS+C+rIRAP | N=17 | 16 (94.1%) | N=9 | 9 (100%) | N=8 | 7 (87.5%) |
| P | 0.14 | 0.27 | 0.06 | |||
| Effect size (ω) | 0.17 | 0.18 | 0.30 | |||
| Power (1-β) | 0.45 | 0.33 | 0.49 | |||
N, number of hips; OA, Osteoarthritis; Mild, Kellgren/Lawrence (K/L) hip grade 2-3; Severe=K/L hip grade 4; VAS, Visual Analogue Scale (0=no pain, 10=most severe pain).
Success was defined by VAS pain reduction, i.e. a positive difference between a patient’s pre- and post-treatment VAS score, of at least 20%. The figures represent absolute frequencies (%), P values were calculated by Fisher’s exact tests with the effect size ω(≥0.50=large, ≥0.30=moderate, ≥0.10=small). Power ≥0.80=large.
This pattern of results was confirmed by a mixed-design ANOVA. Treatment did not interact significantly with changes in the VAS score over time, either overall (P=0.86, ɳ2<0.01, 1-β=0.07) or in patients with mild OA (P=0.57, ɳ2=0.01, 1- β=0.14) or severe OA (P=0.31, ɳ2=0.04, 1- β=0.25).
In order to determine differential treatment effects most directly, an ANCOVA was calculated using pre-treatment VAS scores as a covariate.
This test compares post-treatment scores after controlling for the significantly different pretreatment scores (Table 3). Adjusted mean posttreatment VAS scores did not vary across treatments, either overall (P=0.09, ɳ2=0.03, 1-β=0.49) or in patients with mild OA (P=0.81, ɳ2<0.01, 1-β=0.08). In patients with severe OA, however, there was a significant difference (P<0.05, ɳ2=0.12): ACS patients (2.54±0.54) reported significantly less pain than ACS+C patients (4.31±0.40), but neither group (i.e. ACS and ACS+C) differed significantly from ACS+C+rIRAP patients (3.09±0.75).
Table 3.
Mean post-treatment Visual Analogue Scale (VAS) scores in the Autologous Conditioned Serum (ACS), ACS+Cortisone (ACS+C), and ACS+C+recombinant IL-1 receptor antagonist protein (ACS+C+rIRAP) groups after controlling for the pre-treatment VAS scores.
| Total | Post hoc | Mild OA | Post hoc | Severe OA | Post hoc | ||||
|---|---|---|---|---|---|---|---|---|---|
| Overall | N=150 | 3.15±0.22 | N=96 | 3.05±0.30 | N=54 | 3.31±0.33 | |||
| ACS | N=62 | 2.83±0.29 | N=46 | 2.90±0.34 | N=6 | 2.54±0.54 | |||
| ACS+C | N=71 | 3.70±0.27 | N=41 | 3.23+0.36 | N=30 | 4.31±0.40 | |||
| ACS+C+rlRAP | N=17 | 2.92±0.54 | N=9 | 3.01±0.76 | N=8 | 3.09±0.75 | |||
| P | 0.09 | - | 0.81 | - | N=54 | <0.05 | ACS <ACS+C | ||
| Effect size (ɳ2) | 0.03 | <0.01 | 0.12 | ||||||
| Power (1-β) | 0.49 | 0.08 | 0.62 | ||||||
N, number of hips; OA, osteoarthritis; Mild, Kellgren/Lawrence (K/L) hip grade 2-3; Severe, K/L hip grade 4; VAS, Visual Analogue Scale (0=no pain, 10=most severe pain). The figures represent M±SE, P values were calculated by analysis of covariance using the pre-treatment VAS scores as a covariate with the effect size ɳ2 (≥0.14=large, ≥0.06=moderate, ≥0.01=small) and Bonferroni-corrected post hoc tests. Power ≥0.80=large.
Discussion
The present study demonstrates for the first time in the literature that the intra-articular injection of ACS is an effective treatment for hip OA, both alone and combined with other treatments. Patients in all intervention groups (ACS, ACS+C, ACS+C+rIRAP) reported a large, statistically significant reduction in pain. In addition, VAS scores correlated with K/L grade only prior to treatment; after treatment, this correlation was smaller and nonsignificant. As a result of treatment, objective degeneration (as indicated by K/L grade) no longer significantly predicted patients’ subjective pain perception (and vice versa). Interestingly, subgroup analyses showed that these effects were valid not only for mild OA, but also for severe OA. This pattern of results implies that even patients who could previously be helped only with endoprothetic surgery can benefit from a far less invasive, potentially disease-modifying treatment that can possibly prevent, or at least delay, surgery.
Apart from the overall and severity-independent positive effect of ACS, the second main finding is that additional injections of steroids or steroids and rIRAP did not enhance the beneficial effect of ACS. There was neither a significant interaction between pain reduction over time and intervention, nor a relevant difference between post-treatment scores across interventions after controlling for the (numerically different) pre-treatment VAS scores. According to one analysis in patients with severe OA, the application of ACS alone was even moderately more effective than combining ACS with cortisone. Apparently, the combination of IL-1 antagonists does not clinically increase the positive effects of IL-1 antagonism. On the contrary, adding further agents, such as cortisone, may even reduce treatment success under certain circumstances, such as in severe OA eventually caused by blocking the local immune response by administering cortisone.
Attention must be drawn to some limitations of the present non-blinded and non-randomized study in terms of conclusions for clinical practice. i) Most importantly, treatment effects as measured by the VAS for pain were neither placebo-controlled nor blinded. It is well known, however, that pain is an inherently subjective phenomenon that can be influenced by many factors, including, among others, the evaluation of the observer.28 Research efforts have repeatedly shown that even supposedly established surgical procedures can sometimes turn out to be placebos. As early as 1959, Cobb et al.29 demonstrated that the ligation of the internal thoracic artery for the treatment of angina was no more effective than placebo surgery with regard to patients’ subjective improvement, reduction in nitroglycerin use, and increase in exercise tolerance. In patients with knee OA, Moseley et al.30 found that neither arthroscopic débridement nor arthroscopic lavage was superior to placebo surgery with regard to knee pain or function. Nevertheless, there is reason to believe that our present results do not constitute pure placebo effects: first, pain reduction was not only statistically significant, but was also very large.26 Second, the large reduction in pain scores (almost 50%) and the high percentage of responders (almost 80%) across treatments exceed the average placebo effects typically reported in hip OA patients of approximately 30% (pain reduction) and 33% (responders).2,31 ii) Despite our attempts to compensate for the lack of randomization by comparing success rates, analyzing interaction effects, and controlling for the significantly different pre-treatment scores, the treatment groups were not completely comparable, e.g. concerning the average number of cortisone injections or the percentage of patients with a hip prosthesis. Such problems can always occur in a clinical, retrospective design, thereby limiting the generalizability of its results. iii) Due to the limited sample size, the statistical power was not large in some instances. iv) Although we observed no adverse effects in the course of approximately 14 months of treatment, future studies should use longer and/or additional follow- up intervals in order to assess long-term effects more precisely. Finally, v) ideally, outcome measures are multimodal, whereas we have focused here on the subjective VAS scores. It seems desirable to extend the assessment of treatment success to other measures of patients’ Quality of Life and to complement these self-reports with additional clinical and objective data.
Conclusions
The present exploratory, yet promising results on the efficacy of ACS in hip OA require and warrant replication in larger, prospective, randomized, placebo-controlled, clinical trials, as already reported for ACS in knee OA.1,16 Since the clinical potential of cortisone in OA therapy seems exhausted, both as a single agent and in combination with other treatment options as in our study (where it was more likely to deteriorate outcome than to improve it),3 future research should focus on the remaining allegedly disease-modifying agents, rIRAP and ACS. We used rIRAP only in combination with ACS and cortisone due to its hitherto unproven clinical efficacy as a single agent in human OA.7 Our results thus cannot allow for any final assessment of rIRAP. On a positive note, rIRAP may have mitigated the negative effects of cortisone in patients with severe OA. To overcome the problem of low therapeutic drug levels presumably responsible for the previous clinical failures of rIRAP as a single OA drug, alternative and possibly more effective methods of application might be considered, 7 for example, the gene therapeutic use of viral vectors. This approach proved quite successful in the treatment of both RA and OA in pre-clinical and animal studies,8,17 but its clinical progress has slackened despite its initial success.8,13 Regarding ACS, a direct comparison of the two commercially available ACS products, Orthokin® versus Onoccomed®, would appear to be worthwhile investigating in future research.
References
- 1.Baltzer A, Moser C, Jansen S, Krauspe R. Autologous conditioned serum (Orthokine) is an effective treatment for knee osteoarthritis. Osteoarthritis Cartilage 2009;17:152-60 [DOI] [PubMed] [Google Scholar]
- 2.Richette P, Ravaud P, Conrozier T, et al. Effect of hyaluronic acid in symptomatic hip osteoarthritis: a multicenter, randomized, placebo-controlled trial. Arthritis Rheum 2009;60:824-30 [DOI] [PubMed] [Google Scholar]
- 3.Sadowski T, Steinmeyer J. Effects of non-steroidal antiinflammatory drugs and dexamethasone on the activity and expression of matrix metalloproteinase-1, matrix metalloproteinase-3 and tissue inhibitor of metalloproteinases-1 by bovine articular chondrocytes. Osteoarthritis Cartilage 2001;9:407-15 [DOI] [PubMed] [Google Scholar]
- 4.Kruse D. Intraarticular cortisone injection for osteoarthritis of the hip. Is it effective? Is it safe? Curr Rev Musculoskelet Med 2008;1:227-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abate M, Pelotti P, De A, et al. Viscosupplementation with hyaluronic acid in hip osteoarthritis (a review). Ups J Med Sci 2008;113:261-77 [DOI] [PubMed] [Google Scholar]
- 6.Fernandez L, Ruano-Ravina A. Efficacy and safety of intraarticular hyaluronic acid in the treatment of hip osteoarthritis: a sys-tematic review. Osteoarthritis Cartilage 2006;14:1306-11 [DOI] [PubMed] [Google Scholar]
- 7.Chevalier X. Intraarticular treatments for osteoarthritis: new perspectives. Curr Drug Targets 2010;11:546-60 [DOI] [PubMed] [Google Scholar]
- 8.Evans CH, Ghivizzani SC, Robbins PD. Getting arthritis gene therapy into the clinic. Nat Rev Rheumatol 2010;7:244-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kraus V, Birmingham J, Stabler T, et al. Effects of intraarticular IL1-Ra for acute anterior cruciate ligament knee injury: a randomized controlled pilot trial (NCT00332254). Osteoarthritis Cartilage 2012;20:271-8 [DOI] [PubMed] [Google Scholar]
- 10.Frisbie DD, Ghivizzani SC, Robbins PD, et al. Treatment of experimental equine osteoarthritis by in vivo delivery of the equine interleukin-1 receptor antagonist gene. Gene Ther 2002;9:12-20 [DOI] [PubMed] [Google Scholar]
- 11.Fernandes J, Tardif G, Martel-Pelletier J, et al. In vivo transfer of interleukin-1 receptor antagonist gene in osteoarthritic rabbit knee joints: prevention of osteoarthritis progression. Am J Pathol 1999;154:1159-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rutgers M, Saris DBF, Dhert WJA, Creemers LB. Cytokine profile of autologous conditioned serum for treatment of osteoarthritis, in vitro effects on cartilage metabolism and intra-articular levels after injection. Arthritis Res Ther 2010;12:R114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wehling P, Reinecke J, Baltzer A, et al. Clinical responses to gene therapy in joints of two subjects with rheumatoid arthritis. Hum Gene Ther 2009;20:97-101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burmester GR, Grifka J. [Revision of the recommendations of the Commission on the Pharmacotherapy of the German Society for Rheumatology. Comment on the use of Orthokin]. Z Rheumatol 2007;66:83-4 [Article in German] [DOI] [PubMed] [Google Scholar]
- 15.Frisbie D, Kawcak C, Werpy N, et al. Clinical, biochemical, and histologic effects of intra-articular administration of autologous conditioned serum in horses with experimentally induced osteoarthritis. Am J Vet Res 2007;68:290-6 [DOI] [PubMed] [Google Scholar]
- 16.Yang K, Raijmakers NJ, van Arkel ER, et al. Autologous interleukin-1 receptor antagonist improves function and symptoms in osteoarthritis when compared to placebo in a prospective randomized controlled trial. Osteoarthritis Cartilage 2008;16:498-505 [DOI] [PubMed] [Google Scholar]
- 17.Evans C. Novel biological approaches to the intra-articular treatment of osteoarthritis. Bio Drugs 2005;19:355-62 [DOI] [PubMed] [Google Scholar]
- 18.Bresnihan B, Cobby M. Clinical and radiological effects of anakinra in patients with rheumatoid arthritis. Rheumatology (Oxford) 2003;42 Suppl 2:ii22-8 [DOI] [PubMed] [Google Scholar]
- 19.Chevalier X, Giraudeau B, Conrozier T, et al. Safety study of intraarticular injection of interleukin 1 receptor antagonist in patients with painful knee osteoarthritis: a multicenter study. J Rheumatol 2005;32:1317-23 [PubMed] [Google Scholar]
- 20.Bacconnier L, Jorgensen C, Fabre S. Erosive osteoarthritis of the hand: clinical experience with anakinra. Ann Rheum Dis 2009;68:1078-9 [DOI] [PubMed] [Google Scholar]
- 21.Goupille P, Mulleman D, Chevalier X. Is interleukin-1 a good target for therapeutic intervention in intervertebral disc degeneration: lessons from the osteoarthritic experience. Arthritis Res Ther 2007;9:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gravetter FJ, Forzano LB. Research methods for the behavioral sciences. 3rd ed Belmont, CA: Wadsworth Cenage Learning; 2009 [Google Scholar]
- 23.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis 1957;16:494-502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freyd M. The graphic rating scale. J Educ Psychol 1923;14:83-102 [Google Scholar]
- 25.Goldsmith CH, Boers M, Bombardier C, Tugwell P. Criteria for clinically important changes in outcomes: development, scoring and evaluation of rheumatoid arthritis patient and trial profiles. OMERACT Committee. J Rheumatol 1993;20:561-5 [PubMed] [Google Scholar]
- 26.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. New York: Psychology Press; 1988 [Google Scholar]
- 27.Faul F, Erdfelder E, Lang A, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007;39:175-91 [DOI] [PubMed] [Google Scholar]
- 28.Horng S, Miller FG. Is placebo surgery unethical? N Engl J Med 2002;347:137-9 [DOI] [PubMed] [Google Scholar]
- 29.Cobb LA, Thomas GI, Dillard DH, et al. An evaluation of internal-mammary-artery ligation by a double-blind technic. N Engl J Med 1959;260:1115-8 [DOI] [PubMed] [Google Scholar]
- 30.Moseley JB, O’Malley K, Petersen NJ, et al. Controlled trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med 2002;347:81-8 [DOI] [PubMed] [Google Scholar]
- 31.Schnitzer T, Dattani I, Seriolo B, et al. A 13-week, multicenter, randomized, doubleblind study of lumiracoxib in hip osteoarthritis. Clin Rheumatol 2011;30:1433-46 [DOI] [PubMed] [Google Scholar]


