Abstract
Prosthesis-related infection is a serious complication for patients after orthopedic joint replacement, which is currently difficult to treat with antibiotic therapy. Consequently, in most cases, removal of the infected prosthesis is the only solution to cure the infection. It is, therefore, important to understand the comprehensive interaction between the microbiological situation and the host immune responses that lead to prosthesis infections. Evidence indicates that prosthesis infections are actually biofilm-correlated infections that are highly resistant to antibiotic treatment and the host immune responses. The authors reviewed the related literature in the context of their clinical experience, and discussed the possible etiology and mechanism leading to the infections, especially problems related to bacterial biofilm, and prophylaxis and treatment of infection, including both microbiological and surgical measures. Recent progress in research into bacterial biofilm and possible future treatment options of prosthesis-related infections are discussed.
Key words: prosthesis-related infections, biofilms, diagnosis, prevention and control
Introduction
Osteoarthrosis (OA), also known as osteoarthritis, is a degenerative joint disease that is caused by wearing or breakdown of the cartilage covering the ends of the bones in a joint due to chronic inflammation, leading to local pain and limitation of joint motion. OA most commonly affects middle-aged and elderly people resulting in disability and reduces Quality of Life.
In 2005, it was estimated that over 10% of the adult US population (27 million adult Americans) had clinical OA, and in 2009, OA became the fourth most common cause of hospitalization. 1 OA is the major indication for joint replacement surgery and in 2009 more than 905,000 joint replacements were performed in the US with a total cost of 42.3 billion US dollars. In France, there were 6 million new OA patients diagnosed in 1993,2 and in England and Wales, there are approximately 1.3 and 1.75 million people suffering from OA.3 The economic burden of such a disease accounts for up to 1-2.5% of the gross national product of Western nations.1,4
OA treatment is aimed at reducing pain, maintaining or improving joint function, and minimizing disability. For patients with significant joint dysfunctions, artificial joint replacement becomes the only solution. The procedure is widely recognized as one of the most successful interventions in medicine,5,6 and its impact on Quality of Life has been well documented. 6 Today, it is possible to perform total joint replacement to treat osteoarthrosis in most of the major joints in humans, such as hip, knee, shoulder, elbow, wrist, ankle, spine, etc. It is reported that, in the United States, there has been a rapid increase in the number of operations of joint replacement. The number of primary total hip arthroplasties (THA) increased from 119,000 in 1990 to 193,000 in 2002, while the number of primary total knee arthroplasties (TKA) increased from 129,000 in 1990 to 381,000 in 2002.7 The number of joint replacements in the US reached 905,000 cases in 2009.1 In Germany, the number of THA and TKA in 2008 was 159,000 and 146,000, respectively; this represented a 15% increase in THA and a 33% increase in TKA compared to 2004.8 According to a 2007 estimate, approximately 1.5 million joint arthroplastic operations are performed annually worldwide.5
Following this great success, there are also some failures of arthroplasty, which lead to operative revision. In 2000, there were 28,000 revision THAs and 31,000 revision TKAs performed in US and each year, more than one billion dollars are spent there on THA and TKA revisions.9 Causes that lead to failure of arthroplasty might include aseptic loosening, dislocations, prosthesis infections, massive bone loss, fractures and metal allergy.5,10-14 After total joint replacement operations, the total infection rates of the implants are reported to be less than 1%,15,16 and the patients with rheumatoid arthritis could have a higher infection rate of up to 3.7%.17 However, bacterial infection of a joint prosthesis is a severe complication that is currently difficult to cure with antibiotic treatment. In most cases, the infected prosthesis implant has to be removed in order to cure the infection. It is, therefore, important, and necessary, to improve our understanding of the pathogenesis of prosthesis infections after orthopedic joint replacement.
Possible causes leading to prosthesis-related infections
Air quality control of the operating room
Orthopedic operations require an ultra clean environment. Therefore, the air supply to the operating rooms requires filtration. An airsampling test demonstrated that there was a clear microbiological difference in the air quality before and after air filtration (Figure 1). Airborne bacteria are able to cause an infection during operations including orthopedic procedures leading to implant infection.18 Applications of laminar air flow and ultraviolet light in the operating theaters significantly help to reduce the prevalence of periprosthetic joint infection.19 Most airborne bacteria are of human origin. A person releases approximately 10 million particles/day, equal to 10,000 particles/min when walking, and 5-10% of these particles carry bacteria.20,21 It is, therefore, important to limit the number of persons in the operating theaters and the activities of the staff.22 Staff traffic and material transport during an operation might increase the risk of orthopedic infection.
Figure 1.
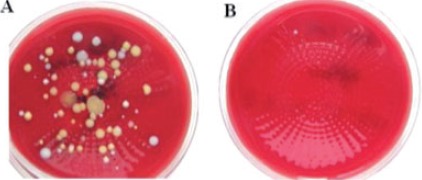
Bacterial colonies on 5% blood agar plates seeded with room air sampling (1 M3) in a Danish hospital (A) before and (B) after air-filtration.
Incomplete skin disinfection and infections related to materials or staff
Incomplete skin disinfection and contamination of the surgical instruments or orthopedic materials play an important role in postoperative infections.23 In addition, contamination from the operating staff should also be taken into consideration. For example, hair or sweat from surgeons or nurses, wound rinsing solution spreading from the operating site to the staff and dropping down in the operating area, coughing or sneezing by the operating staff, etc. These situations are not common, but in an orthopedic setting all potential risk factors are important.
Omission of perioperative antibiotic prophylaxis
Bacterial infection in prosthesis can easily result in wound sepsis and failure of the joint replacement. Therefore, joint replacements require an ultra-clean environment for surgery. Because of the risk factors mentioned in points above, antibiotic prophylaxis is recommended; this should be applied systemically four times a day on the day of surgery and in combination with the application of cementcontaining antibiotic substance. This measure has been demonstrated to be efficient in minimizing the risk of infection.24-27 The details are discussed below (Prophylaxis of the prosthesisrelated infections).
Impairment of immune system
The immune system plays a key role in defending our body against microbial infections. Any compromised factor that weakens the immune system will actually enhance the development of an infection. In patients with diabetes,28 AIDS,29 and other immunocompromised diseases, risk for infections increases significantly.
Bacterial biofilm formation
A biofilm is an accumulation of microorganisms embedded in a self-produced polysaccharide matrix and adherent to a solid biological or non-biological surface (Figure 2).30,31 Biofilms are medically important, accounting for over 80% of microbial infections in the body, including prostheses and internal fixation devices.30 In vitro study has shown that Pseudomonas aeruginosa, Staphylococcus aureus and Staphylococcus epidermidis can easily form biofilms on stainless steel and titanium orthopedic screws.32 Another in vitro study demonstrated that S. aureus, S. epidermidis and P. aeruginosa possess strong forces of adhesion to foreign bodies.33 In studies of implants from patients having revision for total joint arthroplasty of hip or knee without clinical and routine microbiological evidence of infection, bacterial biofilms including coagulase- negative staphylococci were isolated from the removed implants by using ultrasonication and other sensitive methods,34,35 indicating a biofilm infection of the implant. Bacteria usually form biofilm on foreign bodies that are placed in patients for medical reasons, such as peripheral and central venous catheters, heart valves, ventricular assisting devices, coronary stents, arthro-prostheses, fracture-fixation devices, breast implants, intraocular lenses, dental implants, etc.36,37 Orthopedic biomaterials are foreign bodies that provide surfaces for bacteria to adhere to and subsequently form biofilms. Inside bacterial biofilm there is a high density of bacterial population that activates a cell-densitydependent mechanism called quorum sensing (QS). There are QS systems in both Gram positive and Gram negative bacterial populations and these regulate the expression of adhesion mechanisms and virulent factors.38 It has been demonstrated that QS also control the differentiation of the biofilm and can lead to killing of the leukocytes in some Gram negative bacteria. 39,40 Due to the protection offered by the extracellular polymeric substances produced by bacteria themselves and the changed physiology of the biofilm bacteria, it is difficult for the immune system and antibiotics to eradicate the bacterial cells embedded in the biofilm, and, therefore, the biofilm infection becomes chronic.34,36 On the other hand, the biofilm bacterial cells usually elicit less inflammatory response than the planktonic bacterial cells34,36 which makes it difficult for clinicians to diagnose such an infection.
Figure 2.
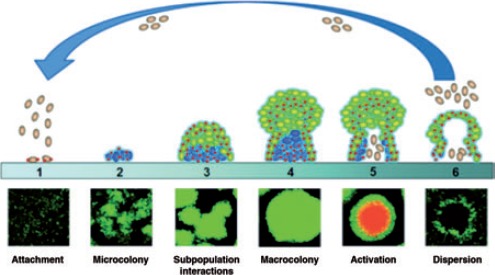
Development stages of bacterial biofilm.
Pathogens causing prosthesis-related infections
It has been reported that many bacteria can cause prosthesis-related infections, such as S. aureus, including methicillin-resistant strain (MRSA), coagulase-negative staphylococci (CNS) (e.g. S. epidermidis, S. haemolyticus, S hominis, S warneri), Propionibacterium acnes, P. aeruginosa, Haemophilus influenzae, Providencia, Enterococci, Streptococcus viridans, Escherichia coli, Citrobacter, Lactobacillus, Acinetobacter, Serratia marcescens, Klebsiella pneumoniae, and Corynebacterium.35,37,41-46 Among those pathogens, S. aureus and coagulase- negative staphylococci are the most common bacteria responsible for prosthesis-related infections, accounting for approximately half of the infections or more.41,42,45 Infections occurring in the first three months after surgery are usually caused by virulent microorganisms such as S. aureus, whereas delayed infections (3-24 months after surgery) are in most of the cases caused by low virulent microorganisms such as coagulase-negative staphylococci.44 Poly-saccharide intercellular adhesin (PIA) produced by staphylococci has been demonstrated to be a crucial virulent factor that helps staphylococci to form biofilm in implants or orthopedic biomaterials. 47,48
Diagnosis of the prosthesis-related infections
It is not difficult to diagnose a clinical infection of prosthesis after joint replacement, if there are local and systemic symptoms such as localized pain, swelling, and congestion with increased inflammatory parameters (high leukocyte count, elevated C-reactive protein, and fever) plus positive culture of the joint fluid or biopsies. However, it is difficult to find evidence for some delayed prosthetic infections or aseptic loosening of the prostheses employing the conventional clinical microbiological methods, and it has been demonstrated that such infections are quite often caused by bacterial biofilms which show little or no systemic symptoms and mild local inflammatory responses.32,34,41,49,50
In recent years, new techniques have been developed to increase the detection rate of infection, especially biofilm infections. In many countries, as well as in our own laboratory, synovial fluid and 5 samples from periimplant tissues are recommended for microbiological diagnosis of orthopedic implant-associated infections.51 In addition, the orthopedic implants removed from patients can be placed in sterile saline, vortexed and sonicated in an ultrasonic bath. The fluid from sonication is then cultured and sent to 16s or 18s polymerase chain reaction (PCR) detection.34,49,51-55 It is reported that sonication cultures improved the microbiological detection of the implant infection.51-53,55 PCR detection is a more sensitive molecule tool, which is also applied in diagnosis of orthopedic implant infection. 49,51,54,55 Furthermore, detection of serum IgM against Staphylococcal slime polysaccharide antigens was used recently for diagnosis of staphylococcal periprosthetic joint infections with 89.7% sensitivity and 95.1% specificity. 51,56 Sometimes blood leukocyte count, Creactive protein, interleukin-6 and procalcitonin also give indications.51 The combination of microbiological routine and new methods, together with clinical symptoms and blood inflammatory markers, will give us a better picture of orthopedic implant infections.
Treatment of prosthesis-related infections
Antibiotic treatments
Prostheses-related infections are now thought to be biofilm-associated infections30,32,34-37,57 which are highly resistant to antibiotic treatment.58-62 The mechanisms for the biofilm bacterial cells to become resistant to antibiotics are not fully understood. It is believed that in addition to conventional resistance mechanisms such as beta-lactamase and efflux pumps,63,64 poor antibiotic penetration, nutrient limitation, slow growth, adaptive stress responses and formation of persister cells are involved.65 In addition, in vitro and in vivo studies of antibiotic pharmacokinetics/pharmacodynamics in bacterial biofilms have indicated that, biofilm bacteria are significantly more resistant than their planktonic counterparts, and antibiotic treatment, therefore, requires a higher dose and combination.58,59 It is, therefore, not recommended to treat implant infections with antibiotics only. On the basis of appropriate surgical intervention, if the clinical signs and symptoms of implant infection have been observed for less than three weeks, the implant is stable and the surrounding tissue is in a good condition, antibiotic treatment becomes crucial.66 Due to the integrated resistance of bacterial biofilm, it is important to choose highly active, better penetrating and combined antibiotic treatment. For the infections caused by staphylococci, Zimmerli et al. performed a randomized, placebo-controlled, double-blind clinical trial on 33 patients with proven staphylococcal infection and stable orthopedic implants from 1992 through 1997. They found that patients treated with initial debridement and 2-week intravenous flucloxacillin (2 g q.i.d. for methicillin- sensitive) or vancomycin (1 g b.i.d. for methicillin-resistant) together with rifampin (450 mg p.o., b.i.d.), followed by three (hip implants) or six (knee implants) months of ciprofloxacin (750 mg p.o., b.i.d.) and rifampin treatment had a 100% cure rate compared with the ciprofloxacin-placebo group (58% cure rate).67 It is, therefore, recommended to treat staphylococcal implant infections with 2-4 weeks intravenous beta (β)-lactam (for methicillin- sensitive) or glycopeptide (for methicillin- resistant) in combination with rifampicin to minimize the bacterial burden and risk of antibiotic resistance, followed by long-term rifampicin (450 mg p.o. b.i.d.) and levofloxacin (750 mg p.o., q.d. to 500 mg b.i.d.) or other fluoroquinolones.44,66,68 For details, please refer to the new protocol of antibiotic treatment up-dated in 2012 by Zimmerli et al.68 Application of the combination with rifampicin (20 mg/kg) and fluoroquinolone showed good results in a French clinical study.69 Fusidic acid was recently recommended as an efficient antibiotic for the treatment of bone and joint infections caused by S. aureus and MRSA.70 In our clinical practice, cefuroxim 1.5 g i.v., t.i.d. and fusidic acid (Fucidin) 500 mg p.o., t.i.d. are used as initial treatment followed by dicloxacillin 1g p.o., q.i.d. together with fucidin 500 mg p.o., t.i.d. or rifamicin 600 mg p.o., b.i.d. for the treatment of Staphylococcus aureus infection. For methicillin-resistant staphylococal infections, vancomycin 1g i.v., b.i.d and fucidin or rifampicin p.o. are applied initially followed by rifampicin and fucidin or moxifloxacin 400 mg p.o., q.d. or linezolid 600 mg p.o. b.i.d. according to the sensitivity results. Spanish colleagues recently reported that combination treatment with rifampicin and linezolid showed a 69.4% success rate (34 of 49 patients) for prosthetic joint infection with retention of the implant after two years.71 Recently, daptomycin has also been recommended as a new option for the treatment of implant infections,68 due to its good effect systemically and locally against methicillin-resistant staphylococci and enterococci in patients with implant-associated infections.72,73 By using such antibiotic treatment, prosthetic knee-associated infections in many patients could be well controlled.42 Soriano et al.,74 in a study of 85 patients with orthopedic implant infections, reported that 47- and 60-day treatment with linezolid showed a 72.2% and a 42.8% success rate in acute and chronic infections, respectively, when the implant was not removed. However, in a clinical study of 112 patients with prosthetic joint infection carried out in the UK, arthroscopic debridement and empirical treatment with vancomycin 1g i.v. every 12 h, plus meropenem 500 mg i.v. t.i.d. for inpatients and ceftriaxone 1 g i.v. q.d. plus teicoplanin 400 mg i.v. q.d. for outpatients, followed by oral rifampicin and quinolones could not avoid failure (18% recurred infection over 2.3 years).75 The authors concluded that antibiotic therapy may simply postpone, rather than prevent failure.75 But arthroscopic debridement might not be sufficient to remove the infected tissue, and, in our opinion, the doses of meropenem and ceftriaxone used in the study were too low.
Surgical interventions
Antibiotic treatment alone is quite often inadequate to treat prosthesis-related infections, especially when it comes to biofilm infections. In most cases, a combination of antibiotic therapy with surgical interventions, which can be divided into debridement with retention of the prostheses and staged exchange of the prostheses, is required.
Debridement with retention of the prostheses
The purpose of debridement is to remove the necrotic tissues, pus or foreign material around the orthopedic implant or the infected implant, which helps to minimize the local infection by means of removing the infectious focus and local application of antibiotics in high concentration. There are different reports regarding the effects of debridement with retention of the prostheses in treatment of the prosthetic infections. The 5-year success rate was reported by Berbari et al. to be 32%,76 whereas the 3-year success rate given by Trebse et al. was 86%.77 The main difference between these two reports might be associated with the selection of the patients for debridement. The patients involved in the study of Berbari were those with rheumatoid arthritis, and the patients in Trebse’s study had stable implants with infection symptoms of less than one year, no sinus tract and the known pathogens were susceptible to the antibiotics used. Zimmerli summarized the indications for debridement57 which included: a stable implant, a pathogen with susceptibility to antimicrobial agents active against surface-adhering microorganisms, absence of a sinus tract or an abscess, and a duration of infection with symptoms of less than three weeks. He believed that both patients with early or late acute-onset hematogenous infection were eligible for this procedure.
Staged exchange of the prostheses
If the prosthetic implants are loosening due to infection, staged exchange of prostheses is required. The decision of 1-stage or 2-stage exchange is made on the basis of an evaluation of the infected prosthesis. Patients with nearly normal or mildly compromised soft tissue can be selected for 1-stage exchange. If the patient is infected with resistant bacteria and the tissue damage is significant, a 2-stage revision will be considered. The interval between the two stages is usually 4-8 weeks with antibiotic treatment, depending on the resistance of the pathogen.57 After staged exchange of the prostheses, treatment with sensitive and well penetrating antibiotics in combination is crucial, and 12-week antibiotic treatment is recommended.68
Prophylaxis of the prosthesis-related infections
Prosthesis-related infections after joint replacement are disastrous for patients because, in most cases, removal of the infected prosthesis is the only way to cure the infection. It becomes, therefore, important to prevent prosthesis infection. The pathogenesis of prosthesis-related infections could be explained by the interactions of three basic factors, i.e. the number of invading bacteria and their virulence, the host’s immune responses, and the properties of the implant materials.
Bacteria
It is believed that approximately 60% of the prosthesis-related infections are caused by direct contamination during the operative procedures. Bacteria come from the patient’s skin, and/or the staff in the operating theater, or airborne pathogens in the operating environment. 41 It is, therefore, important to disinfect the skin of the operating field thoroughly, pay attention to the sterile performance, and prevent any direct contamination from the operating staff. To minimize the possibility of implant and wound infections, it is helpful to use broad-spectrum β-lactam antibiotic prophylaxis intravenously before, during and after surgery. In our orthopedic surgical procedures, cefuroxime 1.5 g is given before and after the operation, and local application of gentamicin solution around the implant before closing the incision is routine practice. Use of orthopedic cement containing antibiotics in joint replacement surgery is one of the prophylaxis measures. In a long-term Norwegian study involving 22,170 THA patients, systemic antibiotic (cephalosporin or semisynthetic penicillinaseresistant penicillin) prophylaxis combined with cement containing gentamicin gave the lowest risk of revision, aseptic loosening and infection, compared to those patients who received only systemic antibiotic prophylaxis,27 indicating the importance of cement with antibiotic. In addition, on the basis of antibiotic- cement application, systemic antibiotic prophylaxis given 4 times on the day of surgery showed significantly lower rates for revision, aseptic loosening and infection27 compared to antibiotic prophylaxis given 1, 2 and 3 times on the day of surgery. Longer antibiotic prophylaxis did not show better results.27 Based on the clinical evidence, application of antibiotic embedded cement in combination with intravenous antibiotic prophylaxis of cefuroxime (1.5 g, q.i.d.) or in case of bβ-lactam allergy, vancomycin (1 g, t.i.d.) is recommended for orthopedic surgery with joint replacement if patient renal function is normal.26 The systemic antibiotic prophylaxis is recommended for the day of surgery only.
To reduce density of the airborne bacteria, use laminar airflow and ultraviolet light is required in the operating rooms and the bacterial concentrations in the air must be monitored regularly. It is also important to actively treat any systemic or local infection to prevent a secondary hematogenous implant infection.57
Host
The innate and acquired immune systems are the main mechanisms defending our bodies against microbial infections. Any impairment or defects in the immune system will naturally increase the susceptibility to infections. It is well known that patients with diabetes, cancer, AIDS and viral infections, or patients under treatment of adrenal cortical hormones or immunosuppressive drugs, are susceptible to bacterial infections because of the impairment of their immune functions. The decision to proceed with implant-related operations in such patients should be taken after careful deliberation; the operation may be performed after a successful immune reconstructing therapy.
Prostheses
As foreign bodies, the implanted prostheses are easily colonized by bacteria. The presence of a foreign body decreases more than 100,000- fold the minimal infecting dose of S. aureus leading to a permanent abscess.57 Animal study demonstrated that 108 colony-forming units (CFU) of S. aureus could not produce any abscesses in the absence of foreign material, whereas 102 CFU was sufficient to infect 95% of the subcutaneous implants.45 The host neutrophils could not effectively clear the bacteria that attached to an implant due to the biofilm mode of growth of the bacteria.57,78 The application of antibiotic cements and the antibioticcoated implants partly down-regulate the risk of prosthetic-related infection due to the slow release of antibiotics from the cements and the formation of a relatively high antibiotic concentration locally.79,80 Animal studies demonstrated that application of antimicrobial-coated devices in a short time significantly reduced biofilm formation and the risk of implant-correlated infection against Staphylococcus aureus.81-83 However, this is not sufficient to solve the problem, and long-term application of antibiotics can also lead to serious problems of antibiotic resistance. To better protect orthopedic prostheses from infections, new materials or techniques with anti-microbe properties for the manufacture of orthopedic prostheses are needed.
Recent progress in laboratory investigations of alternative treatment of biofilm infections
Traditionally, bacterial infections are treated clinically by antibiotic chemotherapy which controls the infections by means of a direct bactericidal effect or by inhibiting bacterial growth. However, antibiotic treatment also leads to the development of antibiotic resistance that becomes a serious clinical problem. Animal studies have shown that artificial bacterial biofilm infections could be controlled effectively by altering the type of immune responses.84-87 The progress achieved in research into bacterial QS systems offers a promising clinical perspective. It has been demonstrated that the virulence of both Gram positive and Gram negative bacteria, and infections caused by these pathogens, could be significantly attenuated by QS inhibitors.38,88-92 Animal studies have shown that implant-related biofilm formations of Staphylococcus aureus, MRSA and Staphylococcus epidermidis could be successfully prevented by using QS inhibitor RIP.88,89 It has been demonstrated that QS inhibitors do not interfere with the growth of bacteria; however, they increase the susceptibility of the biofilm bacterial cells to antibiotics in vitro and lead to a much faster clearance of the bacteria in an animal infection model.91 It has also been found that some extractions from natural plants could interfere with bacterial QS, modulate immune response against biofilm infections and induce dispersal of biofilm bacterial cells, which might benefit the effects of both antibiotics and immune defense mechanisms.86,87,93,94 Therefore, modulation of immune responses and use of QS inhibitors might be a potentially promising therapy for the prosthesis-related infections in the near future.
References
- 1.Murphy L, Helmick CG. The impact of osteoarthritis in the United States: a population- health perspective: a populationbased review of the fourth most common cause of hospitalization in U.S. adults. Orthop Nurs 2012;31:85-91 [DOI] [PubMed] [Google Scholar]
- 2.Levy E, Ferme A, Perocheau D, Bono I. [Socioeconomic costs of osteoarthritis in France]. Rev Rhum Ed Fr 1993;60:63S-7S[Article in French] [PubMed] [Google Scholar]
- 3.Watson M. Management of patients with osteoarthritis. Pharm J 1997;259:296-7 [Google Scholar]
- 4.Reginster JY. The prevalence and burden of arthritis. Rheumatology (Oxford) 2002;41 Supp 1:3-6 [PubMed] [Google Scholar]
- 5.Drees P, Eckardt A, Gay RE, Gay S, Huber LC. Mechanisms of disease: molecular insights into aseptic loosening of orthopedic implants. Nat Clin Pract Rheumatol 2007;3:165-71 [DOI] [PubMed] [Google Scholar]
- 6.Laupacis A, Bourne R, Rorabeck C, et al. The effect of elective total hip replacement on health-related quality of life. J Bone Joint Surg Am 1993;75:1619-26 [DOI] [PubMed] [Google Scholar]
- 7.Kurtz S, Mowat F, Ong K, et al. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. J Bone Joint Surg Am 2005;87:1487-97 [DOI] [PubMed] [Google Scholar]
- 8.Kim SH, Gaiser S, Meehan JP. Epidemiology of primary hip and knee arthroplasties in Germany: 2004 to 2008. J Arthroplasty 2012 [DOI] [PubMed] [Google Scholar]
- 9.Teeny SM, York SC, Mesko JW, Rea RE. Long-term follow-up care recommendations after total hip and knee arthroplasty: results of the American Association of Hip and Knee Surgeons’ member survey. J Arthroplasty 2003;18:954-62 [DOI] [PubMed] [Google Scholar]
- 10.Korovessis P, Petsinis G, Repanti M, Repantis T. Metallosis after contemporary metal-on-metal total hip arthroplasty. Five to nine-year follow-up. J Bone Joint Surg Am 2006;88:1183-91 [DOI] [PubMed] [Google Scholar]
- 11.Lie SA, Hallan G, Furnes O, et al. Isolated acetabular liner exchange compared with complete acetabular component revision in revision of primary uncemented acetabular components: a study of 1649 revisions from the Norwegian Arthroplasty Register. J Bone Joint Surg Br 2007;89:591-4 [DOI] [PubMed] [Google Scholar]
- 12.Nelson CL, McLaren AC, McLaren SG, et al. Is aseptic loosening truly aseptic? Clin Orthop Relat Res 2005;25-30 [DOI] [PubMed] [Google Scholar]
- 13.Ulrich SD, Seyler TM, Bennett D, et al. Total hip arthroplasties: what are the reasons for revision? Int Orthop 2008;32:597-604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fabi D, Levine B, Paprosky W, et al. Metalon- metal total hip arthroplasty: causes and high incidence of early failure. Orthopedics 2012;35:e1009-16 [DOI] [PubMed] [Google Scholar]
- 15.Blom AW, Taylor AH, Pattison G, et al. Infection after total hip arthroplasty. The Avon experience. J Bone Joint Surg Br 2003;85:956-9 [DOI] [PubMed] [Google Scholar]
- 16.Grimer RJ, Abudu A. Infection after total hip arthroplasty. J Bone Joint Surg Br 2005;87:588. [DOI] [PubMed] [Google Scholar]
- 17.Bongartz T, Halligan CS, Osmon DR, et al. Incidence and risk factors of prosthetic joint infection after total hip or knee replacement in patients with rheumatoid arthritis. Arthritis Rheum 2008;59:1713-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gosden PE, MacGowan AP, Bannister GC. Importance of air quality and related factors in the prevention of infection in orthopaedic implant surgery. J Hosp Infect 1998;39:173-80 [DOI] [PubMed] [Google Scholar]
- 19.Evans RP. Current concepts for clean air and total joint arthroplasty: laminar airflow and ultraviolet radiation: a systematic review. Clin Orthop Relat Res 2011;469:945-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chow TT, Yang XY. Ventilation performance in the operating theatre against airborne infection: numerical study on an ultra-clean system. J Hosp Infect 2005;59:138-47 [DOI] [PubMed] [Google Scholar]
- 21.Noble W. C. Dispersal of bacteria from human skin. The International Symposium on Contamination Control. Proceedings of the International Symposium on Contamination Control, Copenhagen, Denmark, 16-24 1976 [Google Scholar]
- 22.Stocks GW, Self SD, Thompson B, et al. Predicting bacterial populations based on airborne particulates: a study performed in nonlaminar flow operating rooms during joint arthroplasty surgery. Am J Infect Control 2010;38:199-204 [DOI] [PubMed] [Google Scholar]
- 23.Dancer SJ, Stewart M, Coulombe C, et al. Surgical site infections linked to contaminated surgical instruments. J Hosp Infect 2012;81:231-8 [DOI] [PubMed] [Google Scholar]
- 24.Jahoda D, Nyc O, Pokorny D, Landor I, Sosna A. [Antibiotic treatment for prevention of infectious complications in joint replacement]. Acta Chir Orthop Traumatol Cech 2006;73:108-14 [Article in Czech]. [PubMed] [Google Scholar]
- 25.Rodriguez-Merchan EC. Preventing surgical site infection in haemophilia patients undergoing total knee arthroplasty. Blood Coagul Fibrinolysis 2012;23:477-81 [DOI] [PubMed] [Google Scholar]
- 26.Bratzler DW, Houck PM. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Am J Surg 2005;189:395-404 [DOI] [PubMed] [Google Scholar]
- 27.Engesaeter LB, Lie SA, Espehaug B, et al. Antibiotic prophylaxis in total hip arthroplasty: effects of antibiotic prophylaxis systemically and in bone cement on the revision rate of 22,170 primary hip replacements followed 0-14 years in the Norwegian Arthroplasty Register. Acta Orthop Scand 2003;74:644-51 [DOI] [PubMed] [Google Scholar]
- 28.Pozzilli P, Leslie RD. Infections and diabetes: mechanisms and prospects for prevention. Diabet Med 1994;11:935-41 [DOI] [PubMed] [Google Scholar]
- 29.Harrison WJ. HIV/AIDS in trauma and orthopaedic surgery. J Bone Joint Surg Br 2005;87:1178-81 [DOI] [PubMed] [Google Scholar]
- 30.NIH, National Heart Lung and Blood Institute. Research on microbial biofilms (PA-03-047) Available from: http://grants. nih.gov/grants/guide/pa-files/PA-03-047.html/ Accessed on: 20-12-2002.
- 31.Yang L, Liu Y, Wu H, et al. Combating biofilms. FEMS Immunol Med Microbiol 2012;65:146-57 [DOI] [PubMed] [Google Scholar]
- 32.Stoodley P, Kathju S, Hu FZ, et al. Molecular and imaging techniques for bacterial biofilms in joint arthroplasty infections. Clin Orthop Relat Res 2005;437:31-40 [DOI] [PubMed] [Google Scholar]
- 33.Muszanska AK, Nejadnik MR, Chen Y, et al. Bacterial adhesion forces with substratum surfaces and the susceptibility of biofilms to antibiotics. Antimicrob Agents Chemother 2012;56:4961-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Costerton JW. Biofilm theory can guide the treatment of device-related orthopaedic infections. Clin Orthop Relat Res 2005;437:7-11 [DOI] [PubMed] [Google Scholar]
- 35.Nguyen LL, Nelson CL, Saccente M, et al. Detecting bacterial colonization of implanted orthopaedic devices by ultrasonication. Clin Orthop Relat Res 2002;403:29-37 [DOI] [PubMed] [Google Scholar]
- 36.Costerton JW, Montanaro L, Arciola CR. Biofilm in implant infections: its production and regulation. Int J Artif Organs 2005;28:1062-8 [DOI] [PubMed] [Google Scholar]
- 37.Gristina AG, Costerton JW. Bacterial adherence to biomaterials and tissue. The significance of its role in clinical sepsis. J Bone Joint Surg Am 1985;67:264-73 [PubMed] [Google Scholar]
- 38.Costerton JW, Montanaro L, Arciola CR. Bacterial communications in implant infections: a target for an intelligence war. Int J Artif Organs 2007;30:757-63 [DOI] [PubMed] [Google Scholar]
- 39.De Kievit TR, Gillis R, Marx S, et al. Quorum-sensing genes in Pseudomonas aeruginosa biofilms: their role and expression patterns. Appl Environ Microbiol 2001;67:1865-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jensen PO, Bjarnsholt T, Phipps R, et al. Rapid necrotic killing of polymorphonuclear leukocytes is caused by quorumsensing- controlled production of rhamnolipid by Pseudomonas aeruginosa. Microbiology 2007;153:1329-38 [DOI] [PubMed] [Google Scholar]
- 41.Barberan J. Management of infections of osteoarticular prosthesis. Clin Microbiol Infect 2006;12 Suppl 3:93-101 [DOI] [PubMed] [Google Scholar]
- 42.Laffer RR, Graber P, Ochsner PE, Zimmerli W. Outcome of prosthetic knee-associated infection: evaluation of 40 consecutive episodes at a single centre. Clin Microbiol Infect 2006;12:433-9 [DOI] [PubMed] [Google Scholar]
- 43.Soriano A, Garcia S, Bori G, et al. Treatment of acute post-surgical infection of joint arthroplasty. Clin Microbiol Infect 2006;12:930-3 [DOI] [PubMed] [Google Scholar]
- 44.Trampuz A, Widmer AF. Infections associated with orthopedic implants. Curr Opin Infect Dis 2006;19:349-56 [DOI] [PubMed] [Google Scholar]
- 45.Montanaro L, Speziale P, Campoccia D, et al. Scenery of Staphylococcus implant infections in orthopedics. Future Microbiol 2011;6:1329-49 [DOI] [PubMed] [Google Scholar]
- 46.Singh JA, Sperling JW, Schleck C, et al. Periprosthetic infections after total shoulder arthroplasty: a 33-year perspective. J Shoulder Elbow Surg 2012;21:1534-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kristian SA, Golda T, Ferracin F, et al. The ability of biofilm formation does not influence virulence of Staphylococcus aureus and host response in a mouse tissue cage infection model. Microb Pathog 2004;36:237-45 [DOI] [PubMed] [Google Scholar]
- 48.Olson ME, Garvin KL, Fey PD, Rupp ME. Adherence of Staphylococcus epidermidis to biomaterials is augmented by PIA. Clin Orthop Relat Res 2006;451:21-4 [DOI] [PubMed] [Google Scholar]
- 49.Dempsey KE, Riggio MP, Lennon A, et al. Identification of bacteria on the surface of clinically infected and non-infected prosthetic hip joints removed during revision arthroplasties by 16S rRNA gene sequencing and by microbiological culture. Arthritis Res Ther 2007;9:R46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hall-Stoodley L, Stoodley P, Kathju S, et al. Towards diagnostic guidelines for biofilmassociated infections. FEMS Immunol Med Microbiol 2012;65:127-45 [DOI] [PubMed] [Google Scholar]
- 51.Vergidis P, Patel R. Novel approaches to the diagnosis, prevention, and treatment of medical device-associated infections. Infect Dis Clin North Am 2012;26:173-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holinka J, Bauer L, Hirschl AM, et al. Sonication cultures of explanted components as an add-on test to routinely conducted microbiological diagnostics improve pathogen detection. J Orthop Res 2011;29:617-22 [DOI] [PubMed] [Google Scholar]
- 53.Vergidis P, Greenwood-Quaintance KE, Sanchez-Sotelo J, et al. Implant sonication for the diagnosis of prosthetic elbow infection. J Shoulder Elbow Surg 2011;20:1275-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Esteban J, Alonso-Rodriguez N, del-Prado G, et al. PCR-hybridization after sonication improves diagnosis of implant-related infection. Acta Orthop 2012;83:299-304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Achermann Y, Vogt M, Leunig M, et al. Improved diagnosis of periprosthetic joint infection by multiplex PCR of sonication fluid from removed implants. J Clin Microbiol 2010;48:1208-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Artini M, Romano C, Manzoli L, et al. Staphylococcal IgM enzyme-linked immunosorbent assay for diagnosis of periprosthetic joint infections. J Clin Microbiol 2011;49:423-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zimmerli W. Infection and musculoskeletal conditions: Prosthetic-joint-associated infections. Best Pract Res Clin Rheumatol 2006;20:1045-63 [DOI] [PubMed] [Google Scholar]
- 58.Hengzhuang W, Wu H, Ciofu O, et al. Pharmacokinetics/pharmacodynamics of colistin and imipenem on mucoid and nonmucoid Pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother 2011;55:4469-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hengzhuang W, Wu H, Ciofu O, et al. In vivo pharmacokinetics/pharmacodynamics of colistin and imipenem in Pseudomonas aeruginosa biofilm infection. Antimicrob Agents Chemother 2012;56:2683-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hoiby N, Krogh JH, Moser C, et al. Pseudomonas aeruginosa and the in vitro and in vivo biofilm mode of growth. Microbes Infect 2001;3:23-35 [DOI] [PubMed] [Google Scholar]
- 61.Hoiby N, Ciofu O, Johansen HK, et al. The clinical impact of bacterial biofilms. Int J Oral Sci 2011;3:55-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hoiby N, Bjarnsholt T, Givskov M, et al. Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents 2010;35:322-32 [DOI] [PubMed] [Google Scholar]
- 63.Bagge N, Hentzer M, Andersen JB, et al. Dynamics and spatial distribution of betalactamase expression in Pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother 2004;48:1168-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rouveix B. Clinical implications of multiple drug resistance efflux pumps of pathogenic bacteria. J Antimicrob Chemother 2007;59:1208-9 [DOI] [PubMed] [Google Scholar]
- 65.Stewart PS. Mechanisms of antibiotic resistance in bacterial biofilms. Int J Med Microbiol 2002;292:107-13 [DOI] [PubMed] [Google Scholar]
- 66.Trampuz A, Zimmerli W. Antimicrobial agents in orthopaedic surgery: prophylaxis and treatment. Drugs 2006;66:1089-105 [DOI] [PubMed] [Google Scholar]
- 67.Zimmerli W, Widmer AF, Blatter M, et al. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. Foreign-Body Infection (FBI) Study Group. JAMA 1998;279:1537-41 [DOI] [PubMed] [Google Scholar]
- 68.Zimmerli W, Moser C. Pathogenesis and treatment concepts of orthopaedic biofilm infections. FEMS Immunol Med Microbiol 2012;65:158-68 [DOI] [PubMed] [Google Scholar]
- 69.Senneville E, Joulie D, Legout L, et al. Outcome and predictors of treatment failure in total hip/knee prosthetic joint infections due to Staphylococcus aureus. Clin Infect Dis 2011;53:334-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang JL, Tang HJ, Hsieh PH, et al. Fusidic acid for the treatment of bone and joint infections caused by meticillin-resistant Staphylococcus aureus. Int J Antimicrob Agents 2012;40:103-7 [DOI] [PubMed] [Google Scholar]
- 71.Gomez J, Canovas E, Banos V, et al. Linezolid plus rifampin as a salvage therapy in prosthetic joint infections treated without removing the implant. Antimicrob Agents Chemother 2011;55:4308-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Corona Perez-Cardona PS, Barro OV, Rodriguez PD, et al. Clinical experience with daptomycin for the treatment of patients with knee and hip periprosthetic joint infections. J Antimicrob Chemother 2012;67:1749-54 [DOI] [PubMed] [Google Scholar]
- 73.Rosslenbroich SB, Raschke MJ, Kreis C, et al. Daptomycin: local application in implant-associated infection and complicated osteomyelitis. Sci Wrld J 2012;2012:578251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Soriano A, Gomez J, Gomez L, et al. Efficacy and tolerability of prolonged linezolid therapy in the treatment of orthopedic implant infections. Eur J Clin Microbiol Infect Dis 2007;26:353-6 [DOI] [PubMed] [Google Scholar]
- 75.Byren I, Bejon P, Atkins BL, et al. One hundred and twelve infected arthroplasties treated with DAIR (debridement, antibiotics and implant retention): antibiotic duration and outcome. J Antimicrob Chemother 2009;63:1264-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Berbari EF, Osmon DR, Duffy MC, et al. Outcome of prosthetic joint infection in patients with rheumatoid arthritis: the impact of medical and surgical therapy in 200 episodes. Clin Infect Dis 2006;42:216-23 [DOI] [PubMed] [Google Scholar]
- 77.Trebse R, Pisot V, Trampuz A. Treatment of infected retained implants. J Bone Joint Surg Br 2005;87:249-56 [DOI] [PubMed] [Google Scholar]
- 78.Zimmerli W, Waldvogel FA, Vaudaux P, Nydegger UE. Pathogenesis of foreign body infection: description and characteristics of an animal model. J Infect Dis 1982;146:487-97 [DOI] [PubMed] [Google Scholar]
- 79.Langlais F, Belot N, Ropars M, et al. Antibiotic cements in articular prostheses: current orthopaedic concepts. Int J Antimicrob Agents 2006;28:84-9 [DOI] [PubMed] [Google Scholar]
- 80.Schmidmaier G, Lucke M, Wildemann B, et al. Prophylaxis and treatment of implantrelated infections by antibiotic-coated implants: a review. Injury 2006;37 Suppl 2:S105-S112 [DOI] [PubMed] [Google Scholar]
- 81.Darouiche RO, Mansouri MD, Zakarevicz D, et al. In vivo efficacy of antimicrobialcoated devices. J Bone Joint Surg Am 2007;89:792-7 [DOI] [PubMed] [Google Scholar]
- 82.Stewart S, Barr S, Engiles J, et al. Vancomycin-modified implant surface inhibits biofilm formation and supports bone-healing in an infected osteotomy model in sheep: a proof-of-concept study. J Bone Joint Surg Am 2012;94:1406-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hickok NJ, Shapiro IM. Immobilized antibiotics to prevent orthopaedic implant infections. Adv Drug Deliv Rev 2012;64:1165-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Johansen HK, Espersen F, Cryz SJ, Jr, et al. Immunization with Pseudomonas aeruginosa vaccines and adjuvant can modulate the type of inflammatory response subsequent to infection. Infect Immun 1994;62:3146-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Johansen HK, Hougen HP, Rygaard J, Hoiby N. Interferon-gamma (IFN-gamma) treatment decreases the inflammatory response in chronic Pseudomonas aeruginosa pneumonia in rats. Clin Exp Immunol 1996;103:212-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Song Z, Johansen HK, Faber V, et al. Ginseng treatment reduces bacterial load and lung pathology in chronic Pseudomonas aeruginosa pneumonia in rats. Antimicrob Agents Chemother 1997;41:961-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Song Z, Moser C, Wu H, et al. Cytokine modulating effect of ginseng treatment in a mouse model of Pseudomonas aeruginosa lung infection. J Cyst Fibros 2003;2:112-9 [DOI] [PubMed] [Google Scholar]
- 88.Anguita-Alonso P, Giacometti A, Cirioni O, et al. RNAIII-inhibiting-peptide-loaded polymethylmethacrylate prevents in vivo Staphylococcus aureus biofilm formation. Antimicrob Agents Chemother 2007;51:2594-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Balaban N, Stoodley P, Fux CA, et al. Prevention of staphylococcal biofilm-associated infections by the quorum sensing inhibitor RIP. Clin Orthop Relat Res 2005;437:48-54 [DOI] [PubMed] [Google Scholar]
- 90.Bjarnsholt T, Jensen PO, Rasmussen TB, et al. Garlic blocks quorum sensing and promotes rapid clearing of pulmonary Pseudomonas aeruginosa infections. Microbiology 2005;151:3873-80 [DOI] [PubMed] [Google Scholar]
- 91.Hentzer M, Wu H, Andersen JB, et al. Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J 2003;22:3803-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wu H, Song Z, Hentzer M, et al. Synthetic furanones inhibit quorum-sensing and enhance bacterial clearance in Pseudomonas aeruginosa lung infection in mice. J Antimicrob Chemother 2004;53:1054-61 [DOI] [PubMed] [Google Scholar]
- 93.Song Z, Kong KF, Wu H, et al. Panax ginseng has anti-infective activity against opportunistic pathogen Pseudomonas aeruginosa by inhibiting quorum sensing, a bacterial communication process critical for establishing infection. Phytomedicine 2010;17:1040-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wu H, Lee B, Yang L, et al. Effects of ginseng on Pseudomonas aeruginosa motility and biofilm formation. FEMS Immunol Med Microbiol 2011;62:49-56 [DOI] [PubMed] [Google Scholar]


