Abstract
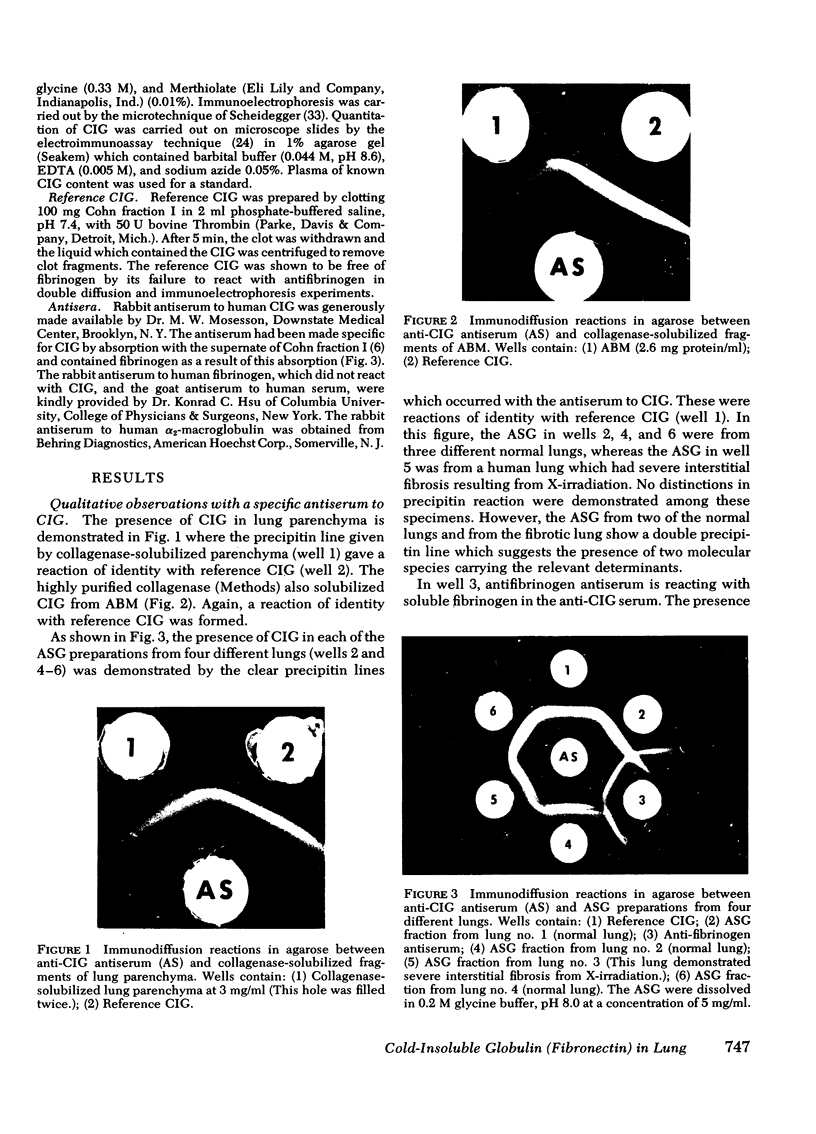
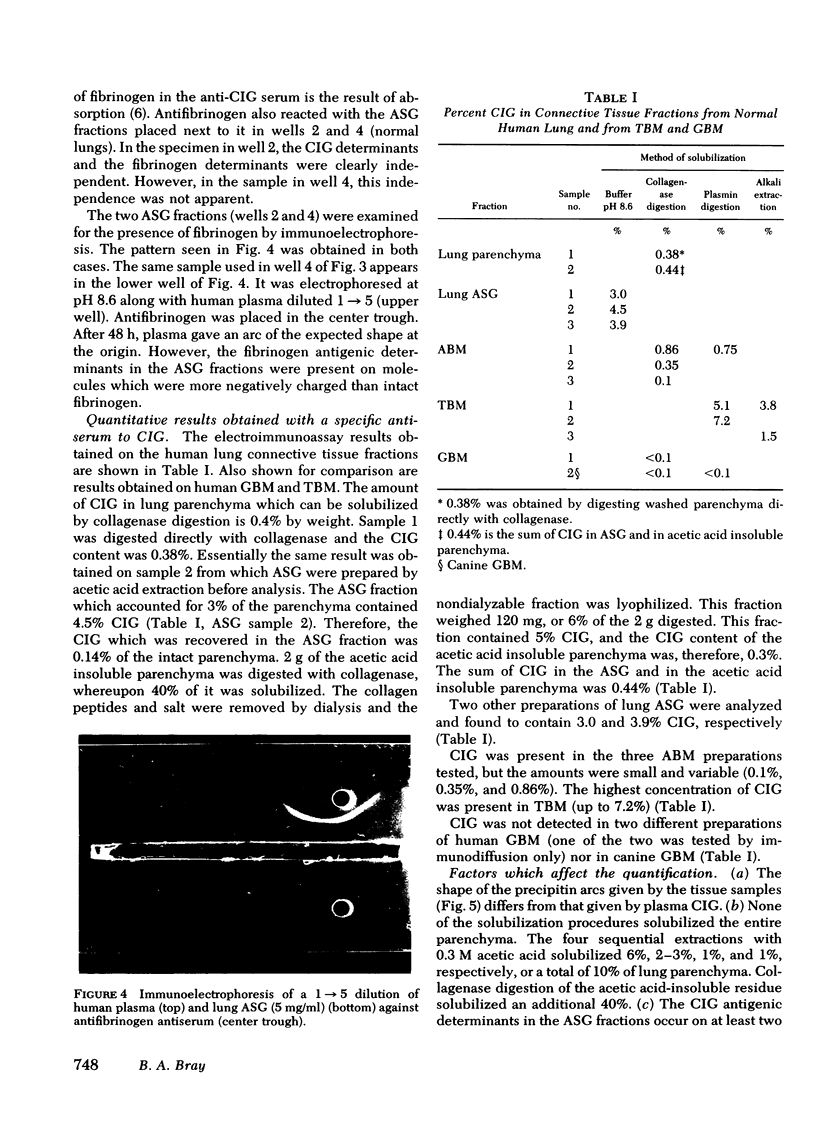
Cold-insoluble globulin (CIG), which is immunochemically indistinguishable from the fibroblast surface protein known as large external transformation-sensitive glycoprotein and fibronectin, was detected immunologically in connective tissue fractions from adult human lung. The fractions tested were (a) intact parenchyma, (b) acidic structural glycoproteins (ASG) extracted from lung parenchyma with 0.3 M acetic acid, and (c) isolated alveolar basement membrane (ABM). For comparison with ABM, preparations of human glomerular basement membrane and human trophoblast basement membrane (TBM) were tested. CIG was not detected in glomerular basement membrane but was present in large amounts in TBM. The CIG antigen could be solubilized from the parenchyma and from ABM by collagenase digestion which indicates that CIG occurs in lung connective tissue in association with collagen. Fibrinogen antigenic determinants were present in the ASG fraction, but the question of whether CIG and fibrin(ogen) are associated in lung connective tissue requires further study. When CIG was quantified by electroimmunoassay, intact lung parenchyma contained approximately equal to 0.4% CIG, ASG contained 3-4.5% CIG, ABM contained 0.1-0.9% CIG and TBM contained 1.5%-7.2% Cg. the evidence suggests that CIG is a chemical constituent of lung connective tissue matrix where it may influence the function of alveoli.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali I. U., Mautner V., Lanza R., Hynes R. O. Restoration of normal morphology, adhesion and cytoskeleton in transformed cells by addition of a transformation-sensitive surface protein. Cell. 1977 May;11(1):115–126. doi: 10.1016/0092-8674(77)90322-1. [DOI] [PubMed] [Google Scholar]
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Bornstein P., Ash J. F. Cell surface-associated structural proteins in connective tissue cells. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2480–2484. doi: 10.1073/pnas.74.6.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray B. A., Hsu K. C., Wigger H. J., LeRoy E. C. Association of fibrinogen and microfibrils with trophoblast basement membrane. Connect Tissue Res. 1975;3(1):55–71. doi: 10.3109/03008207509152342. [DOI] [PubMed] [Google Scholar]
- Bray B. A., LeRoy E. C. Human alveolar basement membrane. Chemical and immunologic comparisons with glomerular basement membrane and trophoblast basement membrane. Microvasc Res. 1976 Jul;12(1):77–89. doi: 10.1016/0026-2862(76)90009-1. [DOI] [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977 Jul 15;20(1):1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- Francis G., Thomas J. Isolation and characterization of acidic structural glycoproteins in pulmonary tissues. Biochem J. 1975 Feb;145(2):299–304. doi: 10.1042/bj1450299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furthmayr H., Timpl R. Acidic structural proteins of connective tissue. II. Evidence for disulfide cross-links. Biochim Biophys Acta. 1970 Nov 17;221(2):396–398. doi: 10.1016/0005-2795(70)90286-2. [DOI] [PubMed] [Google Scholar]
- Gahmberg C. G., Hakomori S. I. Altered growth behavior of malignant cells associated with changes in externally labeled glycoprotein and glycolipid. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3329–3333. doi: 10.1073/pnas.70.12.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg N. M. A comparison of membrane proteins of normal and transformed cells by lactoperoxidase labeling. Proc Natl Acad Sci U S A. 1974 Feb;71(2):489–492. doi: 10.1073/pnas.71.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Alteration of cell-surface proteins by viral transformation and by proteolysis. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3170–3174. doi: 10.1073/pnas.70.11.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O., Destree A. Extensive disulfide bonding at the mammalian cell surface. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2855–2859. doi: 10.1073/pnas.74.7.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadar A., Robert B., Robert L. Etude biochimique et électromicroscopique des microfibrilles du tissu conjonctif. Pathol Biol (Paris) 1973 Nov;21(Suppl):80–88. [PubMed] [Google Scholar]
- Kefalides N. A., Denduchis B. Structural components of epithelial and endothelial basement membranes. Biochemistry. 1969 Nov;8(11):4613–4621. doi: 10.1021/bi00839a057. [DOI] [PubMed] [Google Scholar]
- Keski-Oja J., Mosher D. F., Vaheri A. Cross-linking of a major fibroblast surface-associated glycoprotein (fibronectin) catalyzed by blood coagulation factor XIII. Cell. 1976 Sep;9(1):29–35. doi: 10.1016/0092-8674(76)90049-0. [DOI] [PubMed] [Google Scholar]
- Keski-Oja J., Mosher D. F., Vaheri A. Dimeric character of fibronectin, a major cell surface-associated glycoprotein. Biochem Biophys Res Commun. 1977 Jan 24;74(2):699–706. doi: 10.1016/0006-291x(77)90359-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laurell C. B. Quantitative estimation of proteins by electrophoresis in agarose gel containing antibodies. Anal Biochem. 1966 Apr;15(1):45–52. doi: 10.1016/0003-2697(66)90246-6. [DOI] [PubMed] [Google Scholar]
- Linder E., Stenman S., Lehto V. P., Vaheri A. Distribution of fibronectin in human tissues and relationship to other connective tissue components. Ann N Y Acad Sci. 1978 Jun 20;312:151–159. doi: 10.1111/j.1749-6632.1978.tb16800.x. [DOI] [PubMed] [Google Scholar]
- Linder E., Vaheri A., Ruoslahti E., Wartiovaara J. Distribution of fibroblast surface antigen in the developing chick embryo. J Exp Med. 1975 Jul 1;142(1):41–49. doi: 10.1084/jem.142.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashburn T. A., Jr, Hoffman P. Automated analysis of polysaccharide and protein in cartilage. Anal Biochem. 1970 Jul;36(1):213–221. doi: 10.1016/0003-2697(70)90350-7. [DOI] [PubMed] [Google Scholar]
- Matsuda M., Yoshida N., Aoki N., Wakabayashi K. Distribution of cold-insoluble globulin in plasma and tissues. Ann N Y Acad Sci. 1978 Jun 20;312:74–92. doi: 10.1111/j.1749-6632.1978.tb16794.x. [DOI] [PubMed] [Google Scholar]
- Miller R. L., Udenfriend S. Hydroxylation of proline residues in collagen nascent chains. Arch Biochem Biophys. 1970 Jul;139(1):104–113. doi: 10.1016/0003-9861(70)90051-2. [DOI] [PubMed] [Google Scholar]
- Mosesson M. W., Chen A. B., Huseby R. M. The cold-insoluble globulin of human plasma: studies of its essential structural features. Biochim Biophys Acta. 1975 Apr 29;386(2):509–524. doi: 10.1016/0005-2795(75)90294-9. [DOI] [PubMed] [Google Scholar]
- Mosesson M. W., Umfleet R. A. The cold-insoluble globulin of human plasma. I. Purification, primary characterization, and relationship to fibrinogen and other cold-insoluble fraction components. J Biol Chem. 1970 Nov 10;245(21):5728–5736. [PubMed] [Google Scholar]
- Mosher D. F. Cross-linking of cold-insoluble globulin by fibrin-stabilizing factor. J Biol Chem. 1975 Aug 25;250(16):6614–6621. [PubMed] [Google Scholar]
- Mosher D. F., Saksela O., Vaheri A. Synthesis and secretion of alpha-2-macroglobulin by cultured adherent lung cells. Comparison with cell strains derived from other tissues. J Clin Invest. 1977 Nov;60(5):1036–1045. doi: 10.1172/JCI108854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher D. F., Wing D. A. Synthesis and secretion of alpha2-macroglobulin by cultured human fibroblasts. J Exp Med. 1976 Feb 1;143(2):462–467. doi: 10.1084/jem.143.2.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlstein E. Plasma membrane glycoprotein which mediates adhesion of fibroblasts to collagen. Nature. 1976 Aug 5;262(5568):497–500. doi: 10.1038/262497a0. [DOI] [PubMed] [Google Scholar]
- Peterkofsky B., Diegelmann R. Use of a mixture of proteinase-free collagenases for the specific assay of radioactive collagen in the presence of other proteins. Biochemistry. 1971 Mar 16;10(6):988–994. doi: 10.1021/bi00782a009. [DOI] [PubMed] [Google Scholar]
- Robert L., Robert B., Moczar E., Moczar M. Les glycoprotéines de structure du tissu conjonctif. Pathol Biol (Paris) 1972 Dec;20(23):1001–1012. [PubMed] [Google Scholar]
- Ruoslahti E., Vaheri A. Interaction of soluble fibroblast surface antigen with fribrinogen and fibrin. J Exp Med. 1975 Feb 1;141(2):497–501. doi: 10.1084/jem.141.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHEIDEGGER J. J. Une micro-méthode de l'immuno-electrophorèse. Int Arch Allergy Appl Immunol. 1955;7(2):103–110. [PubMed] [Google Scholar]
- Sjöberg I., Fransson L. A. Synthesis of glycosaminoglycans by human embryonic lung fibroblasts. Different distribution of heparan sulphate, chondroitin sulphate and dermatan sulphate in various fractions of cell culture. Biochem J. 1977 Nov 1;167(2):383–392. doi: 10.1042/bj1670383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenman S., Vaheri A. Distribution of a major connective tissue protein, fibronectin, in normal human tissues. J Exp Med. 1978 Apr 1;147(4):1054–1064. doi: 10.1084/jem.147.4.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpl R., Wolff I., Weiser M. Acidic structural proteins of connective tissue. I. Solubilization and preliminary chemical characterization. Biochim Biophys Acta. 1969 Nov 11;194(1):112–120. doi: 10.1016/0005-2795(69)90186-x. [DOI] [PubMed] [Google Scholar]
- Vaheri A., Ruoslahti E. Disappearance of a major cell-type specific surface glycoprotein antigen (SF) after transformation of fibroblasts by Rous sarcoma virus. Int J Cancer. 1974 May 15;13(5):579–586. doi: 10.1002/ijc.2910130502. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Wolff I., Fuchswans W., Weiser M., Furthmayr H., Timpl R. Acidic structural proteins of connective tissue. Characterization of their heterogeneous nature. Eur J Biochem. 1971 Jun 11;20(3):426–431. doi: 10.1111/j.1432-1033.1971.tb01409.x. [DOI] [PubMed] [Google Scholar]
- Yamada K. M., Schlesinger D. H., Kennedy D. W., Pastan I. Characterization of a major fibroblast cell surface glycoprotein. Biochemistry. 1977 Dec 13;16(25):5552–5559. doi: 10.1021/bi00644a025. [DOI] [PubMed] [Google Scholar]
- Yamada K. M., Weston J. A. Isolation of a major cell surface glycoprotein from fibroblasts. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3492–3496. doi: 10.1073/pnas.71.9.3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. M., Yamada S. S., Pastan I. Cell surface protein partially restores morphology, adhesiveness, and contact inhibition of movement to transformed fibroblasts. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1217–1221. doi: 10.1073/pnas.73.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]