Abstract
Background
Intimate partner violence (IPV) is common worldwide and is an important consideration in couples HIV voluntary counseling and testing (CVCT), especially for HIV serodiscordant couples (i.e., in which only one member is HIV infected).
Design
Prospective study of 3408 HIV serodiscordant couples (2299 in which the HIV infected partner was female) from 7 countries from East and Southern Africa.
Methods
At quarterly visits during up to 2 years of follow-up, participants were asked, separately, about IPV perpetrated against them by their partner during the prior 3 months. Correlates of IPV were determined by generalized estimating equations.
Results
The majority of couples were married and living together, with an average duration of partnership of approximately 5 years. More than 39,000 quarterly visits were recorded. IPV was reported in 2.7% of visits by HIV infected women, 2.2% by HIV uninfected women, 0.9% by HIV infected men, and 0.7% by HIV uninfected men. The majority of IPV reports were verbal or a combination of verbal and physical violence. Those who were HIV infected were more likely to report IPV (for women adjusted odds ratio [AOR] 1.33, p=0.043; for men AOR 2.20, p=0.001), but IPV was not significantly associated with risk of HIV seroconversion in HIV uninfected participants. IPV incidence decreased during follow-up (p<0.001).
Conclusions
During up to 2 years of prospective follow-up, most persons in stable HIV serodiscordant partnerships who had undergone CVCT did not report IPV. A modest increased risk of IPV was seen for HIV infected partners, both female and male.
Keywords: intimate partner violence, HIV serodiscordant couples, women, Africa
Introduction
Intimate partner violence (IPV), including physical, emotional and sexual abuse perpetrated by an intimate partner, has been documented frequently in populations worldwide. In sub-Saharan Africa, studies have reported high lifetime experience of IPV [1–3]. For example, one study from South Africa found that 32% of young men reported having perpetrated some form of abuse on their partner [4] and a study from Kenya found a lifetime prevalence of physical violence was 26% in women, of which 74% was perpetrated by intimate partners [3]. A number of studies have found a correlation between IPV and risk of HIV infection in African women [2, 5–7].
One group in which HIV risk from IPV could be especially important is HIV serodiscordant couples (i.e., in which one member is HIV infected and the other is HIV uninfected). In sub-Saharan Africa, the likelihood that the stable partner of an HIV-infected person will be HIV uninfected is approximately 50%, with women as likely as men to be the infected partner in a serodiscordant couple [8]. Moreover, HIV risk is high in serodiscordant couples, ranging from 2–6% per year when partner status is known to the couple [9] to 10–15% per year in couples unaware of their serodiscordant status [10, 11]. A substantial fraction of new HIV infections in Africa have been estimated to occur among HIV serodiscordant married or cohabiting couples [12].
Becoming aware of being in a serodiscordant relationship itself could lead to IPV, and knowledge of serodiscordancy could contribute to ongoing IPV risk in couples. Prevention of blame and IPV is an important consideration in HIV voluntary counseling and testing, especially in the setting of couples voluntary counseling and testing (CVCT). Higher uptake of HIV prevention strategies, such as safer sex practices and antiretrovirals for prevention of mother-to-child HIV transmission, by persons undergoing CVCT compared to those using only individual VCT have made CVCT a priority strategy for HIV prevention [13–15]. However, few data on IPV have been reported among HIV serodiscordant couples who have undergone CVCT.
CVCT and facilitated disclosure of results offers an important opportunity to prevent HIV transmission. Efforts to expand CVCT as an HIV prevention strategy need to be informed by data on possible consequences of knowledge of serodiscordant HIV status, especially potential increased risk of IPV. We assessed the frequency and correlates of IPV by partners in a multinational prospective study of HIV serodiscordant African couples.
Methods
Population and Procedures
Between November 2004 and May 2007, 3408 HIV serodiscordant couples from seven African countries in East (Kenya, Rwanda, Tanzania, Uganda) and Southern Africa (Botswana, South Africa, Zambia) were enrolled in the Partners in Prevention HSV/HIV Transmission Study, a randomized, double-blind, placebo-controlled, clinical trial of acyclovir herpes simplex virus type 2 (HSV-2) suppressive therapy for the prevention of HIV transmission [9, 16, 17]. All couples were aware of their HIV serodiscordant status; most became aware at the time of screening for the study, during the month prior to enrollment. Eligible couples were those in which both members were ≥18 years of age, sexually active, and intended to remain as a couple for the duration of the study. HIV infected partners were also seropositive for HSV-2 and had a CD4 count above national cut-offs for antiretroviral therapy initiation at the time (i.e., ≥250 cells/mm3); CD4 counts were monitored every 6 months and those who came to meet national criteria for antiretroviral therapy were referred to HIV treatment clinics. HIV infected partners visited the study clinic monthly and HIV uninfected partners had visits scheduled quarterly. Couples were encouraged to attend study visits together, as often as they found possible, to allow ongoing counseling together and to address any emerging challenges related to living together as an HIV serodiscordant couple. Follow-up was for up to a maximum of 24 months, with earlier completion of follow-up for some couples as a result of site closure at the end of the study (October 2008). HSV-2 suppressive therapy with acyclovir did not decrease the risk of HIV transmission [9].
Laboratory procedures included serology for HIV (at baseline and then quarterly for HIV uninfected participants), serology for HSV-2 (baseline), and nucleic acid amplification for sexually transmitted infections (STIs, Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis) from samples collected at study enrollment, as described previously [16]. CD4 counts were measured by standard flow cytometry.
All participants received pre- and post-test HIV counseling, risk reduction counseling (both individual and couple), free condoms, and management of STIs according to World Health Organization guidelines at each study visit. The study protocol was approved by the University of Washington Human Subjects Review Committee and ethics review boards at each study site. All participants provided written informed consent.
Assessment of IPV
The present report is a secondary analysis of the Partners in Prevention HSV/HIV Transmission Study dataset, evaluating the frequency of IPV at enrollment into the study and incidence of IPV during study prospective follow-up. At enrollment and at quarterly follow-up visits thereafter, all HIV infected and uninfected participants were asked, separately and in the setting of a private counseling room, about any abuse – verbal or physical – against them by their study partner during the prior 3 months. Study staff were provided with examples of verbal (“yelling, name calling, or threatening”) and physical abuse (“hitting, slapping, or forcing someone to have sex against their wishes”) to facilitate data collection. The question about IPV was asked in local languages and in a manner considered culturally appropriate for each study site. Interviewers were experienced in couples counseling and were trained through multiple role plays to ask the question as a part of a risk-reduction counseling process. If IPV was reported, a standard data collection form was completed by the interviewer, detailing the nature of the abuse. In addition, if other reports of IPV were made by participants at interim visits occurring between the quarterly visits, the same data form was collected. Information about IPV was obtained from the participant who reported the abuse (i.e., the survivor) rather than the perpetrator; those accused of being perpetrators of IPV were not asked about abuse reported by their partner.
Statistical Analysis
Medians and inter-quartile ranges (IQR) were provided for continuous variables. Counts and percentages were provided for categorical variables. IPV incidence during follow-up was calculated by dividing the number of IPV reports by person-time in follow-up. The cumulative proportion of persons ever reporting IPV, including at enrollment and follow-up, was determined using Kaplan-Meier analysis. Univariate and multivariate generalized estimating equations with robust standard errors were used to assess correlates of IPV. Variables statistically significant in univariate analyses at p<0.10 were included in multivariate adjusted models. For analyses of correlates of IPV, HIV uninfected participants who seroconverted to HIV during follow-up were censored thereafter; separate descriptions of IPV after HIV seroconversion were then calculated. Data were analyzed using Intercooled STATA version 11.0.
Results
Participant characteristics and prevalence of IPV at study enrollment
Of 3408 HIV serodiscordant couples enrolled, 2299 were couples in which the HIV infected partner was female and 1109 were couples in which the HIV infected partner was male (Table 1). Two-thirds of the couples were from the East Africa region. The majority were married and living together. The average duration of the partnerships was 4.9 years for couples in which the HIV-infected partner was female and 6.7 years for couples in which the HIV-infected partner was male. The average age was in the mid-30s, with male partners on average older than female partners. Most couples had at least one child together. Most participants had attended school for ≥8 years and were either low or no income earners. Couples reported a median of 4 sex acts (IQR 2 – 8) in the month prior to enrollment with about one quarter reporting unprotected sex.
Table 1.
Enrollment Characteristics
| Median (IQR) or number (%) | ||||
|---|---|---|---|---|
| Couples with HIV infected women (n=2299) | Couples with HIV infected men (n=1109) | |||
| HIV infected woman |
HIV uninfected man |
HIV infected man |
HIV uninfected woman |
|
| Couple Characteristics* | ||||
| East Africa (vs. Southern Africa) | 1487 (64.7%) | 763 (68.8%) | ||
| Married | 1691 (73.6%) | 895 (80.7%) | ||
| Living together | 2050 (89.2%) | 1032 (93.1%) | ||
| Duration of partnership, years | 4.9 (2.1, 9.4) | 6.7 (2.9, 13.8) | ||
| Number of living children with study partner | 1 (0, 2) | 2 (1, 3) | ||
| Number of sex acts, prior month | 4 (2, 8) | 4 (2, 8) | ||
| Any unprotected sex with study partner, prior month | 700 (30.5%) | 294 (26.5%) | ||
| Demographic characteristics | ||||
| Age, years | 30 (25.2, 34.7) | 35.1 (29.6, 42.1) | 37.2 (32.0, 44.9) | 30.6 (25.4, 37.7) |
| Education, years | 8 (6, 10) | 9 (7, 12) | 8 (7, 11) | 8 (6, 10) |
| Monthly income | ||||
| None | 1734 (75.4%) | 923 (40.2%) | 444 (40.0%) | 821 (74.0%) |
| < median income for the study site | 343 (14.9%) | 576 (25.1%) | 266 (24.0%) | 170 (15.3%) |
| >= median income for the study site | 221 (9.6%) | 800 (34.8%) | 399 (36.0%) | 118 (10.6%) |
| Health characteristics | ||||
| Any STI | 416 (18.1%) | 220 (9.6%) | 73 (6.6%) | 144 (13.0%) |
| Current hormonal contraceptive use | 455 (19.8%) | N/A | N/A | 197 (17.8%) |
| Other sexual partners in past month | 32 (1.4%) | 89 (3.9%) | 85 (7.7%) | 6 (0.5%) |
| Verbal or physical abuse by study partner, past 3 months | 65 (2.8%) | 23 (1.0%) | 22 (2.0%) | 34 (3.1%) |
Couple characteristics based on data reported by the HIV uninfected partner.
IQR, interquartile range; STI, sexually transmitted infection (Chlamydia trachomatis, Neisseria gonorrhea, and Trichomonas vaginalis)
At the enrollment visit, 2.8% and 3.1% of HIV infected and uninfected women and 2.0% and 1.0% of HIV infected and uninfected men, respectively, reported having experienced IPV during the prior 3 months. Approximately 36.9% of IPV reports at enrollment in HIV infected women were assessed by the study staff as definitely or probably related to the couples’ learning their of serodiscordant HIV serostatus, compared to only 14.7% of IPV reports from HIV uninfected women. Similar percentages were observed in men: 36.4% of reports were probably/definitely related to discovery of serodiscordancy in HIV infected men compared to 13.0% in HIV uninfected men.
IPV during follow-up
Median follow-up was 18 months (IQR 15–24) for HIV infected and 18 months (IQR 12–24) for HIV uninfected participants. During follow-up, women completed a total of 19,985 assessments of IPV (13,392 HIV infected, 6593 HIV uninfected) and men completed a total of 19,813 assessments (6674 HIV infected, 13,139 HIV uninfected).
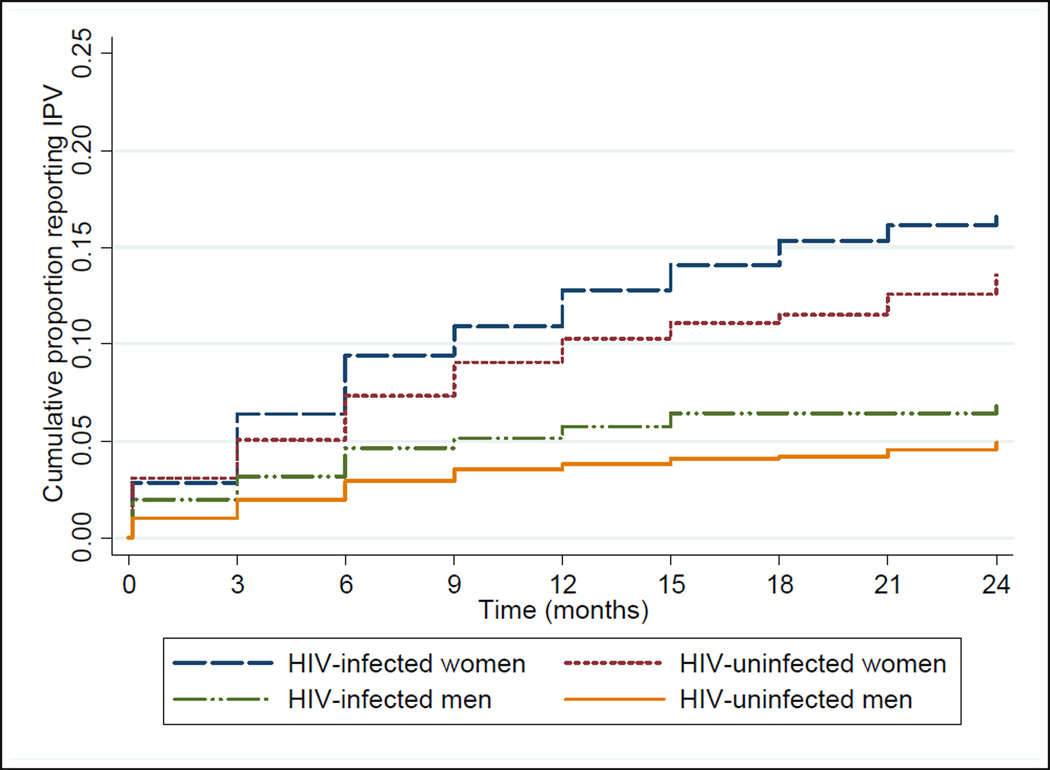
IPV was reported at 2.7% of quarterly visits by HIV infected women, 2.2% of visits by HIV uninfected women, 0.9% of visits by HIV infected men, and 0.7% of visits by HIV uninfected men (Table 2). By Kaplan-Meier analysis, the cumulative proportion of persons ever reporting IPV by 24 months of follow-up was 18.0% in HIV infected women, 14.4% in HIV uninfected women, 7.1% in HIV infected men, and 5.0% in HIV uninfected men (Figure 1). For both HIV infected and uninfected participants, women had a higher cumulative risk of IPV than men (log-rank p<0.0001). In addition, report of IPV at enrolment was strongly and significantly associated with reporting IPV during follow-up, for both women (odds ratio [OR] 5.75, 95% confidence interval [CI] 4.19–7.89, p<0.001) and men (OR 7.90, 95% CI 4.41–14.14, p<0.001).
Table 2.
Incidence of Intimate Partner Abuse during Follow-up Visits by Gender, HIV status, Region & Type of Abuse*
| WOMEN | MEN | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HIV-infected | HIV-uninfected | HIV-infected | HIV-uninfected | |||||||||
| Intimate Partner abuse reports / visits |
% of visits |
Incidence per 100 py** |
Intimate partner abuse reports |
% of visits |
Incidence per 100 py** |
Intimate partner abuse reports |
% of visits |
Incidence per 100 py** |
Intimate partner abuse reports |
% of visits |
Incidence per 100 py** |
|
| East Africa | 231 / 9397 | 2.5% | 9.7 | 89 / 4829 | 1.8% | 7.1 | 37 / 4938 | 0.7% | 3.0 | 42 / 9248 | 0.5% | 1.7 |
| Verbal only | 100 | 1.1% | 4.2 | 38 | 0.8% | 3.0 | 32 | 0.6% | 2.6 | 31 | 0.3% | 1.3 |
| Physical only | 22 | 0.2% | 0.9 | 10 | 0.2% | 0.8 | 1 | 0.0% | 0.1 | 2 | 0.0% | 0.1 |
| Both | 108 | 1.1% | 4.5 | 40 | 0.8% | 3.2 | 4 | 0.1% | 0.3 | 9 | 0.1% | 0.4 |
| Southern Africa | 137 / 3995 | 3.4% | 13.5 | 53 / 1764 | 3.0% | 11.4 | 21 / 1715 | 1.2% | 4.8 | 53 / 3891 | 1.4% | 5.1 |
| Verbal only | 55 | 1.4% | 5.4 | 17 | 1.0% | 3.6 | 16 | 0.9% | 3.7 | 39 | 1.0% | 3.8 |
| Physical only | 11 | 0.3% | 1.1 | 11 | 0.6% | 2.4 | 0 | 0.0% | 0.0 | 1 | 0.0% | 0.1 |
| Both | 71 | 1.8% | 7.0 | 25 | 1.4% | 5.4 | 5 | 0.3% | 1.1 | 12 | 0.3% | 1.2 |
| TOTAL | 368 / 13392 | 2.7% | 10.8 | 142 / 6593 | 2.2% | 8.3 | 58 / 6653 | 0.9% | 3.4 | 95 / 13139 | 0.7% | 2.7 |
| Verbal only | 155 | 1.2% | 15.3 | 55 | 0.8% | 3.2 | 48 | 0.7% | 2.8 | 70 | 0.5% | 2.0 |
| Physical only | 33 | 0.2% | 3.3 | 21 | 0.3% | 1.2 | 1 | 0.0% | 0.1 | 3 | 0.0% | 0.1 |
| Both | 179 | 1.3% | 17.7 | 65 | 1.0% | 3.8 | 9 | 0.1% | 0.5 | 21 | 0.2% | 0.6 |
Type of abuse was unknown for 3 IPV reports, thus columns may not add up to totals.
Person years (PY): HIV infected women (2383 East Africa [EA], 1012 Southern Africa [SA]), HIV uninfected women (1253 EA, 467 SA), HIV infected men (1250 EA, 437 SA), HIV uninfected men (2427 EA, 1040 SA).
Figure 1. Cumulative probability of reporting IPV, by gender and HIV status.
Cumulative proportion of persons reporting IPV during study follow-up, by HIV status and gender, as calculated by Kaplan-Meier analysis. At 24 months, the cumulative probability of any IPV report was 18.0% for HIV infected women, 14.4% for HIV uninfected women, 7.1% for HIV infected men, and 5.0% for HIV uninfected men.
For both men and women, the majority of the IPV reports were verbal or a combination of verbal and physical abuse. For women, 41.2% of IPV reports were verbal only, 47.8% were both verbal and physical, and this distribution was similar for both HIV infected and HIV uninfected women. For men, about three-quarters of reports were verbal abuse only. Compared to men, women were significantly more likely to report physical abuse only (10.6% vs. 2.6% of reports, p=0.002) or a combination of verbal and physical abuse (47.8% vs. 19.6% of reports, p<0.001).
In total, there were 536 couples (15.7% of 3408 couples) in which one or both members ever reported IPV during the study (at enrollment or during follow-up). Among couples who reported IPV, 369 (68.8%) had reports by the woman only, 71 (13.2%) had reports by the man only, and 96 (17.9%) had reports by both the man and the woman. Overall, for 64 (66.7%) of the 96 couples in which both members ever reported IPV, both members had at least one IPV report from the same study visit, demonstrating that IPV was bidirectional during that study time interval.
Correlates of IPV
Correlates of IPV during the study were assessed, separately for women and men, in multivariate models.
Among women (Table 3), IPV was significantly more frequent in those who were HIV infected (AOR 1.33, p=0.043), those who were unmarried (AOR 1.42, p=0.026), and those living with their partner (AOR 2.57, p<0.001). Notably, IPV was also reported more commonly at visits at which women reported unprotected sex with their partner (AOR 1.86, p<0.001). There were statistical trends (p<0.1) for younger women (<45 years, AOR 1.6–1.7), individuals whose duration of relationship with their serodiscordant partner was <10 years (AOR 1.2–1.6), and those from Southern Africa sites (AOR 1.28, p=0.068) to be more likely to report IPV. There was no statistically significant relationship between pregnancy and risk of IPV, and women who had sex partners in addition to their study partner were no more likely to report IPV. For HIV infected women, IPV was less frequently reported by those with CD4 counts <350 (AOR 0.72, p=0.027, compared to those with CD4 counts ≥500) and those who were on antiretroviral therapy (AOR 0.42, p=0.091).
Table 3.
Correlates of IPV among Women
| Study visits n=23393 |
Visits IPV reported n=609 |
|||||
|---|---|---|---|---|---|---|
| Correlates | n(%)* | n(%) | OR (95%CI) | P | AOR (95%CI) | P |
| Socio-Demographic Characteristics | ||||||
| Age (years) | ||||||
| <25 | 5021 (21.5) | 147 (2.9) | 1.87 (1.12, 3.14) | 0.017 | 1.63 (0.86, 3.08) | 0.134 |
| 25–34 | 11266 (48.2) | 306 (2.7) | 1.73 (1.05, 2.84) | 0.030 | 1.71 (0.96, 3.05) | 0.069 |
| 35–44 | 5509 (23.6) | 129 (2.3) | 1.51 (0.89, 2.56) | 0.131 | 1.64 (0.92, 2.92) | 0.095 |
| 45+ | 1592 (6.8) | 26 (1.6) | Reference | Reference | ||
| Region | ||||||
| East Africa | 16476 (70.4) | 368 (2.2) | Reference | Reference | ||
| Southern Africa | 6917 (29.6) | 241 (3.5) | 1.58 (1.30, 1.93) | <0.001 | 1.28 (0.98, 1.66) | 0.068 |
| Education (years) | ||||||
| None | 1269 (5.4) | 47 (3.7) | 1.4 (0.92, 2.13) | 0.117 | ||
| 1–8 | 12839 (54.9) | 318 (2.5) | 0.95 (0.77, 1.16) | 0.594 | ||
| >8 | 9285 (39.7) | 244 (2.6) | Reference | |||
| Monthly income | ||||||
| None | 17386 (74.3) | 463 (2.7) | 1.32 (0.92, 1.89) | 0.138 | ||
| < median site income | 3619 (15.5) | 94 (2.6) | 1.30 (0.84, 2.01) | 0.243 | ||
| ≥median site income | 2377 (10.2) | 48 (2.0) | Reference | |||
| Couple Characteristics | ||||||
| Age difference (years , man’s age –woman’s age) | ||||||
| ≤−5 | 761 (3.3) | 27 (3.6) | 1.63 (0.94, 2.84) | 0.084 | 1.53 (0.84, 2.78) | 0.166 |
| −5 to 5 | 10437 (44.6) | 283 (2.7) | 1.21 (0.93, 1.57) | 0.149 | 1.14 (0.86, 1.51) | 0.351 |
| 5 to 10 | 6513 (27.8) | 157 (2.4) | 1.08 (0.81, 1.44) | 0.609 | 1.14 (0.84, 1.54) | 0.407 |
| 10+ | 5641 (24.1) | 127 (2.3) | Reference | Reference | ||
| Married | ||||||
| Yes | 18322 (78.3) | 434 (2.4) | Reference | Reference | ||
| No | 5071 (21.7) | 175 (3.5) | 1.47 (1.18, 1.82) | <0.001 | 1.42 (1.04, 1.92) | 0.026 |
| Living together | ||||||
| Yes | 21323 (91.2) | 573 (2.7) | 1.55 (1.03, 2.33) | 0.035 | 2.57 (1.59, 4.15) | <0.001 |
| No | 2070 (8.9) | 36 (1.7) | Reference | Reference | ||
| Partnership duration (years) | ||||||
| <2 | 4701 (20.1) | 123 (2.6) | 1.41 (1.03, 1.93) | 0.032 | 1.22 (0.82, 1.81) | 0.326 |
| 2 to 5 | 5998 (25.6) | 211 (3.5) | 1.93 (1.45, 2.56) | <0.001 | 1.59 (1.12, 2.26) | 0.010 |
| 5 to 10 | 6239 (26.7) | 153 (2.5) | 1.35 (1.00, 1.82) | 0.054 | 1.26 (0.90, 1.77) | 0.186 |
| 10+ | 6226 (26.6) | 115 (1.9) | Reference | Reference | ||
| Children together | ||||||
| Yes | 16470 (70.4) | 410 (2.5) | Reference | |||
| No | 6914 (29.6) | 199 (2.9) | 1.14 (0.92, 1.40) | 0.230 | ||
| Self-reported Sexual Behavior | ||||||
| Unprotected sex with study partner, prior month | ||||||
| Yes | 2775 (11.9) | 132 (4.8) | 1.98 (1.59, 2.45) | <0.001 | 1.86 (1.46, 2.37) | <0.001 |
| No | 20593 (88.0) | 463 (2.3) | Reference | Reference | ||
| Other sex partners, prior month | ||||||
| Yes | 765 (3.3) | 26 (3.4) | 1.11 (0.69, 1.79) | 0.674 | ||
| No | 22060 (94.3) | 548 (2.5) | Reference | |||
| Medical Characteristics | ||||||
| STI at enrollment | ||||||
| Yes | 3523 (15.1) | 111 (3.2) | 1.29 (1.01, 1.67) | 0.045 | 1.14 (0.88, 1.48) | 0.326 |
| No | 17636 (75.4) | 432 (2.5) | Reference | Reference | ||
| Pregnant during study | ||||||
| Yes | 5809 (24.8) | 171 (2.9) | 1.22 (0.98, 1.52) | 0.072 | 1.21 (0.94, 1.56) | 0.135 |
| No | 17569 (75.1) | 424 (2.4) | Reference | Reference | ||
| Hormonal contraceptive use | ||||||
| Yes | 5016 (21.4) | 148 (3.0) | 1.15 (0.93, 1.43) | 0.201 | ||
| No | 18362 (78.5) | 447 (2.4) | Reference | |||
| HIV Characteristics | ||||||
| HIV infected | 15691 (67.1) | 433 (2.8) | 1.22 (0.98, 1.53) | 0.081 | 1.33 (1.01, 1.76) | 0.043 |
| HIV uninfected | 7702 (32.9) | 176 (2.3) | Reference | Reference | ||
| CD4 count** | ||||||
| <350 | 4450 (28.4) | 92 (2.1) | 0.64 (0.49, 0.84) | 0.001 | 0.72 (0.54, 0.96) | 0.027 |
| 350–499 | 4493 (28.6) | 113 (2.5) | 0.79 (0.62, 1.01) | 0.058 | 0.81 (0.63, 1.05) | 0.116 |
| ≥500 | 6712 (42.8) | 214 (3.2) | Reference | Reference | ||
| WHO stage of disease** | ||||||
| I & II | 14898 (95.0) | 398 (2.7) | Reference | |||
| III & IV | 757 (4.8) | 21 (2.8) | 1.05 (0.69, 1.60) | 0.812 | ||
| ART*** | ||||||
| Yes | 616 (3.9) | 4 (0.7) | 0.24 (0.09, 0.61) | 0.003 | 0.42 (0.16, 1.15) | 0.091 |
| No | 15039 (95.8) | 415 (2.8) | Reference | Reference | ||
| Seroconverted to HIV during study*** | ||||||
| Yes | 260 (3.4) | 13 (5.0) | 2.71 (1.26, 5.82) | 0.011 | 1.62 (0.59, 4.47) | 0.348 |
| No | 7442 (96.6) | 163 (2.2) | Reference | Reference | ||
| Time in study | ||||||
| Enrollment | 3408 (14.6) | 99 (2.9) | Reference | Reference | ||
| 1–6 months | 6502 (27.8) | 237 (3.7) | 1.27 (1.01, 1.58) | 0.039 | 1.53 (1.17, 1.97) | 0.002 |
| 7–12 months | 5993 (25.6) | 153 (2.6) | 0.88 (0.69, 1.13) | 0.318 | 1.14 (0.86, 1.51) | 0.360 |
| 13–18 months | 4610 (19.7) | 80 (1.7) | 0.61 (0.46, 0.80) | <0.001 | 0.70 (0.50, 0.97) | 0.031 |
| 19–24 months | 2880 (12.3) | 40 (1.4) | 0.47 (0.32, 0.67) | <0.001 | 0.63 (0.42, 0.96) | 0.031 |
Column totals may not add up to total number of study visits due to missing data.
Limited to 15,691 study visits by 2,299 HIV-infected women.
Limited to 1,109 initially HIV-uninfected participants (61 women seroconverted).
OR, odds ratio; AOR, adjusted odds ratio; STI, sexually transmitted infection; IPV, intimate partner violence; WHO, World Health Organization; ART, antiretroviral therapy
In men (Table 4), HIV seropositivity (AOR 2.20, p=0.001), younger age (<45 years, AOR ∼1.9), being from the Southern African region (AOR 2.19, p<0.001), and having other sexual partners (AOR 2.57, p<0.001) were associated with increased likelihood of reporting IPV. Men who had an STI diagnosed at enrollment were at decreased likelihood of reporting IPV (AOR 0.45, p=0.04). Marital status and other couple characteristics were not associated with IPV in men. For HIV infected men, CD4 count and ART use were not significantly associated with IPV.
Table 4.
Correlates of IPV Among Men
| Study visits n=23221 |
Visits IPV reported n=198 |
|||||
|---|---|---|---|---|---|---|
| Correlates | n(%)* | n(%) | OR (95%CI) | P | AOR (95%CI) | P |
| Socio �Demographic Characteristics | ||||||
| Age (years) | ||||||
| <25 | 1260 (5.4) | 14 (1.1) | 2.47 (1.12, 5.45) | 0.025 | 1.99 (0.77, 5.14) | 0.154 |
| 25–34 | 8350 (36.0) | 86 (1.0) | 2.30 (1.36, 3.90) | 0.002 | 1.85 (0.96, 3.55) | 0.065 |
| 35–44 | 8270 (35.6) | 74 (0.9) | 2.01 (1.18, 3.42) | 0.010 | 1.90 (1.04, 3.45) | 0.036 |
| 45+ | 5325 (22.9) | 24 (0.5) | Reference | Reference | ||
| Region | ||||||
| East Africa | 16436 (70.8) | 99 (0.6) | Reference | Reference | ||
| Southern Africa | 6785 (29.2) | 99 (1.5) | 2.40 (1.74, 3.31) | <0.001 | 2.19 (1.46, 3.28) | <0.001 |
| Education (years) | ||||||
| None | 805 (3.5) | 8 (1.0) | 0.98 (0.45, 2.14) | 0.955 | 1.21 (0.52, 2.83) | 0.655 |
| 1–8 | 10577 (45.6) | 75 (0.7) | 0.72 (0.51, 1.00) | 0.048 | 0.88 (0.62, 1.25) | 0.482 |
| >8 | 11832 (51.0) | 115 (1.0) | Reference | Reference | ||
| Monthly income | ||||||
| None | 9166 (39.5) | 68 (0.7) | 0.80 (0.54, 1.18) | 0.265 | ||
| < median site income | 5900 (25.4) | 52 (0.9) | 0.95 (0.65, 1.41) | 0.807 | ||
| ≥ median site income | 8153 (35.1) | 77 (0.9) | Reference | |||
| Couple Characteristics | ||||||
| Age difference (years, man’s age –woman’s age) | ||||||
| ≤−5 | 738 (3.2) | 9 (1.2) | 2.00 (0.90, 4.43) | 0.087 | 1.32 (0.55, 3.16) | 0.540 |
| −5 to 5 | 10272 (44.2) | 102 (1.0) | 1.65 (1.06, 2.59) | 0.028 | 1.23 (0.74, 2.07) | 0.426 |
| 5 to 10 | 6484 (27.9) | 50 (0.8) | 1.27 (0.78, 2.08) | 0.331 | 1.04 (0.62, 1.76) | 0.874 |
| 10+ | 5703 (24.6) | 35 (0.6) | Reference | Reference | ||
| Married | ||||||
| Yes | 18275 (78.7) | 125 (0.7) | Reference | Reference | ||
| No | 4945 (21.3) | 73 (1.5) | 2.19 (1.57, 3.06) | <0.001 | 1.25 (0.78, 2.01) | 0.351 |
| Living together | ||||||
| Yes | 21223 (91.4) | 186 (0.9) | 1.51 (0.81, 2.82) | 0.191 | ||
| No | 1997 (8.6) | 12 (0.6) | Reference | |||
| Partnership Duration (years) | ||||||
| <2 | 4611 (19.9) | 51 (1.1) | 1.87 (1.14, 3.05) | 0.013 | 1.36 (0.69, 2.69) | 0.378 |
| 2 to 5 | 5926 (25.5) | 56 (0.9) | 1.57 (0.98, 2.51) | 0.059 | 1.22 (0.64, 2.34) | 0.546 |
| 5 to 10 | 6090 (26.2) | 51 (0.8) | 1.40 (0.85, 2.29) | 0.182 | 1.45 (0.80, 2.62) | 0.217 |
| 10+ | 6342 (27.3) | 38 (0.6) | Reference | Reference | ||
| Children together | ||||||
| Yes | 16322 (70.3) | 138 (0.9) | Reference | |||
| No | 6894 (26.7) | 60 (0.9) | 1.05 (0.74, 1.49) | 0.779 | ||
| Self-reported Sexual Behavior | ||||||
| Unprotected sex with partner, prior month | ||||||
| Yes | 2754 (11.9) | 30 (1.1) | 1.26 (0.81, 1.98) | 0.305 | ||
| No | 20454( 88.1) | 166 (0.8) | Reference | |||
| Other sex partners, prior month | ||||||
| Yes | 2271 (9.8) | 32 (1.4) | 1.75 (1.11, 2.76) | 0.016 | 2.57 (1.61, 4.10) | <0.001 |
| No | 19723 (84.9) | 147 (0.8) | Reference | Reference | ||
| Medical Characteristics | ||||||
| STI at enrollment | ||||||
| Yes | 1854 (8.0) | 8 (0.4) | 0.48 (0.22, 1.04) | 0.063 | 0.45 (0.21, 0.97) | 0.041 |
| No | 20947 (90.2) | 184 (0.9) | Reference | Reference | ||
| HIV Characteristics | ||||||
| HIV infected | 7783 (33.5) | 80 (1.0) | 1.34 (0.97, 1.85) | 2.20 (1.40, 3.44) | 0.001 | |
| HIV uninfected | 15438 (66.48) | 118 (0.8) | Reference | Reference | ||
| CD4 count** | ||||||
| <350 | 2,793 (35.9) | 22 (0.8) | 0.59 (0.33, 1.06) | 0.076 | 0.66 (0.37, 1.18) | 0.164 |
| 350–499 | 2,456 (31.6) | 23 (0.9) | 0.69 (0.39, 1.22) | 0.202 | 0.66 (0.36, 1.19) | 0.166 |
| ≥500 | 2526 (32.5) | 33 (1.3) | Reference | Reference | ||
| WHO stage of disease** | ||||||
| I & II | 7373 (94.7) | 75 (1.0) | Reference | |||
| III & IV | 402 (5.2) | 3 (0.8) | 0.69 (0.19, 2.50) | 0.571 | ||
| ART*** | ||||||
| Yes | 422 (5.4) | 2 (0.5) | 0.46 (0.13, 1.69) | 0.243 | ||
| No | 7353 (94.5) | 76 (1.0) | Reference | |||
| Seroconverted to HIV during study*** | ||||||
| Yes | 371 (2.4) | 6 (1.6) | 2.09 (0.93, 4.69) | 0.070 | 1.69 (0.62, 4.64) | 0.305 |
| No | 15067 (97.6) | 112 (0.7) | Reference | Reference | ||
| Time in study | ||||||
| Enrollment | 3408 (14.7) | 45 (1.3) | Reference | Reference | ||
| 1–6 months | 6429 (27.7) | 81 (1.3) | 0.95 (0.67, 1.35) | 0.776 | 0.99 (0.65, 1.52) | 0.981 |
| 7–12 months | 5937 (25.6) | 42 (0.7) | 0.52 (0.35, 0.79) | 0.002 | 0.52 (0.32, 0.83) | 0.006 |
| 13–18 months | 4547 (19.6) | 18 (0.4) | 0.29 (0.17, 0.52) | <0.001 | 0.29 (0.16, 0.55) | <0.001 |
| 19–24 months | 2900 (12.5) | 12 (0.4) | 0.28 (0.13, 0.59) | 0.001 | 0.31 (0.14, 0.69) | 0.004 |
Column totals may not add up to total number of study visits due to missing data.
Limited to 7,783 study visits by HIV-infected men.
Limited to 2,299 initially-HIV uninfected participants (90 men seroconverted).
OR, odds ratio; AOR, adjusted odds ratio; STI, sexually transmitted infection; IPV, intimate partner violence; WHO, World Health Organization; ART, antiretroviral therapy
For both women and men, those who experienced HIV seroconversion did not have statistically significant increased risk of IPV prior to acquiring HIV (AOR 1.62, p=0.348 for women and AOR 1.69, p=0.305 for men). Similarly, IPV was not reported significantly more often at visits at which HIV seroconversion was detected (data not shown). During 497 post-seroconversion visits by 146 HIV seroconverters, a total of eight (1.6% of visits) reports of IPV were obtained, by 7 women: 4 had not reported IPV and 3 had reported IPV before seroconversion. No men reported IPV after HIV seroconversion.
Finally, for both women and men, the incidence of IPV decreased significantly during study follow-up (test for trend p<0.001).
Consequences of IPV
Relationship dissolution was the most common consequence of IPV during the follow-up period, occurring in 31.3% and 22.5% of HIV infected and uninfected women and 20.7% and 35.8% of HIV infected and uninfected men reporting IPV. The second most common consequence associated with IPV was change of residence, also occurring in slightly over 20% of cases. Other consequences included loss of economic support (18.8% of HIV infected women, <10% of others) and loss of custody of children (6.3% of HIV uninfected men, <5% of others). For 24.5% and 47.2% of IPV reports by HIV infected and uninfected women, and 34.5% and 31.6% of reports by HIV infected and uninfected men, participants reported that there were no consequences to the relationship or the individual as a result of IPV.
Discussion
In this large prospective study of HIV serodiscordant couples from East and Southern Africa who were aware of their serodiscordant status, we found less than 20% of women and less than 10% of men experienced verbal or physical IPV over up to 24 months of prospective follow-up. IPV was reported at less than 3% of quarterly follow-up visits by women and less than 1% of visits by men; the frequency of IPV decreased during follow-up. Thus, among HIV serodiscordant couples who had recently become aware of their serodiscordant serostatus and who received frequent couples counseling, frequency of IPV was similar to or lower than background levels in the general population in the region [1–3, 7, 18–20].
We had hypothesized that IPV might be common among HIV serodiscordant couples, particularly couples in which the HIV infected partner was female. Other studies have recorded higher vulnerability to abuse of women who are HIV infected [20, 21], and one Nigerian study reported a 3-fold rise in reports of partner abuse after HIV diagnosis among pregnant HIV infected women who had an uninfected partner [22]. In our study, women more frequently reported IPV than men, and HIV infected women had a 33% higher risk of IPV compared with uninfected women and had the highest cumulative risk of IPV (18.0% ever reporting by 24 months).
IPV has been associated with increased HIV risk in some [2], but not all studies [23], with the strongest associations likely reflecting physical abuse accompanied by forced sex [3, 6, 7, 24]. Much of this literature points at the role of partner violence in transmission of HIV through concurrent partnerships, transactional sex, and coercive sex without condoms [2, 25, 26]. For HIV uninfected women, risk of HIV acquisition could be increased due to reduced capacity to negotiate condom use in an abusive partnership [27, 28]. We found that women were more prone to report partner violence that included physical abuse than men, and that IPV was more common among women reporting recent sex unprotected by condoms. However, we found no statistically significant relationship between IPV and subsequent HIV seroconversion, for women or for men.
Among women in HIV serodiscordant partnerships, not being married to the partner was associated with a 44% higher risk of IPV report and living together was associated with a 62% higher risk. For unmarried couples, the relative instability of the relationship may lead to greater risk of IPV; couples living together may have more frequent opportunity for IPV. For both women and men, we found those from Southern African sites were more likely to report IPV than those from East Africa, which may reflect differences in gender power or relationship stability on a regional level.
Our data are unique in documenting the frequency of IPV against men by their female partners. Similar to findings for women, men also had increased risk of intimate partner abuse report if they were HIV infected. There may have been underreporting of IPV among men due to social desirability bias in the patriarchal societies of Africa [29].
Our study had several strengths. First, it analyzed data from a large prospective cohort. Many of the available published works are either from cross-sectional studies, such as national demographic and health surveys. Second, we recorded intimate partner abuse reports over relatively short recall periods (3 months), which likely increased the accuracy of our data compared to reports recalled over longer periods. Third, we examined the role of both HIV status and the gender in IPV incidents. Finally, our data were from 14 study sites in East and Southern Africa, which may increase the generalizability of our findings. We did not collect data on alcohol use, which is a limitation as alcohol has been associated with IPV in other settings [30, 31]. Our clinical trial population may not be fully representative of HIV serodiscordant couples, particularly those unaware of their serodiscordant status and those who do not receive ongoing couples counseling and support services. Ongoing individual and couples counseling, by experienced staff, may have maximized condom use and other risk reductions in the study population, and decreased the frequency of IPV.
In summary, we found that most members of known HIV serodiscordant couples followed for up to two years do not report IPV. Overall, the rate of IPV was lower than or similar to that expected in the general population of the countries in which this study was done; HIV infected partners, both women and men, were at modest increased IPV risk. Men and women experienced IPV differently, with women reporting physical and verbal abuse compared to men who generally reported verbal abuse alone. The decrease in IPV incidence with follow-up could also be an indicator of the effectiveness of ongoing counseling in addressing couples’ need for support and thereby reducing the risk of IPV report over time. Further studies are warranted to determine the frequency and focus of counseling for HIV serodiscordant couples in order to reduce HIV transmission and minimize IPV.
Acknowledgments
We gratefully acknowledge the invaluable contributions of the HIV serodiscordant couples who participated in this study. We thank the teams at the study sites and at the University of Washington for work on data collection and management.
Funding source: This study was supported through research grants from the Bill & Melinda Gates Foundation (grant ID # 26469) and the US National Institutes of Health (National Institute of Allergy and Infectious Diseases grant R01 AI083034).
Role of the Funding Source: The authors designed and executed the study, had full access to the raw data, performed all analyses, wrote the manuscript, and had final responsibility for the decision to submit for publication. The funder had no role in design, data collection, analysis, interpretation, or writing of the report.
Partners in Prevention HSV/HIV Transmission Study Team
University of Washington Coordinating Center and Central Laboratories, Seattle, USA: Connie Celum (principal investigator), Anna Wald (protocol co-chair), Jairam Lingappa (medical director), Jared M. Baeten, Mary Campbell, Lawrence Corey, Robert W. Coombs, James P. Hughes, Amalia Magaret, M. Juliana McElrath, Rhoda Morrow, James I. Mullins
Study sites and site principal investigators:
Cape Town, South Africa (University of Cape Town): David Coetzee; Eldoret, Kenya (Moi University, Indiana University): Kenneth Fife, Edwin Were; Gaborone, Botswana (Botswana Harvard Partnership): Max Essex, Joseph Makhema; Kampala, Uganda (Infectious Disease Institute, Makerere University): Elly Katabira, Allan Ronald; Kigali, Rwanda (Rwanda Zambia HIV Research Group, and Emory University): Susan Allen, Kayitesi Kayitenkore, Etienne Karita; Kisumu, Kenya (Kenya Medical Research Institute, University of California San Francisco): Elizabeth Bukusi, Craig Cohen; Kitwe, Zambia (Rwanda Zambia HIV Research Group, and Emory University): Susan Allen, William Kanweka; Lusaka, Zambia (Rwanda Zambia HIV Research Group, and Emory University): Susan Allen, Bellington Vwalika; Moshi, Tanzania (Kilimanjaro Christian Medical College, Harvard University): Saidi Kapiga, Rachel Manongi; Nairobi, Kenya (University of Nairobi, University of Washington): Carey Farquhar, Grace John-Stewart, James Kiarie; Ndola, Zambia (Rwanda Zambia HIV Research Group, and Emory University): Susan Allen, Mubiana Inambao; Orange Farm, South Africa (Reproductive Health Research Unit, University of the Witwatersrand): Sinead Delany-Moretlwe, Helen Rees; Soweto, South Africa (Perinatal HIV Research Unit, University of the Witwatersrand): Guy de Bruyn, Glenda Gray, James McIntyre; Thika, Kenya (University of Nairobi, University of Washington): Nelly Rwamba Mugo Data management was provided by DF/Net Research, Inc. (Seattle, USA) and site laboratory oversight was provided by Contract Lab Services (University of the Witwatersrand, Johannesburg, South Africa).
Footnotes
Conflict of interest: No authors report conflicts of interest regarding content for this manuscript.
Author contributions: EW conceived the study, with EN-J, and wrote the first draft of the manuscript, along with KC and JMB. KC and JMB performed the statistical analysis. SD-M, EN-J, NRM, JK, EB, and CC contributed critical revisions to the analysis and interpretation. All authors contributed to the writing of the final draft.
References
- 1.Ntaganira J, Muula AS, Masaisa F, Dusabeyezu F, Siziya S, Rudatsikira E. Intimate partner violence among pregnant women in Rwanda. BMC Womens Health. 2008;8:17. doi: 10.1186/1472-6874-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jewkes RK, Dunkle K, Nduna M, Shai N. Intimate partner violence, relationship power inequity, and incidence of HIV infection in young women in South Africa: a cohort study. Lancet. 2010;376:41–48. doi: 10.1016/S0140-6736(10)60548-X. [DOI] [PubMed] [Google Scholar]
- 3.Fonck K, Leye E, Kidula N, Ndinya-Achola J, Temmerman M. Increased risk of HIV in women experiencing physical partner violence in Nairobi, Kenya. AIDS Behav. 2005;9:335–339. doi: 10.1007/s10461-005-9007-0. [DOI] [PubMed] [Google Scholar]
- 4.Dunkle KL, Jewkes RK, Nduna M, Levin J, Jama N, Khuzwayo N, et al. Perpetration of partner violence and HIV risk behaviour among young men in the rural Eastern Cape, South Africa. AIDS. 2006;20:2107–2114. doi: 10.1097/01.aids.0000247582.00826.52. [DOI] [PubMed] [Google Scholar]
- 5.Campbell JC. Health consequences of intimate partner violence. Lancet. 2002;359:1331–1336. doi: 10.1016/S0140-6736(02)08336-8. [DOI] [PubMed] [Google Scholar]
- 6.Andersson N, Cockcroft A, Shea B. Gender-based violence and HIV: relevance for HIV prevention in hyperendemic countries of southern Africa. AIDS. 2008;22(Suppl 4):S73–S86. doi: 10.1097/01.aids.0000341778.73038.86. [DOI] [PubMed] [Google Scholar]
- 7.Jewkes R, Dunkle K, Nduna M, Levin J, Jama N, Khuzwayo N, et al. Factors associated with HIV sero-status in young rural South African women: connections between intimate partner violence and HIV. Int J Epidemiol. 2006;35:1461–1468. doi: 10.1093/ije/dyl218. [DOI] [PubMed] [Google Scholar]
- 8.Eyawo O, de Walque D, Ford N, Gakii G, Lester RT, Mills EJ. HIV status in discordant couples in sub-Saharan Africa: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:770–777. doi: 10.1016/S1473-3099(10)70189-4. [DOI] [PubMed] [Google Scholar]
- 9.Celum C, Wald A, Lingappa JR, Magaret AS, Wang RS, Mugo N, et al. Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2. N Engl J Med. 2010;362:427–439. doi: 10.1056/NEJMoa0904849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wawer MJ, Gray RH, Sewankambo NK, Serwadda D, Li X, Laeyendecker O, et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis. 2005;191:1403–1409. doi: 10.1086/429411. [DOI] [PubMed] [Google Scholar]
- 11.Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. Rakai Project Study Group. N Engl J Med. 2000;342:921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 12.Dunkle KL, Stephenson R, Karita E, Chomba E, Kayitenkore K, Vwalika C, et al. New heterosexually transmitted HIV infections in married or cohabitating couples in urban Zambia and Rwanda: an analysis of survey and clinical data. Lancet. 2008;371:2183–2191. doi: 10.1016/S0140-6736(08)60953-8. [DOI] [PubMed] [Google Scholar]
- 13.Farquhar C, Mbori-Ngacha DA, Bosire RK, Nduati RW, Kreiss JK, John GC. Partner notification by HIV-1 seropositive pregnant women: association with infant feeding decisions [letter] AIDS. 2001;15:815–817. doi: 10.1097/00002030-200104130-00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamenga M, Ryder RW, Jingu M, Mbuyi N, Mbu L, Behets F, et al. Evidence of marked sexual behavior change associated with low HIV-1 seroconversion in 149 married couples with discordant HIV-1 serostatus: experience at an HIV counselling center in Zaire. AIDS. 1991;5:61–67. doi: 10.1097/00002030-199101000-00009. [DOI] [PubMed] [Google Scholar]
- 15.The Voluntary HIV-1 Counseling and Testing Efficacy Study Group. Efficacy of voluntary HIV-1 counselling and testing in individuals and couples in Kenya, Tanzania, and Trinidad: a randomised trial. Lancet. 2000;356:103–112. [PubMed] [Google Scholar]
- 16.Lingappa JR, Kahle E, Mugo N, Mujugira A, Magaret A, Baeten J, et al. Characteristics of HIV-1 discordant couples enrolled in a trial of HSV-2 suppression to reduce HIV-1 transmission: the Partners Study. PLoS ONE. 2009;4:e5272. doi: 10.1371/journal.pone.0005272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lingappa JR, Lambdin B, Bukusi EA, Ngure K, Kavuma L, Inambao M, et al. Regional differences in prevalence of HIV-1 discordance in Africa and enrollment of HIV-1 discordant couples into an HIV-1 prevention trial. PLoS ONE. 2008;3:e1411. doi: 10.1371/journal.pone.0001411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCloskey LA, Williams C, Larsen U. Gender inequality and intimate partner violence among women in Moshi, Tanzania. Int Fam Plan Perspect. 2005;31:124–130. doi: 10.1363/3112405. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Moreno C, Jansen HA, Ellsberg M, Heise L, Watts CH. Prevalence of intimate partner violence: findings from the WHO multi-country study on women's health and domestic violence. Lancet. 2006;368:1260–1269. doi: 10.1186/1471-2458-11-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Straten A, King R, Grinstead O, Vittinghoff E, Serufilira A, Allen S. Sexual Coercion, Physical Violence, and HIV Infection Among Women in Steady Relationships in Kigali, Rwanda. AIDS and Behavior. 1998;2:61–73. [Google Scholar]
- 21.Maman S, Mbwambo JK, Hogan NM, Kilonzo GP, Campbell JC, Weiss E, Sweat MD. HIV-positive women report more lifetime partner violence: findings from a voluntary counseling and testing clinic in Dar es Salaam, Tanzania. Am J Public Health. 2002;92:1331–1337. doi: 10.2105/ajph.92.8.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ezechi OC, Gab-Okafor C, Onwujekwe DI, Adu RA, Amadi E, Herbertson E. Intimate partner violence and correlates in pregnant HIV positive Nigerians. Arch Gynecol Obstet. 2009;280:745–752. doi: 10.1007/s00404-009-0956-9. [DOI] [PubMed] [Google Scholar]
- 23.Harling G, Msisha W, Subramanian SV. No association between HIV and intimate partner violence among women in 10 developing countries. PLoS ONE. 2010;5:e14257. doi: 10.1371/journal.pone.0014257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silverman JG, Decker MR, Saggurti N, Balaiah D, Raj A. Intimate partner violence and HIV infection among married Indian women. JAMA. 2008;300:703–710. doi: 10.1001/jama.300.6.703. [DOI] [PubMed] [Google Scholar]
- 25.Decker MR, Seage GR, 3rd, Hemenway D, Gupta J, Raj A, Silverman JG. Intimate partner violence perpetration, standard and gendered STI/HIV risk behaviour, and STI/HIV diagnosis among a clinic-based sample of men. Sex Transm Infect. 2009;85:555–560. doi: 10.1136/sti.2009.036368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dunkle KL, Jewkes RK, Brown HC, Gray GE, McIntryre JA, Harlow SD. Gender-based violence, relationship power, and risk of HIV infection in women attending antenatal clinics in South Africa. Lancet. 2004;363:1415–1421. doi: 10.1016/S0140-6736(04)16098-4. [DOI] [PubMed] [Google Scholar]
- 27.Wingood GM, DiClemente RJ. The effects of an abusive primary partner on the condom use and sexual negotiation practices of African-American women. Am J Public Health. 1997;87:1016–1018. doi: 10.2105/ajph.87.6.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bogart LM, Collins RL, Cunningham W, Beckman R, Golinelli D, Eisenman D, Bird CE. The association of partner abuse with risky sexual behaviors among women and men with HIV/AIDS. AIDS Behav. 2005;9:325–333. doi: 10.1007/s10461-005-9006-1. [DOI] [PubMed] [Google Scholar]
- 29.Courtenay-Quirk C, Pals SL, Colfax G, McKirnan D, Gooden L, Eroglu D. Factors associated with sexual risk behavior among persons living with HIV: gender and sexual identity group differences. AIDS Behav. 2008;12:685–694. doi: 10.1007/s10461-007-9259-y. [DOI] [PubMed] [Google Scholar]
- 30.Zablotska IB, Gray RH, Koenig MA, Serwadda D, Nalugoda F, Kigozi G, et al. Alcohol use, intimate partner violence, sexual coercion and HIV among women aged 15–24 in Rakai, Uganda. AIDS Behav. 2009;13:225–233. doi: 10.1007/s10461-007-9333-5. [DOI] [PubMed] [Google Scholar]
- 31.Kalichman SC, Simbayi LC, Kaufman M, Cain D, Jooste S. Alcohol use and sexual risks for HIV/AIDS in sub-Saharan Africa: systematic review of empirical findings. Prev Sci. 2007;8:141–151. doi: 10.1007/s11121-006-0061-2. [DOI] [PubMed] [Google Scholar]