Abstract
Background
Patients with un-witnessed stroke represent over 25% of all strokes. Clinical trials enrolling patients with un-witnessed stroke onset have conservatively used ‘last known normal’ (LKN) as the time of stroke onset. We explored the impact of alternative methods of selecting onset time in un-witnessed strokes on eligibility into an acute stroke therapeutic trial using a representative sample of acute stroke subjects.
Methods
We analyzed data on 641 consecutive patients with suspected stroke presenting to our hospital from January 2007 to July 2008. Time of onset was calculated by three methods: (1) LKN, (2) first known abnormal (FKA), (3) midpoint between LKN and FKA. Subjects with incomplete data or a non-ischemic stroke final diagnosis were excluded. Rates of trial eligibility based on different onset times were compared for several inclusion time windows.
Results
Onset time was known in 440 subjects (69%). Of the remaining 201 patients with un-witnessed onset, 114 (57%) were ‘wake-up’ strokes. Among un-witnessed stroke subjects, eligibility increased from 18% using LKN to 57% using FKA at 4.5 hours and 42% to 81% at 9 hours (p<0.001), respectively. Overall enrollment eligibility for the full cohort increased from 36% to 48% at 4.5 hours and 61% to 73% at 9 hours (p<0.001), respectively.
Conclusion
Given potential advantages in safety evaluation and the historical inadequate enrollment rates in acute stroke trials, alternative onset time definitions, perhaps in combination with advanced neuroimaging methods (e.g. FLAIR-DWI mismatch), should be considered for early-phase trials.
Keywords: stroke, trials, time, onset, enrollment
Background
Ischemic stroke is a dynamic process of structural and metabolic disturbances that evolve in a non-uniform fashion over time and space. Brain tissue viability correlates with time elapsed since onset of ischemia, so the duration of symptoms has become a critical determinant of treatment eligibility for many trials. All of the major thrombolysis trials have either excluded patients with unknown time of onset or conservatively assigned time of onset as the time last known normal (LKN). Since the earliest of these trial publications, the LKN method of assigning onset time has become firmly entrenched.
We conducted a NINDS-funded phase II acute stroke trial of normobaric oxygen to evaluate the safety of this intervention by clinical assessments, brain imaging and molecular biomarkers. The midpoint between LKN and the time the patient was first known to be abnormal (FKA) was used for the time of onset. It was hypothesized that this onset time enrollment criterion would positively impact trial enrollment and enable a more thorough evaluation of safety. The aim of this study is to explore the impact of different methods of determining time of onset in un-witnessed stroke on therapeutic trial eligibility in a large, representative sample of patients presenting with symptoms of acute cerebral ischemia.
Methods
Patient Selection and Data Acquisition
Consecutive patients presenting with symptoms of acute stroke to the Massachusetts General and Brigham and Women’s Hospitals from January 19, 2007 to July 6, 2008 were prospectively entered into a registry. Data recorded for each patient included whether the onset of symptoms was witnessed or not. When witnessed, time and date of onset was recorded. If un-witnessed, the time and date the subject was LKN and FKA was recorded, along with whether the patient awoke with symptoms present. The best available information from the subject, their relatives and friends, and emergency medical services personnel was used. Patients with incomplete onset-time data and whose eventual diagnosis was not ischemic stroke, were excluded. The data was collected with approval of the Institutional Review Board and in compliance with the Health Insurance Portability and Accountability Act.
Analysis
TO for subjects with un-witnessed onset was assigned by three different methods: (1) LKN, (2) the midpoint between LKN and FKA (i.e. the mean of LKN and FKA), and (3) FKA. Time since onset is the elapsed time between the time of onset and time of initial assessment at the hospital emergency department. Chi square tests were used to compare the proportion of patients eligible for our normobaric oxygen stroke trial based solely on the 3 possible onset times, the other variables for determining inclusion-exclusion remaining stable. Proportions were calculated for 2 inclusion time windows, 4.5 hours and 9 hours.
Results
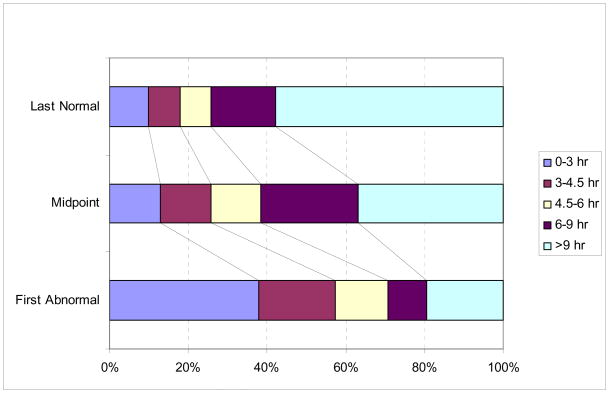
The analysis included 641 patients. Time of onset was known exactly in 440 patients (68.6%) and unknown in the remaining 201 patients, 114 (57%) of whom reported symptoms present upon awakening. Figure 1 shows the percentage of patients with unknown time of onset in this study that would be assigned to commonly used time since onset groupings based on the three methods of calculating onset time. The percentage of patients with unknown time of onset who would be eligible for a therapy restricted to within 4.5 hours since onset increased from 17.9% using LKN, 25.9% using the midpoint, and to 57.2% using FKA (p<0.001), and using a 9 hour restriction, from 42.2% to 63.2% to 80.6%, respectively (p<0.001).
Figure 1. Time Since Onset in Patients with Unwitnessed Onset.
The distribution of patients into time since onset categories is shown according to the methods of assigning time of onset (LKN, midpoint and FKA). A significant shift toward lower time since onset categories is observed.
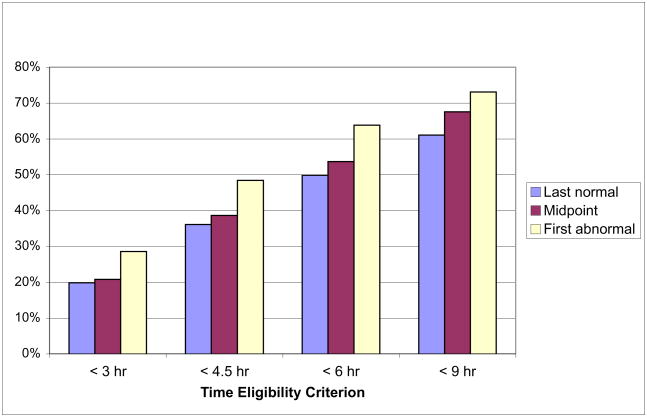
Due to the fact that only 31.4% of subjects presented with unknown onset time, the effect on total enrollment was modest. As depicted in Figure 2, the percentage of all patients eligible for the same hypothetical 4.5 hour restricted therapy would increase from 36.0% to 38.5% to 48.4% (p<0.001), and 61.0% to 67.6% to 73.0% (p<0.001) at 9 hours using the three different time of onset calculation methods.
Figure 2.
Total Percent of Patients Eligible
Discussion
Ineligibility for acute stroke therapies due to elapsed time since onset is of major importance. In the NINDS tPA trial where a LKN criterion was used, 17,324 patients were screened to recruit 624 eligible subjects, and most of the ineligible patients were excluded due to the time since onset criterion.1 In an analysis of patients presenting to Calgary-area hospitals, 32.9% were ineligible for IV tPA because of uncertain onset time.2 Our results suggest that different methods of assigning time since onset for patients with unknown onset time, can influence the proportion of trial-eligible subjects by approximately 12%. Thrombolysis trials have contributed important wisdom to the design of later stroke trials, but may now be exerting an exaggerated impact on the design of trials that require different considerations, such as biomarker and neuroprotective agent studies.
Prior studies have shown that stroke onset in ‘wake-up’ strokes is usually closer to the waking hours, and that the use of a rigid and universal time window is incongruent with the physiological state of the ischemic tissue.3,4 Alternative methods for characterizing tissue state have been inadequate.5 Significant interest is now focused on the use of FLAIR-DWI mismatch in estimating onset time.6,7 Aside from the equivocal data to support its use in establishing time of onset,6,7 multimodal imaging remains expensive, limited in availability and is cumbersome to implement.
A conservative approach to selecting time of onset may be appropriate for efficacy trials, but suboptimal for thorough safety studies. Phase I and II treatment trials could benefit from more liberal time inclusion criteria. Collecting LKN and FKA data allows for time-related safety characteristics to be more thoroughly explored and builds in a margin of error. Use of a midpoint calculation will underestimate the time since onset in a small number of patients, allowing them to undergo an intervention and thus expose any time-variant risks. Furthermore, using the midpoint calculation method would allow investigators to report a true mean time since onset and a more accurate statistical representation of the relationship between time and measured outcomes. Based on these considerations, the EXTEND Trial is currently enrolling patients for thrombolysis treatment using a midpoint calculation for its time inclusion criterion.8 This study demonstrates that relaxing time since onset from LKN to either a midpoint or FKA method would increase enrollment eligibility. Our phase II study of oxygen therapy enrolling patients within 9 hours based on the midpoint method of time since onset, appears to be the first acute stroke treatment trial to use an alternative to the LKN method.9 Use of the midpoint method in that study is expected to facilitate accurate correlation between stroke duration and the radiographic and molecular biomarkers being measured in the trial.
Conclusions
A fixed time limit for all stroke-related therapies is inconsistent with the highly individualized duration of the ischemic penumbra.1 Exclusion from acute stroke therapeutic trials is frequently due to time ineligibility. The time of symptoms data from this representative sample of patients demonstrates the recruitment advantage of using alternate time since onset definitions. Given the potential advantages in safety evaluation and the failure of many acute stroke trials due to inadequate enrollment, alternative time since onset definitions should be considered for early phase trials.
Acknowledgments
Sources of Funding: Supported by National Institutes of Health grants P50NS051343 and 5R01NS051412.
Footnotes
Conflicts of Interest and Disclosures:
Matthew B. Maas, MD- none.
Aneesh B. Singhal, MD- none
Author contributions:
Dr. Maas- Drafting and revising the manuscript for content, study concept, analysis and interpretation of data, statistical analysis.
Dr. Singhal- Drafting and revising the manuscript for content, study concept, analysis and interpretation of data, study supervision, obtaining funding.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gladstone DJ, Black SE, Hakim AM Heart and Stroke Foundation of Ontario Centre of Excellence in, Stroke Recovery. Toward wisdom from failure: lessons from neuroprotective stroke trials and new therapeutic directions. Stroke. 2002;33:2123–36. doi: 10.1161/01.str.0000025518.34157.51. [DOI] [PubMed] [Google Scholar]
- 2.Barber PA, Zhang J, Demchuk AM, Hill MD, Buchan AM. Why are stroke patients excluded from TPA therapy? An analysis of patient eligibility. Neurology. 2001;56:1015–1020. doi: 10.1212/wnl.56.8.1015. [DOI] [PubMed] [Google Scholar]
- 3.Casetta I, Granieri E, Fallica E, la Cecilia O, Paolino E, Manfredini R. Patient demographic and clinical features and circadian variation in onset of ischemic stroke. Arch Neurol. 2002;59:48–53. doi: 10.1001/archneur.59.1.48. [DOI] [PubMed] [Google Scholar]
- 4.Serena J, Davalos A, Segura T, Mostacero E, Castillo J. Stroke on awakening: looking for a more rational management. Cerebrovasc Dis. 2003;16:128–133. doi: 10.1159/000070592. [DOI] [PubMed] [Google Scholar]
- 5.Baron JC, von Kummer R, del Zoppo GJ. Treatment of acute ischemic stroke. Challenging the concept of a rigid and universal time window. Stroke. 1995;26:2219–2221. doi: 10.1161/01.str.26.12.2219. [DOI] [PubMed] [Google Scholar]
- 6.Thomalla G, Rossbach P, Rosenkranz M, Siemonsen S, Krützelmann A, Fiehler J, Gerloff C. Negative fluid-attenuated inversion recovery imaging identifies acute ischemic stroke at 3 hours or less. Ann Neurol. 2009;65:724–32. doi: 10.1002/ana.21651. [DOI] [PubMed] [Google Scholar]
- 7.Ebinger M, Galinovic I, Rozanski M, Brunecker P, Endres M, Fiebach JB. Fluid-attenuated inversion recovery evolution within 12 hours from stroke onset: a reliable tissue clock? Stroke. 2010;41:250–5. doi: 10.1161/STROKEAHA.109.568410. [DOI] [PubMed] [Google Scholar]
- 8.Extending the Time for Thrombolysis in Emergency Neurological Deficits (EXTEND) [Accessed 7/19, 2011]; Available at: http://www.clinicaltrials.gov/ct2/show/NCT00887328.
- 9.Normobaric Oxygen Therapy in Acute Ischemic Stroke Trial. [Accessed 5/20, 2009];2009 Available at: http://www.clinicaltrials.gov/ct2/show/NCT00414726.