SUMMARY
We measured quality of life (QOL) among individuals receiving treatment for human immunodeficiency virus (HIV; n = 45), active tuberculosis (TB; n = 44) and both TB and HIV (n = 9) in Rio de Janeiro, Brazil. Active treated TB was associated with lower physical health (absolute decrease of 0.95 standard deviation in summary score), but not mental health, among people being treated for HIV. Visual analogue scale scores were similar across all three populations, and corresponded closely to standard disability weights used in the literature. Among patients receiving treatment, those with HIV, active TB and both conditions together appear to have similar QOL.
Keywords: tuberculosis, human immunodeficiency virus, quality of life, Brazil
Globally, 13% of people with active tuberculosis (TB) are living with the human immunodeficiency virus (HIV). The joint effect of these diseases on quality of life (QOL)—defined as ‘individuals’ perception of their position in life in the context of the [ir] culture and value systems’1—is poorly understood. QOL has been studied separately in people living with HIV2,3 and active TB,4,5 but rarely in co-infected individuals.6,7 Many economic evaluations assume additive morbidity from HIV and TB, an assumption that may bias cost-effectiveness estimates and that can be tested efficiently. We thus conducted a small cross-sectional QOL survey among individuals being treated for HIV and TB in Rio de Janeiro, Brazil.
METHODS
We identified patients receiving antiretroviral therapy and/or TB chemotherapy across five public sector clinics in Rio de Janeiro, selected for administrative convenience from a larger trial.8 Routine health providers asked consecutive patients to participate until they reached the target sample size (nine patients each with TB and HIV); we did not enroll our target sample of 45 TB-HIV co-infected participants, and routine providers did not disclose refusal rates. Consenting participants completed an in-person interview, which included the 35-item Medical Outcomes Study HIV Health Survey (MOS-HIV) and a visual analogue scale (VAS) to rate health from 0 (worst imaginable state) to 100 (best imaginable). The MOS-HIV is a validated instrument for QOL assessment among persons living with HIV.9,10 It has 10 subscales that are combined statistically to generate summary scores describing physical and mental health. These summary scores were transformed such that 50 represented the mean score, and 10 the standard deviation (SD); thus, 68% of scores during initial validation fell between 40 and 60. We added a VAS for comparison with other utility assessments. In contrast to the disability weights used to construct disability-adjusted life years (DALYs, a globally representative measure),11 these instruments assessed QOL specific to the Brazilian context.
The study protocol was approved by the institutional review boards of the Johns Hopkins University School of Medicine and the City of Rio de Janeiro.
We assessed univariate differences using Fisher’s exact test for categorical variables, Spearman’s correlation coefficients for continuous variables and one-way analysis of variance (ANOVA) for differences by clinic. We evaluated multivariable associations using linear regression on summary measures.
RESULTS
Of the 98 participants who completed the interview, 45 were being treated for HIV, 44 for active TB and 9 for both HIV and active TB. Participants with TB were younger than those with HIV (median age 32 vs. 42, P < 0.01), but were similar by sex (45% vs. 31% female, P = 0.30). Among TB patients, MOS-HIV sub-scores did not differ according to HIV status, nor did sub-scores among persons receiving HIV treatment differ by TB status (P > 0.05 for all comparisons).
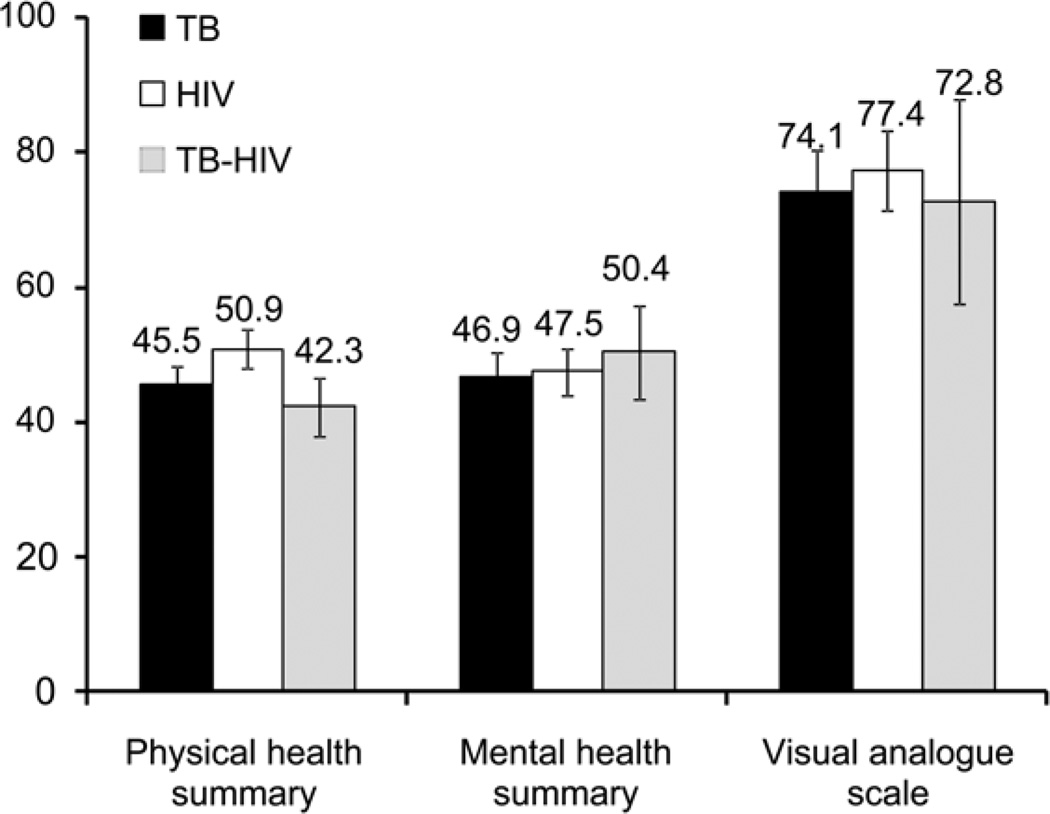
The Figure shows differences in MOS-HIV summary scores and VAS responses. Among persons being treated for HIV, those with active TB reported significantly lower physical health (mean summary score 42.3, 95% confidence interval [CI] 37.2–47.4 vs. 50.9, 95%CI 47.9–53.9), but no difference in mental health or VAS. Persons being treated for active TB and HIV otherwise had no discernible differences in QOL. These findings persisted after adjustment for age and sex: active TB was associated with lower physical health summary scores (difference in the HIV-infected subset: 9.5 points, 95% CI 2.8–16.2; 10 points = 1 SD), but there was no discernible difference in mental health or VAS utility (Table).
Figure.
Health-related quality of life among patients treated for HIV and TB in Rio de Janeiro. Physical and mental health summaries are measured by the Medical Outcomes Study HIV Health Survey; a score of 50 corresponds to the normative value among persons living with HIV, with a standard deviation of 10. Visual analogue scale scores are scaled from 0 (worst health state imaginable) to 100 (best health state imaginable). Mean values are shown; error bars correspond to 95% confidence intervals. TB = tuberculosis; HIV = human immunodeficiency virus.
Table.
Multivariable predictors of health-related quality of life among patients treated for HIV or TB in Rio de Janeiro, Brazil*
| Variable | Physical health summary† score (95%CI) |
Mental health summary† score (95%CI) |
Visual analogue scale‡ score (95%CI) |
|---|---|---|---|
| All patients | |||
| Age (per 10 years) | −1.7 (−3.2 to −0.2)§ | 0.2 (−1.7 to 2.1) | −0.8 (−4.2 to 2.7) |
| Female sex | 1.7 (−2.2 to 5.7) | −3.2 (−8.2 to 1.7) | −1.1 (−10.0 to 7.7) |
| HIV infection | −2.5 (−9.4 to 4.4) | 3.1 (−5.5 to 11.6) | −1.3 (−16.6 to 14.1) |
| Active TB | −9.1 (−16.0 to −2.2)§ | 3.0 (−5.6 to 11.5) | −4.8 (−20.1 to 10.5) |
| Patients with HIV only | |||
| Age (per 10 years) | −3.1 (−5.4 to −0.7)§ | 2.9 (0.0 to 5.8) | 0.7 (−19.9 to 11.0) |
| Female sex | 2.7 (−2.8 to 8.1) | −3.5 (−10.5 to 3.4) | −0.2 (−12.9 to 12.4) |
| Active TB | −9.5 (−16.2 to −2.8)§ | 3.7 (−4.8 to 12.2) | −4.4 (−19.9 to 11.0) |
| Patients with TB only | |||
| Age (per 10 years) | −0.8 (−2.7 to 1.1) | −0.9 (−3.2 to 1.4) | −1.9 (−6.2 to 2.5) |
| Female sex | 0.9 (−4.4 to 6.2) | −2.2 (−8.8 to 4.3) | 2.7 (−9.5 to 14.9) |
| HIV co-infection | −2.8 (−9.8 to 4.2) | 3.5 (−5.2 to 12.1) | −0.5 (−16.6 to 15.6) |
Values reflect the change in score, adjusted for other variables in the Table. 95% confidence intervals are shown in parentheses.
A 10-point reduction corresponds to one standard deviation in a normative population.
Scored between 0 (worst imaginable health state) and 100 (best imaginable health state).
Statistically significant results (P < 0.05).
HIV = human immunodeficiency virus; TB = tuberculosis; CI = confidence interval.
DISCUSSION
In this cross-sectional evaluation of 98 individuals treated in public health clinics in Rio de Janeiro, Brazil, persons receiving treatment for HIV, active TB and both conditions together had similar QOL. Among participants with HIV, those with active TB had lower physical QOL scores but similar mental QOL and health utility.
Others have described QOL12 and social stigma13 among Latin American populations with TB; we evaluated the simultaneous impact of TB and HIV on QOL. Unlike our study, which evaluated patients on TB treatment, Deribew et al. found that HIV-infected Ethiopian patients had lower QOL at the time of TB diagnosis.7 Further studies should describe QOL trajectories as symptoms resolve during the course of TB chemotherapy.
Although our population may not be representative of all persons treated for TB and HIV in Rio de Janeiro, our results are consistent with global estimates of QOL in TB and HIV. Our participants with HIV alone had similar physical and mental health to those of normative populations from industrialized countries.14 Furthermore, VAS-assessed QOL decrements closely matched corresponding standard disability weights: 0.259 vs. 0.264 for active TB, and 0.226 vs. 0.236 for treated HIV.11 In our population, HIV co-infection did not further reduce the QOL among people being treated for active TB. Evaluations of interventions (e.g., cost-effectiveness analyses) should not assume that the QOL decrement from TB-HIV coinfection is simply the sum of independent decrements from HIV and TB.
This study has important limitations. Our sample size (n = 98) was small, but was sufficient to exclude differences of large magnitude. For example, an additive effect of TB and HIV on QOL was excluded with 95% confidence. Our analysis was also cross-sectional in nature, precluding longitudinal assessment (e.g., how QOL improves during the course of TB or HIV treatment) and evaluation of mortality (e.g., those who die of TB are also likely to have poor QOL preceding death). We did not assess comorbidities, which may impact QOL, and we recruited patients through non-study personnel, limiting our ability to estimate non-response bias. Finally, the MOS-HIV does not consider important factors (e.g., social context, unique HIV support programs15) that may have a strong influence on QOL in Brazil and, as an HIV-specific instrument, may not fully describe TB-related influences on QOL.
In conclusion, we found similar QOL decrements among people being treated for HIV and active TB in five public health clinics in urban Brazil, with no evidence of an additive effect among people with TB-HIV co-infection. These findings provide a contextual baseline for evaluations of TB-HIV-specific interventions and their impact on QOL.
Footnotes
Conflict of interest: none declared.
References
- 1.World Health Organization Programme on Mental Health. Geneva, Switzerland: WHO; 1997. WHOQOL: measuring quality of life. WHO/MSA/MNH/PSF/97.4. [Google Scholar]
- 2.Wu AW, Hays RD, Kelly S, Malitz F, Bozzette SA. Applications of the Medical Outcomes Study health-related quality of life measures in HIV/AIDS. Qual Life Res. 1997;6:531–554. doi: 10.1023/a:1018460132567. [DOI] [PubMed] [Google Scholar]
- 3.Robberstad B, Olsen JA. The health-related quality of life of people living with HIV/AIDS in sub-Saharan Africa—a literature review and focus group study. Cost Effect Resource Alloc. 2010;8:5. doi: 10.1186/1478-7547-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo N, Marra F, Marra CA. Measuring health-related quality of life in tuberculosis: a systematic review. Health Qual Life Outcomes. 2009;7:14. doi: 10.1186/1477-7525-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang B, Wu AW, Hansel NN, Diette GB. Quality of life in tuberculosis: a review of the English language literature. Qual Life Res. 2004;13:1633–1642. doi: 10.1007/s11136-004-0374-1. [DOI] [PubMed] [Google Scholar]
- 6.Babikako HM, Neuhauser D, Katamba A, Mupere E. Feasibility, reliability and validity of health-related quality of life questionnaire among adult pulmonary tuberculosis patients in urban Uganda: cross-sectional study. Health Qual Life Outcomes. 2010;8:93. doi: 10.1186/1477-7525-8-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deribew A, Tesfaye M, Hailmichael Y, et al. Tuberculosis and HIV co-infection: its impact on quality of life. Health Qual Life Outcomes. 2009;7:105. doi: 10.1186/1477-7525-7-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Durovni B, Cavalcante SC, Saraceni V, et al. The implementation of isoniazid preventive therapy in HIV clinics: the experience from the TB/HIV in Rio (THRio) Study. AIDS. 2010;24(Suppl 5):S49–S56. doi: 10.1097/01.aids.0000391022.95412.a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu AW, Revicki DA, Jacobson D, Malitz FE. Evidence for reliability, validity and usefulness of the Medical Outcomes Study HIV Health Survey (MOS-HIV) [Accessed November 2012];Qual Life Res. 1997 6:481–493. doi: 10.1023/a:1018451930750. http://www.jhsph.edu/mos-hiv/index.html. [DOI] [PubMed] [Google Scholar]
- 10.de Soarez PC, Castelo A, Abrao P, Holmes WC, Ciconelli RM. Brazilian-Portuguese translation and validation of the HIV/AIDS-targeted quality of life instrument. Rev Panam Salud Publica. 2009;25:69–76. doi: 10.1590/s1020-49892009000100011. [Spanish] [DOI] [PubMed] [Google Scholar]
- 11.Murray CJL, Lopez AD. Boston, MA, USA: Harvard School of Public Health (on behalf of the World Health Organization and the World Bank); 1996. The global burden of disease: a comprehensive assessment of mortality and disability from diseases, injuries, and risk factors in 1990 and projected to 2020. [Google Scholar]
- 12.Fernandez-Plata Mdel R, Garcia-Sancho MC, Perez-Padilla JR. A case-control study of the impact of tuberculosis on the quality of life of patients. Rev Invest Clin. 2011;63:39–45. [PubMed] [Google Scholar]
- 13.Macq J, Solis A, Martinez G, Martiny P, Dujardin B. An exploration of the social stigma of tuberculosis in five ‘municipios’ of Nicaragua to reflect on local interventions. Health Policy. 2005;74:205–217. doi: 10.1016/j.healthpol.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Revicki DA, Sorensen S, Wu AW. Reliability and validity of physical and mental health summary scores from the Medical Outcomes Study HIV Health Survey. Med Care. 1998;36:126–137. doi: 10.1097/00005650-199802000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Okie S. Fighting HIV—lessons from Brazil. N Engl J Med. 2006;354:1977–1981. doi: 10.1056/NEJMp068069. [DOI] [PubMed] [Google Scholar]