Abstract
Mutations in ferroportin (Fpn) result in iron overload. We correlate the behavior of three Fpn mutants in vitro with patients’ phenotypes. Patients with Fpn mutations A77D or N174I showed macrophage iron loading. In cultured cells, FpnA77D did not reach the cell surface and cells did not export iron. Fpn mutant N174I showed plasma membrane and intracellular localization, and did not transport iron. Fpn mutation G80S was targeted to the cell surface and was transport competent, however patients showed macrophage iron. We suggest that FpnG80S represents a class of Fpn mutants whose behavior in vitro does not explain the patients’ phenotype.
Keywords: ferroportin, hemochromatosis, hepcidin, iron transport
HFE-related hemochromatosis (HC) is prevalent hereditary iron overload disorder in humans.1 Hereditary iron loading syndromes, due to mutations in other genes have, however, recently been reported. Ferroportin-associated iron overload (termed ferroportin disease) is increasingly recognized as a cause of hereditary hyperferritinemia.2 Ferroportin disease was recognized in 1999 as an autosomal dominant form of hereditary iron overload with unusually high reticuloendothelial iron stores and normal-low transferrin saturation.3 This pathologic phenotype was linked to the A77D mutation of ferroportin (Fpn)4 in 2001 as well as other Fpn mutations.5–11
Fpn is a transmembrane protein that exports iron in many tissues.12–14 Fpn is the receptor for hepcidin, a hormone produced by the liver in response to iron and inflammation. Hepcidin binds to Fpn resulting in Fpn internalization and degradation in lysosomes resulting in reduced iron egress.15 Patients with ferroportin disease present with either reticuloendothelial iron overload and relative plasma iron deficiency, consistent with a lack of iron export activity2 or increased transferrin saturation and parenchymal cell iron overload.16 Recent in vitro studies have shown that a subgroup of Fpn mutations expressed in cultured cells are hepcidin resistant and show increased rather than diminished iron export activity.17–20 The paucity of good clinical data on ferroportin disease has been an obstacle to understanding its pathogenesis, as pointed out by Liu et al.20 In this study we set out to characterize the biochemical and metabolic properties of Fpn mutants A77D, N174I, G80S in vitro and verify their clinical correlates in patients with ferroportin disease.
Design and Methods
Fpn mutations
We studied three human Fpn mutations: FpnA77D, FpnN174I and FpnG80S. The coding regions of the hemochromatosis (HFE), transferrin receptor 2, hemojuvelin and hepcidin genes were also analyzed in Fpn patients. Clinical data of the patients can be found elsewhere.21
Cells and media
Mouse Fpn cDNA was cloned into pEGFP-N1 (Clontech). This vector expresses Fpn as a fusion protein with a carboxyl terminal green fluorescent protein (GFP). All cell lines were maintained in Dulbecco’s minimal essential media (DMEM) with 10% fetal bovine serum and transfected with pFpn-EGFP-N1 or pFpn(mutations)-EGFP-N1 using Nucleofector (Amaxa, Gaithersburg, MD, USA). Mouse bone marrow macrophages were cultured as previously described22 and transfected using Nucleofector technology.
Generation of Fpn constructs
All human Fpn mutations were generated in pFpn-EGFP-N1 using a QuikChange Site-Directed Mutagenesis Kit (Stratagene)TM.
Other procedures
Hepcidin was synthesized, iodinated and used in binding assays as described elsewhere.15 Fpn-GFP expressing cells were solubilized in 150 mM NaCl/10 mM EDTA/10 mM Tris, pH 7.4/1% Triton X-100/protease inhibitor mixture (Roche Applied Science) and samples analyzed by SDS-PAGE followed by western blotting19 using rabbit anti-GFP (1:10,000, Abcam #ab6556) or goat anti-human actin (1:1,000 Santa Cruz Biotechnology) followed by either peroxidase-conjugated goat anti-rabbit IgG (1:12,500, Jackson ImmunoResearch Labs) or peroxidase- conjugated donkey anti-goat IgG (1:5,000, Santa Cruz Biotechnology). Densitometric analysis was performed using Biorad FluorMax with Quantity One software. For ferritin analysis, cells expressing GFP only, Fpn-GFP or mutant Fpn-GFP were incubated with 10 μM ferric ammonium citrate (FAC) for 24 hours, harvested and ferritin content determined by enzyme-linked immunosorbent assay (ELISA) (Laguna Scientific). All western blots were normalized for protein using the bicinchoninic acid assay (Pierce).
Results and Discussion
Clinical data
Clinical and biochemical data of patients carrying the FpnA77D mutation have been reported and discussed previously.3,4,21 Patients present with high serum ferritin and low transferrin saturation. Transferrin saturation increases with age, with values above 50% in older subjects.3,4 We studied six patients with FpnG80S and three patients with FpnN174I (Table 1). All these patients were negative for mutations in HFE, transferrin receptor 2, hemojuvelin and hepcidin genes. Perls’ Prussian blue liver staining showed iron accumulation in Kupffer cells in subjects with A77D, G80S and N174I mutations (data not shown).
Table 1.
Biochemical parameters in patients with the ferroportin disease.
| Patient | Age (years) | Sex (M/F) | Hemoglobin g/dL | Transferrin saturation (%) | Serum ferritin g/L | Ferroportin mutation |
|---|---|---|---|---|---|---|
| Normal | M/F | 12–18 | 20–50 | 12–300 | none | |
| 1. | 66 | F | 14.9 | 48 | 5815 | N174I |
| 2. | 38 | F | 13.4 | 45 | 5430 | N174I |
| 3. | 45 | M | 14.7 | 39 | 3200 | N174I |
| 4. | 34 | M | 15.8 | 60 | 4420 | G80S |
| 5. | 52 | M | 16.1 | 42 | 1540 | G80S |
| 6. | 55 | M | 14.8 | 30 | 2309 | G80S |
| 7. | 51 | F | 12.5 | 27 | 1727 | G80S |
| 8. | 17 | M | 15.9 | 23 | 1122 | G80S |
| 9. | 57 | M | 14.4 | 34 | 1434 | G80S |
Clinical data associated with iron overload disorder due to unique mutations in FPN. Hemoglobin, transferrin saturation and serum ferritin levels were determined as previously described.3
Subcellular localization of mutant Fpn
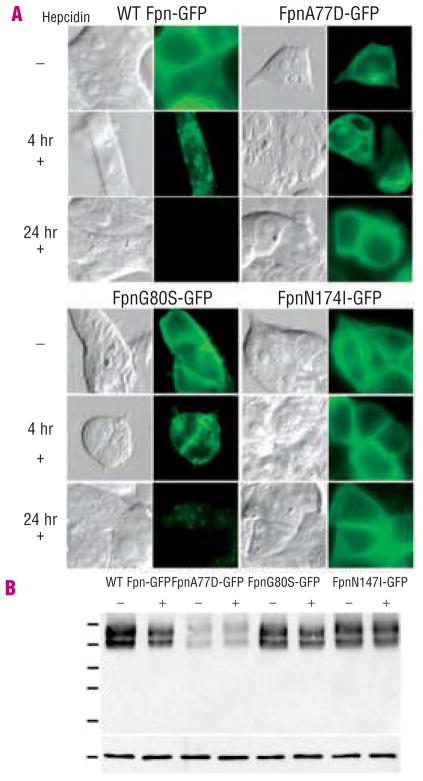
Mouse and human Fpn are highly conserved (90% identity) and we previously showed that mouse Fpn-GFP was functional for iron export when expressed in human cells.15,19 We generated known human Fpn mutations in mouse Fpn-GFP expressed under the control of the cytomegalovirus promoter, transfected cultured HEK293T cells and examined Fpn-GFP cellular localization. Wild type Fpn localized to the cell surface (Figure 1A, Fpn-GFP). Fpn mutant A77D was predominantly intracellular (Figure 1A, FpnA77D-GFP). Fpn mutant G80S showed cell surface localization (Figure 1A, FpnG80S-GFP). FpnN174I-GFP showed both intracellular and cell surface localization (Figure 1A, FpnN174I-GFP). The subcellular distribution of the Fpn mutants was unaffected by the type of cells transfected (data not shown).
Figure 1.
Human Fpn mutations affect Fpn localization and hepcidin-induced internalization. A. HEK293T cells were transiently transfected with plasmids containing wild type (WT) Fpn-GFP, FpnA77D-GFP, FpnG80S-GFP or FpnN174I-GFP. Eighteen to 24 h after transfection, localization of Fpn-GFP and response to hepcidin were assessed by epifluorescent microscopy. Cells were incubated with or without 1 μg/mL hepcidin for 4 and 24 h to assess hepcidin response. B. 18–24 h after transfection cells were incubated with or without 1 μg/mL hepcidin for 4 h, and extracts were analyzed by western blot analysis using antibody to GFP and actin as a loading control, as described in the Design and Methods.
Response of mutant Fpn to hepcidin
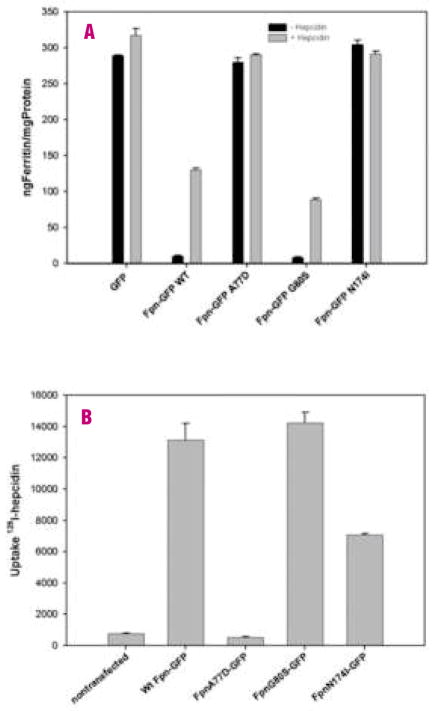
Addition of hepcidin to cells expressing wild type Fpn-GFP results in the internalization and degradation of Fpn-GFP. In a 4-hour incubation with hepcidin, most of the wild type Fpn was internalized. FpnA77D-GFP did not show any response to hepcidin. Some FpnG80S-GFP remained on the cell surface after 4 hours of hepcidin (Figure 1A). After 24-hours most of the mutant G80S Fpn had been internalized. Fpn N714I-GFP localization remained unchanged after incubation with hepcidin. Western blot analysis showed that N174I and G80S were expressed at concentrations comparable to wild type Fpn (Figure 1B), whereas FpnA77D-GFP expression was lower. Sequence analysis showed that the decreased expression observed in FpnA77D-GFP could not be ascribed to incidental mutations in the coding sequence or the promoter and may reflect the stability of the FpnA77D protein. Western blot analysis of cells expressing Fpn-GFP detected two bands associated with Fpn-GFP. The two Fpn-GFP bands were observed independently of the Fpn mutants introduced. Fpn-GFP has a predicted molecular mass of 97kDa. The lower band may represent a degradation product of Fpn-GFP. Densitometric analysis of Fpn-GFP westerns blots showed 77% of wild type Fpn-GFP degraded after 4-hours of treatment with hepcidin while Fpn A77D showed a 14% reduction in protein levels, Fpn G80S showed a 66% reduction and Fpn mutant N174I showed a 10% decrease in Fpn. Cells incubated in high iron increase cytosolic iron and accumulate the iron storage protein ferritin (Figure 2A, GFP black bars). Expression of Fpn-GFP decreased cytosolic iron and ferritin levels, even in the presence of iron-containing media (Figure 2A, WT Fpn-GFP). Cells expressing FpnA77D-GFP had high levels of ferritin. Expression of FpnG80S-GFP resulted in ferritin levels similar to that of cells expressing wild type Fpn-GFP. In cells expressing FpnN174I-GFP, ferritin levels were high suggesting a defect in iron transport. This is the first report of an Fpn mutant protein that localizes to the cell surface but does not export iron.
Figure 2.
Fpn mutations affect intracellular ferritin levels and 125I-hepcidin uptake. A. HEK293T cells were transiently transfected with plasmids containing wild type (WT) Fpn-GFP, FpnA77D-GFP, FpnG80S-GFP or FpnN174I-GFP. Eighteen hours after transfection, cells were cultured with ferric ammonium citrate (FAC) (20 μM iron) for 24 h. Cells were incubated with 100 μM cycloheximide for 1 h followed by 1 μg/mL hepcidin for 4 h. Ferritin levels were determined by ELISA and normalized to total protein concentration. Error bars represent the standard error of the mean of three independent experiments. B. Eighteen hours after transfection, 125I-hepcidin was added to HEK293T and 125I-hepcidin uptake measured as described previously.15,19 Error bars represent the average of three separate experiments in triplicate (n=9).
Addition of hepcidin to cells expressing wild type Fpn resulted in an increase in ferritin (Figure 2A gray bars). FpnA77D cells showed no change in ferritin levels in response to hepcidin. Cells expressing FpnG80S showed a modest increasein ferritin after incubation with hepcidin, although the increase was less than that observed for wild type Fpn-GFP cells (p<0.01). The decreased response to hepcidin could indicate impaired hepcidin binding or an altered response subsequent to hepcidin binding. To distinguish between these possibilities we measured the binding of 125I-hepcidin to cells expressing wild type or Fpn mutants (Figure 2B). Cells expressing FpnA77D-GFP did not bind 125I-hepcidin. Cells expressing FpnG80S-GFP bound 125I-hepcidin similarly to wild type but FpnN174I-GFP-expressing cells bound 125I-hepcidin less efficiently.
Ferroportin is an iron exporter, expressed in macrophages recycling iron from senescent erythrocytes, enterocytes absorbing dietary iron and hepatocytes which store iron.23 The concentration of Fpn on the cell surface is controlled by hepcidin. Hepcidin binds to Fpn, causing its internalization and degradation thus blocking cellular iron efflux.15 Autosomal-dominant mutations in Fpn result in iron overload with heterogeneous phenotypes.2 Studies suggest that Fpn mutations fall into two classes: mutant Fpn molecules that fail to reach the plasma membrane or mutant Fpn that is capable of exporting iron but is resistant to hepcidin-mediated down-regulation.17–19 This latter class of mutations contradicts the paradigm that Fpn-related iron overload or ferroportin disease is always due to loss of protein function. This class of Fpn mutations suggests that resistance to hepcidin could result in high iron egress from the intestine and macrophages, increased transferrin saturation and progressive parenchymal cell iron overload.
Here we describe the in vitro behavior of two Fpn mutants and a previously described FpnA77D mutant, and correlate these observations with clinical findings. All patients showed the ferroportin disease phenotype with macrophage iron loading, but the three Fpn mutants showed striking differences when expressed in cultured cells. Fpn A77D and N174I were unable to export iron consistent with the patients’ phenotype of increased macrophage iron retention. That Fpn N174I leads to intra-cellular iron accumulation was surprising because approximately half of Fpn N174I-GFP was targeted to the cell surface. Our data suggest that Fpn mutant N174I is transport-incompetent. Decreased function of Fpn is limiting for macrophage iron export but not for intestinal iron export.4,15
Fpn G80S-GFP was expressed at the cell surface and exported iron at levels similar to those of wild type Fpn. FpnG80S-GFP showed a slower rate of internalization compared to Fpn-GFP. Fpn mutation Q182H, also showed a slower rate of hepcidin-mediated Fpn internalization.19 Fpn mutations have been described that are not internalized in response to hepcidin, and patients with these mutations show hepatocyte ir loading. Surprisingly, patients with FpnG80S were almost indistinguishable from those with Fpn A77D or N174I Fpn. The extent of transferrin saturation in patients carrying the G80S mutation was inappropriately low compared with the levels of serum ferritin (Table 1). The discrepancy between the in vitro findings and the clinical phenotypes was not due to the specific cellular context. It is possible that in vitro expression of Fpn regulated by the cytomegalovirus promoter (constitutive high expression) obscures trafficking defects that are seen when Fpn is expressed at endogenous levels. Published studies suggest that in macrophages, Fpn may predominantly localize to an intracellular compartment and is targeted to the cell surface upon iron loading.24 This translocation step may not be appropriately modeled in non-macrophage cell lines or even in macrophages overexpressing Fpn. This study clearly demonstrates that several mechanisms may lead to abnormal trafficking and/or function of Fpn.
Acknowledgments
Funding: This work was supported by NIH grant DK70947 to J.K and Telethon grant #GGP030308 to A.P.
Footnotes
IDD, DMW: conducted experiments and wrote the manuscript; EN: conducted experiments; TG: wrote the manuscript; EC: trans-ferrin and liver biopsies; FF: transferrin and liver biopsies; GM: wrote the manuscript; AP: patient profiles and wrote the manuscript; JK: wrote the manuscript.
References
- 1.Feder JN, Gnirke A, Thomas W, Tsuchihashi Z, Ruddy DA, Basava A, et al. A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis [see comments] Nat Genet. 1996;13:399–408. doi: 10.1038/ng0896-399. [DOI] [PubMed] [Google Scholar]
- 2.Pietrangelo A. The ferroportin disease. Blood Cells Mol Dis. 2004;32:131–8. doi: 10.1016/j.bcmd.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Pietrangelo A, Montosi G, Totaro A, Garuti C, Conte D, Cassanelli S, et al. Hereditary hemochromatosis in adults without pathogenic mutations in the hemochromatosis gene. N Engl J Med. 1999;341:725–32. doi: 10.1056/NEJM199909023411003. [DOI] [PubMed] [Google Scholar]
- 4.Montosi G, Donovan A, Totaro A, Garuti C, Pignatti E, Cassanelli S, et al. Autosomal-dominant hemochromatosis is associated with a mutation in the ferroportin (SLC11A3) gene. J Clin Invest. 2001;108:619–23. doi: 10.1172/JCI13468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Njajou OT, de Jong G, Berghuis B, Vaessen N, Snijders PJ, Goossens JP, et al. Dominant hemochromatosis due to N144H mutation of SLC11A3: clinical and biological characteristics. Blood Cells Mol Dis. 2002;29:439–43. doi: 10.1006/bcmd.2002.0581. [DOI] [PubMed] [Google Scholar]
- 6.Devalia V, Carter K, Walker AP, Perkins SJ, Worwood M, May A, et al. Autosomal dominant reticuloendothelial iron overload associated with a 3-base pair deletion in the ferroportin 1 gene (SLC11A3) Blood. 2002;100:695–7. doi: 10.1182/blood-2001-11-0132. [DOI] [PubMed] [Google Scholar]
- 7.Wallace DF, Pedersen P, Dixon JL, Stephenson P, Searle JW, Powell LW, et al. Novel mutation in ferroportin 1 is associated with autosomal dominant hemochromatosis. Blood. 2002;100:692–4. doi: 10.1182/blood.v100.2.692. [DOI] [PubMed] [Google Scholar]
- 8.Roetto A, Merryweather-Clarke AT, Daraio F, Livesey K, Pointon JJ, Barbabietola G, et al. A valine deletion of ferroportin 1: a common mutation in hemochromastosis type 4. Blood. 2002;100:733–4. doi: 10.1182/blood-2002-03-0693. [DOI] [PubMed] [Google Scholar]
- 9.Cazzola M, Cremonesi L, Papaioannou M, Soriani N, Kioumi A, Charalambidou A, et al. Genetic hyperferritinaemia and reticuloendothelial iron overload associated with a three base pair deletion in the coding region of the ferroportin gene (SLC11A3) Br J Haematol. 2002;119:539–46. doi: 10.1046/j.1365-2141.2002.03946.x. [DOI] [PubMed] [Google Scholar]
- 10.Hetet G, Devaux I, Soufir N, Grand-champ B, Beaumont C. Molecular analyses of patients with hyperferritinemia and normal serum iron values reveal both L ferritin IRE and 3 new ferroportin (slc11A3) mutations. Blood. 2003;102:1904–10. doi: 10.1182/blood-2003-02-0439. [DOI] [PubMed] [Google Scholar]
- 11.Courselaud B, Pigeon C, Inoue Y, Inoue J, Gonzalez FJ, Leroyer P, et al. C/EBPα regulates hepatic transcription of hepcidin, an antimicrobial peptide and regulator of iron metabolism. Cross-talk between C/EBP pathway and iron metabolism. J Biol Chem. 2002;277:41163–70. doi: 10.1074/jbc.M202653200. [DOI] [PubMed] [Google Scholar]
- 12.Donovan A, Brownlie A, Zhou Y, Shepard J, Pratt SJ, Moynihan J, et al. Positional cloning of zebrafish ferroportin 1 identifies a conserved vertebrate iron exporter. Nature. 2000;403:776–81. doi: 10.1038/35001596. [DOI] [PubMed] [Google Scholar]
- 13.McKie AT, Marciani P, Rolfs A, Brennan K, Wehr K, Barrow D, et al. A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol Cell. 2000;5:299–309. doi: 10.1016/s1097-2765(00)80425-6. [DOI] [PubMed] [Google Scholar]
- 14.Abboud S, Haile DJ. A novel mammalian iron-regulated protein involved in intra-cellular iron metabolism. J Biol Chem. 2000;275:19906–12. doi: 10.1074/jbc.M000713200. [DOI] [PubMed] [Google Scholar]
- 15.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–3. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 16.Sham RL, Phatak PD, West C, Lee P, Andrews C, Beutler E. Autosomal dominant hereditary hemochromatosis associated with a novel ferroportin mutation and unique clinical features. Blood Cells Mol Dis. 2005;34:157–61. doi: 10.1016/j.bcmd.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Drakesmith H, Schimanski LM, Ormerod E, Merryweather-Clarke AT, Viprakasit V, Edwards JP, et al. Resistance to hepcidin is conferred by hemochromatosis-associated mutations of ferroportin. Blood. 2005;106:1092–7. doi: 10.1182/blood-2005-02-0561. [DOI] [PubMed] [Google Scholar]
- 18.Schimanski LM, Drakesmith H, Merryweather-Clarke AT, Viprakasit V, Edwards JP, Sweetland E, et al. In vitro functional analysis of human ferroportin (FPN) and hemochromatosis-associated FPN mutations. Blood. 2005;105:4096–102. doi: 10.1182/blood-2004-11-4502. [DOI] [PubMed] [Google Scholar]
- 19.De Domenico I, Ward DM, Nemeth E, Vaughn MB, Musci G, Ganz T, et al. The molecular basis of ferroportin-linked hemochromatosis. Proc Natl Acad Sci USA. 2005;102:8955–60. doi: 10.1073/pnas.0503804102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu XB, Yang F, Haile DJ. Functional consequences of ferroportin 1 mutations. Blood Cells Mol Dis. 2005;35:33–46. doi: 10.1016/j.bcmd.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Corradini E, Montosi G, Ferrara F, Caleffi A, Pignatti E, Barelli S, et al. Lack of enterocyte iron accumulation in the ferroportin disease. Blood Cells Mol Dis. 2005;35:315–8. doi: 10.1016/j.bcmd.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 22.Perou CM, Kaplan J. Chediak-Higashi syndrome is not due to a defect in micro-tubule-based lysosomal mobility. J Cell Sci. 1993;106:99–107. doi: 10.1242/jcs.106.1.99. [DOI] [PubMed] [Google Scholar]
- 23.Donovan A, Lima CA, Pinkus JL, Pinkus GS, Zon LI, Robine S, et al. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 2005;1:191–200. doi: 10.1016/j.cmet.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 24.Knutson MD, Oukka M, Koss LM, Aydemir F, Wessling-Resnick M. Iron release from macrophages after erythrophagocytosis is up-regulated by ferroportin 1 overexpression and down-regulated by hepcidin. Proc Natl Acad Sci USA. 2005;102:1324–8. doi: 10.1073/pnas.0409409102. [DOI] [PMC free article] [PubMed] [Google Scholar]