Abstract
OBJECTIVE
Decline in visuospatial abilities with advancing age has been attributed to a demise of bottom-up and top-down functions involving sensory processing, selective attention, and executive control. These functions may be differentially affected by age-related volume shrinkage of subcortical and cortical nodes subserving the dorsal and ventral processing streams and the corpus callosum mediating interhemispheric information exchange.
METHOD
55 healthy adults (25–84 years) underwent structural MRI and performed a visual search task to test perceptual and attentional demands by combining feature-conjunction searches with ‘gestalt’ grouping and attentional cueing paradigms.
RESULTS
Poorer conjunction, but not feature, search performance was related to older age and volume shrinkage of nodes in the dorsolateral processing stream. When displays allowed perceptual grouping through distractor homogeneity, poorer conjunction-search performance correlated with smaller ventrolateral prefrontal cortical and callosal volumes. An alerting cue attenuated age effects on conjunction search, and the alertness benefit was associated with thalamic, callosal, and temporal cortex volumes.
CONCLUSION
Our results indicate that older adults can capitalize on early parallel stages of visual information processing, whereas age-related limitations arise at later serial processing stages requiring self-guided selective attention and executive control. These limitations are explained in part by age-related brain volume shrinkage and can be mitigated by external cues.
Keywords: Aging, neuroimaging, regional brain volumes, single-feature and feature-conjunction search, perceptual grouping, attentional cueing
INTRODUCTION
It can be particularly challenging for older people to find small personal items such as keys or glasses, because the environment is typically cluttered with many potential targets as they look for specific items. Processes involved in such visual search include alertness, perceptual grouping, visuospatial attention, and executive control, which are associated with thalamofrontal, ventral frontotemporal, and dorsal frontoparietal brain networks (Hopf, Boelmans, Schoenfeld, Luck, and Heinze, 2004; Kellermann et al., 2011; Schulte et al., 2009; Sturm et al., 2006; Xu et al., 2010). Regional gray matter volume shrinkage that occurs with advancing age (Benedetti et al., 2006; Burzynska et al., 2011; Driscoll et al., 2009; Jernigan et al., 2001; Peelle, Cusack, and Henson, 2012; Pfefferbaum et al., 1994; Raz, Rodrigue, and Haacke, 2007; Sigurdsson et al., 2011; Sullivan, Rosenbloom, Serventi, and Pfefferbaum, 2004; Taki et al., 2011) may provide neural substrate correlates for functional declines in the elderly involving activities of daily living requiring visual search.
Visual search comprises both single-feature and feature-conjunction elements. When search is defined by a single feature (e.g., looking for something red), target detection time is largely unaffected by visual load (set size). That is, the number of distracting objects in a display does not affect single-feature detection because the salient feature “pops out” of its surrounding and involuntarily captures attention. Thus, feature search is assumed to rely on spatially parallel, preattentive processing mechanisms (Treisman and Gelade, 1980) and thought to be resistant to age-related deterioration (Plude and Doussard-Roosevelt, 1989; Burton-Danner, Owsley, and Jackson, 2001). Brain regions that have been associated with single-feature search comprise primary visual and visual association areas in occipitotemporal cortices and subcortical regions including superior colliculus in the midbrain and the pulvinar in the thalamus (Anderson and Rees, 2011; Corbetta, Miezin, Shulman, and Petersen, 1991; Himmelbach, Erb, and Karnath, 2007; Peelen and Kastner, 2011; Shipp, 2004).
In contrast with single-feature search, conjunction search, defined by the combination of two or more primary visual features such as color and form (e.g., looking for something red and round), requires focused attention to integrate features for target identification among distractors (e.g., red and triangular, or yellow and round). Conjunction search is affected by visual load, because attention is directed selectively and serially from one item to the next (Treisman and Gelade, 1980), and can decline with normal aging (Folk and Lincourt, 1996; Madden, Gottlob, and Allen, 1999; Plude and Doussard-Roosevelt, 1989; but see Kramer, Martin-Emerson, Larish, and Andersen 1996). Conjunction search is influenced by distractor homogeneity that allows perceptual grouping, i.e., distractors that share the same features can be grouped via preattentive mechanisms that are receptive to the gestalt property of similarity (Duncan and Humphreys, 1989; Wilkinson, Halligan, Henson, and Dolan, 2002).
McMains and Kastner (2011) recently studied the effects of this bottom-up influence on neural competition by parametrically varying the degree of perceptual grouping in displays. Their findings suggest that the strength of attentional modulation in the visual system is constrained by the degree to which distractor competition can be resolved by bottom-up processes related to the segmentation of scenes into candidate objects. Because younger subjects typically benefit from distractor homogeneity in conjunction search more and show faster reaction times than older subjects, it has been assumed that elderly have difficulties in both perceptual grouping (bottom-up processes) and inhibition of distractors (top-down processes) (Gilmore, Tobias, and Royer, 1985; Hommel, Li, and Li, 2004; Humphrey and Kramer, 1997; Madden, Pierce, and Allen, 1996, Scialfa, Esau, and Joffe, 1998). However, the effect of aging and age-related regional brain volume shrinkage on the interaction of automatic attentional processes (“pop-out” feature search, bottom-up), perceptual grouping in conjunctions search (interaction of bottom-up and top-down), and selective attention (conjunction search, top-down) processes has not been systematically investigated.
We developed a paradigm that allows systematic modulation of task demands at a perceptual level and an attentional control level. Combining visual conjunction search with attentional cueing tested top-down attentional control mechanisms. Thus, we tested selective attentional control by means of visual load in conjunction search (vs. feature search) and by means of the validity effect, i.e., longer responses to invalidly than validly-cued trials, which reflects the ability to disengage and shift attention, a mechanism associated with temporo-parietal junction (TPJ) and inferior parietal lobe (IPL) functions (Posner and Petersen, 1990). Bottom-up preattentive processing in aging was tested with single feature “pop-out” search, and its role in conjunction search was explored via distractor grouping and phasic alertness, i.e., capability to enhance response readiness following a warning stimulus (Sokolov, 1963; Posner, 2008). Alertness has been associated with activation of thalamic brain circuits (Coull, Frith, Büchel, and Nobre, 2000; Kellermann et al., 2011; Sturm and Willmes, 2001) that are partly overlapping with nodes of the frontoparietal orienting and executive control network (Fernandez-Duque and Posner, 1997; Harter and Guido, 1980; Shipp, 2004). For example, in a functional MRI study, Kellermann et al. (2011) found thalamic and prefrontal activation in response to an alerting cue.
Our overarching hypotheses guided examination of the relations among age, brain volume, and cognition: (1) Older age would be associated with slower processing speed and smaller brain tissue volumes. Age-related regional brain volumes shrinkage would differentially affect component visual search processes: dorsal frontoparietal volumes for top-down attentional control, ventral frontotemporal volumes for stimulus identification and perceptual grouping, thalamofrontal volumes for cue-induced response readiness (phasic alertness), and callosal volume for interhemispheric integration of information from both visual hemifields (Davis, Kragel, Madden and Cabeza, 2011; Müller-Oehring, Schulte, Raassi, Pfefferbaum, and Sullivan, 2007). (2) Older age would be associated with a specific pattern of sparing and impairment of component visual search processes: sparing of bottom-up processes as they relate to saliency in single-feature pop-out search, and impairment of self-guided selective attention and executive control processes in conjunction search, with less difficulty when displays allow perceptual grouping. (3) Older adults would profit from external attentional guidance by visual cues.
METHODS
2.1. Participants
The study sample comprised 55 healthy adults (30 women, 25 men), age 25 to 84 years. The overall mean age was 52 ± 16 years; women and men did not differ in age (women: 54 ± 17 years, men: 50 ± 15 years, t(53) = .96, ns). Table 1 presents descriptive statistics of the subjects including handedness (Crovitz and Zener, 1962), years of education, socioeconomic status (SES), and verbal intelligence (ANART IQ, American National Adult Reading Test; Grober and Sliwinski, 1991). SES was determined using a 2-factor scale that includes both education and lifetime occupation (Hollingshead and Redlich, 1958) and can be considered a representative measure of the highest functioning achieved (Sassoon et al., 2007). Verbal intelligence, commonly considered a measure of premorbid intelligence in clinical studies, can be used in aging studies for group description of highest cognitive ability in addition to demographic variables SES and education. All participants underwent color vision (Ishihara, 1917) and visual acuity screening (Freiburg Visual Acuity and Contrast Test – FrACT; Bach, 1996). None of the included subjects was color-blind, and subjects were tested with normal or corrected-to-normal vision. All participants received a Structural Clinical Interview for DSM-IV diagnosis (American Psychiatric Association, 2001) by trained clinicians to rule out psychiatric and neurological disease. Written informed consent was obtained from all participants, and the Institutional Review Boards of SRI International and Stanford University approved the study.
Table 1.
Demographic data of the study group
| N | Women/ Men | Age (years) | Age range | Education (years) | SES | Handedness | Verbal IQ ANART | |
|---|---|---|---|---|---|---|---|---|
| ALL | 55 | 30/25 | 52 (±16) | 25 – 84 | 16 (±2.5) | 25 (±11) | 22 (±11.5) | 117 (±9.4) |
|
| ||||||||
| 1.5 T | 41 | 23/18 | 51 (±17) | 25 – 79 | 16 (±2.7) | 25 (±10) | 21 (±9.7) | 117 (±9.1) |
| 3 T | 10 | 6/4 | 57 (±14) | 42 – 84 | 17 (±1.9) | 27 (±15.6) | 29 (±17.8) | 117 (±11.6) |
| t-tests, p < | ns1 | ns | ns | ns | ns | ns | ||
Demographic data for the study group of 55 healthy adults, both totals and separated by MRI acquisition at 1.5T vs. 3T field strength. SES: socioeconomic status, higher scores represent lower SES (range 11 – 77); handedness inventory: scores 14–32 right-handed, 50–70 left-handed. ANART: age-corrected IQ, American National Adult Reading Test. Note: MRI data were available from 51 of 55 participants.
Chi-square statistic
2.2. Stimuli and procedures
We devised a computerized visual search task that systematically manipulated perceptual and attentional demands. For perceptual manipulation, the task involved three complexity factors: single-feature search (target=red tomato; distractors=yellow tomatoes); color-form conjunction search (target=red tomato; distractors=yellow tomatoes and red or yellow strawberries); and two visual array types (organized and disorganized). In organized conjunction search displays, items were spatially grouped by color, whereas items in disorganized displays were spatially unevenly distributed and mixed in color-form conjunctions (Figure 1). Attentional demands varied by presence of a cue that was valid, neutral, or invalid in directing attention to the target location within disorganized color-form conjunction search arrays. The cue was a red square and appeared for 500 ms prior to the conjunction search display with an inter-stimulus interval of 100, 150, or 200 ms between cue and search display. The red square was presented at the location of 1) the target for valid trials, 2) a distractor for invalid trials, and 3) the fixation point for neutral conditions. Neutral conditions included search arrays with (target trials) and without targets (non-target trials). Each visual search task contained a visual load component of either 4 or 8 stimuli in a search array (Figure 1).
Figure 1.
Design of the visual search task with four conditions: feature search and organized, disorganized and cued conjunction search. For cued conjunction search for display purposes, cue and search display are shown here superimposed, whereas in the actual task the cue appeared 150 ms prior (and not simultaneously) to the search display. Specific effects were visual load calculated as the difference between high load (8 set size) and low load (4 set size) [visual load = 8 – 4 items], cue validity calculated as the difference between invalid and valid trials [validity = invalid –valid], with benefits for valid cueing calculated as the difference between neutral and valid trials [benefit = neutral – valid], cueing costs as the difference between invalid and neutral trials [cost = invalid – neutral], and alertness calculated for low and high visual-load conditions as the difference between uncued and neutrally-cued trials [alertness = no cue – neutral cue].
Stimuli were presented in four blocks of trials. In the feature search block, participants searched for a red tomato among yellow tomatoes. In the conjunction search blocks, participants searched for a red tomato among yellow tomatoes and red and yellow strawberries. Participants were instructed to identify the red tomato among distractors and pressed a YES button with the index finger of their dominant hand when a red tomato was present, and a NO button with the middle finger of the same hand when no target was displayed. Response buttons were the N and M keys on a regular computer keyboard that was aligned to the participants’ body midline. The visual search display stayed on the screen until a button was pressed, which initiated the next trial, to ensure sufficient processing time for older participants. As a result, the task was ‘self-timed’ meaning that the next trial n+1 would come up 500 ms after button press to trial n, and thus would differ for each trial and subject. Target and no-target trials were randomly intermixed. Reaction times (RTs) and errors were collected for each trial. Errors describe the number of incorrect responses (inverse of accuracy, the number of correct responses), i.e., when there was a target in the search display (red tomato) but the subject pressed the NO-button.
A total of 216 stimuli were presented. Feature, organized, and disorganized conjunction search tasks each comprised 48 stimuli with 12 stimuli in each of the four conditions. Cued conjunction search comprised 72 stimuli with a 3:1 ratio of valid and invalid trials resulting in 36 valid, 12 invalid, 12 neutral target, and 12 neutral non-target trials (Figure 1) (for valid-to-invalid trial ratio see Posner and Peterson, 1990). Participants performed short practice blocks of trials for each attention condition before testing.
Overall RT indexed search task difficulty. Mean RTs were calculated for each condition, and RTs > 3 SD from the individual’s mean were excluded from analyses. Specific visual search effects were visual load, cue validity, and alertness (see Figures 1 and 2) and were calculated as difference RT scores (diff RT) and Z-scores. RT difference values are direct measures of performance and provide information on the effect of an experimental manipulation in milliseconds, but can interact with task difficulty. To disentangle task difficulty from specific processing demands, we calculated Z-scores in addition to RT measures. Z-score transformation was obtained by taking each individual’s condition means, subtracting their overall mean, and dividing by the standard deviation of their condition means (Faust et al., 1999). Z-score transformation controlled for individual difference in overall response latency, i.e., processing rate, and for difference in condition difficulty (Faust et al., 1999), thereby allowing us to study age effects on specific visual search components such as visual load, validity, and alertness independent of response latency and task difficulty.
Figure 2.
Visual-load, validity and alertness effects: Z-transformed mean reaction times for A) feature search and organized, disorganized, and cued conjunction searches; and for B) valid, neutral, invalid and uncued conditions. Data were collapsed over target and non-target trials.
Visual load effects were calculated for feature and conjunction searches by subtracting performance measures (mean RT, Z-scores) on low-load displays (4 items) from those for high-load displays (8 items). Validity effects were calculated for cued conjunction-search displays by subtracting performance measures (mean RT, Z-scores) for valid trials from those for invalid trials; benefits from valid cueing by subtracting performance measures (mean RT, Z-scores) for valid trials from those for neutral trials, and costs from invalid cueing by subtracting performance measures (mean RT, Z-scores) for neutral trials from those for invalid trials. Alertness effects were calculated for disorganized conjunction-search displays for both high and low visual-load conditions by subtracting performance measures (mean RT, Z scores) for neutrally cued trials from those for uncued trials.
2.3. Cortical and Subcortical Tissue Quantification
Magnetic Resonance Imaging (MRI) acquisition protocol
MRI data were acquired on General Electric 1.5T and 3T clinical systems. Images were acquired using volumetric SPoiled Gradient Recalled (SPGR) sequences (1.5T scan parameters: 94, 2-mm-thick slices; skip=0 mm; TR=26 ms; TE=5 ms; flip angle=30°; matrix=256 × 192; 3T scan parameters: 124, 1.25-mm-thick slices; skip=0 mm; TR=6.5 ms; TE=1.54 ms; flip angle=15°; matrix=256 × 256) for morphometry and dual-echo fast spin-echo (FSE) sequences (1.5T scan parameters: 47, 4-mm-thick slices, skip=0 mm; TR=7150 ms; TE=17/85 ms; matrix=256 × 192; 3T scan parameters: 62, 2.5-mm-thick slices; skip=0 mm; TR=8585 ms; TE=17/102 ms; matrix=256 × 192) for automated fluid-tissue delineation and brain extraction. All images were read by a clinical neuroradiologist to identify space-occupying lesions or other dysmorphology indicative of neuropathology that could interfere with morphometric analysis. Additional review of images identified scans with quality too poor for quantification. Images from 51 of the 55 study participants were determined to be of sufficient quality for further analyses; of these, 41 scans were acquired at 1.5T and 10 scans at 3T. Subjects scanned at 1.5T did not differ in demographics from those scanned at 3T (Table 1).
MRI quantification
The same atlas-based parcellation procedure was applied to analyze both the 1.5T and 3T data (Pfefferbaum et al., 2012). Potential bias of the results due to field strength (e.g., related to different image resolution, SNR, or bias field effects) was dealt with at the statistical modeling level by modeling field strength as a nuisance variable (see description below). As previously described (Chanraud, Pitel, Rohlfing, Pfefferbaum, and Sullivan, 2010), a parcellated template was first created using an average of 24 normal controls spanning the adult age range (Rohlfing, Zahr, Sullivan, and Pfefferbaum, 2010). The template was semiautomatically parcellated using an already published description of 116 anatomical brain regions (Tzourio-Mazoyer et al., 2002). Structural volumes were estimated using the following protocol for each subject: (1) Intensity bias-field correction was performed separately on the SPGR and early-echo FSE image. The early-echo FSE bias field was also applied to the late-echo FSE image. (2) The bias-corrected early-echo FSE image was registered to the bias-corrected SPGR image. (3) The bias-corrected SPGR and early- and late-echo FSE images were then each passed independently through the FSL Brain Extraction Tool (Smith, 2002) to extract the brain and exclude dura, skull, scalp, and other non-brain tissue. The final brain mask for the SPGR data was then created from the three separate, co-registered channel brain masks using majority voting (i.e., a pixel was labeled ‘brain’ if two out of the three input masks labeled it as such). (4) The brain-extracted SPGR data were registered to the SPGR channel of the SRI24 atlas. (5) All atlas regions of interest (ROIs) were reformatted to subject SPGR coordinate space and resolution. (6) Local tissue volumes for regional statistics were based on a three-compartment segmentation (cerebrospinal fluid (CSF), gray matter (GM), and white matter (WM)) map of that subject created with FSL-FAST (Zhang et al., 2001) from the brain-extracted SPGR image. Gray matter, white matter, and CSF volume were determined for each reformatted atlas ROI in subject image space. For cortical volumes, we included the GM local tissue, except for the thalamus, striatum, and medial temporal ROIs, for which we included the total local tissue (GM+WM) in the analyses, because tissue contrast limitations precluded accurate separation of the two tissue types. For the corpus callosum ROIs, we included the WM local tissue in the analyses.
Brain regions of interest (ROIs)
For the dorsal processing stream, ROIs were the lateral parietal lobe (lPL: superior and inferior parietal lobe, supramarginal and angular gyri), the dorsolateral prefrontal cortex (dlPFC: superior and middle frontal gyri), and the medial prefrontal cortex (mPFC: anterior cingulate cortex and medial superior frontal gyrus). For the ventral processing stream, ROIs were visual association areas (VAA: lingual and fusiform gyri) in occipitotemporal regions, medial and lateral temporal lobes (mTL: hippocampus, parahippocampus, amygdala; lTL: superior and middle temporal gyri and pole), and ventrolateral prefrontal cortex (vlPFC: inferior frontal, orbitofrontal, and triangular gyri). Subcortical ROIs included the thalamus and the striatum (caudate nucleus, putamen). Interhemispheric ROIs were corpus callosum white matter regions connecting frontal (genu), parietal, and occipito-temporal (splenium) cortices between the two hemispheres (Figure 4). Units of analyses were z-transformed regional brain volumes that were corrected for supratentorial volume.
Figure 4.
Correlations between regional brain volumes (ROIs) and specific conjunction-search effects: Visual load for organized and disorganized displays, cue validity, and alertness for high load disorganized conjunction search conditions. Removal of outliers did not affect statistical significances.
2.4. Statistical analysis
Non-paired Student’s t-tests and χ2 tests were used for group comparison (men vs. women) of demographic data. RT analysis of visual search processing was based on correct responses only. First, analyses of variance (ANOVA) with repeated measures tested for load and task effects. The alpha significance level was set to 0.05 for all hypotheses tested. Secondly, relationships between age and regional brain volumes and with visual load, validity, and alertness were tested with Pearson correlations, which permitted age to be modeled as a continuous rather than discrete variable. We calculated significance values for multiple comparisons to control the false discovery rate (FDR) with alpha significance levels set at pFDR-corrected<0.05 (Benjamini, Drai, Elmer, Kafkafi, and Golani, 2001). Relationships involving age, brain volumes, and visual load and alertness were tested one-tailed, assuming smaller brain volumes and greater visual load and alertness effects with older age (SPSS 15.0).
RESULTS
3.1. Visual search performance – accuracy
Overall accuracy was high; for single-feature and organized-conjunction search number of errors committed ranged between 0 and 4 (mean ± SD feature search errors = 1.9 ± 2.4; organized-uncued conjunction search errors = 1.2 ± 1.9), for disorganized-uncued conjunction search between 0 and 2 errors (0.8 ± 1.2), and for disorganized-cued conjunction search between 0 and 5 errors (0.9 ± 1.3).
Despite the overall high accuracy, significantly more errors were committed during feature than organized-conjunction search, which in turn had more errors than cued and uncued disorganized-conjunction search (ANOVA; F(1,53)=8.84, p=0.004). Older age correlated moderately with greater accuracy, i.e., fewer errors, in the most difficult disorganized (uncued) conjunction search task (Rho=−.24, p=0.04). Accuracy and overall reaction time for each visual search task did not significantly correlate (all p>0.1), indicating an absence of a speed-accuracy trade-off.
3.2. Specific visual search effects – reaction time analyses
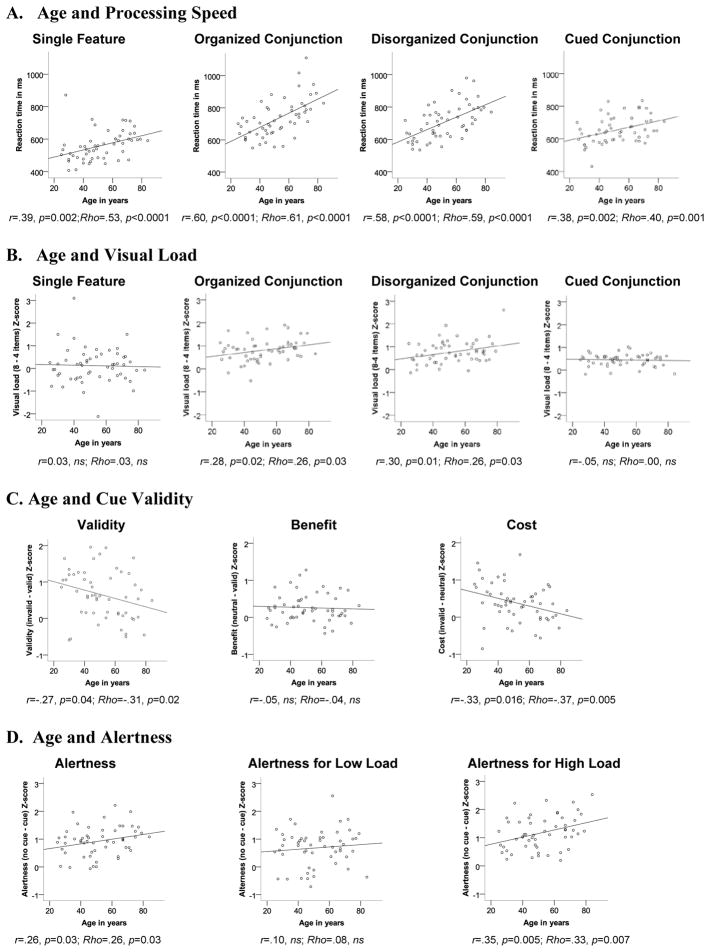
We first examined relationships between age and processing speed for each visual search task: Older age correlated with slower responses in all visual search tasks (Figure 3A).
Figure 3.
Correlation between age and processing speed (A) and visual search-specific effects: (B) visual load, (C) cue validity, and (D) alertness. Removal of outliers did not affect statistical significances.
3.2.1. Visual load
We tested the hypothesis that older age would be related to greater visual load effects during conjunction but not feature search, especially for disorganized rather than organized conjunction search displays, which would allow perceptual grouping of distractors.
We used Z-scores to control for the potential of disproportionate effects of age on task difficulty. This possibility was evident because greater difference RT scores (indexing visual load effects) correlated with higher overall RTs (indexing task difficulty) for conjunction searches (organized: r=.53, p<0.001; disorganized: r=.42, p=0.002), but not feature (r=.24, ns) or cued conjunction searches (r=.10, ns).
An ANOVA with search task (single-feature, organized, and disorganized conjunction), visual load (4-items, 8-items), and target (yes, no) as within-subjects factors revealed a significant 3-way interaction (F(1,54)=9.78, p=0.003). Longer RTs occurred in both conjunction search conditions than in the feature search condition (task main effect F(1,54)=168.47, p<0.0001) with high relative to low loads (load main effect F(1,54)=153.72, p<0.0001), and for non-target relative to target trials (target main effect F(1,54)=264.39, p<0.0001). Load effects were less pronounced in the feature search than in either conjunction search (load-by-task interaction F(1,54)=29.93, p<0.0001) and in target than non-target trials (load-by-target interaction F(1,54)=12.4, p=0.001) (Figure 2A). Target effects, i.e. faster responses to target than no-target displays, were less pronounced in the feature search than either conjunction search (task-by-target interaction F(1,54)=98.1, p<0.0001).
Following-up on significant task and load main effects with paired t-tests showed longer RTs for organized conjunction than feature search (t(54)=12.98, p<0.001), for disorganized conjunction than feature search (t(54)=10.8, p<0.001), and somewhat longer RTs for organized than disorganized conjunction search conditions (t(54)=2.58, p=0.013). Load effects, i.e., longer RTs for 8 than 4 item displays, were observed for organized (t(54)=12.48, p<0.001) and disorganized conjunction search (t(54)=11.47, p<0.001), but not for feature search (t(54)=1.2, ns).
Age and visual load
We tested the hypothesis that older age would affect the serial processing component of visual search. Specifically, older age would be related to greater visual load effects for conjunction but not for feature searches. We found a moderate correlation between older age and higher visual load Z-scores for organized (r=.28, p=0.02; Rho=.26, p=0.026) and disorganized conjunction searches (r=.30, p=0.012; Rho=.26, p=0.027), but not for feature or cued conjunction searches (Figure 3B).
3.2.2
We next tested the hypothesis that age-related difficulties in conjunction search would be minimized when the search was externally guided by a visual cue that modulated aspects of attention (1) by cue validity and (2) by an alerting (neutral) cue.
(1) Cue validity
We tested the hypothesis that visuospatial attention is modulated by cue validity with faster responses for validly cued trials and slower responses for invalidly-cued trials relative to neutrally-cued trials. An ANOVA with cue validity (valid, neutral, invalid) and visual load (4-items, 8-items) as within-subjects factors revealed a significant validity effect (F(1,53)=49.45, p<0.0001) with greatest Z-scores (longest RTs) for invalid-cue and smallest Z-scores (shortest RTs) for valid-cue conditions, with neutral-cue Z-scores falling in between. Significant load effects indicated longer RTs to 8-item than 4-item set sizes (F(1,53)=69.32, p<0.0001). A load-by-cue validity interaction (F(1,53)=26.08, p<0.0001) revealed greater load effects for invalidly than neutrally and validly cued target trials (Figure 2B).
Age and cue validity
We tested the hypothesis that all subjects regardless of age would show similar response time benefits from valid cueing and similar costs from invalid cueing. Although all subjects benefitted similarly from valid cueing (i.e., no correlation between age and cue benefit: r=−.05, ns; Rho=−.04, ns), we found a moderate correlation between older age and smaller cue-validity effects (Z-scores) (r=−.27, p=0.04; Rho=−.31, p=0.02), with lower cost from invalid cueing (r=−33, p=0.016; Rho=−.37, p=0.005) (Figure 3C).
(2) Alertness
For alertness effects, we tested whether responses would be faster to trials with a neutral cue than to trials with no cue at all. An ANOVA with alertness (neutral cue, no cue), visual load (4, 8), and target (yes, no) as within-subjects factors revealed an effect for alertness (F(1,53)=10.65, p=0.002), i.e., smaller Z-scores, from being alerted by the presence of a neutral cue than no cue (Figure 2B). An alertness-by-load interaction indicated greater benefit for high-load than low-load trials (F(1,53)=5.52, p=0.023). A significant load-by-target interaction (F(1,53)=26.68, p<0.0001) indicated greater load effects (F(1,53)=254.5, p<0.0001) for non-target than target trials (F(1,53)=583.2, p<0.0001).
Age and alertness
Older age correlated with greater alertness effects for the high visual load (r=.35, p=0.01; Rho=.33, p=0.014) but not the low visual load (r=.10, ns) (Figure 3D).
3.3. Age, visual search, and regional brain volumes
Here, we tested the hypothesis that older age would be associated with slower processing speed and smaller brain tissue volumes. Regional brain volumes would mediate age effects on component visual search processes: dorsal frontoparietal volumes for top-down attentional control, ventral frontotemporal volumes for stimulus identification and perceptual grouping, thalamofrontal volumes for cue-induced response readiness (phasic alertness), and callosal volume for interhemispheric integration of information. For the cognitive variables visual load, cue validity, and alertness, the units of analysis were difference RTs (diff RT) and Z-scores (Z) (Table 2). We used both diff RT and Z-scores to test whether slowed processing speed (overall reaction time, RT), as a sign of enhanced task difficulty and cognitive slowing with aging, contributes to the relationship between brain volume and specific visual search effects (visual load, validity, alertness). The null hypothesis was that processing speed does not influence the relationship between brain volume and specific effects. When structure-function relations are driven by cognitive slowing, significant correlations should emerge for difference RT scores but not Z-scores, whereas correlations for Z-scores indicate specificity for brain volume-specific effect relations over and above the effect of slowed processing.
Table 2.
Correlation of regional brain volumes with age and search task performance: overall RT, visual load, cue validity, and alertness
| Feature search | Conjunction search | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||||||
| Processing stream | ROIs | Age | ORGANIZED | DISORGANIZED | CUED | Alertness High load | ||||||||||||||
| RT | Load | RT | Load | RT | Load | RT | Load | Validity | Cost | |||||||||||
| Diff RT | Z | Diff RT | Z | Diff RT | Z | Diff RT | Z | Diff RT | Z | Diff RT | Z | Diff RT | Z | |||||||
| dorsal | lPL | −.72 | −.30 | −.56 | −.32 | −.54 | −.36 | −.28a | −.37 | .30a | .27a | .38a | −.28a | |||||||
|
| ||||||||||||||||||||
| dlPFC | −.71 | −.39 | −.53 | −.27 | −.50 | −.25 | −.28a | −38 | .30a | .35a | ||||||||||
|
| ||||||||||||||||||||
| mPFC | −.64 | −.32a | −.46 | −.25 | −.43 | −.34 | −.25a | −.38 | .28a | .29a | .31a | .29a | ||||||||
|
| ||||||||||||||||||||
| ventral | VAA | −.35 | −.34 | −.25 | −.28 | −.27a | ||||||||||||||
|
| ||||||||||||||||||||
| lTL | −.64 | −.44 | −.28 | −.40 | −.25 | −.31 | −.26a | |||||||||||||
|
| ||||||||||||||||||||
| mTL | −.28 | −.35 | −.29 | −.29 | −.42 | −.33a | ||||||||||||||
|
| ||||||||||||||||||||
| vlPFC | −.60 | −.25a | −.56 | −.36 | −.25a | −.45 | −.26 | .31a | ||||||||||||
|
| ||||||||||||||||||||
| subcortical | striatum | −.37 | −.25a | −.38 | −.42 | −.30 | −.24a | −.28 | −.25a | |||||||||||
|
| ||||||||||||||||||||
| thalamus | −.56 | −.24a | −.52 | −.33 | −.48 | −.34 | .29a | −.31 | −.31a | |||||||||||
|
| ||||||||||||||||||||
| corpus callosum | splenium | −.40 | −.29a | −.38 | −.34 | −.35 | −.29a | −.38 | −.27a | |||||||||||
|
| ||||||||||||||||||||
| parietal | −.46 | −.35 | −.31 | −.38 | −.28 | −.29 | −.31a | |||||||||||||
|
| ||||||||||||||||||||
| genu | −.47 | −.32a | −.38 | −.29 | −.36 | −.32 | −.34 | −.52 | ||||||||||||
Pearson’s correlation coefficients; significance level was pFDR-corrected<0.05 corrected for multiple comparisons by controlling the false discovery rate (FDR) and, for additional information, values significant at puncorrected<0.05 are marked with an a; N=51.
Correlation values and significances held with partial correlations using MRI scanner (1.5T, 3T) as covariate; only the correlation between VAA volume / feature load (Diff RT) attenuated to r=−.23, puncorrected=0.05, and the correlation between mPFC volume / disorganized conjunction load (Z score) attenuated to r=−.22, puncorrected=0.06.
Abbreviations: RT=reaction time; Diff RT=difference RT value; Z=Z-transformed RT score; ROI= region of interest; VAA=visual association area, TL= temporal lobe, PL=parietal lobe, PFC=prefrontal cortex, l=lateral, dl=dorsolateral, vl=ventrolateral, m=medial.
Age and ROI volumes
Older age was associated with smaller gray and white matter volumes in all ROIs measured (Table 2).
Processing speed and ROI volumes
Higher RTs in feature and conjunction search conditions were associated with smaller brain volumes (Table 2).
Visual load and ROI volumes
Relationships emerged for conjunction search (Table 2 and Figure 4): For organized displays, greater visual-load effects (diff RT and Z-scores) were related to smaller volumes in the parietal sector of the corpus callosum and the ventrolateral prefrontal cortex. For disorganized displays, visual-load effects (diff RT and Z-scores) correlated with lPL, dlPFC, and mPFC volumes of the dorsal processing stream. Visual load for feature search calculated as difference RT scores correlated significantly with VAA volume, but not with Z-score correction for response latency. Similarly, the difference RT, but not Z-score for visual load for cued conjunction search was related striatal volume.
To test whether these relations were modulated by the effects of age on ROI volume, we used partial correlation with age as the covariate and found that correlation coefficients remained similar for feature (load diff. RT–VAA rp=−.31, p=0.013; load Z–CC splenium rp=.33, p=0.010) and cued conjunction searches (cued conj. load diff RT–striatum rp=−.29, p=0.021), but age as a covariate attenuated correlation coefficients for uncued conjunction searches (organized conj. load Z–vlPFC rp=−.14, ns; CC parietal rp=−.31, p=0.014; disorganized conj. load Z–lPL rp=−.10, ns; dlPFC rp=−.09, ns; mPFC rp=−.07, ns; VAA rp=−.18, ns; striatum rp=−.15, ns).
Cue validity
Smaller cue-validity effects (Z-scores), in particular lower cost from invalid cueing, were related to smaller lPL, dlPFC, and mPFC volumes, which are nodes of the dorsal processing stream (Table 2). To test whether age modulated these correlations, we used partial correlation coefficients with age as covariate and found attenuated correlation coefficients for validity costs–ROI volume relations (validity costs Z–lPL rp=.23, p=0.056; dlPFC rp=.18, ns; mPFC rp=.12, ns; vlPFC rp=.16, v; thalamus rp=.14, ns).
Alertness
Smaller callosal, thalamic, medial and lateral temporal lobe (mTL, lTL), and lateral parietal lobe (lPL) volumes predicted greater alertness effects (diff RT and Z-scores) (Table 2). Also for alertness-ROI volume relations, age as covariate attenuated partial correlation coefficients (alertness 8-load Z–lPL rp=.01, ns; lTL rp=.00, ns; mTL rp=−.25, p=0.043; thalamus rp=−.10. ns; CC splenium rp=−.13, ns; CC body rp=−.16, ns; CC genu rp=−.40, p=0.002).
DISCUSSION
Older age was associated with slower processing speed and with brain volume shrinkage that was most pronounced in frontoparietal regions of the dorsal processing stream and least pronounced in medial temporal cortices and visual association areas. Dissociations emerged for relationships between age, regional brain volumes, and particular visual search effects. Frontoparietal regions of the dorsal processing stream were associated with visual load effects, especially during uncued disorganized conjunction search and with cueing costs during cued disorganized conjunction search. Both measures are assumed to index component top-down attentional control processes required for searching disorganized displays and for disengaging and shifting attention in cued conjunction search (Bisley, Mirpour, Arcizet, and Ong, 2011; Donner et al., 2000; Li, Gratton, Yao, and Knight, 2010; Madden et al., 2007). By contrast, ventrolateral prefrontal lobe and parietal corpus callosum volumes were associated with processes invoked by organized conjunction search, enabling perceptual grouping through distractor similarity.
Volume of visual association area (VAA) correlated negatively with individual variability in processing speed for high relative to low feature load conditions. Because relationships were observed for diff RT values but not Z-scores (Table 2), feature load effects in individuals with smaller VAA volume were likely driven by individuals’ processing speed (see also Hopf et al., 2004). Smaller thalamic, callosal, and temporal cortical volumes were associated with enhanced phasic alertness (see also Kellermann et al., 2011). In addition, callosal volume mediated the relationship between age and response readiness after cueing (phasic alertness) in high-load disorganized conjunction search, and between age and smaller visual-load effects in organized conjunction search. This pattern suggests a useful role for interhemispheric connectivity under challenging conditions in aging when bilateral processing enables compensation (Cabeza, 2002) and when integrating bihemispheric visual information facilitates perceptual grouping.
Robust visual-load effects were observed in conjunction but not feature search. Conjunction searches became slower with older age specifically for higher relative to lower item loads and for disorganized distractor grouping. However, attentional allocation, i.e., the ability to direct attention according to a cue, was preserved and served to reduce conjunction-load effects in older age. Thus, when a visual cue guided attention to the target location, older adults performed similarly to their younger counterparts even under difficult disorganized search conditions; when a cue predicted the target’s location incorrectly, older adults were less affected than younger adults. Also phasic alertness, i.e., the ability to enhance response readiness following a cue, was preserved in aging and resulted in search time benefits in older age, especially for high-load displays. These findings support the hypothesis that age-related decrements in visual search abilities can be minimized by reducing attentional demands on control processes with alerting cues.
Age-related regional brain volume decline predicted component visual-search effects, adding to findings showing functional decline associations with age-related brain volume shrinkage (Burzynska et al., 2011; Kennedy, Rodrigue, Head, Gunning-Dixon, and Raz, 2009; Nyberg et al., 2010). Both structural (Kochunov et al., 2011) and functional (Pardo et al., 2007; Tomasi and Volkow, 2011; Voineskos et al., 2010) brain changes occur gradually over the life span and become more apparent with older age (Fjell and Walhovd, 2010). However, some functions are more affected by the untoward effects of aging on brain structure than others. For example, selective attention in conjunction search, which requires serial processing, appears to be more affected by aging than the automatic processes underlying feature search (Plude and Doussard-Roosevelt, 1989). In single-feature search, the detection of the red tomato among yellow tomatoes is effortless, independent of the number of distractors (visual load), and thus is assumed to rely on preattentive, spatially parallel processing mechanisms (Treisman and Gelade, 1980). The observation that feature-search performance correlated with volumes of visual association areas (VAA) comports with the assumption that pop-out feature search draws on preattentive, perceptual processing mechanisms (Treisman and Gelade, 1980) that are likely occurring early in the visual processing stream (Töllner et al., 2011). In our study, VAA volume may have mediated the speed of such preattentive, perceptual processes, and was independent of the effects of age. By contrast, in color-form conjunction search, detection of the red tomato among yellow tomatoes and red and yellow strawberries is dependent on the number of distractors present (visual load) and requires top-down selective-attention mechanisms. Indeed, we found that age-related shrinkage of dorsal frontoparietal brain regional volumes predicted greater conjunction-load effects, i.e., prolonged responses to higher relative to lower item loads. Although age-related brain volume shrinkage was related to slower processing speed independent of brain region and visual search task, age effects on brain volume did not always adversely affect brain structure-function relations. When conjunction search was cued, load effects were not related to age or brain volume. Nonetheless, we found smaller cue validity effects, especially lower cost from invalid cueing, with older age and smaller dorsal frontoparietal brain volumes. Thus, although smaller dorsolateral frontoparietal volumes were correlated with greater conjunction search load effects with older age, they were also related to reduced vulnerability to invalid cues with older age and independent of processing speed. This dissociation clearly demonstrates the differential effect of age on brain structure and function, that is, age-related fronto-parietal volume shrinkage selectively impaired self-guided selective attention, but not externally-guided attention during cued conjunction search. In contrast to our results, Greenwood, Parasuraman, and Alexander (1997) observed that the validity of cues has a strong effect on conjunction-search performance, owing to response time benefits from valid cues that were attenuated with advanced age. It is possible that differences in task conditions such as inter-stimulus-interval, viewing time, and the simple and concrete nature of our task contributed to the older subjects’ ability to overcome invalid cueing effects and benefit from valid cues as did the young adults. Thus, it appears that older adults can accelerate their visual search and perform similarly to their young counterparts, provided external guidance reduces attentional demands on frontoparietal network functions.
In young adults, the frontoparietal attention system is involved in top-down visuospatial attention and executive control required during conjunction search (Corbetta, Miezin, Shulman, and Petersen, 1993; Corbetta and Shulman, 1998; Donner et al., 2000; Weidner, Krummenacher, Reimann, Müller, and Fink, 2009). When distractor similarity is high, however, part-whole grouping (gestalt) mechanisms can be invoked. Conjunction search can rely on both serial and parallel processing (Duncan and Humphreys, 1989; Mordkoff, Yantis, and Egeth, 1990; Nakayama and Silverman, 1986; Wolfe, 1994) and can involve the ventral frontotemporal in addition to the dorsal frontoparietal processing stream (Wilkinson et al., 2002). Accordingly, we found for organized conjunction search that smaller ventrolateral, prefrontal, and callosal (parietal sector) volumes with older age were related to greater visual-load effects. Functional imaging studies have shown that older adults recruit different brain systems from their young counterparts during visual conjunction search, probably to compensate for difficulties in distractor inhibition, and they often show bilateral processing patterns for tasks that are processed unilaterally by younger adults (Cabeza, 2002; Reuter-Lorenz et al., 1999). This age-related reduction in functional lateralization may be mediated by shrinkage of the corpus callosum, thereby enabling top-down functional compensation (Cabeza, 2002) needed for disorganized conjunction searches. Age-related callosal shrinkage predicted performance in organized conjunction search, implying a role for the corpus callosum in perceptual grouping of symmetrical displays, probably via parallel processes in extrastriate cortices (Han, 2004; Schulte, Müller-Oehring, Rohlfing, Pfefferbaum, and Sullivan, 2010; Töllner et al., 2011) that require the integration of visual information between hemifields (Knyazeva, Fornari, Meuli, Innocenti, and Maeder, 2006). Although callosal macrostructure is typically robust to aging (Sullivan et al., 2010), the subject sample of the current study exhibited age-related callosal shrinkage that was functionally meaningful in predicting visual search performance.
We conclude that older adults can capitalize on early parallel stages of visual information processing by means of stimulus salience, perceptual grouping, and cue-induced enhancement of response readiness (phasic alertness). Age-related limitations in visual search arise as a result of the later, serial stage of processing requiring self-guided selective attention and executive control. These limitations are explained – at least in part – by age-related brain volume shrinkage and can be mitigated through the use of external cues.
Acknowledgments
This work was supported by National Institute on Aging grant AG017919, National Institute on Alcohol Abuse and Alcoholism grants AA018022, AA010723, and AA017168, and National Institute of Biomedical Imaging and Bioengineering grant EB008381. The authors thank Margaret J. Rosenbloom, M.A., for comments on this manuscript. We also thank Stephanie Sassoon, Ph.D., and Megan Thompson, B.A., for help with recruiting and screening study participants and assistance in data collection. All in-house image processing software used in this study is available from http://nitrc.org/projects/cmtk/; the SRI24 atlas is available from http://nitrc.org/projects/sri24/. The information in this manuscript and the manuscript itself are new and original and have never been published either electronically or in print.
Footnotes
There are no financial or other relationships that could be interpreted as a conflict of interest affecting this manuscript.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 2001. text revision. [Google Scholar]
- Anderson EJ, Rees G. The neural correlates of spatial orienting in the human superior colliculus. Journal of Neurophysiology. 2011 doi: 10.1152/jn.00286.2011. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach M. The “Freiburg Visual Acuity Test” – Automatic measurement of visual acuity. Optometry and Vision Science. 1996;73:49–53. doi: 10.1097/00006324-199601000-00008. [DOI] [PubMed] [Google Scholar]
- Benedetti B, Charil A, Rovaris M, Judica E, Valsasina P, Sormani MP, Filippi M. Influence of aging on brain gray and white matter changes assessed by conventional, MT, and DT MRI. Neurology. 2006;66:535–539. doi: 10.1212/01.wnl.0000198510.73363.c6. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behavioural Brain Research. 2001;125:279–284. doi: 10.1016/s0166-4328(01)00297-2. [DOI] [PubMed] [Google Scholar]
- Bisley JW, Mirpour K, Arcizet F, Ong WS. The role of the lateral intraparietal area in orienting attention and its implications for visual search. The European Journal of Neuroscience. 2011;33:1982–1990. doi: 10.1111/j.1460-9568.2011.07700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burzynska AZ, Nagel IE, Preuschhof C, Gluth S, Bäckman L, Li SC, Lindenberger U, Heekeren HR. Cortical thickness is linked to executive functioning in adulthood and aging. Human Brain Mapping. 2011 doi: 10.1002/hbm.21311. [Jul 7, 2011; Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton-Danner K, Owsley C, Jackson GR. Aging and feature search: the effect of search area. Experimental Aging Research. 2001;27:1–18. doi: 10.1080/03610730125782. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychology and Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. Review. [DOI] [PubMed] [Google Scholar]
- Chanraud S, Pitel AL, Rohlfing T, Pfefferbaum A, Sullivan EV. Dual tasking and working memory in alcoholism: relation to frontocerebellar circuitry. Neuropsychopharmacology. 2010;35:1868–1878. doi: 10.1038/npp.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Miezin FM, Shulman GL, Petersen SE. Selective attention modulates extrastriate visual regions in humans during visual feature discrimination and recognition. Ciba Foundation symposium. 1991;163:165–180. doi: 10.1002/9780470514184.ch10. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Miezin FM, Shulman GL, Petersen SE. A PET study of visuospatial attention. Journal of Neuroscience. 1993;13:1202–1226. doi: 10.1523/JNEUROSCI.13-03-01202.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Human cortical mechanisms of visual attention during orienting and search. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 1998;353:1353–1362. doi: 10.1098/rstb.1998.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JT, Frith CD, Büchel C, Nobre AC. Orienting attention in time: behavioural and neuroanatomical distinction between exogenous and endogenous shifts. Neuropsychologia. 2000;38:808–819. doi: 10.1016/s0028-3932(99)00132-3. [DOI] [PubMed] [Google Scholar]
- Crovitz HF, Zener K. A group-test for assessing hand- and eye-dominance. American Journal of Psychology. 1962;75:271–276. [PubMed] [Google Scholar]
- Davis SW, Kragel JE, Madden DJ, Cabeza R. The Architecture of Cross-Hemispheric Communication in the Aging Brain: Linking Behavior to Functional and Structural Connectivity. Cerebral Cortex. 2011 doi: 10.1093/cercor/bhr123. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner T, Kettermann A, Diesch E, Ostendorf F, Villringer A, Brandt SA. Involvement of the human frontal eye field and multiple parietal areas in covert visual selection during conjunction search. The European Journal of Neuroscience. 2000;12:3407–3414. doi: 10.1046/j.1460-9568.2000.00223.x. [DOI] [PubMed] [Google Scholar]
- Driscoll I, Davatzikos C, An Y, Wu X, Shen D, Kraut M, Resnick SM. Longitudinal pattern of regional brain volume change differentiates normal aging from MCI. Neurology. 2009;72:1906–1913. doi: 10.1212/WNL.0b013e3181a82634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J, Humphreys GW. Visual search and stimulus similarity. Psychological Review. 1989;96:433–458. doi: 10.1037/0033-295x.96.3.433. [DOI] [PubMed] [Google Scholar]
- Faust ME, Balota DA, Spieler DH, Ferraro FR. Individual differences in information-processing rate and account: implications for group differences in response latency. Psychological Bulletin. 1999;125:777–799. doi: 10.1037/0033-2909.125.6.777. [DOI] [PubMed] [Google Scholar]
- Fernandez-Duque D, Posner MI. Relating the mechanisms of orienting and alerting. Neuropsychologia. 1997;35:477–486. doi: 10.1016/s0028-3932(96)00103-0. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB. Structural brain changes in aging: courses, causes and cognitive consequences. Reviews in the Neurosciences. 2010;21:187–221. doi: 10.1515/revneuro.2010.21.3.187. Review. [DOI] [PubMed] [Google Scholar]
- Folk CL, Lincourt AE. The effects of age on guided conjunction search. Experimental Aging Research. 1996;22:99–118. doi: 10.1080/03610739608254000. [DOI] [PubMed] [Google Scholar]
- Gilmore G, Tobias T, Royer F. Aging and similarity grouping in visual search. Journal of Gerontology. 1985;40:586–592. doi: 10.1093/geronj/40.5.586. [DOI] [PubMed] [Google Scholar]
- Greenwood PM, Parasuraman R, Alexander GE. Controlling the focus of spatial attention during visual search: effects of advanced aging and Alzheimer disease. Neuropsychology. 1997;11:3–12. doi: 10.1037//0894-4105.11.1.3. [DOI] [PubMed] [Google Scholar]
- Grober E, Sliwinski M. Development and validation of a model for estimating premorbid verbal intelligence in the elderly. Journal of Clinical and Experimental Neuropsychology. 1991;13:933–949. doi: 10.1080/01688639108405109. [DOI] [PubMed] [Google Scholar]
- Han S. Interactions between proximity and similarity grouping: an event-related brain potential study in humans. Neuroscience Letters. 2004;367:40–43. doi: 10.1016/j.neulet.2004.05.098. [DOI] [PubMed] [Google Scholar]
- Harter MR, Guido W. Attention to pattern orientation: negative cortical potentials, reaction time, and the selection process. Electroencephalography and Clinical Neurophysiology. 1980;49:461–475. doi: 10.1016/0013-4694(80)90389-2. [DOI] [PubMed] [Google Scholar]
- Himmelbach M, Erb M, Karnath HO. Activation of superior colliculi in humans during visual exploration. BMC Neuroscience. 2007;8:66. doi: 10.1186/1471-2202-8-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead A, Redlich F. Social Class and Mental Illness: A Community Sample. John Wiley and Sons; New York: 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommel B, Li KZ, Li SC. Visual search across the life span. Developmental Psychology. 2004;40:545–558. doi: 10.1037/0012-1649.40.4.545. [DOI] [PubMed] [Google Scholar]
- Hopf JM, Boelmans K, Schoenfeld MA, Luck SJ, Heinze HJ. Attention to features precedes attention to locations in visual search: evidence from electromagnetic brain responses in humans. Journal of Neuroscience. 2004;24:1822–1832. doi: 10.1523/JNEUROSCI.3564-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey DG, Kramer AF. Age differences in visual search for feature, conjunction, and triple-conjunction targets. Psychology of Aging. 1997;12:704–717. doi: 10.1037//0882-7974.12.4.704. [DOI] [PubMed] [Google Scholar]
- Ishihara S. Tests for colour-blindness. Handaya, Tokyo: Hongo Harukicho; 1917. [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Gamst AC, Stout JC, Bonner J, Hesselink JR. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiology of Aging. 2001;22:581–594. doi: 10.1016/s0197-4580(01)00217-2. [DOI] [PubMed] [Google Scholar]
- Kellermann T, Reske M, Jansen A, Satrapi P, Shah NJ, Schneider F, Habel U. Latencies in BOLD response during visual attention processes. Brain Research. 2011;1386:127–138. doi: 10.1016/j.brainres.2011.02.023. [DOI] [PubMed] [Google Scholar]
- Kennedy KM, Rodrigue KM, Head D, Gunning-Dixon F, Raz N. Neuroanatomical and cognitive mediators of age-related differences in perceptual priming and learning. Neuropsychology. 2009;23:475–491. doi: 10.1037/a0015377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, Glahn DC, Lancaster J, Thompson PM, Kochunov V, Rogers B, Fox P, Blangero J, Williamson DE. Fractional anisotropy of cerebral white matter and thickness of cortical gray matter across the lifespan. Neuroimage. 2011;58:41–49. doi: 10.1016/j.neuroimage.2011.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knyazeva MG, Fornari E, Meuli R, Innocenti G, Maeder P. Imaging of a synchronous neuronal assembly in the human visual brain. Neuroimage. 2006;29:593–604. doi: 10.1016/j.neuroimage.2005.07.045. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Martin-Emerson R, Larish JF, Andersen GJ. Aging and filtering by movement in visual search. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences. 1996;51:201–216. doi: 10.1093/geronb/51b.4.p201. [DOI] [PubMed] [Google Scholar]
- Li L, Gratton C, Yao D, Knight RT. Role of frontal and parietal cortices in the control of bottom-up and top-down attention in humans. Brain Research. 2010;1344:173–184. doi: 10.1016/j.brainres.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Pierce TW, Allen PA. Adult age differences in the use of distractor homogeneity during visual search. Psychology of Aging. 1996;11:454–474. doi: 10.1037//0882-7974.11.3.454. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Gottlob LR, Allen PA. Adult age differences in visual search accuracy: attentional guidance and target detectability. Psychology of Aging. 1999;14:683–694. doi: 10.1037//0882-7974.14.4.683. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Spaniol J, Whiting WL, Bucur B, Provenzale JM, Cabeza R, White LE, Huettel SA. Adult age differences in the functional neuroanatomy of visual attention: a combined fMRI and DTI study. Neurobiology of Aging. 2007;28:459–476. doi: 10.1016/j.neurobiolaging.2006.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMains S, Kastner S. Interactions of top-down and bottom-up mechanisms in human visual cortex. Journal of Neuroscience. 2011;31:587–597. doi: 10.1523/JNEUROSCI.3766-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordkoff JT, Yantis S, Egeth HE. Detecting conjunctions of color and form in parallel. Perception & Psychophysics. 1990;48:157–168. doi: 10.3758/bf03207083. [DOI] [PubMed] [Google Scholar]
- Müller-Oehring EM, Schulte T, Raassi C, Pfefferbaum A, Sullivan EV. Local-global interference is modulated by age, sex and anterior corpus callosum size. Brain Research. 2007;1142:189–205. doi: 10.1016/j.brainres.2007.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K, Silverman GH. Serial and parallel processing of visual feature conjunctions. Nature. 1986;320:264–265. doi: 10.1038/320264a0. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Salami A, Andersson M, Eriksson J, Kalpouzos G, Kauppi K, Lind J, Pudas S, Persson J, Nilsson LG. Longitudinal evidence for diminished frontal cortex function in aging. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:22682–22686. doi: 10.1073/pnas.1012651108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo JV, Lee JT, Sheikh SA, Surerus-Johnson C, Shah H, Munch KR, Carlis JV, Lewis SM, Kuskowski MA, Dysken MW. Where the brain grows old: decline in anterior cingulate and medial prefrontal function with normal aging. Neuroimage. 2007;35:1231–1237. doi: 10.1016/j.neuroimage.2006.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelen MV, Kastner S. A neural basis for real-world visual search in human occipitotemporal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:12125–12130. doi: 10.1073/pnas.1101042108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelle JE, Cusack R, Henson RN. Adjusting for global effects in voxel-based morphometry: Gray matter decline in normal aging. Neuroimage. 2012 doi: 10.1016/j.neuroimage.2011.12.086. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Archives of Neurology. 1994;51:874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rohlfing T, Rosenbloom MJ, Sullivan EV. Combining atlas-based parcellation of regional brain data acquired across scanners at 1.5 T and 3.0 T field strengths. Neuroimage. 2012;60:940–951. doi: 10.1016/j.neuroimage.2012.01.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plude DJ, Doussard-Roosevelt JA. Aging, selective attention, and feature integration. Psychology of Aging. 1989;4:98–105. doi: 10.1037/0882-7974.4.1.98. [DOI] [PubMed] [Google Scholar]
- Posner MI. Measuring alertness. Annals of the New York Academy of Sciences. 2008;1129:193–199. doi: 10.1196/annals.1417.011. Review. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annual Review of Neuroscience. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Haacke EM. Brain aging and its modifiers: insights from in vivo neuromorphometry and susceptibility weighted imaging. Annals of the New York Academy of Sciences. 2007;1097:84–93. doi: 10.1196/annals.1379.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Stanczak L, Miller A. Neural recruitment and cognitive aging: Two hemispheres are better than one especially as you age. Psychological Science. 1999;10:494–500. [Google Scholar]
- Rohlfing T, Zahr NM, Sullivan EV, Pfefferbaum A. The SRI24 multichannel atlas of normal adult human brain structure. Human Brain Mapping. 2010;31:798–819. doi: 10.1002/hbm.20906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassoon SA, Fama R, Rosenbloom MJ, O’Reilly A, Pfefferbaum A, Sullivan EV. Component cognitive and motor processes of the digit symbol test: differential deficits in alcoholism, HIV infection, and their comorbidity. Alcoholism: Clinical and Experimental Research. 2007;31:1315–1324. doi: 10.1111/j.1530-0277.2007.00426.x. [DOI] [PubMed] [Google Scholar]
- Schulte T, Müller-Oehring EM, Chanraud S, Rosenbloom MJ, Pfefferbaum A, Sullivan EV. Age-related reorganization of functional networks for successful conflict resolution: A combined functional and structural MRI study. Neurobiology of Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.12.002. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte T, Müller-Oehring EM, Rohlfing T, Pfefferbaum A, Sullivan EV. White matter fiber degradation attenuates hemispheric asymmetry when integrating visuomotor information. Journal of Neuroscience. 2010;30:12168–12178. doi: 10.1523/JNEUROSCI.2160-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scialfa CT, Esau SP, Joffe KM. Age, target-distractor similarity, and visual search. Experimental Aging Research. 1998;24:337–358. doi: 10.1080/036107398244184. [DOI] [PubMed] [Google Scholar]
- Sigurdsson S, Aspelund T, Forsberg L, Fredriksson J, Kjartansson O, Oskarsdottir B, Jonsson PV, Eiriksdottir G, Harris TB, Zijdenbos A, van Buchem MA, Launer LJ, Gudnason V. Brain tissue volumes in the general population of the elderly The AGES-Reykjavik Study. Neuroimage. 2011 doi: 10.1016/j.neuroimage.2011.11.024. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipp S. The brain circuitry of attention. Trends in Cognitive Sciences. 2004;8:223–230. doi: 10.1016/j.tics.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolov EN. Perception and the conditioned reflex. New York: Macmillan; 1963. [Google Scholar]
- Sturm W, Willmes K. On the functional neuroanatomy of intrinsic and phasic alertness. Neuroimage. 2001;14:76–84. doi: 10.1006/nimg.2001.0839. [DOI] [PubMed] [Google Scholar]
- Sturm W, Schmenk B, Fimm B, Specht K, Weis S, Thron A, Willmes K. Spatial attention: more than intrinsic alerting? Experimental Brain Research. 2006;171:16–25. doi: 10.1007/s00221-005-0253-1. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom M, Serventi KL, Pfefferbaum A. Effects of age and sex on volumes of the thalamus, pons, and cortex. Neurobiology of Aging. 2004;25:185–192. doi: 10.1016/s0197-4580(03)00044-7. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rohlfing T, Pfefferbaum A. Longitudinal study of callosal microstructure in the normal adult aging brain using quantitative DTI fiber tracking. Developmental Neuropsychology. 2010;35:233–256. doi: 10.1080/87565641003689556. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taki Y, Kinomura S, Sato K, Goto R, Kawashima R, Fukuda H. A longitudinal study of gray matter volume decline with age and modifying factors. Neurobiology of Aging. 2011;32:907–915. doi: 10.1016/j.neurobiolaging.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Töllner T, Zehetleitner M, Krummenacher J, Müller HJ. Perceptual basis of redundancy gains in visual pop-out search. Journal of Cognitive Neuroscience. 2011;23:137–150. doi: 10.1162/jocn.2010.21422. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. Aging and functional brain networks. Molecular Psychiatry. 2011 doi: 10.1038/mp.2011.81. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman AM, Gelade G. A feature-integration theory of attention. Cognitive Psychology. 1980;12:97–136. doi: 10.1016/0010-0285(80)90005-5. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Voineskos AN, Rajji TK, Lobaugh NJ, Miranda D, Shenton ME, Kennedy JL, Pollock BG, Mulsant BH. Age-related decline in white matter tract integrity and cognitive performance: A DTI tractography and structural equation modeling study. Neurobiology of Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.02.009. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner R, Krummenacher J, Reimann B, Müller HJ, Fink GR. Sources of top-down control in visual search. Journal of Cognitive Neuroscience. 2009;21:2100–2113. doi: 10.1162/jocn.2008.21173. [DOI] [PubMed] [Google Scholar]
- Wilkinson DT, Halligan PW, Henson RN, Dolan RJ. The effects of interdistracter similarity on search processes in superior parietal cortex. Neuroimage. 2002;15:611–619. doi: 10.1006/nimg.2001.0993. [DOI] [PubMed] [Google Scholar]
- Wolfe JM. Visual search in continuous, naturalistic stimuli. Vision Research. 1994;34:1187–1195. doi: 10.1016/0042-6989(94)90300-x. [DOI] [PubMed] [Google Scholar]
- Xu GQ, Lan Y, Huang DF, Rao DZ, Pei Z, Chen L, Zeng JS. Visuospatial attention deficit in patients with local brain lesions. Brain Research. 2010;1322:153–159. doi: 10.1016/j.brainres.2010.01.072. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation maximization algorithm. IEEE Transactions on Medical Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]