Abstract
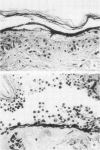
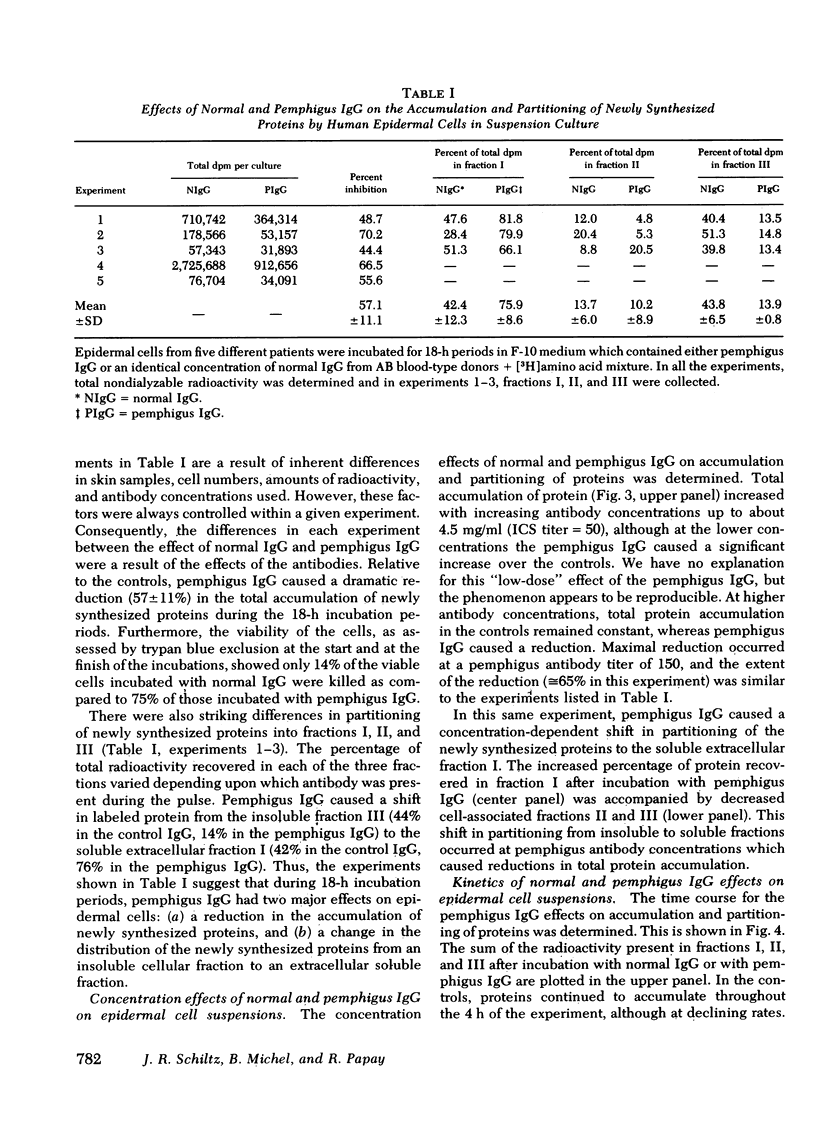
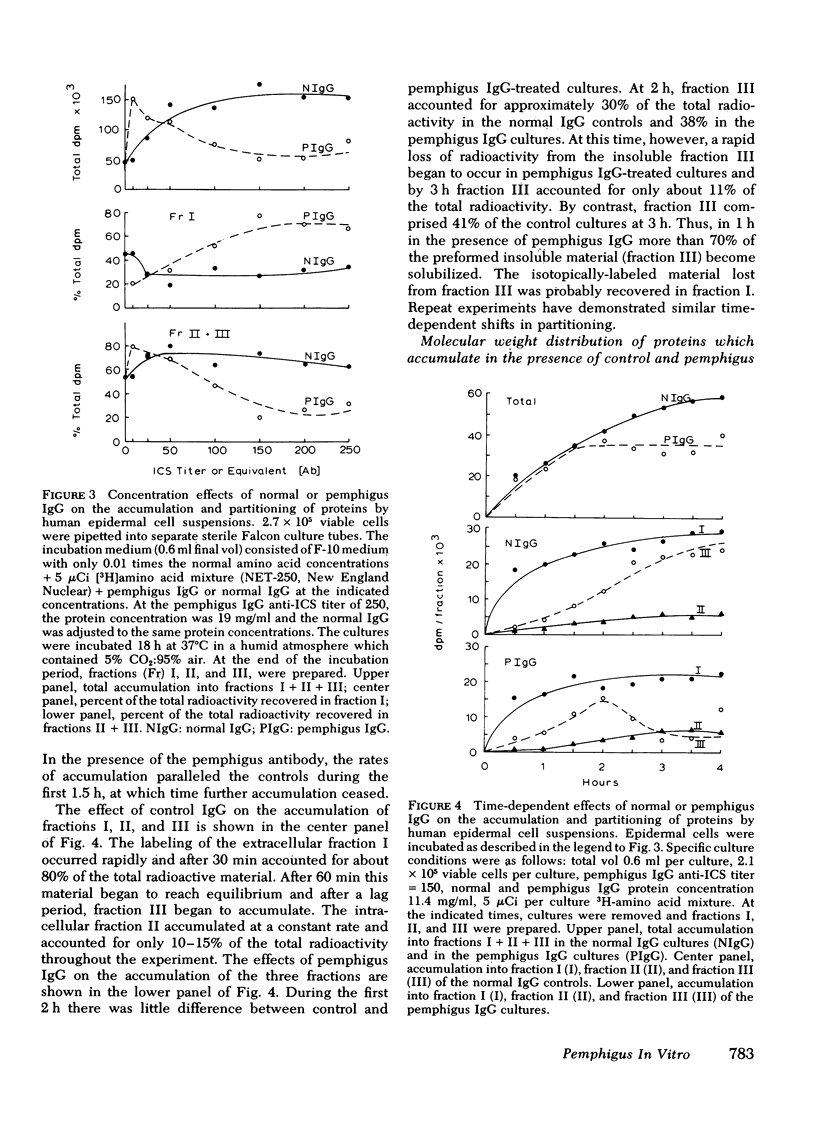
The mechanism of pemphigus acantholysis has been studied with an in vitro system. Freshly prepared human skin epidermal cells were incubated in F-10 medium which contained the immunoglobulin G fraction from either pemphigus serum or normal human serum. During 18-h incubation periods, the pemphigus antibody became bound to the surface of the epidermal cells, caused the destruction of 75% of the viable cells as compared to only 14% in the normal immunoglobulin G controls (trypan blue exclusion), prevented the accumulation of newly synthesized proteins by nearly 60% as determined by radioactive tracer studies, and caused a dramatic shift in distribution of the newly synthesized proteins from an insoluble cell-associated fraction to an extracellular soluble fraction. These effects on the accumulation and partitioning of newly synthesized proteins were antibody concentration-dependent. Kinetic studies showed that at a fixed pemphigus antibody concentration the inhibition of protein accumulation preceded solubilization by about 1 h, at which time rapid solubilization of up to 70% of the insoluble cellular material occurred. Several lines of evidence suggested that this phenomenon was caused by enzymatic activity. Epidermal extracts solubilized a prepared substrate of radioactivity labeled insoluble epidermal cell material. This activity was heat labile and pH dependent, with pH optima ranging from 4.5 to 6.5. Enzymes with pH optima between 6 and 6.5 were recovered in the culture medium after a 2-day incubation of pure, intact epidermis with the pemphigus antibody. We proposed the following hypothesis to account for pemphigus acantholysis. The pemphigus antibody reacts with the epidermal cell surface and produces such a severe disturbance that the integrity of the cell surface is lost. As a result of these primary perturbations, the cell is killed and during the process, responds by release or activiation of soluble hydrolytic enzymes. This autolytic process results in the characteristic acantholysis of pemphigus.
Full text
PDF



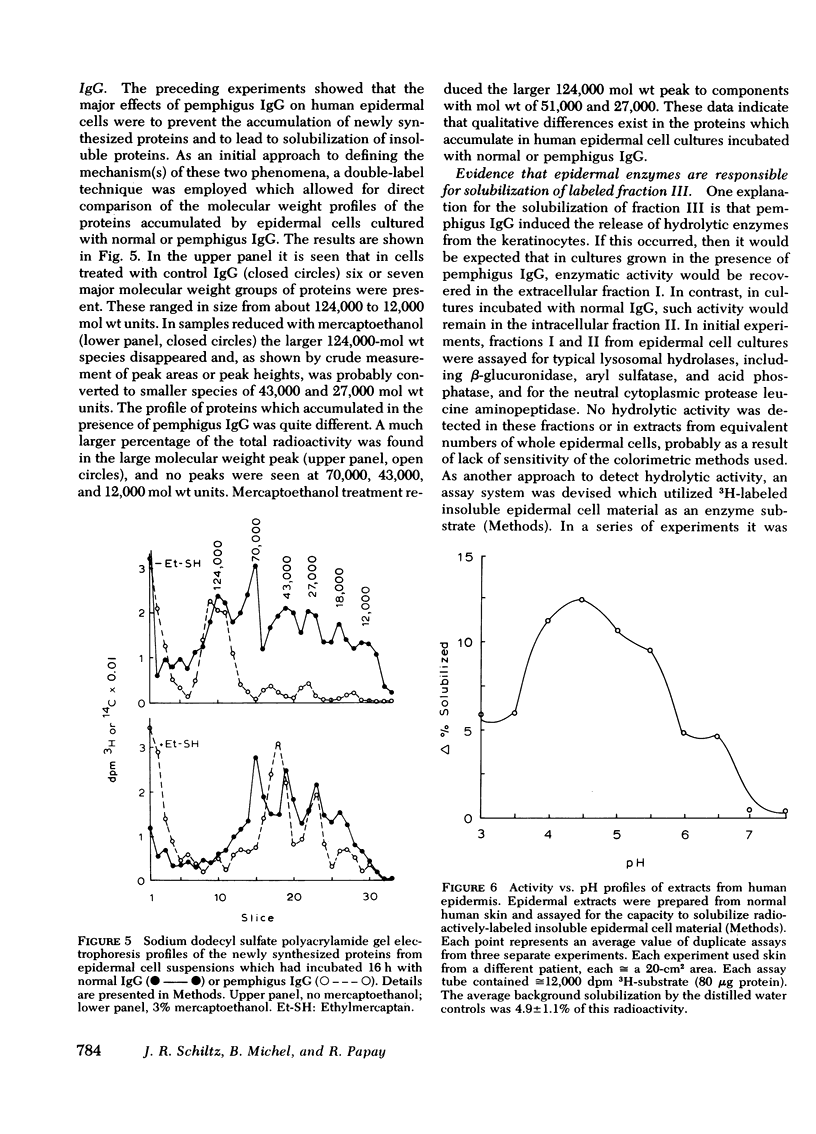
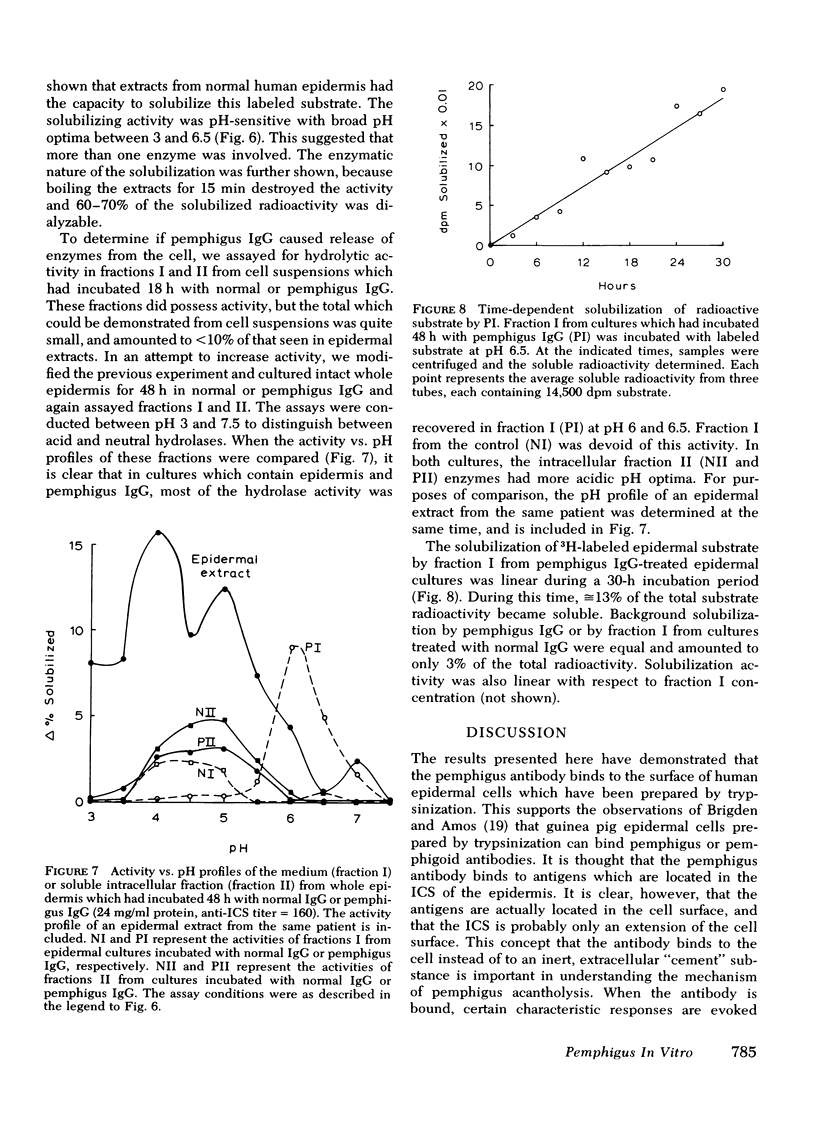



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BELLONE A. G., LEONE V. Ricerche sull'influenza esercitata da sieri di soggetti sani o affetti da pemfigo su pelle umana normale e pemfigosa coltivata in vitro. Soc Ital Dermatol Sifilogr Sezioni Interprov Soc Ital Dermatol Sifilogr. 1956 Mar-Apr;97(2):97–109. [PubMed] [Google Scholar]
- BEUTNER E. H., JORDON R. E. DEMONSTRATION OF SKIN ANTIBODIES IN SERA OF PEMPHIGUS VULGARIS PATIENTS BY INDIRECT IMMUNOFLUORESCENT STAINING. Proc Soc Exp Biol Med. 1964 Nov;117:505–510. doi: 10.3181/00379727-117-29622. [DOI] [PubMed] [Google Scholar]
- BEUTNER E. H., LEVER W. F., WITEBSKY E., JORDON R., CHERTOCK B. AUTOANTIBODIES IN PEMPHIGUS VULGARIS: RESPONSE TO AN INTERCELLULAR SUBSTANCE OF EPIDERMIS. JAMA. 1965 May 24;192:682–688. doi: 10.1001/jama.1965.03080210026006. [DOI] [PubMed] [Google Scholar]
- Barnett M. L., Beutner E. H., Chorzelski T. P. Organ culture studies of pemphigus antibodies. II. Ultrastructural comparison between acantholytic changes in vitro and human pemphigus lesions. J Invest Dermatol. 1977 May;68(5):265–271. doi: 10.1111/1523-1747.ep12494207. [DOI] [PubMed] [Google Scholar]
- Brigden W. D., Amos H. E. The differential binding of antibody from the sera of patients with pemphigus and pemphigoid to isolated guinea-pig epidermal cells. Br J Dermatol. 1975 Oct;93(4):425–430. doi: 10.1111/j.1365-2133.1975.tb06516.x. [DOI] [PubMed] [Google Scholar]
- Briggaman R. A., Wheeler C. E., Jr Epidermal-dermal interactions in adult human skin: role of dermis in epidermal maintenance. J Invest Dermatol. 1968 Dec;51(6):454–465. doi: 10.1038/jid.1968.155. [DOI] [PubMed] [Google Scholar]
- Deng J. S., Beutner E. H., Shu S., Chorzelski T. P. Pemphigus antibody action on skin explants: kinetics of acantholytic changes and stability of antigens in tissue cultures of normal monkey skin explants. Arch Dermatol. 1977 Jul;113(7):923–926. doi: 10.1001/archderm.113.7.923. [DOI] [PubMed] [Google Scholar]
- Eisinger M., Kucarova O., Sarkar N. H., Good R. A. Propagation of human wart virus in tissue culture. Nature. 1975 Jul 31;256(5516):432–434. doi: 10.1038/256432a0. [DOI] [PubMed] [Google Scholar]
- Farb R. M., Dykes R., Lazarus G. S. Anti-epidermal-cell-surface pemphigus antibody detaches viable epidermal cells from culture plates by activation of proteinase. Proc Natl Acad Sci U S A. 1978 Jan;75(1):459–463. doi: 10.1073/pnas.75.1.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMBRICK G. W., Jr, BLANK H. Whole mounts for the study of skin and its appendages. J Invest Dermatol. 1954 Dec;23(6):437–453. doi: 10.1038/jid.1954.126. [DOI] [PubMed] [Google Scholar]
- HAM R. G. CLONAL GROWTH OF MAMMALIAN CELLS IN A CHEMICALLY DEFINED, SYNTHETIC MEDIUM. Proc Natl Acad Sci U S A. 1965 Feb;53:288–293. doi: 10.1073/pnas.53.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K., Lever W. F. An electron microscopic study on pemphigus vulgaris of the mouth and the skin with special reference to the intercellular cement. J Invest Dermatol. 1967 Jun;48(6):540–552. doi: 10.1038/jid.1967.86. [DOI] [PubMed] [Google Scholar]
- Hu C. H., Michel B., Schiltz J. R. Epidermal acantholysis induced in vitro by pemphigus autoantibody. An ultrastructural study. Am J Pathol. 1978 Feb;90(2):345–362. [PMC free article] [PubMed] [Google Scholar]
- Jordan R. E., Day N. K., Luckasen J. R., Good R. A. Complement activation in pemphigus vulgaris blister fluid. Clin Exp Immunol. 1973 Sep;15(1):53–63. [PMC free article] [PubMed] [Google Scholar]
- Jordon R. E., Sams W. M., Jr, Diaz G., Beutner E. H. Negative complement immunofluorescence in pemphigus. J Invest Dermatol. 1971 Dec;57(6):407–410. doi: 10.1111/1523-1747.ep12293273. [DOI] [PubMed] [Google Scholar]
- Jordon R. E., Schroeter A. L., Rogers R. S., 3rd, Perry H. O. Classical and alternate pathway activation of complement in pemphigus vulgaris lesions. J Invest Dermatol. 1974 Sep;63(3):256–259. doi: 10.1111/1523-1747.ep12680098. [DOI] [PubMed] [Google Scholar]
- Michel B., Ko C. S. An organ culture model for the study of pemphigus acantholysis. Br J Dermatol. 1977 Mar;96(3):295–302. doi: 10.1111/j.1365-2133.1977.tb06141.x. [DOI] [PubMed] [Google Scholar]
- SCOTT A. A study of the action of chymotrypsin on the skin. J Invest Dermatol. 1958 Apr;30(4):201–205. doi: 10.1038/jid.1958.38. [DOI] [PubMed] [Google Scholar]
- STOUGHTON R. B., BAGATELL F. The nature of cantharidin acantholysis. J Invest Dermatol. 1959 Nov;33:287–292. doi: 10.1038/jid.1959.152. [DOI] [PubMed] [Google Scholar]
- STOUGHTON R. B. Enzymatic cytolysis of epithelium by filtrates of feces from patients with ulcerative colitis. Science. 1952 Jul 11;116(3002):37–39. [PubMed] [Google Scholar]
- STOUGHTON R. B., NOVAK N. Distribution of tonofibrils and intercellular bridges by disulfide-splitting agents. J Invest Dermatol. 1956 Feb;26(2):127–136. doi: 10.1038/jid.1956.17. [DOI] [PubMed] [Google Scholar]
- Sams W. M., Jr, Schur P. H. Studies of the antibodies in pemphigoid and pemphigus. J Lab Clin Med. 1973 Aug;82(2):249–254. [PubMed] [Google Scholar]
- Schiltz J. R., Michel B. Production of epidermal acantholysis in normal human skin in vitro by the IgG fraction from pemphigus serum. J Invest Dermatol. 1976 Aug;67(2):254–260. doi: 10.1111/1523-1747.ep12513454. [DOI] [PubMed] [Google Scholar]
- Schiltz J. R., Rosenbloom J., Levenson G. E. The effects of ascorbic acid deficiency on collagen synthesis by mouse molar tooth germs in organ culture. J Embryol Exp Morphol. 1977 Feb;37(1):49–57. [PubMed] [Google Scholar]