Abstract
Previous studies have shown large differences in taste responses to several sweeteners between mice from the C57BL/6ByJ (B6) and 129P3/J (129) inbred strains. The goal of this study was to compare behavioral responses of the B6 and 129 mice to a wider variety of sweeteners. Seventeen sweeteners were tested using two-bottle preference tests with water. Three main patterns of strain differences were evident. First, sucrose, maltose, saccharin, acesulfame, sucralose and SC-45647 were preferred by both strains, but the B6 mice had lower preference thresholds and higher solution intakes. Second, the amino acids D-phenylalanine, D-tryptophan, L-proline and glycine were highly preferred by the B6 mice, but not by the 129 mice. Third, glycyrrhizic acid, neohesperidin dihydrochalcone, thaumatin and cyclamate did not evoke strong preferences in either strain. Aspartame was neutral to all 129 mice and some B6 mice, but other B6 mice strongly preferred it. Thus, compared with the 129 mice, the B6 mice had higher preferences for sugars, sweet-tasting amino acids and several but not all non-caloric sweeteners. Glycyrrhizic acid, neohesperidin, thaumatin and cyclamate are not palatable to B6 or 129 mice.
Keywords: sweet, behavior, preference, genetics, mice
Introduction
Genetic variation among inbred mouse strains provides a tool to identify genetic loci underlying variable traits (Lush, 1991; Whitney and Harder, 1994). Mouse strains have large differences in consumption of sweeteners (substances that evoke sweet taste sensation in humans). Previous studies using two-bottle choice tests indicate that mice from C57BL/6 strains have high avidity and mice from 129 strains have low avidity for sweeteners, although only a few sweeteners have been examined (Lush, 1989; Belknap et al., 1993; Capeless and Whitney, 1995; Lush et al., 1995). We therefore used mice from C57BL/6ByJ (B6) and 129P3/J (129; formerly named 129/J) inbred strains in this study to represent a range of the genetic variation in sweetener preferences among laboratory mice.
Using two-bottle tests, we examined a wide range of sweeteners, which were selected based on the following criteria. First, all of them evoke sweet taste sensations in humans. Second, they represent various chemical groups: sugars (sucrose and maltose), sugar alcohols (sorbitol and erythritol), a chlorinated sugar analog (sucralose), amino acids (D-phenylalanine, D-tryptophan, L-proline and glycine), a dipeptide (aspartame), a protein (thaumatin), N-sulfonyl amides (saccharin and acesulfame-K), a sulfamate (cyclamate), a guanidinacetic acid-based sweetener (SC-45647), a triterpenoid glycoside (glycyrrhizic acid), and a dihydrochalcone glycoside (neohesperidin dihydrochalcone). Third, previous psychophysical and neurophysiological studies have revealed differences in responses to these sweeteners (Plata-Salaman et al., 1993; Schiffman and Gatlin, 1993; DuBois, 1995; Naim et al., 1998). Fourth, comparative studies have shown that several of these compounds are sweet to some mammalian species but not to others (Beauchamp et al., 1977; Jakinovich, 1981; Naim et al., 1982; Ferrell, 1984; Schiffman and Gatlin, 1993; Hellekant et al., 1994; Glaser et al., 1995; Nofre et al., 1996; Danilova et al., 1998).
In this paper, we describe the results of testing the B6 and 129 mice with the sweeteners using two-bottle tests. An accompanying paper (Inoue et al., submitted) describes an electrophysiological study of multiunit chorda tympani responses to lingual application of sweeteners in these two mouse strains.
Methods
Animals
Male mice of the C57BL/6ByJ (B6; n = 66) and 129P3/J (129; formerly named 129/J; n = 72) strains were obtained from The Jackson Laboratory (Bar Harbor, ME). Six groups of the B6 and 129 mice were tested with different compounds. Group 1 (8 B6 and 12 129 6.5-month old mice) was tested with sucrose; group 2 (11 B6 and 12 129 2-month old mice) with saccharin, D-phenylalanine, glycine, aspartame, L-proline and D-tryptophan; group 3 (12 B6 and 12 129 2-month old mice) with glycyrrhizic acid, SC-45647, thaumatin and neohesperidin hydrochalcone; group 4 (12 B6 and 12 129 2-month old mice) with maltose, cyclamate and sorbitol; group 5 (11 B6 and 12 129 3-month old mice) with acesulfame K; and group 6 (12 B6 and 12 129 2-month old mice) with sucralose and erythritol. These compounds were tested in each group in the order listed.
During the experiments, the mice were housed in individual cages in a temperature-controlled room at 23°C on a 12-h light: 12-h dark cycle (7:00 a.m. on, 7:00 p.m. off). They had free access to deionized water and Teklad Rodent Diet 8604 (Harlan Teklad, Madison, WI; 24.5% protein, 50.3% carbohydrate and 4.4% fat; 3.93 Kcal/g gross energy; 0.31 % sodium, 0.99 % potassium and 1.46 % calcium).
Taste solutions
Sweetener solutions were prepared in deionized water. We tested sucrose, maltose, sorbitol, saccharin, D-phenylalanine, D-tryptophan, L-proline, glycine, cyclamate (cyclamic acid, hemicalcium salt), aspartame (Asp-Phe methyl ester), neohesperidin dihydrochalcone (Sigma Chemical Company, St. Louis, MO); glycyrrhizic acid, monoammonium salt (Aldrich Chemical Company, Milwaukee, WI); acesulfame K (Hoechst Food Ingredients, Edison, NJ); erythritol (M&C Sweeteners/Mitsubishi Chemical and Cargill, Blair, NE); sucralose (McNeil Specialty, New Brunswick, NJ); SC-45647 [a Nutrasweet compound (Tinti and Nofre, 1991; DuBois, 1995)] and thaumatin (gifts of Dr. G. DuBois). Detailed information about most of these sweeteners can be found elsewhere (Schiffman and Gatlin, 1993). The order of testing of the sweeteners within each group of mice is described above (Animals).
The range of solution concentrations (Table 1) was selected so that the weakest solutions would be below the human detection threshold, and the strongest solutions would be above the human threshold and would approximately match the sweetness of 2 - 7.5% sucrose in humans (Schiffman and Gatlin, 1993). In some cases, limited compound solubility or sweetness potency precluded matching the upper level of sweetness.
Table 1.
Sweetener concentrations tested, preferred and avoided by B6 and 129 mice
| Solution | Units | Range tested | Preferreda | Avoidedb | ||
|---|---|---|---|---|---|---|
|
| ||||||
| B6 | 129 | B6 | 129 | |||
| Sucrose | mM | 29 - 935 | 58 – 935 | 234 – 935 | - | - |
| % | 1 - 32 | 2 – 32 | 8 – 32 | - | - | |
| Maltose | mM | 0.08 - 833 | 28 – 833 | 83 – 833 | - | - |
| % | 0.003 - 30 | 1 – 30 | 3 – 30 | - | - | |
| Sorbitol | mM | 1.7 - 1647 | 16.5 – 549 | 55 – 549 | 1647 | 1098 -1647 |
| % | 0.03 - 30 | 0.3 – 10 | 1 – 10 | 30 | 20 - 30 | |
| Erythritol | mM | 2.5 - 2457 | 246 - 491 | - | 1638 - 2457 | 819 - 2457 |
| % | 0.03 - 30 | 3 - 6 | - | 20 - 30 | 10 – 30 | |
| Saccharin | mM | 0.43 - 255 | 0.43 – 85 | 4.3 – 85 | 255 | 170 - 255 |
| g/l | 0.1 - 62 | 0.1 – 20.5 | 1 – 20.5 | 62 | 41 - 62 | |
| Acesulfame K | mM | 0.01 - 100 | 0.3c – 100 | 10 – 100 | - | - |
| g/l | 0.002 - 20 | 0.06c – 20 | 2 – 20 | - | - | |
| Sucralose | mM | 0.0008 - 25 | 0.25 – 25 | 2.5 - 25 | - | - |
| mg/l | 0.3 - 10000 | 0.1 – 10 | 1 – 10 | - | - | |
| SC-45647 | mM | 0.0003 - 0.9 | 0.009 – 0.9 | 0.09 – 0.9 | - | - |
| mg/l | 0.1 - 300 | 3 – 300 | 30 – 300 | - | - | |
| D-phenylalanine | mM | 3 - 100 | 10 - 100 | - | - | - |
| g/l | 0.5 - 16.5 | 1.7 – 17 | - | - | - | |
| D-tryptophan | mM | 0.3 - 50 | 3 - 50 | - | - | - |
| g/l | 0.06 - 10.2 | 0.6 – 10 | - | - | - | |
| L-proline | mM | 1 - 1000 | 100 – 1000 | 100 | - | 1000 |
| g/l | 0.12 - 115 | 11.5 – 115 | 11.5 | - | 115 | |
| Glycine | mM | 0.1 - 1000 | 0.1 – 1000 | 100 - 300 | - | - |
| g/l | 0.008 - 75 | 0.008 – 75 | 7.5 - 22.5 | - | - | |
| Glycyrrhizic acid | mM | 0.0011 - 3.4 | - | - | - | - |
| mg/l | 1 - 3000 | - | - | - | - | |
| Neohesperidin | mM | 0.00016 - 0.16 | - | - | - | - |
| mg/l | 0.1 - 100 | - | - | - | - | |
| Thaumatin | mM | 0.0000013 - 0.0045 | - | - | - | - |
| mg/l | 0.03 - 100 | - | - | - | - | |
| Cyclamate | mM | 0.15 - 151 | - | 0.5 | 151 | - |
| g/l | 0.03 - 30 | - | 0.1 | 30 | - | |
| Aspartame | mM | 0.03 - 10 | - | - | - | - |
| mg/l | 8.8 - 2943 | - | - | - | - | |
Consumed in significantly greater amounts than water. The lowest concentration in the range represents preference threshold.
Consumed in significantly smaller amounts than water. The lowest concentration in the range represents avoidance threshold.
See explanation in Results.
‘-’ No significant preference/avoidance; solution intake did not differ significantly from water intake.
Measurement of fluid intake
Fluid intake was measured using two-bottle preference tests of individually caged mice. Construction of drinking tubes and other experimental details have been described previously (Bachmanov et al., 1996b). The drinking tubes were positioned to the right of the feeder with their tips 15 mm apart, and each extended 25 mm into the cage. Each tube had a stainless steel tip with a 3.175-mm diameter hole from which the mice could lick fluids.
The mice were presented with one tube containing a solution and the other tube containing deionized water. The solutions were tested in increasing order of concentrations. For most sweeteners, the concentrations were increased by approximately half log-steps. Each concentration was tested for 48 h. The positions of the tubes were switched every 24 h in order to control for side preferences. Daily measurements were made in the middle of the light period by reading fluid volume to the nearest 0.2 ml. There were no breaks between testing different concentrations of the same sweetener, but between testing different sweeteners, the mice received deionized water in both drinking tubes for at least two days.
Data analyses
Average daily (24-h) fluid intakes were calculated for each mouse for each solution concentration. Preference scores were calculated as the ratio of the average daily solution intake to average daily total fluid (solution + water) intake, in percent. The B6 mice were heavier than were the 129 mice (28.8 ± 0.3 and 24.9 ± 0.3 g, respectively; means ± standard errors; p < 0.0001, t-test). To account for this, body weights (BW) of individual mice were measured before and after each test series, averaged and used to calculate intakes per 30 g of BW (the approximate weight of an adult mouse). The relationships between BW and fluid intakes have been discussed in detail previously (Bachmanov et al., 1998).
The data for each sweetener were analyzed separately using two-way ANOVAs with strain as the between-group factor and concentration as the within-group factor (Table 2). Scheffé post-hoc tests were used to evaluate differences between individual means. These statistical tests used a two-tailed criterion for significance of p < 0.05.
The significance of preference or avoidance of a taste solution in the two-bottle tests was determined by comparing the solution and water intakes using paired t-tests. Because this comparison was made for each sweetener concentration for each strain, the total number of comparisons was 136. To avoid potential false positives due to multiple testing, we applied a Bonferroni correction, which set the two-tailed critical level of statistical significance at p < 0.05/136 or 0.000368. Preference or avoidance thresholds were defined as the lowest solution concentrations that were consumed in significantly larger or smaller amounts than water, respectively.
Results
For most sweeteners, concentration significantly affected solution intakes and preferences [in all cases F(3-11, 63-231) ≥ 3.9, p < 0.01, main effect of concentration in two-way ANOVA]. Exceptions were neohesperidin hydrochalcone (ns) and aspartame [effect of concentration was significant for intakes, F(5,105) = 3.2, p < 0.05, but not for preferences]. Four typical patterns of preference scores as a function of increasing concentration were observed (Figure 1): (i) indifference – preference; (ii) indifference – preference – avoidance; (iii) indifference – avoidance; (iv) only indifference. In some cases, the concentration-response curves were similarly shaped in the B6 and 129 mice, with the 129 preferences being shifted towards the higher concentrations (e.g., sugars, saccharin, acesulfame, sucralose, SC-45647), or with both strains being indifferent (e.g., glycyrrhizic acid, neohesperidin, thaumatin). In other cases, the two strains had differently shaped concentration-response curves (e.g., amino acids, erythritol).
Figure 1.
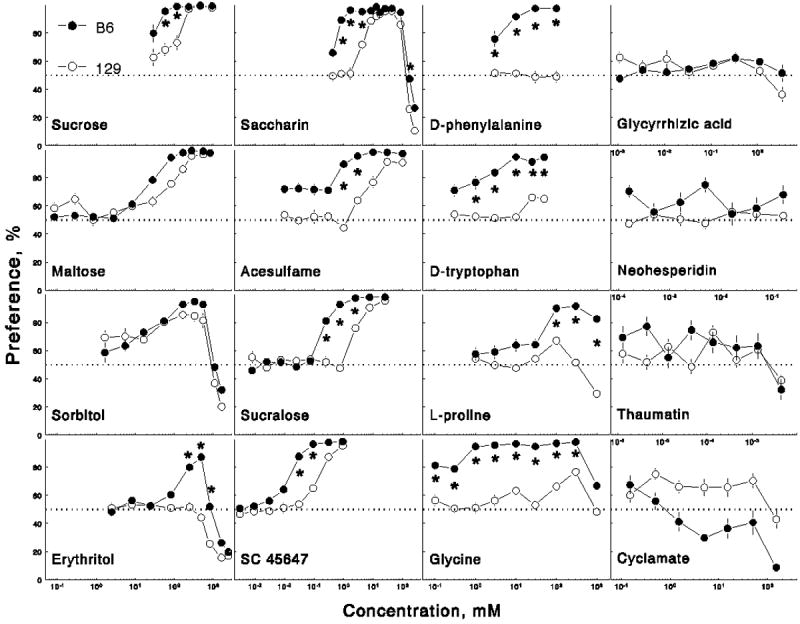
Preferences of B6 and 129 mice for 16 sweeteners. Vertical bars represent SE. *Significant difference between B6 and 129 strains, p < 0.05, Scheffé post-hoc tests.
For most sweeteners, strain differences in preferences (except for sorbitol, glycyrrhizic acid and thaumatin) and intakes (except for sorbitol, glycyrrhizic acid, neohesperidin hydrochalcone and thaumatin) were significant [in all cases F(1, 18-22) ≥ 4.9, p < 0.05, main effect of strain in two-way ANOVA]. Similarly, strain × concentration interaction effects were significant [in all cases F(3-11, 63-231) ≥ 2.2, p < 0.05), except for neohesperidin hydrochalcone and aspartame preferences and intakes.
Sucrose
Compared with the 129 mice, the B6 mice had lower preference thresholds (Table 1), higher preferences for 58 and 117 mM (2 and 4% respectively) solutions (Figure 1), and they drank more 117 – 467 mM (4 – 16%) solutions (Figure 2).
Figure 2.
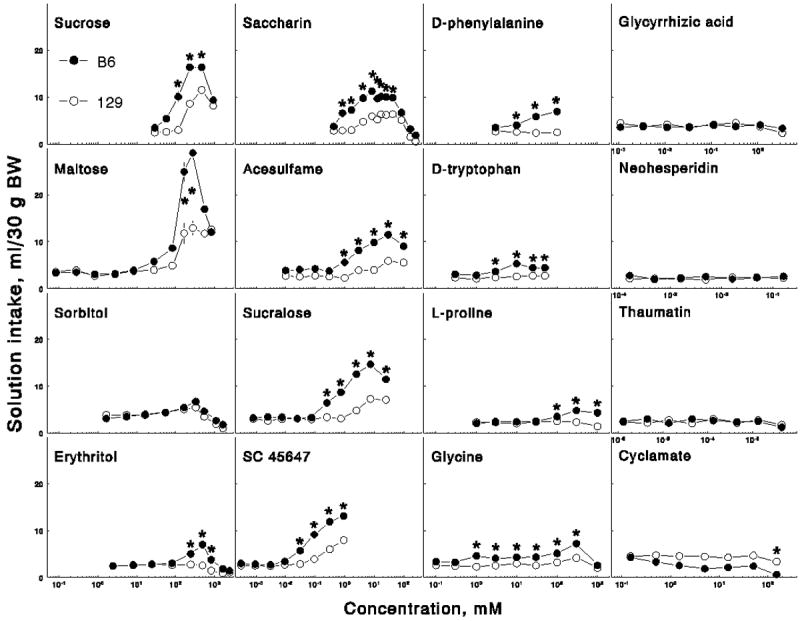
Consumption of 16 sweeteners by B6 and 129 mice. Explanations are the same as in the Figure 1.
Maltose
Compared with the 129 mice, the B6 mice had lower preference thresholds and they drank more 167 and 278 mM (6 and 10%) solutions. Maltose preference depended on strain [the B6 mice had a higher overall preference than did the 129 mice; effect of strain, F(1,22) = 4.9, p < 0.05, ANOVA], and on a strain × concentration interaction [F(10,220) = 6.5, p < 0.001]; however, no significant strain differences for individual concentrations were detected in post-hoc tests.
Sorbitol
Compared with the 129 mice, the B6 mice had lower preference thresholds and higher avoidance thresholds. Although sorbitol preference and intake depended on a strain × concentration interaction [F(8,176) ≥ 3.0, p < 0.01], no significant strain differences for individual concentrations were detected.
Erythritol
Unlike the B6 mice, the 129 mice did not prefer any erythritol concentration. The B6 mice had higher erythritol avoidance thresholds than did the 129 mice. Compared with the 129 mice, the B6 mice had higher preferences for, and intakes of, 246 – 819 mM (3 – 10%) erythritol.
Saccharin
Compared with the 129 mice, the B6 mice had lower preference and higher avoidance thresholds, higher preference scores for 0.85 – 4.3 mM (0.2 – 1 g/l) and 170 mM (41 g/l) solutions, and they drank more 0.85 - 43 mM (0.2 – 10.3 g/l) solutions.
Acesulfame K
The B6 mice preferred 0.01, 0.03 and 0.3 – 100 mM solutions relative to water. Because preferences for 0.01 – 0.3 mM solutions by the B6 mice fluctuated near the threshold of statistical significance and may have exceeded it by chance, a more conservative estimation of preference threshold for the B6 strain would be 0.3 – 1 mM. Compared with the 129 mice, the B6 mice had lower preference thresholds, higher preferences for 1 and 3 mM (0.2 and 0.6 g/l) solutions, and they drank more 1 – 100 mM (0.2 – 20 g/l) solutions.
Sucralose
Compared with the 129 mice, the B6 mice had lower preference thresholds, higher preferences for 0.25 – 2.5 mM (0.1 – 1 g/l) solutions, and they drank more 0.25 - 25 mM (0.1 – 10 g/l) solutions.
SC-45647
Compared with the 129 mice, the B6 mice had lower preference thresholds, higher preferences for 0.03 and 0.09 mM (10 and 30 mg/l) solutions, and they drank more 0.03 – 0.9 mM (10 – 300 mg/l) solutions.
D-phenylalanine
Unlike the B6 mice, the 129 mice did not prefer any D-phenylalanine concentration. Compared with the 129 mice, the B6 mice had higher preferences for 3 – 100 mM (0.5 – 17 g/l) solutions and intakes of 10 – 100 mM (1.7 – 17 g/l) solutions.
D-tryptophan
Unlike the B6 mice, the 129 mice did not prefer any D-tryptophan concentration. Compared with the 129 mice, the B6 mice had higher preferences for 1 – 50 mM (0.2 – 10 g/l) solutions and intakes of 3 - 50 mM (0.6 – 10 g/l) solutions.
L-proline
The preference threshold concentration was 100 mM (11.5 g/l) for both B6 and 129 mice, but preference scores of the 129 mice for this solution were lower and did not exceed 70%. Compared with the 129 mice, the B6 mice had higher preferences for and intakes of 100 – 1000 mM (11.5 – 115 g/l) solutions.
Glycine
The B6 mice had lower preference thresholds than did the 129 mice. Although the 129 mice preferred 100 and 300 mM solutions over water, their preference scores for these solutions were lower than those of the B6 mice and did not exceed 80%. Compared with the 129 mice, the B6 mice had higher preferences for 0.1 – 300 mM (0.008 – 22.5 g/l) solutions and intakes of 1 – 300 mM (0.08 – 22.5 g/l) solutions.
Glycyrrhizic acid
Both B6 and 129 mice were indifferent to all concentrations tested. Although preference scores and intakes depended on a strain × concentration interaction [F(7,154) ≥ 2.7, p < 0.05], post-hoc tests did not detect strain differences for individual concentrations.
Neohesperidin dihydrochalcone
Both B6 and 129 mice were indifferent to all concentrations tested. The only significant effect found was a strain difference in neohesperidin preference scores [F(1,22) = 8.1, p < 0.01]: they were higher overall in the B6 mice than in the 129 mice, but they did not exceed 75% in either strain.
Thaumatin
Both B6 and 129 mice were indifferent to all concentrations tested. Although preference scores and intakes depended on a strain × concentration interaction [F(7,154) ≥ 2.2, p < 0.05], post-hoc tests did not detect strain differences for individual concentrations.
Cyclamate
The 129 mice preferred 0.5 mM cyclamate solution over water but were indifferent to other concentrations. The B6 mice avoided the highest (151 mM) cyclamate concentration but were indifferent to other concentrations. The preference scores did not exceed 75% in either strain. The preference scores depended on strain [the B6 mice had lower preferences than did the 129 mice, F (1,22) = 25.4, p < 0.001], and on a strain × concentration interaction [F(6,132) = 3.6, p < 0.01], but post-hoc tests did not detect strain differences for individual concentrations. The B6 mice had lower 151 mM (30 g/l) cyclamate intakes than did the 129 mice.
Aspartame
On average, neither B6 nor 129 strain preferred or avoided any concentration tested (Table 1), but the B6 mice had higher overall aspartame preference scores and intakes [effect of strain, F(1,21) ≥ 5.7, p < 0.05; Figure 3A, B]. Within the B6 strain, there was a substantial individual variation in aspartame preferences: some B6 mice displayed strong preferences over the range of aspartame concentrations, whereas other B6 mice were indifferent (Figure 3C). No 129 mice showed strong preference or avoidance of aspartame (Figure 3D).
Figure 3.
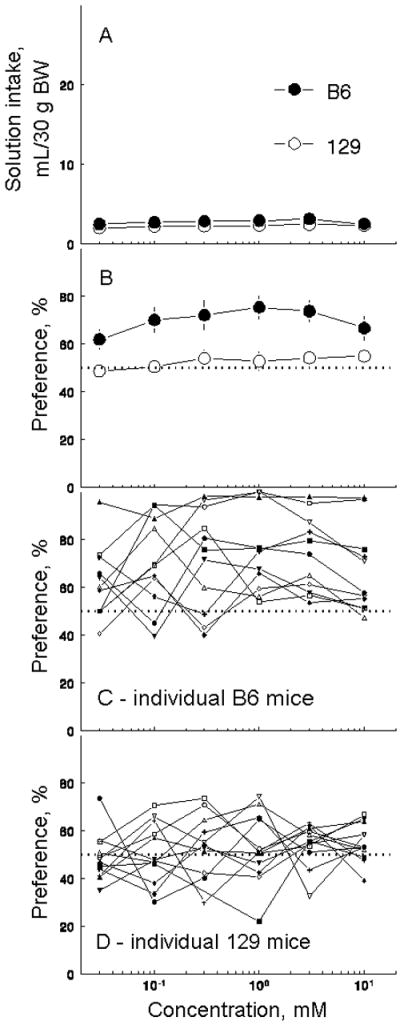
Aspartame solutions intakes and preferences by B6 and 129 mice. (A) Solution intakes, strain means ± SE. (B) Solution preferences, strain means ± SE. (C) Solution preferences by individual B6 mice. (D) Solution preferences by individual 129 mice.
Discussion
In this study, we used two-bottle tests to characterize taste responses to seventeen sweeteners by B6 and 129 mice. High preference scores and large sweetener solution intakes suggest that many of these sweeteners are highly palatable to the mice. Although the two-bottle tests do not characterize taste quality perception directly, such high preferences and intakes in replete animals are usually observed only in tests with sweeteners. In addition, several of these compounds have been characterized using conditioned taste aversion tests or single-fiber recordings from gustatory nerves in mice (Ninomiya et al., 1984a; Ninomiya et al., 1984b) and other species (Hellekant et al., 1997; Danilova et al., 1998), and they were shown to have sensory properties similar to those of sucrose.
We observed three main patterns of strain differences in taste responses to sweeteners. First, sucrose, maltose, saccharin, acesulfame, sucralose and SC-45647 were preferred by both strains, but the B6 mice had lower preference thresholds and higher intakes. Second, the amino acids D-phenylalanine, D-tryptophan, L-proline and glycine were highly preferred by the B6 mice, but not by the 129 mice. Third, glycyrrhizic acid, neohesperidin, thaumatin, cyclamate and aspartame did not evoke strong preferences in either strain. In the last group of sweeteners, the responses to cyclamate and aspartame had some unique features. Cyclamate was more aversive to the B6 mice than to the 129 mice, which were relatively indifferent to it. Whereas all 129 mice and some B6 mice were indifferent to aspartame, other B6 mice strongly preferred it. Sugar alcohols produced a somewhat different pattern of strain differences compared with sugars, despite their chemical similarity. Lower concentrations of sorbitol were preferred by mice from both B6 and 129 strains. Lower concentrations of erythritol were preferred by the B6 mice, but were neutral to the 129 mice. Mice from both strains avoided high (≥ 800 mM, or 10%) concentrations of sugar alcohols, whereas they strongly preferred similar concentrations of sugars.
Although properties of sweetener solutions other than sweetness (e.g., bitterness or postingestive effects) probably affected the results of our tests, we believe that peripheral perception of sweet taste was a major determinant of the strain differences. First, compared with the 129 mice, preferences and intakes of the B6 mice were higher for a wide variety of sweet compounds with different non-sweet sensory and/or post-ingestive properties. This suggests that the pattern of the strain differences is explained by a common attribute of these solutions (i.e., sweetness), rather than by the variable attributes (e.g., bitterness or postingestive effects). Second, greater sweetener consumption by B6 mice is genetically related to larger responses of their gustatory nerves to sweeteners (Bachmanov et al., 1997b; Bachmanov et al., 1997a; Li et al., 2001), suggesting that differences in peripheral sensory input are involved in the genetic variation in behavioral responses to sweeteners.
Nevertheless, non-sweet sensory (e.g., bitterness, viscosity, osmolality, or coolness resulting from endothermic reactions with saliva) and postingestive factors probably affected the results for some sweeteners. In particular, avoidance of concentrated solutions of saccharin, L-proline and cyclamate may be due to their predominant bitter taste at these concentrations [see also (Dess, 1993)]. Cyclamate probably does not taste sweet to either mouse strain, as was found in other species (Hellekant et al., 1997; Danilova et al., 1998), but the B6 mice may be more sensitive to its bitterness compared with the 129 mice, resulting in a stronger avoidance by the B6 mice. The B6 and 129 mice differ in behavioral taste responses to bitter, salty, sour and umami solutions, although these differences are genetically unrelated to their differential sweetener responsiveness (Bachmanov et al., 1996a; Bachmanov et al., 1996b; Bachmanov et al., 2000). The caloric value of the sugars tested (sucrose and maltose) may account for their higher consumption compared with the non-nutritive sweeteners (e.g., saccharin, acesulfame, sucralose, SC-45647) in both strains. Sugar alcohols are metabolized differently than sugars, and may also cause discomfort because of their intestinal osmotic effects (Schiffman and Gatlin, 1993). This may have inhibited the mice from consuming large volumes of these solutions and resulted in avoidance of the more concentrated solutions. It is possible that the B6 and 129 mice differ not only in perceived sweetness of the sugar alcohols, but also in their postingestive handling (e.g., intestinal absorption, metabolism, or excretion). Thus, the strain differences in consumption of the sugar alcohols may depend on an interaction between the perception of their sweetness and their postingestive properties.
Because we tested several different sweeteners in the same groups of animals, the results of our tests might have been affected by ‘carry-over’ effects from testing previous compounds. Although we cannot exclude them completely, their possible contribution was most likely very small. First, the mice were given only water to drink for at least two days between testing different sweeteners. Second, taste responses clearly depended on solution concentration within a series of a particular sweetener, rather than being correlated with responses to a previously tested compound. There were no instances when strain differences were present for the first (weakest) concentration tested, but then disappeared. Third, we tested some of the sweeteners used in this study in other published (Bachmanov et al., 1996a; Bachmanov et al., 1996b; Bachmanov et al., 1997b; Bachmanov et al., 1997a; Bachmanov et al., 2000; Li et al., 2001) and unpublished experiments and found similar results.
A distinctive feature of aspartame preference was a substantial variation among the B6 mice, which is consistent with previous data from rats (Sclafani and Abrams, 1986; De Francisco and Dess, 1998). A few B6 mice consistently preferred aspartame to water over the range of concentrations tested, whereas some other B6 mice did not.
The sweet-tasting amino acids were strongly preferred by the B6 mice at some concentrations, but the 129 mice were generally neutral to them and did not display preferences greater than 80% at any concentration tested. This contrasted with responses to sugars and several other sweeteners, which at certain concentrations were strongly preferred by both B6 and 129 mice. Electrophysiological experiments also show different patterns for these two groups of sweeteners [see accompanying MS (Inoue et al., submitted)]. The B6 mice had greater chorda tympani nerve responses to sucrose, maltose, saccharin, sorbitol, acesulfame K, SC-45647 and sucralose. However, among the amino acids tested, only L-proline evoked stronger responses in the chorda tympani nerve of the B6 mice, whereas responses to D-phenylalanine and glycine were similar in the two strains [see discussion in accompanying manuscript (Inoue et al., submitted)].
The indifference of the 129 mice to the amino acids may be explained by a non-functional mechanism for detecting amino acids sweetness in this strain. However, it can also be explained by other reasons, for example by different sweetness potency of the stimuli. Sweetness might not have reached threshold level for the 129 strain even at the highest concentrations of the amino acids tested (limited by solubility), so that if it was possible to test higher concentrations, they might be preferred by the 129 mice. This can be illustrated by comparing intakes of solutions of the amino acids and other sweeteners. When the highest intakes of the amino acids by the B6 mice were matched with intakes of other sweeteners, the corresponding concentrations of these other sweeteners were below preference thresholds for the 129 mice (compare two middle columns in Figure 2). Another possible reason for the indifference of the 129 mice to the amino acids may be that they are more sensitive to their unpleasant sensory or postingestive properties compared with the B6 mice.
The differences between the B6 and 129 strains in behavioral taste responses to at least some sweeteners are due to allelic variation of a few genes (Bachmanov et al., 1996a). The largest contribution to the mouse strain variation in responsiveness to sweeteners originates from the Sac locus (Fuller, 1974; Lush, 1989; Phillips et al., 1994; Lush et al., 1995; Bachmanov et al., 1997b; Bachmanov et al., 1997a; Blizard et al., 1999; Li et al., 2001). Other genetic loci, including dpa, also affect mouse strain variation in sweetener responsiveness (Ninomiya et al., 1987; Ninomiya et al., 1991; Phillips et al., 1994; Capeless and Whitney, 1995) and possibly affect the differences between the B6 and 129 strains (Bachmanov et al., 1996a; Bachmanov et al., 1997b).
In this study, we tested mice with compounds that taste sweet to humans. Mice (at least from the B6 strain), as well as many other animals including humans, show high avidity for sugars, sugar alcohols, amino acids and some artificial sweeteners (N-sulfonyl amides, a chlorinated sugar analog, and a guanidinacetic acid-based sweetener). However, some compounds (cyclamate, aspartame, thaumatin and the glycosides) that taste sweet to humans and other Old World simians (Glaser et al., 1995; Hellekant et al., 1996; Nofre et al., 1996) are evidently not sweet to mice and some other species (Jakinovich, 1981; Naim et al., 1982; Sclafani and Abrams, 1986; Hellekant et al., 1994; Hellekant et al., 1997; Danilova et al., 1998; De Francisco and Dess, 1998). The absence of a consistent preference for aspartame and thaumatin by the B6 and 129 mice corresponds to equally weak chorda tympani responses to them in both strains [see accompanying manuscript (Inoue et al., submitted)].
In summary, this study shows that compared with the 129 mice, B6 mice have higher preferences for, and intakes of, several compounds tasting sweet to humans. Cyclamate, aspartame, thaumatin, glycyrrhizic acid and neohesperidin dihydrochalcone are not palatable to mice.
Supplementary Material
Acknowledgments
The data for sucrose intakes and preferences presented in Figs. 1 and 2 have been published previously (Bachmanov et al., 1996b). Supported by NIH grants R01DC00882 (GKB) and R01AA11028 (MGT). We thank the following persons and organizations for providing us with sweeteners as gifts: Dr. Grant DuBois (SC-45647 and thaumatin); Hoechst Food Ingredients, Edison, NJ (acesulfame K); Mr. Hank Wong of the M&C Sweeteners, Blair, NE (erythritol); McNeil Specialty, New Brunswick, NJ (sucralose). We thank Drs. Stuart McCaughey and Masashi Inoue for comments on an earlier version of the manuscript.
References
- Bachmanov AA, Reed DR, Ninomiya Y, Inoue M, Tordoff MG, Price RA, Beauchamp GK. Genetic locus on mouse chromosome 4 influencing taste responses to sweeteners. Chemical Senses. 1997a;22:642. Abstract. [Google Scholar]
- Bachmanov AA, Reed DR, Ninomiya Y, Inoue M, Tordoff MG, Price RA, Beauchamp GK. Sucrose consumption in mice: major influence of two genetic loci affecting peripheral sensory responses. Mammalian Genome. 1997b;8:545–548. doi: 10.1007/s003359900500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov AA, Reed DR, Tordoff MG, Price RA, Beauchamp GK. Intake of ethanol, sodium chloride, sucrose, citric acid, and quinine hydrochloride solutions by mice: a genetic analysis. Behavior Genetics. 1996a;26:563–573. doi: 10.1007/BF02361229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov AA, Tordoff MG, Beauchamp GK. Ethanol consumption and taste preferences in C57BL/6ByJ and 129/J mice. Alcoholism: Clinical and Experimental Research. 1996b;20:201–206. doi: 10.1111/j.1530-0277.1996.tb01630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov AA, Tordoff MG, Beauchamp GK. Voluntary sodium chloride consumption by mice: Differences among five inbred strains. Behavior Genetics. 1998;28:117–124. doi: 10.1023/a:1021471924143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov AA, Tordoff MG, Beauchamp GK. Intake of umami-tasting solutions by mice: A genetic analysis. J Nutr. 2000;130:935S–941S. doi: 10.1093/jn/130.4.935S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp GK, Maller O, Rogers JG. Flavor preferences in cats (Felis catus and Panthera sp.) Journal of Comparative and Physiological Psychology. 1977;91:1118–1127. [Google Scholar]
- Belknap JK, Crabbe JC, Young ER. Voluntary consumption of alcohol in 15 inbred mouse strains. Psychopharmacology. 1993;112:503–510. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- Blizard DA, Kotlus B, Frank ME. Quantitative trait loci associated with short-term intake of sucrose, saccharin and quinine solutions in laboratory mice. Chemical Senses. 1999;24:373–385. doi: 10.1093/chemse/24.4.373. [DOI] [PubMed] [Google Scholar]
- Capeless CG, Whitney G. The genetic basis of preference for sweet substances among inbred strains of mice: Preference ratio phenotypes and the alleles of the Sac and dpa loci. Chemical Senses. 1995;20:291–298. doi: 10.1093/chemse/20.3.291. [DOI] [PubMed] [Google Scholar]
- Danilova V, Hellekant G, Tinti JM, Nofre C. Gustatory responses of the hamster Mesocricetus auratus to various compounds considered sweet by humans. J Neurophysiol. 1998;80:2102–2112. doi: 10.1152/jn.1998.80.4.2102. [DOI] [PubMed] [Google Scholar]
- De Francisco JC, Dess NK. Aspartame consumption in rats selectively bred for high versus low saccharin intake. Physiol Behav. 1998;65:393–396. doi: 10.1016/s0031-9384(98)00215-7. [DOI] [PubMed] [Google Scholar]
- Dess NK. Saccharin’s aversive taste in rats: evidence and implications. Neurosci Biobehav Rev. 1993;17:359–372. doi: 10.1016/s0149-7634(05)80113-7. [DOI] [PubMed] [Google Scholar]
- DuBois GE. New insights on the coding of the sweet taste message in chemical structure. Firmenich Jubilee Symposium 1895-1995 Olfaction and Taste: A Century for the Senses. 1995:32–95. [Google Scholar]
- Ferrell F. Preference for sugars and nonnutritive sweeteners in young beagles. Neurosci Biobehav Rev. 1984;8:199–203. doi: 10.1016/0149-7634(84)90041-1. [DOI] [PubMed] [Google Scholar]
- Fuller JL. Single-locus control of saccharin preference in mice. Journal of Heredity. 1974;65:33–36. doi: 10.1093/oxfordjournals.jhered.a108452. [DOI] [PubMed] [Google Scholar]
- Glaser D, Tinti JM, Nofre C. Evolution of the sweetness receptor in primates. I. Why does alitame taste sweet in all Prosimians and Simians, and Aspartame only in Old World Simians? Chemical Senses. 1995;20:573–584. doi: 10.1093/chemse/20.5.573. [DOI] [PubMed] [Google Scholar]
- Hellekant G, Danilova V, Ninomiya Y. Primate sense of taste: behavioral and single chorda tympani and glossopharyngeal nerve fiber recordings in the rhesus monkey, Macaca mulatta. J Neurophysiol. 1997;77:978–993. doi: 10.1152/jn.1997.77.2.978. [DOI] [PubMed] [Google Scholar]
- Hellekant G, Hard af Segerstad C, Roberts TW. Sweet taste in the calf: III. Behavioral responses to sweeteners. Physiol Behav. 1994;56:555–562. doi: 10.1016/0031-9384(94)90301-8. [DOI] [PubMed] [Google Scholar]
- Hellekant G, Ninomiya Y, DuBois GE, Danilova V, Roberts TW. Taste in chimpanzee: I. The summated response to sweeteners and the effect of gymnemic acid. Physiol Behav. 1996;60:469–479. doi: 10.1016/s0031-9384(96)80021-7. [DOI] [PubMed] [Google Scholar]
- Inoue M, McCaughey SA, Bachmanov AA, Beauchamp GK. Whole-nerve chorda tympani responses to sweeteners in C57BL/6ByJ and 129P3/J mice. Chemical Senses. doi: 10.1093/chemse/26.7.915. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakinovich W. Stimulation of the gerbil’s gustatory receptors by artificial sweeteners. Brain Res. 1981;210:69–81. doi: 10.1016/0006-8993(81)90885-4. [DOI] [PubMed] [Google Scholar]
- Li X, Inoue M, Reed DR, Huque T, Puchalski RB, Tordoff MG, Ninomiya Y, Beauchamp GK, Bachmanov AA. High-resolution genetic mapping of the saccharin preference locus (Sac) and the putative sweet taste receptor (T1R1) gene (Gpr70) to mouse distal chromosome 4. Mammalian Genome. 2001;12:13–16. doi: 10.1007/s003350010236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lush IE. The genetics of tasting in mice. VI. Saccharin, acesulfame, dulcin and sucrose. Genetical Research. 1989;53:95–99. doi: 10.1017/s0016672300027968. [DOI] [PubMed] [Google Scholar]
- Lush IE. The genetics of bitterness, sweetness, and saltiness in strains of mice. In: Wysocki CJ, Kare MR, editors. Genetics of Perception and Communication. Marcel Dekker; New York: 1991. pp. 227–241. [Google Scholar]
- Lush IE, Hornigold N, King P, Stoye JP. The genetics of tasting in mice. VII. Glycine revisited, and the chromosomal location of Sac and Soa. Genetical Research. 1995;66:167–174. doi: 10.1017/s0016672300034510. [DOI] [PubMed] [Google Scholar]
- Naim M, Rogatka H, Yamamoto T, Zehavi U. Taste responses to neohesperidin dihydrochalcone in rats and baboon monkeys. Physiol Behav. 1982;28:979–986. doi: 10.1016/0031-9384(82)90163-9. [DOI] [PubMed] [Google Scholar]
- Naim M, Striem BJ, Tal M. Cellular signal transduction of sweetener-induced taste. Advances in Food and Nutrition Research. 1998;42:211–243. doi: 10.1016/s1043-4526(08)60096-0. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Higashi T, Katsukawa H, Mizukoshi T, Funakoshi M. Qualitative discrimination of gustatory stimuli in three different strains of mice. Brain Research. 1984a;322:83–92. doi: 10.1016/0006-8993(84)91183-1. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Higashi T, Mizukoshi T, Funakoshi M. Genetics of the ability to perceive sweetness of d-phenylalanine in mice. Annals of the New York Academy of Sciences. 1987;510:527–529. [Google Scholar]
- Ninomiya Y, Mizukoshi T, Higashi T, Katsukawa H, Funakoshi M. Gustatory neural responses in three different strains of mice. Brain Research. 1984b;302:305–314. doi: 10.1016/0006-8993(84)90244-0. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Sako N, Katsukawa H, Funakoshi M. Taste receptor mechanisms influenced by a gene on chromosome 4 in mice. In: Wysocki CJ, Kare MR, editors. Genetics of Perception and Communication. Marcel Dekker; New York: 1991. pp. 267–278. [Google Scholar]
- Nofre C, Tinti JM, Glaser D. Evolution of the sweetness receptor in primates. II. Gustatory responses of non-human primates to nine compounds known to be sweet in man. Chem Senses. 1996;21:747–762. doi: 10.1093/chemse/21.6.747. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Crabbe JC, Metten P, Belknap JK. Localization of genes affecting alcohol drinking in mice. Alcoholism: Clinical and Experimental Research. 1994;18:931–941. doi: 10.1111/j.1530-0277.1994.tb00062.x. [DOI] [PubMed] [Google Scholar]
- Plata-Salaman C, Scott T, Smith-Swintosky V. Gustatory neural coding in the monkey cortex: the quality of sweetness. J Neurophysiol. 1993;69:482–493. doi: 10.1152/jn.1993.69.2.482. [DOI] [PubMed] [Google Scholar]
- Schiffman SS, Gatlin CA. Sweeteners: State of knowledge review. Neuroscience and Biobehavioral Reviews. 1993;17:313–345. doi: 10.1016/s0149-7634(05)80015-6. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Abrams M. Rats show only a weak preference for the artificial sweetener aspartame. Physiology and Behavior. 1986;37:253–256. doi: 10.1016/0031-9384(86)90228-3. [DOI] [PubMed] [Google Scholar]
- Tinti J-M, Nofre C. Design of sweeteners: A rational approach. In: Walters DE, Orthoefer FT, DuBois GE, editors. Sweeteners: Discovery, Molecular Design, and Chemoreception. American Chemical Society; Washington, DC: 1991. pp. 88–99. [Google Scholar]
- Whitney G, Harder DB. Genetics of bitter perception in mice. Physiology and Behavior. 1994;56:1141–1147. doi: 10.1016/0031-9384(94)90358-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


