Abstract
Ageing leads to functional deterioration of many brain systems, including the circadian clock - an internal time-keeping system that generates 24 hr rhythms in physiology and behaviour. Numerous clinical studies have established a direct correlation between the severity of neurodegenerative disorders, sleep disturbances and weakening of circadian clock functions. The latest data from model organisms, gene expression studies and clinical trials imply that the dysfunction of the circadian clock may contribute to the progression of ageing and age-associated pathologies, suggesting a functional link between the circadian clock, and age-associated decline of brain functions. Potential molecular mechanisms underlying this link include the circadian control of brain metabolism, reactive oxygen species homeostasis, hormone secretion, autophagy and stem cell proliferation.
Introduction
The circadian system, or circadian clock, provides a wide range of organisms from archaebacteria to humans with an adaptive advantage to the 24 hour periodicity in the Earth’s rotation 1. The growing list of circadian-clock-controlled physiological processes includes metabolism, hormone secretion and cardiac function, all of which exhibit daily oscillations. The circadian system also maintains the internal coordination of multiple oscillators within and between various organ systems in order to increase the fitness of an organism and provide the most efficient response to the environment 2–5. In humans, dysfunction of the circadian clock induced by shift work, for example, significantly increases the risk of developing metabolic syndromes, cardiovascular diseases and cancer, indicating that these circadian-clock-controlled processes play key roles in human physiology and pathology 6. Although the molecular mechanisms are unknown, it has been suggested that shift work leads to the resynchronization of circadian oscillators, uncoupling different organ systems from each other and from the central pacemaker 5.
A connection between the circadian clock and ageing has been recently proposed 7. Whereas the effect of ageing on the performance of the circadian system has been known for many years8, recent data show that a dysfunctional circadian clock can in turn contribute to ageing and pathologies associated with old age9. Here we will discuss a potential role of the circadian clock in the age-associated decline of brain function, with specific emphasis on age-associated brain disorders and neurodegenerative diseases. While sometimes it is difficult to dissociate the effect of sleep abnormalities and circadian clock dysfunction, multiple lines of evidence suggest that the activity of the circadian clock in the regulation of cognition and mood is far beyond the circadian control of sleep and the rest/activity cycle. Here we will focus on the relationship between the circadian clock and brain ageing with an emphasis on sleep-independent mechanisms. The role of sleep in age-associated cognitive decline has been recently reviewed 10 and is beyond the scope of this article.
The organization of the circadian clock
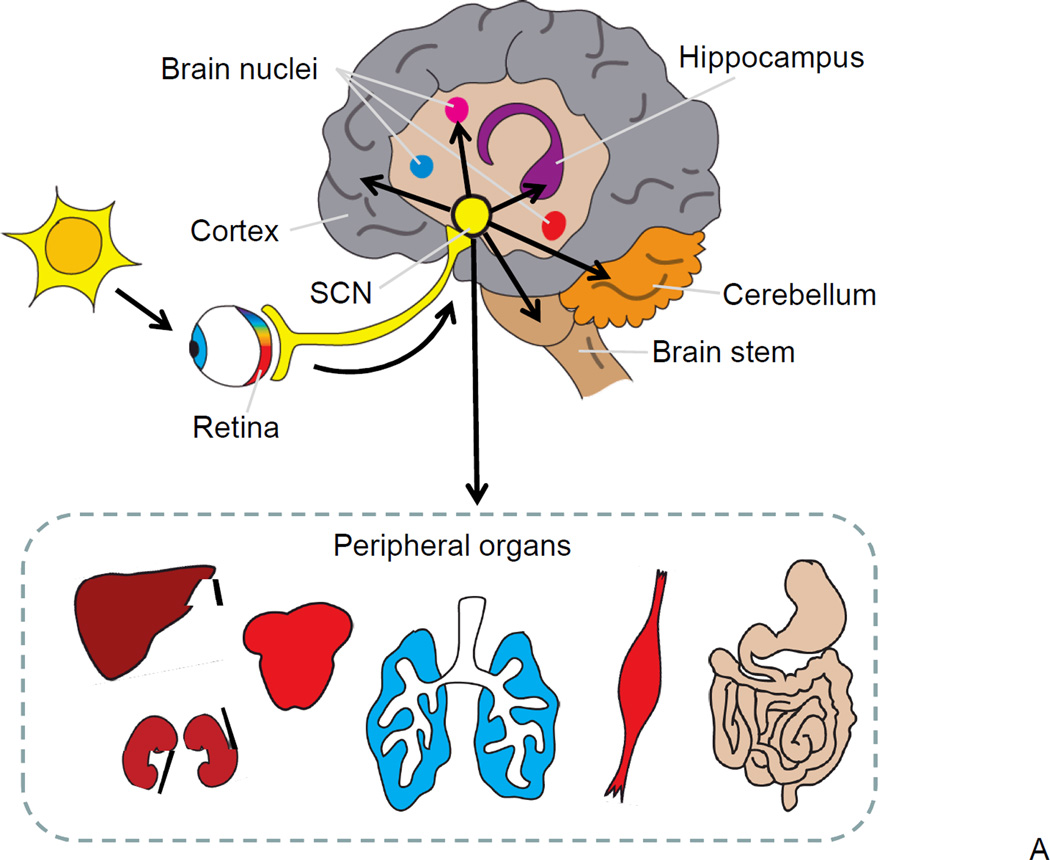
Virtually all tissues in an organism exhibit circadian oscillations in gene expression, underscoring the importance of the circadian clock throughout the body. Multiple reviews on the anatomical and physiological organization of the circadian clock have recently been published 6, 7, 11; therefore, here we provide only a short overview of the circadian clock system and the molecular components that form the circadian oscillator in the mammalian cell (Figure 1A). The central, or master, circadian clock is located in the suprachiasmatic nucleus (SCN) of the anterior hypothalamus 12. SCN neurons generate rhythmic electrical activity and produce synchronizing signals, which control the phases of the oscillations of so-called peripheral clocks operating in other tissues, such as the liver, heart and muscles. The rhythmic activity of the SCN is synchronized with the external light cues through signals from the retina. Peripheral tissues produce rhythmic physiological outputs which are orchestrated by the SCN and synchronized with the environment, thus providing optimal activity or response to an organism’s needs at the specific time of the day 13. Shift work-associated pathologies underscore the importance of such synchronization 5.
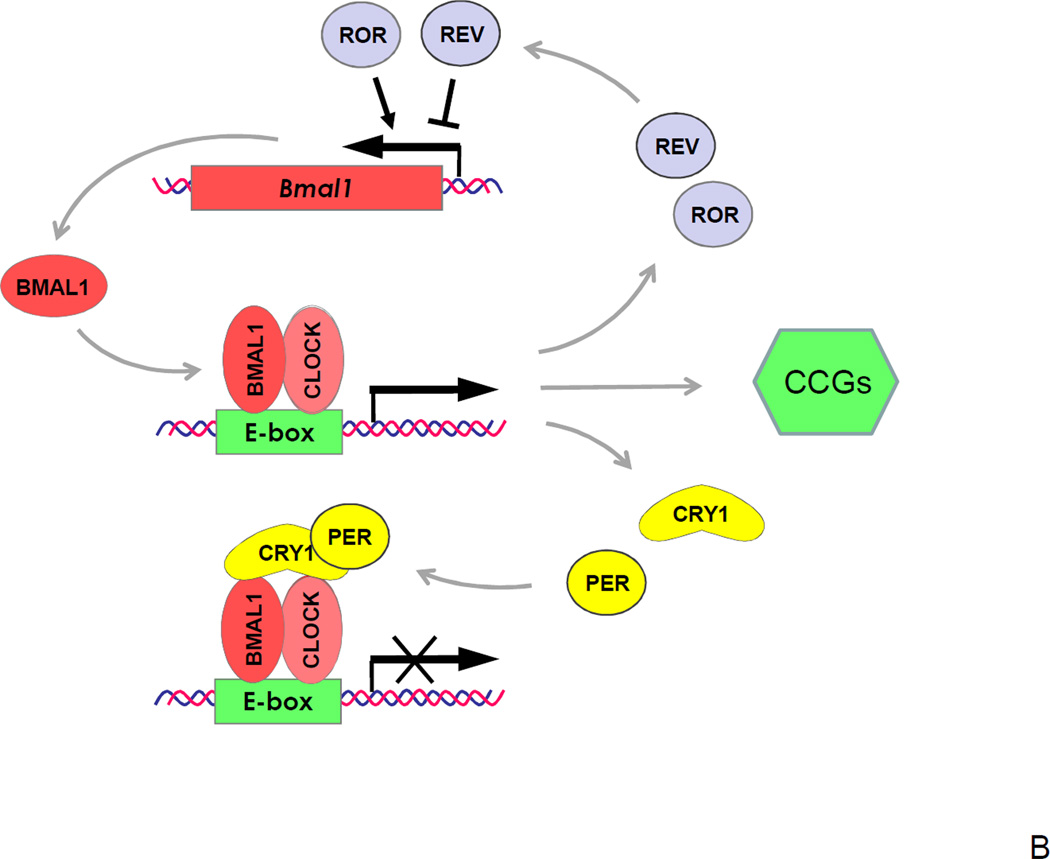
Figure 1. Circadian clock in mammals.
(A) A hierarchical organization of the circadian clock. The master pacemaker is located in the SCN of the hypothalamus and generates internal circadian rhythms in gene expression, electrophysiology and hormone secretion; direct projection from the retina transfers information about light/darkness to the SCN, which synchronizes a phase of SCN rhythms with the outside world. Local circadian clocks are found in different brain regions and throughout the body (peripheral oscillators). Electrical and humoral signals from the SCN synchronize phases of local circadian clocks oscillations. Local clocks generate rhythms in gene expression, metabolism and other physiological activities. (B) The molecular circadian oscillator. For the circadian clock transcriptional-translational negative feedback loop positive elements are shown in red, negative elements in yellow, and components that stabilize the loop in grey. Basic-HLH-PAS domain containing transcriptional factors BMAL1 and CLOCK (or NPAS2) regulate the transcription of genes with circadian E box elements in the promoter and represent positive elements of the transcriptional-translational feedback loop. The BMAL1:CLOCK complex activates the expression of Per and Cry genes. PER and CRY represent negative elements of the loop; they form complexes and inhibit the activity of BMAL1:CLOCK and hence their own expression. Rev-Erb-α and RORs represent an additional loop, these transcriptional factors negatively (Rev-Erb-α) and positively (RORs) regulate the expression of BMAL1. Finally, the BMAL1:CLOCK complex regulates the expression of circadian clock controlled genes (CCGs), which provide a circadian output in physiology.
The mechanisms underlying circadian rhythms involve circadian oscillations in gene expression, protein modifications and secretion. These oscillations are controlled by the products of the core circadian clock genes 14. Experiments in model organisms, in which single or multiple clock genes have been deleted or mutated, have revealed a short list of genes that are critically important for circadian rhythms in locomotion, behaviour, physiology and gene expression. The current model connects these circadian clock genes and their products in transcriptional-translational feedback loops 15, 16 (Figure 1B). Among clock gene products, there are positive regulators of transcription: BMAL1 (Brain and Muscle Arnt Like protein 1), its interacting partners CLOCK (Circadian Locomotor Output Cycles Kaput) or NPAS2 (Neuronal PAS domain-containing protein 2), and RORs (Retinoid-related Orphan Receptors), and negative regulators of transcription: CRYs (Cryptochromes), PERs (Periods) and Rev-Erb-α. The functions of CLOCK and NPAS2 proteins are partially redundant. The BMAL1:CLOCK or BMAL1:NPAS2 complexes specifically interact with and modify chromatin, which results in up regulation of the expression of a set of genes, including Cry and Per genes. Products of these genes, CRY and PER proteins form a complex that inhibits the activity of the BMAL1-containing complexes and thereby down regulate their own expression, thus forming a negative feedback loop. Rhythmic expression of Per1 and Per2 genes at the mRNA level can be detected in almost all tissues and serves as an indicator of circadian clock activity. Additional loops are formed with the help of Rev-Erb-α and RORs, which fine tune the oscillations generated by the main loop 17. Post-translational modifications and degradation of circadian proteins provide additional levels of regulation 18. It is also worth mentioning that although Bmal1 is the only circadian clock gene with a non-redundant clock function 19, it can be functionally substituted by Bmal2, if exogenous Bmal2 is expressed in the SCN of Bmal1 knockout mice 20. However, in contrast to Clock and Npas2 which are redundant, endogenous Bmal2 does not compensate for BMAL1 deficiency in Bmal1 knock out mice 19.
Circadian clock, sleep, mood and cognitive function
The expression of circadian clock genes rhythmically oscillates in different brain structures outside the SCN, suggesting the existence of multiple circadian clocks in the brain. It has been suggested that the synchronized activity of these clocks is critically important for a number of mental processes and that loss of phase coherence between these clocks (under influence of shift work for example) can negatively affect cognitive function 5, 21, 22. The regulation of sleep by the circadian clock is well-established, however, recent work has shown that the clock is also involved in regulation of mood, memory and adaptation to novelty 23.
Sleep is controlled by an interaction between the homeostatic drive for sleep and circadian clock-generated signals. Local circadian clocks are present in the brain stem, thalamus and cortex - the brain structures associated with sleep control. Circadian control of neural activities in these centres is important for daily rhythms of wakefulness/sleep. Deficiency in the circadian clock induced by a targeted disruption of clock genes impairs sleep in mice 5. The importance of the circadian clock in the sleep control in humans has also been demonstrated: a mutation in Per2 gene was found to be responsible for Familiar Advanced Sleep Phase Syndrome, while Delayed Sleep Phase Syndrome have been linked with polymorphisms in clock genes5.
Several lines of evidence link the circadian clock and regulation of mood 24. Linkage analysis studies in humans list circadian clock genes as candidates for the regulation of major depression, bipolar disorder and seasonal affective disorder 25. Another connection has been shown in a study in rodents: mice with mutation in the Clock gene demonstrate mania-like behaviour that has been attributed to increased dopaminergic activity in the ventral tegmental area26. Interestingly, drugs used for treatments of mood-related disorders directly regulate the activity and expression of circadian clock genes 27. These results suggest that an impaired circadian clock significantly affects mood in experimental animals and most likely contributes to mood-related disorders in humans.
The role of the circadian clock in cognition and especially in memory formation and consolidation is currently an active area of research 28. The circadian clock probably regulates memory though several mechanisms. One potential mechanism involves the circadian control of sleep: indeed, consolidation of procedural and declarative memory is sleep-dependent, and quality and duration of sleep is critical for cognitive functions. However, a growing body of evidence indicates the involvement of the circadian clock in memory formation and/or consolidation that is independent from its role in the regulation of sleep. Recent studies in animals in which different components of the circadian clock have been disrupted demonstrated impairment of contextual, spatial and time-related memory, as well as impaired adaptation to a novel environment29–33.Targeted disruption of circadian genes leads to defects in particular types of memory task, but not others. For example, Cry1,2−/− mice exhibit a deficit in time-place learning but not in other types of associated learning 31, Per2−/− mice demonstrate impairment in trace fear memory formation, but not in cued fear memory formation 34, NPAS2 −/− mice have impaired cued and contextual fear memory formation 32, and finally, BMAL1−/− and CRY1−/−,CRY2−/− deficient mice demonstrate opposite novelty-induced behaviours and habituation29.
In humans, cognitive abilities are known to fluctuate throughout the day. To dissect the sleep and the circadian clock-dependent components of this fluctuation, the sleep/wake cycle and the circadian clock cycle were completely desynchronized through a forced non-24-hour day, in which subjects followed 20-hour or 28-hour rest/activity schedules. Under these extreme conditions the internal circadian clock cannot be adjusted to external cues and continues to run at its intrinsic period of about 24 hours. When performance in an addition/subtraction test and a psychomotor vigilance task were evaluated, the authors found that the subjects’ abilities had a clear circadian profile, suggesting that the circadian clock has a sleep-independent effect on cognitive function in humans 35.
What molecular mechanisms could be engaged by the circadian system to control brain function? Long-term potentiation (LTP) requires activation of the transcriptional factor CREB through the MAP kinase and cyclic AMP-dependent pathways. In the mouse hippocampus, the level of cyclic AMP, phosphorylation of MAPK, which is a sign of activation, and CREB-dependent transcription exhibit circadian oscillations that peak during sleep time. Physiological and pharmacological interferences with circadian rhythms in MAPK phosphorylation significantly impair hippocampus-dependent memory formation 36. Thus, the circadian control of the AMP/MAPK/CREB signal transduction pathway provides a potential mechanistic explanation for the circadian control of memory 37.
Another potential mechanism is synchronization of local clocks throughout the brain. Temporal compartmentalization of metabolic and replication cycles in yeast was demonstrated to protect the cells’ genome 38, 39. A similar ‘temporal partitioning’ was suggested for the circadian-clock-dependent control of metabolic processes in peripheral tissues, specifically in the liver. According to this model, the phases of daily cycles of hepatic gene expression are locked by SCN signalling and, thus, local enzymatic activity is synchronized with the metabolic demands of activity and sleep 13, 40–44. Likewise,5 if circadian clock genes are expressed in the hippocampus, cortex, amygdala and other structures with different phases, the internal synchronisation of these local brain clocks imposed by the SCN pacemaker45may affect cognition. Thus, during waking hours, the gene expression pattern must support brain functions such as acquisition, responsiveness and processing of stimuli5; whereas, during sleep, when the process of short-term memory consolidation occurs, the gene expression pattern must support synaptic rewiring 5. However, at present there is no direct experimental support of this model.
Neoneurogenesis is an essential component of cognition46 and may also be a mechanism through which the circadian clock regulates cognitive function. Circadian-clock-dependent regulation of neoneurogenesis has been proposed based on a few observations that neurogenesis among the olfactory projection neurons in lobsters47 as well as cell proliferation in the dentate gyrus of the adult rat48 fluctuates with the light-dark cycle. However, it is unclear if the observed rhythms in cell proliferation are due to true circadian control or they are coupled to daily patterns of activity or sleep.
Circadian clock and ageing
Ageing is a complex biological process affecting activity of many physiological systems including the circadian clock 7, 8. Functional weakening of the circadian system with age has been known for many years. The effect of ageing on circadian clock activity has been reported in different model organisms including invertebrates 49,50,51,52, rodents 53 and primates 54, suggesting that the effect of ageing on the circadian system is highly conserved.
Phase advance and reduced amplitudes of circadian rhythms of temperature and hormone secretion (specifically melatonin and cortisol) have been detected in aged humans 8, 55. However, there are discrepancies between studies on the effect of aging on melatonin and body temperature rhythms 56. A reduction of circadian amplitude was reported in several studies, but predominantly in old males 56. By contrast, no reduction in amplitude was observed in old females 56, 57. The sensitivity of the circadian system to light is dramatically reduced with age as measured by the induction of c-fos expression in the SCN of hamsters 58, mice59 and primates 60. This reduced response to light may contribute to the impaired entrainment of the circadian clock by light cues in aged rats 61.
Ageing does not have an effect on the size or the number of neurons in the SCN 62; however, a decrease in the number of vasopressin expressing neurons has been found in the SCN of ageing rats63. Aging brings about significant changes in electrophysiological and neurochemical outputs of the SCN 64. The amplitude of the SCN cells’ electrophysiological65 and hormone secreting activity66 and their gene expression levels 67, 68 all decline in the ageing brain. These age-associated changes in the SCN have been proposed to be responsible for the impairment of circadian clock synchronization throughout the body8.
In mice, disruption of the circadian system by targeted deletions of clock genes significantly affects the rate of ageing. Deficiency of the transcriptional factor BMAL1 results in development of a premature ageing phenotype, characterised by multiple age-related pathologies and an almost three fold reduction in lifespan 69. Interestingly, flies with a mutation in the cycle gene (a drosophila homolog of Bmal1) have reduced longevity 70, although the effect is gender-specific as lifespan is reduced only in males. These findings suggest that the role of BMAL1 in ageing is conserved in evolution.
In mice, deficiency of CLOCK (Clock−/−) results in an increased rate of inflammation, development of cataracts and a 15% reduction in longevity 71. The lifespan of mice with mutations in both Clock and Per2 genes have not been reported; however, after exposure to non-lethal doses of ionizing irradiation these mutants demonstrate a reduced lifespan and some ageing-associated phenotypes72, 73. They do not develop the accelerated ageing phenotype that is characteristic for Bmal1−/− mice so, although disruption of the circadian clock affects the rate of ageing regardless of the particular mechanism of disruption, the activity of some clock proteins, such as BMAL1, is much more important in the control of ageing than the activity of other proteins, such as CLOCK or PERs. This suggests that circadian clock proteins have non-circadian roles in ageing (see also 53, 74).
Circadian clock and cognitive impairment associated with brain ageing
Both healthy and pathological brain ageing are associated with multiple impairments in cognitive function. Sleep duration, quality and pattern together with phase of sleep and wakefulness are significantly different in elders 75,10. An ageing-related decline in spatial, declarative and other forms of memory is well-documented 10, 76, 77. Furthermore, changes in mood regulation: increased frequency of episodes of depression, mania and anxiety, are also observed with age 78–80. How does the circadian clock contribute to these processes?
A potential role of the clock in brain ageing is illustrated in Figure 2. A decline in clock functions contributes to sleep impairment 21, 22, but as mentioned above, the effect of circadian system ageing on cognitive functions may be both sleep-dependent and sleep-independent. It is difficult to distinguish between these effects. For example, sleep is important for memory consolidation, thus, the disruption of normal sleep patterns as a result of circadian clock ageing can be one of the contributors to memory impairment5, 10. In agreement with this, flattened amplitude of circadian activity in aged rats is associated with a deficit in long-term (sleep-dependent) spatial memory, whereas working memory is unaffected 81. At the same time, sleep-independent mechanisms of clock-controlled memory and mood (discussed above) would also be impaired upon ageing of the circadian clock. Although ageing of the circadian system is not equivalent to the disruption of the circadian clock in circadian clock mutants, we may expect that the effects on cognition observed in circadian clock mutant models reflect to some extent the effects of circadian system impairment in the ageing brain. Further study of the role of specific circadian proteins in the control of cognition, sleep and ageing using circadian mutant animals may provide some clues about how ageing of the circadian clock contributes to age-related cognitive dysfunctions and might help to separate the effects of circadian rhythms and sleep on cognition.
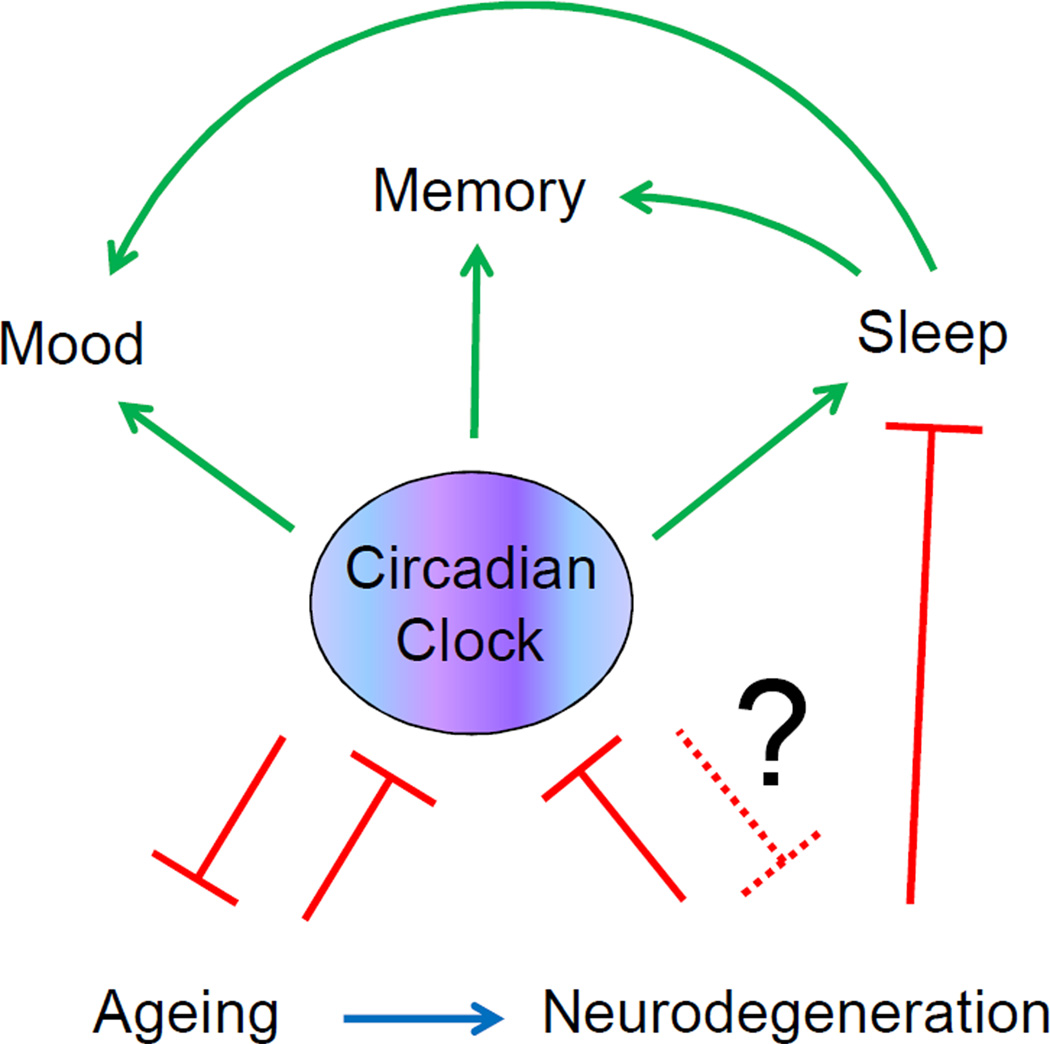
Figure 2. The circadian clock, ageing and cognitive functions.
The circadian clock regulates sleep and other brain cognitive functions such as memory and mood in a sleep-dependent and sleep-independent manner. Ageing is associated with a decline in the activity of the circadian system; this decline in turn can contribute to ageing and to age-associated changes in sleep, memory and mood. Neurodegeneration is also associated with ageing and affects sleep and circadian clock functions, this disruption of sleep and circadian rhythms will affect cognitive functions. It is possible that the circadian clock is involved in control of neurodegeneration. Potential circadian clock independent effects of ageing and neurodegeneration on cognitive functions are omitted for simplicity.
Neurodegenerative diseases and the circadian clock
Ageing of the circadian system contributes to the decline in mental performance of aged brains, but the circadian clock may also be involved in specific age-associated neurodegenerative diseases such as Alzheimer’s, Parkinson’s and Huntington’s diseases (AD, PD and HD)22. Indeed, as it has been demonstrated through multiple clinical reports, while each of these three pathologies have their unique pathological signatures, the disruption of sleep and circadian rhythms is one of the common and earliest signs of Alzheimer’s, Parkinson’s and Huntington’s diseases; abnormalities in the circadian clock and sleep worsen as the disease progresses22, 82 (see details on neurodegeneration associated circadian disruption in Box1). Degeneration of brain nuclei containing sleep circuits and circadian clock-regulating circuits in the SCN 83, 84, hypothalamus85–87, basal forebrain 85, 88 and brain stem 87, 89, 90 is one of the possible causes of sleep defects in patients with neurodegenerative diseases. These brain structures release different neurotransmitters; during wakefulness, excitatory neurotransmitters such as adrenaline, serotonin and histamine are released, whereas the release of inhibitory neurotransmitters such as GABA and galanin is suppressed. Disruption of this neurotransmitter release/suppression pattern with age or as a result of neurodegeneration may contribute to defects in sleep and circadian clock activity22. The negative effect of neurodegeneration on the circadian clock has been confirmed in animal models of AD 91, PD92 and HD93.
Box 1. Circadian disruption in ageing-associated neurodegenerative diseases.
Alzheimer’s disease (AD). AD is the most common neurodegenerative disease in aged humans. It is characterized by progressive dementia, memory impairment, mood change and disrupted circadian rhythms. The anatomical feature of AD is a degeneration of multiple regions of the brain.137 Degeneration in the VLPO, SCN and basal forebrain is, almost certainly, responsible for problems with sleep and circadian rhythms138. Nocturnal production of melatonin is also significantly reduced. Histological hallmarks of AD are extracellular protein deposits where β-amyloid peptide is a main component (senile amyloid plagues) and intraneuronal aggregates of hyperphosphorylated microtubule associated protein tau (neurofibrillary tangles)137. Senile plaques and tangles play essential roles in generation of reactive oxygen species. Oxidative stress is, most likely, responsible for the death of neurons and astrocytes in the brain of AD patients94.
Parkinson’s disease (PD). PD is neurodegenerative disease that significantly affects a patient’s motor functions. Typical pathological features are bradykinesia, tremor and rigidity139. PD is associated with progressive loss of the substantia nigra dopaminergic neurons. Intracytoplasmic inclusions termed Lewy bodies formed by α-synuclein-containing aggregates and dystrophic neuritis are the most common anatomical abnormalities observed in the brain of PD patients140. PD is associated with reduced mitochondria function and increased oxidative damage to lipids, proteins and DNA; protein and lipid aggregates found in Lewy bodies are generally oxidized. Evidence of circadian disruption in PD include: significant sleep disturbance, and problems with the ability to fall and/or stay asleep141. There are also indications for disrupted rhythms of melatonin release.
Huntington’s disease (HD). HD is an autosomal dominant neurodegenerative disorder. Pathological features are cognitive impairment, motor skill impairment and premature death142. The disease is caused by expansion of trinucleotide repeat in the huntingtin gene, which leads to the formation of a polyglutamine tract at the N-terminus of the protein. The mutated protein cannot be efficiently degraded and forms aggregates leading to the accumulation of neuronal inclusion bodies. It was suggested that the inclusion bodies affect mitochondria and increase oxidative stress, which cause death of striatal neurons and astrocytes142. Atrophy of the lateral hypothalamus was suggested to contribute to the disruption circadian timing in HD patients143.
The correlation between neurodegeneration and circadian clock dysfunction raises important questions: first, whether or not the circadian system is involved in the pathophysiology of neurodegenerative diseases, and if it is, how does the circadian clock affect the development of neurodegenerative diseases? On the one hand, the intact circadian system may have neuroprotective functions; on the other hand, disruption of the circadian clock seems to promote neurodegeneration. Hypothetical connections between the circadian clock and neurodegeneration are illustrated in Figure 3 and are discussed in more detail.
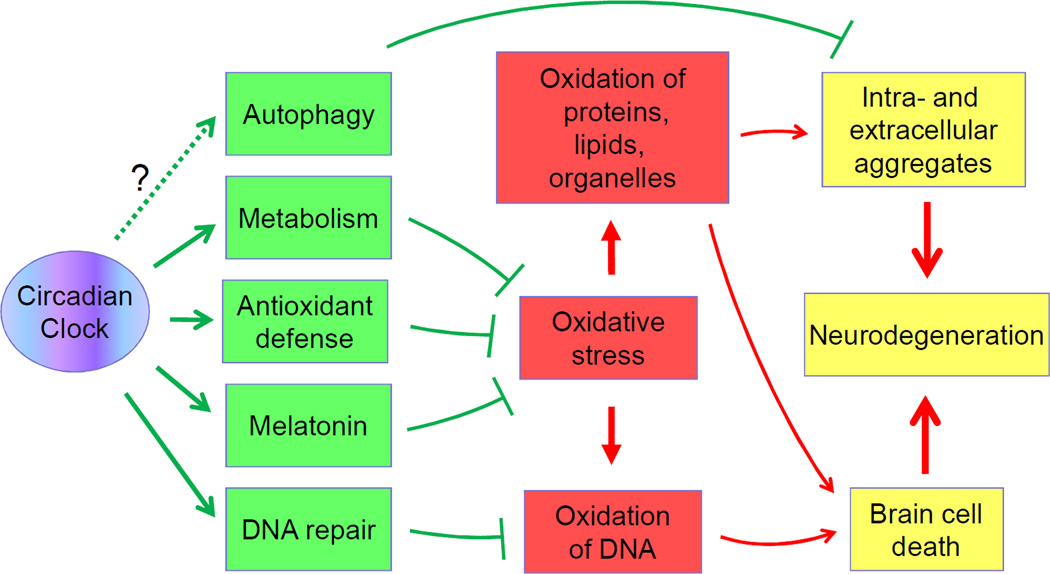
Figure 3. Potential mechanisms of circadian clock-dependent regulation of neurodegeneration.
The circadian clock regulates metabolism, ROS homeostasis, DNA repair and, probably, autophagy (circadian clock controlled systems and pathways are shown in green). Disruption of circadian system function will compromise the activities of these systems, which will lead to oxidative stress (shown in red) and accumulation of intra- and extra-cellular aggregates in the brain. This in turn will lead to brain cell death and degeneration of brain structures (shown in yellow). Similar mechanisms can contribute to the changes in the brain during the normal ageing.
One of the main features of the three above-mentioned neurodegenerative diseases is increased oxidative stress, which most likely plays a role in neurodegeneration94. In AD, oxidative damage leads to an increased accumulation of DNA oxidation products in the nuclei of neurons and elevated levels of oxidized proteins and lipids, which can be found in plaques and neurobrillary tangles. In PD, the products of protein oxidation and lipid peroxidation can be detected in Lewy bodies. Mitochondria dysfunctions are described in HD patients, and increased oxidative stress was reported in a mouse model of this disease. Increased oxidative damage results in the death of neurons, astrocytes and oligodendrocytes, as well as degeneration of normal brain structures. Possible mechanisms include dysfunction of damaged mitochondria, microglia overactivation, protein/lipid plaques formation and reduced antioxidant defence 94.
Weakened antioxidant defence in the ageing brain may contribute to increased oxidative stress; however, the mechanisms involved are unknown 95. The role of the circadian system in the regulation of reactive oxygen species homeostasis is well-established. ROS levels oscillate in different mouse tissues 69 and these rhythmic patterns are disrupted in mice with impaired circadian clocks. ROS misbalance was proposed as a potential mechanism of premature ageing of BMAL1-deficient mice; in agreement with this, treatment with the antioxidant NAC increased the longevity of these animals 96. Daily oscillations in the level of the products of lipid and protein oxidation have been observed 97. The circadian clock can orchestrate antioxidant defence by controlling the daily oscillation in the expression of antioxidant enzymes and low molecular weight antioxidants.
Potential mechanistic links between the circadian clock and ROS homeostasis have been studied in model organisms. In Neurospora crassa, the circadian clock may regulate ROS levels through the small membrane-anchored G-protein RAS-198 and through regulation of catalase expression and activity99. In Drosophila melanogaster, the sensitivity to oxidative stress, protein carbonylation and hydrogen peroxide productionchanges throughout the day and these rhythms are disrupted in circadian mutants bearing a null mutation in the period gene. Reduced activity of catalase in circadian mutants may be responsible for their increased sensitivity to oxidative stress 100. It is possible that a similar mechanism regulates ROS homeostasis in in mammals. In agreement with the importance of the circadian clock in redox state regulation, circadian rhythms of peroxiredoxin oxidation have been observed in nucleated and enucleated (red blood cells) cells 101. Similar transcription independent circadian rhythms were observed in pico-eukaryotic alga Ostreococcus tauri 102, but the underlying mechanisms are unknown in both cases.
Another possibility, although not demonstrated directly, is that the circadian clock regulates ROS production: multiple reports link the circadian clock with daily oscillations in metabolism and body temperature, and in turn, metabolic rhythms are intimately linked with the activity of the mitochondria and ROS generation as a side product of oxidative catabolism. Many metabolic enzymes and metabolism-regulating growth factors and hormones are under the direct control of the circadian clock13; disruption of the circadian clock leads to numerous defects in metabolism and to the development of metabolic syndromes, which are known to be associated with increased oxidative damage. If, similarly to the regulation of metabolism of the liver and other peripheral organs, the clock regulates metabolism in the brain, one can expect that disruption of this regulation will lead to oxidative damage. Death of brain cells, especially neurons and astrocytes, is a hallmark of neurodegeneration. Because differentiated neurons are unable to divide, mechanisms to repair damage are extremely important in the physiology of the neuron. Indeed, accumulation of damaged DNA may lead to activation of apoptotic programmes; damaged proteins and lipids may form aggregates, intra- and extracellular inclusions; damaged organelles, such as mitochondria, can directly induce cell death. Several mechanisms responsible for the elimination of damaged macromolecules may also be under the control of the circadian clock.
ROS are constantly produced in the brain as metabolic byproducts and probably as signal molecules; oxidative stress is one of the main reasons of brain cell death. Repair of oxidized DNA is critical for the survival of brain cells. Nucleotide excision repair systems remove two major products of DNA oxidation, 8-oxoguanine and thymine glycol. Nucleotide excision repair activity rhythmically oscillates during the day with a peak in the early evening 103. In agreement with this, one of the DNA damage recognition proteins, the core excision repair factor xeroderma pigmentosum A exhibits a circadian, time-dependent oscillation. Thus, it was proposed that circadian control of nucleotide excision repair plays an important role in neuroprotection104. This role may be especially important in neurodegenerative diseases, which are associated with increased oxidative stress: disruption of the normal clock functions may result in reduced removal of DNA lesions and increased neuron death.
The three neurodegenerative diseases discussed are associated with the deposition of aggregates in the brain: B-amyloid plaques and neurofibrillary tangles in patients with AD, Lewy bodies in patients with PD and huntingtin-containing neuronal inclusion bodies in patients with HD, suggesting some failure in the systems responsible for the degradation of damaged proteins and other cytoplasmic components. The ubiquitin-proteasome dependent system and autophagy are the two major systems responsible for degradation of cellular proteins, including misfolded and damaged proteins 105, 106. Autophagy is also responsible for the degradation of lipids and non-functional or damaged organelles such as mitochondria and peroxisomes. Although there is a significant difference between pathologies and causes of AD, PD and HD, defects in autophagy have been reported in all of these diseases106. The mechanisms of autophagy deregulation in neurodegeneration are mostly unknown, but some insights have been provided by recent studies106. In patients with AD the clearance of autophagic β-amyloid-containing vacuoles is defective. Increased autophagic degradation of mitochondria is another pathological change observed in AD106. In patients with PD, autophagosomes accumulate, lysosome-dependent degradation of α-synuclein is impaired and targeting of damaged mitochondria for degradation through mitophagy is defective106. Defective autophagy has also been implicated in the degradation of huntingtin aggregates and general protein turnover is reduced in patients with HD. The current model suggests that defects in cargo recognition result in autophagic vacuoles that contain minimal cargo106. However, the relationship between autophagy and neurodegeneration is complex; autophagy must be tightly regulated as both suppression and over activation of autophagy can be dangerous106.
The circadian clock is one of the potential systems for tuning autophagic activity 107. Daily rhythms of autophagy in rat hepatocytes and cardiomyocytes108 (peaking near to resting phase and at a minimum at the time of feeding) have been reported. Similar oscillations have also been also reported in the kidney109 and retina110. The physiological significance of these rhythms is unclear, as is whether the circadian clock is involved in the control of autophagy. The expression of several genes associated with autophagy rhythmically oscillate in the liver in a circadian manner suggesting a molecular connection between the circadian oscillator and autophagy107. C/EBPbeta was identified as a key link between autophagy and the circadian clock; this transcriptional cofactor can activate autophagy in vitro, it is rhythmically expressed in the liver and the disruption of its circadian expression in mice lacking a functional liver clock correlates with disrupted rhythms in autophagy in the liver111. If, like in the liver and the heart, autophagy has rhythmic variations in the brain, the disruption of circadian rhythms could lead to the accumulation of aggregates and damaged mitochondria, contributing to the development of neurodegenerative disease and other age-associated brain dysfunctions.
Another potential link connecting the circadian clock and neurodegeneration is the activity of melatonin112. Melatonin is a peptide hormone produced by the pineal gland in a circadian manner. It has a myriad of activities, such as intracellular signal transduction and regulation of cell death and proliferation. Melatonin is also produced by other body tissues, but pineal gland production is the most relevant action of melatonin on brain function. The most studied melatonin function is its antioxidant activity. It was demonstrated that melatonin can be more efficient than many classical antioxidant scavengers such as vitamins E and C112. Melatonin is a naturally synthesized compound, its high local concentration in the brain and cerebrospinal fluid makes it a highly attractive candidate to provide a molecular connection between the circadian clock and neuroprotection. Indeed, disrupted production of melatonin can significantly compromise the normal brain antioxidant defence. Additionally, melatonin production decreases with age, and could be a predisposing factor for neurodegeneration 113, 114 and mood disorders115. The circadian production of melatonin is affected in Alzheimer’s, Parkinson’s and Huntington disease112. Reduced amplitude, earlier timing of peak and diminished amount of total secretion in melatonin secretion rhythms have been reported for AD patients55, 116. In HD patients, the timing of the evening peak in melatonin level is significantly delayed but total secretion is not different from control group117. Melatonin therapy (oral administration of the hormone) has recently been used for the treatment of AD and PD, but its efficacy is unclear (see next section).
Overactive glia and impaired immune functions have also been proposed to contribute to AD, PD and HD. It was recently demonstrated that disruption of circadian clock function affects the release of inflammatory cytokines in response to endotoxin118 or lipopolysaccharide challenge 119. One of the potential mechanisms underlying this effect is the circadian-clock-dependent production of cortisol, which is a powerful regulator of the activity of the immune system. The daily oscillation of cortisol levels is significantly affected by ageing; thus, a defect in cortisol secretion may contribute to the misbalance of the immune system regulation and to the pathology of age-associated diseases.
Modulation of the circadian clock and brain ageing
Light therapy and melatonin treatments have been used for a long time as mechanisms to reset the circadian clock in humans. Several reports on the effects of light therapy and melatonin treatment on the progression of neuropsychiatric diseases in aged people or people suffering from specific disorders are promising120. In patients with AD, treatment with melatonin has mixed results: no significant improvements in sleep between melatonin treated and control groups were reported in several double-blind randomised placebo controlled studies121–123, but because the effects of melatonin are dependent on the phase of the circadian cycle, combinations of melatonin and bright light therapy significantly improve patients sleep and daytime activity according to other studies124, 125. Mild cognitive impairment (MCI) is an etiologically heterogenous syndrome, with about 12% of MCI cases being associated with AD. Melatonin alone or in combination with light therapy shows significant beneficial effects in patients with MCI112. In mouse models of AD, treatment with melatonin inhibits oxidative and amyloid pathology, protects against cognitive deficits and neurodegeneration and increases survival126, 127. Interestingly, in vitro melatonin interacts with amyloid β-protein and inhibits the formation of amyloid fibrils128.
While melatonin demonstrates a significant neuroprotective role in animal model of PD129, there is a controversy about its role in clinical treatment of PD. Use of melatonin in combination with bright light exposure improves sleep quality in PD patients, suggesting that the circadian clock can be used not only as early marker of the disease, but as a means of potential therapeutic intervention according to one study130, according to other studies melatonin does not demonstrate any therapeutic effects131–133, thus, more study on melatonin as a potential drug for PD is necessary.
Even more impressive are recent studies in experimental organisms, which suggest that it is possible to delay the development of neurodegenerative disease by treatment of sleep abnormalities and improvement of circadian clock functions. In a mouse model of HD, restoration of daily rest/activity cycle with the benzodiazepine alprazolam restored dysfunctional circadian clock gene expression, improved cognitive performance and significantly extended the lifespan of mice134, 135. Scheduled feeding can also restore daily behavioural rhythms in this model, but the effect of this intervention on the development of pathology has not been reported136.
It is also worth mentioning that many pharmacological drugs used for the treatment of neuropsychiatric disorders affect the function of the circadian clock leading to the possibility that they may, at least partially, act by restoring circadian clock function. Will the resetting of the circadian clock help to delay the decline in cognitive functions during normal ageing? To the best of our knowledge, no studies have been published on this subject.
Conclusions and future directions of research
During the last several decades human lifespan has dramatically increased, creating a new level of challenges for biomedical sciences. Chiefly, how to improve the quality of life of the aged population and promote healthy ageing. Diseases of the ageing brain, specifically neurodegenerative diseases, attract significant attention because the incidence of these pathologies is on the rise. To develop rational strategies for the prevention and treatment of these diseases, it is necessary to identify the molecular pathways associated with these pathologies. The circadian clock is one of the body’s physiological systems recently linked to the control of ageing. It is becoming clear that the physiological function of the circadian clock is relevant for multiple brain functions, including sleep, mood, and memory; therefore, ageing of the clock affects these functions and contributes to age-associated cognitive decline. Circadian clock-dependent control of several pathways known to be involved in the pathology of neurodegeneration, such as metabolism, ROS homeostasis and oxidative stress response, DNA damage repair and, potentially, autophagy suggests that defects in circadian clock functions may have causative roles in AD, PD and HD.
Many questions still remain to be answered. The hypothesis that the circadian clock is involved in the regulation of oxidative stress in the brain needs to be tested. Is the circadian clock involved in daily metabolic processes in the brain? How is this related to ROS generation? Is the circadian clock involved in brain antioxidant defence? What are the molecular mechanisms responsible? Is the beneficial effect of melatonin on the pathology of neurodegenerative diseases associated with restoration of circadian clock function, with the antioxidant properties of melatonin, or with some other melatonin function? The use of mouse strains that do not produce significant amounts of melatonin may shed some light on this issue. The therapeutic effect of melatonin was demonstrated in the mouse model of AD, therefore, it is possible to test if circadian clock functions are important for melatonin function by crossing AD transgenic mice with circadian clock mutant mice and investigating the effect of melatonin in the generated hybrids. Is the circadian clock involved in the control of autophagy in the brain? And if the clock is involved, does ageing-associated impairment of the circadian clock contribute to the deposition of extra- and intra-cellular aggregates in neurodegenerative diseases? Finally, recently several potential pharmacological modulators of the circadian clock in vitro have been reported. If the biological activity of these modulators is confirmed in vivo, they need to be tested in animal models of neurodegeneration. It will be important to see if they will improve the function of the clock under pathological conditions and even more important to see whether it will affect the development of the disease.
The evidence available so far warrants further research into the role of the circadian clock in the regulation of normal and pathological brain physiology. Targeting the circadian clock could be a novel direction for diagnosis and treatment of disorders of the ageing brain, including neurodegenerative conditions.
Online 'at-a-glance' summary.
Recent data suggest that the circadian clock regulates cognitive functions such as memory and mood in sleep-dependent and sleep-independent manner.
Some circadian clock proteins are important regulators of longevity.
Circadian rhythms in sleep and behaviour are significantly affected by ageing; this impairment can contribute to the cognitive decline of ageing brain
Circadian rhythms in sleep and behaviour are significantly disturbed in AD, PD and HD patients. Circadian clock disruption can serve as a biomarker of a neurodegenerative disease progression.
Circadian clock dependent control of metabolism, oxidative stress response, DNA repair and autophagy can contribute to brain ageing and neurodegeneration.
Restoration of circadian clock function in animal model of neurodegeneration improves a cognitive performance and increases the survival.
Restoration of circadian rhythms with combined light and melatonin therapy improves sleep quality in humans.
Acknowledgements
We thank Dr. Monica Hoyos Flight and three anonymous referees for critical reading of the manuscript and for suggestions, which help us to improve the manuscript significantly. We thank Willam Samsa for editorial help. This work was supported by 1R03AG033881 and 1R01AG039547 to R.V.K.
Glossary
- Circadian clock
A complex of genetically determined molecular, cellular and physiological processes, which result in the generation of near 24-hrs rhythms (known as circadian rhythms) in organism behaviour, metabolism etc.
- Advanced Sleep Phase Syndrome (ASPS)
A circadian rhythm sleep disorder which causes a few hours advanced phase in circadian rhythms and the sleep/wake cycle. Familial ASPS is genetically determined syndrome associated with mutation in circadian clock gene Per2 or Casein kinase 1 delta.
- Delayed Sleep Phase Syndrome (DSPS)
Also known as Delayed Sleep Phase Disorder, is a circadian rhythm sleep disorder which causes a few hours delayed phase in circadian rhythms and the sleep/wake cycle. Light therapy, sleep phase chronotherapy and melatonin administration have been used for the treatment of DSPS.
- Long-term potentiation (LTP)
LTP is an enhancement in synaptic strength and candidate cellular mechanism of learning and memory.
- Synaptic rewiring
Synaptic rewiring, also known as a synaptic plasticity, is a process of formation and elimination of synapses in the nervous system.
- Mitophagy
Mitophagy is a process of removal of mitochondria through macroautophagic pathways.
- Light therapy
An experimental therapy that has been used to treat disorders associated with disrupted circadian rhythms. At a specific time of the day patients are exposed to light (daylight or artificial light) of defined intensity.
References
- 1.Bell-Pedersen D, et al. Circadian rhythms from multiple oscillators: lessons from diverse organisms. Nat Rev Genet. 2005;6:544–556. doi: 10.1038/nrg1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2011;330:1349–1354. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gery S, Koeffler HP. Circadian rhythms and cancer. Cell Cycle. 2010;9 doi: 10.4161/cc.9.6.11046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young ME. The circadian clock within the heart: potential influence on myocardial gene expression, metabolism, and function. Am J Physiol Heart Circ Physiol. 2006;290:H1–H16. doi: 10.1152/ajpheart.00582.2005. [DOI] [PubMed] [Google Scholar]
- 5.Kyriacou CP, Hastings MH. Circadian clocks: genes, sleep, and cognition. Trends Cogn Sci. 2010;14:259–267. doi: 10.1016/j.tics.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 6.Dardente H, Cermakian N. Molecular circadian rhythms in central and peripheral clocks in mammals. Chronobiol Int. 2007;24:195–213. doi: 10.1080/07420520701283693. [DOI] [PubMed] [Google Scholar]
- 7.Kondratov RV. A role of the circadian system and circadian proteins in aging. Ageing Res Rev. 2007;6:12–27. doi: 10.1016/j.arr.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Hofman MA, Swaab DF. Living by the clock: the circadian pacemaker in older people. Ageing Res Rev. 2006;5:33–51. doi: 10.1016/j.arr.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Tranah GJ, et al. Circadian activity rhythms and mortality: the study of osteoporotic fractures. J Am Geriatr Soc. 2010;58:282–291. doi: 10.1111/j.1532-5415.2009.02674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pace-Schott EF, Spencer RM. Age-related changes in the cognitive function of sleep. Prog Brain Res. 2011;191:75–89. doi: 10.1016/B978-0-444-53752-2.00012-6. [DOI] [PubMed] [Google Scholar]
- 11.Gachon F. & Bonnefont, X Circadian clock-coordinated hepatic lipid metabolism: only transcriptional regulation? Aging (Albany NY) 2010;2:101–106. doi: 10.18632/aging.100123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 13.Green CB, Takahashi JS, Bass J. The meter of metabolism. Cell. 2008;134:728–742. doi: 10.1016/j.cell.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006;15(Spec No 2):R271–R277. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- 15.Shearman LP, et al. Interacting molecular loops in the mammalian circadian clock. Science. 2000;288:1013–1019. doi: 10.1126/science.288.5468.1013. [DOI] [PubMed] [Google Scholar]
- 16.Nakahata Y, Grimaldi B, Sahar S, Hirayama J, Sassone-Corsi P. Signaling to the circadian clock: plasticity by chromatin remodeling. Curr Opin Cell Biol. 2007;19:230–237. doi: 10.1016/j.ceb.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 17.Hirayama J, Sassone-Corsi P. Structural and functional features of transcription factors controlling the circadian clock. Curr Opin Genet Dev. 2005;15:548–556. doi: 10.1016/j.gde.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Harms E, Kivimae S, Young MW, Saez L. Posttranscriptional and posttranslational regulation of clock genes. J Biol Rhythms. 2004;19:361–373. doi: 10.1177/0748730404268111. [DOI] [PubMed] [Google Scholar]
- 19.Bunger MK, et al. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103:1009–1017. doi: 10.1016/s0092-8674(00)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi S, et al. Circadian clock gene Bmal1 is not essential; functional replacement with its paralog, Bmal2. Curr Biol. 2010;20:316–321. doi: 10.1016/j.cub.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reddy AB, O’Neill JS. Healthy clocks, healthy body, healthy mind. Trends Cell Biol. 2010;20:36–44. doi: 10.1016/j.tcb.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wulff K, Gatti S, Wettstein JG, Foster RG. Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease. Nat Rev Neurosci. 2010;11:589–599. doi: 10.1038/nrn2868. [DOI] [PubMed] [Google Scholar]
- 23.Gerstner JR, et al. Cycling behavior and memory formation. J Neurosci. 2009;29:12824–12830. doi: 10.1523/JNEUROSCI.3353-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McClung CA. Circadian genes, rhythms and the biology of mood disorders. Pharmacol Ther. 2007;114:222–232. doi: 10.1016/j.pharmthera.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McClung CA. Circadian rhythms and mood regulation: Insights from pre-clinical models. Eur Neuropsychopharmacol. 2011;21(Suppl 4):S683–S693. doi: 10.1016/j.euroneuro.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Roybal K, et al. Mania-like behavior induced by disruption of CLOCK. Proc Natl Acad Sci U S A. 2007;104:6406–6411. doi: 10.1073/pnas.0609625104. Using animal model authors demonstrated a connection between the circadian clock and mode control. These data can help us to understand mechanisms of such mood disorders as bipolar disorder.
- 27.Bellet MM, Vawter MP, Bunney BG, Bunney WE, Sassone-Corsi P. Ketamine Influences CLOCK:BMAL1 Function Leading to Altered Circadian Gene Expression. PLoS One. 2011;6:e23982. doi: 10.1371/journal.pone.0023982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gerstner JR, Yin JC. Circadian rhythms and memory formation. Nat Rev Neurosci. 2010;11:577–588. doi: 10.1038/nrn2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kondratova AA, Dubrovsky YV, Antoch MP, Kondratov RV. Circadian clock proteins control adaptation to novel environment and memeory formation. Aging (Albany NY) 2010;2:285–297. doi: 10.18632/aging.100142. Mice with deficiency in core clock genes Bmal1 and Clock but not Cry deficient mice demonstrate impaired adaptation to novel environment. This work suggests clock independent role of core clock proteins in regulation of memory formation.
- 30. Lyons LC, Roman G. Circadian modulation of short-term memory in Drosophila. Learn Mem. 2009;16:19–27. doi: 10.1101/lm.1146009. This report demonstrates that short-term associative memory formation but not sensory perception is regulated in circadian manner. These data suggest that central circadian oscillator is important for short-term memory formation.
- 31. Van der Zee EA, et al. Circadian time-place learning in mice depends on Cry genes. Curr Biol. 2008;18:844–848. doi: 10.1016/j.cub.2008.04.077. Mice deficient in core circadian clock genes demonstrate normal performance spatial learning tasks and impairment in time-place associated learning.
- 32.Garcia JA, et al. Impaired cued and contextual memory in NPAS2-deficient mice. Science. 2000;288:2226–2230. doi: 10.1126/science.288.5474.2226. [DOI] [PubMed] [Google Scholar]
- 33. Sakai T, Tamura T, Kitamoto T, Kidokoro Y. A clock gene, period, plays a key role in long-term memory formation in Drosophila. Proc Natl Acad Sci U S A. 2004;101:16058–16063. doi: 10.1073/pnas.0401472101. In flies mutation in Period gene but not in other circadian clock genes affect long term memory formation. This work suggest circadian clock independent role of clock proteins in regulation of memory formation.
- 34.Wang LM, et al. Expression of the circadian clock gene Period2 in the hippocampus: possible implications for synaptic plasticity and learned behaviour. ASN Neuro. 2009;1 doi: 10.1042/AN20090020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wyatt JK, Ritz-De Cecco A, Czeisler CA, Dijk DJ. Circadian temperature and melatonin rhythms, sleep, and neurobehavioral function in humans living on a 20-h day. Am J Physiol. 1999;277:R1152–R1163. doi: 10.1152/ajpregu.1999.277.4.r1152. Cognitive performance in humans demonstrates circadian profile. Experimental design of this study allows to discriminate between the effects of sleep and the circadin clock on cognition.
- 36. Eckel-Mahan KL, et al. Circadian oscillation of hippocampal MAPK activity and cAmp: implications for memory persistence. Nat Neurosci. 2008;11:1074–1082. doi: 10.1038/nn.2174. Authors demonstrated molecular connections between the circadian clock and memory formation. These data help to understand mechanisms of the persistence of hippocampal long-term memory.
- 37.Eckel-Mahan KL, Storm DR. Circadian rhythms and memory: not so simple as cogs and gears. EMBO Rep. 2009;10:584–591. doi: 10.1038/embor.2009.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tu BP, Kudlicki A, Rowicka M, McKnight SL. Logic of the yeast metabolic cycle: temporal compartmentalization of cellular processes. Science. 2005;310:1152–1158. doi: 10.1126/science.1120499. [DOI] [PubMed] [Google Scholar]
- 39.Chen Z, Odstrcil EA, Tu BP, McKnight SL. Restriction of DNA replication to the reductive phase of the metabolic cycle protects genome integrity. Science. 2007;316:1916–1919. doi: 10.1126/science.1140958. [DOI] [PubMed] [Google Scholar]
- 40.Reddy AB, et al. Circadian orchestration of the hepatic proteome. Curr Biol. 2006;16:1107–1115. doi: 10.1016/j.cub.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 41.Albrecht U. Molecular orchestration of the hepatic circadian symphony. Genome Biol. 2006;7:234. doi: 10.1186/gb-2006-7-9-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akhtar RA, et al. Circadian cycling of the mouse liver transcriptome, as revealed by cDNA microarray, is driven by the suprachiasmatic nucleus. Curr Biol. 2002;12:540–550. doi: 10.1016/s0960-9822(02)00759-5. [DOI] [PubMed] [Google Scholar]
- 43.Panda S, et al. Coordinated transcription of key pathways in the mouse by circadian clock. Cell. 2002;109:307–320. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 44.Panda S, Hogenesch JB. It's all in the timing: many clocks, many outputs. J Biol Rhythms. 2004;19:374–387. doi: 10.1177/0748730404269008. [DOI] [PubMed] [Google Scholar]
- 45.Hastings MH, Maywood ES, Reddy AB. Two decades of circadian time. J Neuroendocrinol. 2008;20:812–819. doi: 10.1111/j.1365-2826.2008.01715.x. [DOI] [PubMed] [Google Scholar]
- 46.Nakada D, Levi BP, Morrison SJ. Integrating physiological regulation with stem cell and tissue homeostasis. Neuron. 70:703–718. doi: 10.1016/j.neuron.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goergen EM, Bagay LA, Rehm K, Benton JL, Beltz BS. Circadian control of neurogenesis. J Neurobiol. 2002;53:90–95. doi: 10.1002/neu.10095. [DOI] [PubMed] [Google Scholar]
- 48.Guzman-Marin R, Suntsova N, Bashir T, Szymusiak R, McGinty D. Cell proliferation in the dentate gyrus of the adult rat fluctuates with the light-dark cycle. Neurosci Lett. 2007;422:198–201. doi: 10.1016/j.neulet.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Joshi D, Barnabas R, Martin ER, Parihar V, Kanojiya M. Aging alters properties of the circadian pacemaker controlling the locomotor activity rhythm in males of Drosophila nasuta. Chronobiol Int. 1999;16:751–758. doi: 10.3109/07420529909016942. [DOI] [PubMed] [Google Scholar]
- 50.Rezaval C, et al. A functional misexpression screen uncovers a role for enabled in progressive neurodegeneration. PLoS One. 2008;3:e3332. doi: 10.1371/journal.pone.0003332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zheng X, Yang Z, Yue Z, Alvarez JD, Sehgal A. FOXO and insulin signaling regulate sensitivity of the circadian clock to oxidative stress. Proc Natl Acad Sci U S A. 2007;104:15899–15904. doi: 10.1073/pnas.0701599104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koh K, Evans JM, Hendricks JC, Sehgal A. A Drosophila model for age-associated changes in sleep:wake cycles. Proc Natl Acad Sci U S A. 2006;103:13843–13847. doi: 10.1073/pnas.0605903103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Froy O. Circadian rhythms, aging, and life span in mammals. Physiology (Bethesda) 2011;26:225–235. doi: 10.1152/physiol.00012.2011. [DOI] [PubMed] [Google Scholar]
- 54.Zhdanova IV, et al. Aging of intrinsic circadian rhythms and sleep in a diurnal nonhuman primate, Macaca mulatta. J Biol Rhythms. 2011;26:149–159. doi: 10.1177/0748730410395849. [DOI] [PubMed] [Google Scholar]
- 55.Skene DJ, Swaab DF. Melatonin rhythmicity: effect of age and Alzheimer's disease. Exp Gerontol. 2003;38:199–206. doi: 10.1016/s0531-5565(02)00198-5. [DOI] [PubMed] [Google Scholar]
- 56. Monk TH. Aging human circadian rhythms: conventional wisdom may not always be right. J Biol Rhythms. 2005;20:366–374. doi: 10.1177/0748730405277378. Author reviews a existing clinical data on the effects of ageing on circadian system in humans.
- 57.Monk TH, Buysse DJ, Reynolds CF, 3rd, Kupfer DJ, Houck PR. Circadian temperature rhythms of older people. Exp Gerontol. 1995;30:455–474. doi: 10.1016/0531-5565(95)00007-4. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Y, et al. Effects of aging on lens transmittance and retinal input to the suprachiasmatic nucleus in golden hamsters. Neurosci Lett. 1998;258:167–170. doi: 10.1016/s0304-3940(98)00887-8. [DOI] [PubMed] [Google Scholar]
- 59.Lupi D, Semo M, Foster RG. Impact of age and retinal degeneration on the light input to circadian brain structures. Neurobiol Aging. 2012;33:383–392. doi: 10.1016/j.neurobiolaging.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 60.Aujard F, et al. Artificially accelerated aging by shortened photoperiod alters early gene expression (Fos) in the suprachiasmatic nucleus and sulfatoxymelatonin excretion in a small primate, Microcebus murinus. Neuroscience. 2001;105:403–412. doi: 10.1016/s0306-4522(01)00202-0. [DOI] [PubMed] [Google Scholar]
- 61.Asai M, et al. Circadian profile of Per gene mRNA expression in the suprachiasmatic nucleus, paraventricular nucleus, and pineal body of aged rats. J Neurosci Res. 2001;66:1133–1139. doi: 10.1002/jnr.10010. [DOI] [PubMed] [Google Scholar]
- 62.Madeira MD, Sousa N, Santer RM, Paula-Barbosa MM, Gundersen HJ. Age and sex do not affect the volume, cell numbers, or cell size of the suprachiasmatic nucleus of the rat: an unbiased stereological study. J Comp Neurol. 1995;361:585–601. doi: 10.1002/cne.903610404. [DOI] [PubMed] [Google Scholar]
- 63. Roozendaal B, van Gool WA, Swaab DF, Hoogendijk JE, Mirmiran M. Changes in vasopressin cells of the rat suprachiasmatic nucleus with aging. Brain Res. 1987;409:259–264. doi: 10.1016/0006-8993(87)90710-4. Authors report anatomical and physiological changes induced by ageing in the SCN. These results help us to understand mechanisms of circadian system ageing.
- 64.Colwell CS. Linking neural activity and molecular oscillations in the SCN. Nat Rev Neurosci. 2011;12:553–569. doi: 10.1038/nrn3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nygard M, Hill RH, Wikstrom MA, Kristensson K. Age-related changes in electrophysiological properties of the mouse suprachiasmatic nucleus in vitro. Brain Res Bull. 2005;65:149–154. doi: 10.1016/j.brainresbull.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 66.Beynon AL, Thome J, Coogan AN. Age and time of day influences on the expression of transforming growth factor-beta and phosphorylated SMAD3 in the mouse suprachiasmatic and paraventricular nuclei. Neuroimmunomodulation. 2009;16:392–399. doi: 10.1159/000228914. [DOI] [PubMed] [Google Scholar]
- 67.Davidson AJ, Yamazaki S, Arble DM, Menaker M, Block GD. Resetting of central and peripheral circadian oscillators in aged rats. Neurobiol Aging. 2008;29:471–477. doi: 10.1016/j.neurobiolaging.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kawakami F, et al. Loss of day-night differences in VIP mRNA levels in the suprachiasmatic nucleus of aged rats. Neurosci Lett. 1997;222:99–102. doi: 10.1016/s0304-3940(97)13355-9. [DOI] [PubMed] [Google Scholar]
- 69. Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP. Early aging and age-related pathologies in mice deficient in BMAL1, the core component of the circadian clock. Genes Dev. 2006;20:1868–1873. doi: 10.1101/gad.1432206. Authors demonstrate that deregulation of BMAL1 dependent control of reactive oxygen species homeostasis is associated with premature ageing. These data can help us to understand the role of circadian clock in physiology.
- 70.Hendricks JC, et al. Gender dimorphism in the role of cycle (BMAL1) in rest, rest regulation, and longevity in Drosophila melanogaster. J Biol Rhythms. 2003;18:12–25. doi: 10.1177/0748730402239673. [DOI] [PubMed] [Google Scholar]
- 71.Dubrovsky YV, Samsa WE, Kondratov RV. Deficiency of circadian protein CLOCK reduces lifespan and increases age-related cataract development in mice. Aging (Albany NY) 2010;2:936–944. doi: 10.18632/aging.100241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fu L, Pelicano H, Liu J, Huang P, Lee C. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell. 2002;111:41–50. doi: 10.1016/s0092-8674(02)00961-3. [DOI] [PubMed] [Google Scholar]
- 73.Antoch MP, et al. Disruption of the circadian clock due to the Clock mutation has discrete effects on aging and carcinogenesis. Cell Cycle. 2008;7:1197–1204. doi: 10.4161/cc.7.9.5886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yu EA, Weaver DR. Disrupting the circadian clock: gene-specific effects on aging, cancer, and other phenotypes. Aging (Albany NY) 2010;3:479–493. doi: 10.18632/aging.100323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Colrain IM. Sleep and the brain. Neuropsychol Rev. 2011;21:1–4. doi: 10.1007/s11065-011-9156-z. [DOI] [PubMed] [Google Scholar]
- 76.Merrill DA, Small GW. Prevention in psychiatry: effects of healthy lifestyle on cognition. Psychiatr Clin North Am. 2011;34:249–261. doi: 10.1016/j.psc.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 77.Kaup AR, Mirzakhanian H, Jeste DV, Eyler LT. A review of the brain structure correlates of successful cognitive aging. J Neuropsychiatry Clin Neurosci. 2011;23:6–15. doi: 10.1176/appi.neuropsych.23.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Beekman AT. Neuropathological correlates of late-life depression. Expert Rev Neurother. 2011;11:947–949. doi: 10.1586/ern.11.88. [DOI] [PubMed] [Google Scholar]
- 79.Wolkowitz OM, Reus VI, Mellon SH. Of sound mind and body: depression, disease, and accelerated aging. Dialogues Clin Neurosci. 2011;13:25–39. doi: 10.31887/DCNS.2011.13.1/owolkowitz. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Magee JC, Carmin CN. The relationship between sleep and anxiety in older adults. Curr Psychiatry Rep. 2010;12:13–19. doi: 10.1007/s11920-009-0087-9. [DOI] [PubMed] [Google Scholar]
- 81.George O, et al. Low brain allopregnanolone levels mediate flattened circadian activity associated with memory impairments in aged rats. Biol Psychiatry. 2010;68:956–963. doi: 10.1016/j.biopsych.2010.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Naismith SL, Lewis SJ, Rogers NL. Sleep-wake changes and cognition in neurodegenerative disease. Prog Brain Res. 2011;190:21–52. doi: 10.1016/B978-0-444-53817-8.00002-5. [DOI] [PubMed] [Google Scholar]
- 83.Stopa EG, et al. Pathologic evaluation of the human suprachiasmatic nucleus in severe dementia. J Neuropathol Exp Neurol. 1999;58:29–39. doi: 10.1097/00005072-199901000-00004. [DOI] [PubMed] [Google Scholar]
- 84.Zhou JN, Hofman MA, Swaab DF. VIP neurons in the human SCN in relation to sex, age, and Alzheimer's disease. Neurobiol Aging. 1995;16:571–576. doi: 10.1016/0197-4580(95)00043-e. [DOI] [PubMed] [Google Scholar]
- 85.Gabery S, et al. Changes in key hypothalamic neuropeptide populations in Huntington disease revealed by neuropathological analyses. Acta Neuropathol. 2010;120:777–788. doi: 10.1007/s00401-010-0742-6. [DOI] [PubMed] [Google Scholar]
- 86.Saper CB, German DC. Hypothalamic pathology in Alzheimer's disease. Neurosci Lett. 1987;74:364–370. doi: 10.1016/0304-3940(87)90325-9. [DOI] [PubMed] [Google Scholar]
- 87.Pavese N, Rivero-Bosch M, Lewis SJ, Whone AL, Brooks DJ. Progression of monoaminergic dysfunction in Parkinson's disease: a longitudinal 18F-dopa PET study. Neuroimage. 2011;56:1463–1468. doi: 10.1016/j.neuroimage.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 88.Teipel SJ, et al. The cholinergic system in mild cognitive impairment and Alzheimer's disease: an in vivo MRI and DTI study. Hum Brain Mapp. 2011;32:1349–1362. doi: 10.1002/hbm.21111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yohrling GJt, et al. Analysis of cellular, transgenic and human models of Huntington's disease reveals tyrosine hydroxylase alterations and substantia nigra neuropathology. Brain Res Mol Brain Res. 2003;119:28–36. doi: 10.1016/j.molbrainres.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 90.Brunnstrom H, Friberg N, Lindberg E, Englund E. Differential degeneration of the locus coeruleus in dementia subtypes. Clin Neuropathol. 2011;30:104–110. doi: 10.5414/npp30104. [DOI] [PubMed] [Google Scholar]
- 91.Sterniczuk R, Dyck RH, Laferla FM, Antle MC. Characterization of the 3xTg-AD mouse model of Alzheimer's disease: part 1. Circadian changes. Brain Res. 2011;1348:139–148. doi: 10.1016/j.brainres.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 92.Kudo T, Loh DH, Truong D, Wu Y, Colwell CS. Circadian dysfunction in a mouse model of Parkinson's disease. Exp Neurol. 2011 doi: 10.1016/j.expneurol.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 93.Oakeshott S, et al. Circadian Abnormalities in Motor Activity in a BAC Transgenic Mouse Model of Huntington's Disease. PLoS Curr. 2011;3:RRN1225. doi: 10.1371/currents.RRN1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Grimm S, Hoehn A, Davies KJ, Grune T. Protein oxidative modifications in the ageing brain: consequence for the onset of neurodegenerative disease. Free Radic Res. 2011;45:73–88. doi: 10.3109/10715762.2010.512040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Siqueira IR, et al. Total antioxidant capacity is impaired in different structures from aged rat brain. Int J Dev Neurosci. 2005;23:663–671. doi: 10.1016/j.ijdevneu.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 96.Kondratov RV, Vykhovanets O, Kondratova AA, Antoch MP. Antioxidant N-acetyl-L-cysteine ameliorates symptoms of premature aging associated with the deficiency of the circadian protein BMAL1. Aging (Albany NY) 2009;1:979–987. doi: 10.18632/aging.100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hardeland R, Coto-Montes A, Poeggeler B. Circadian rhythms, oxidative stress, and antioxidative defense mechanisms. Chronobiol Int. 2003;20:921–962. doi: 10.1081/cbi-120025245. [DOI] [PubMed] [Google Scholar]
- 98.Belden WJ, et al. The band mutation in Neurospora crassa is a dominant allele of ras-1 implicating RAS signaling in circadian output. Genes Dev. 2007;21:1494–1505. doi: 10.1101/gad.1551707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yoshida Y, Iigusa H, Wang N, Hasunuma K. Cross-Talk between the Cellular Redox State and the Circadian System in Neurospora. PLoS One. 2011;6:e28227. doi: 10.1371/journal.pone.0028227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Krishnan N, Davis AJ, Giebultowicz JM. Circadian regulation of response to oxidative stress in Drosophila melanogaster. Biochem Biophys Res Commun. 2008;374:299–303. doi: 10.1016/j.bbrc.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.O'Neill JS, Reddy AB. Circadian clocks in human red blood cells. Nature. 2011;469:498–503. doi: 10.1038/nature09702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.O'Neill JS, et al. Circadian rhythms persist without transcription in a eukaryote. Nature. 2011;469:554–558. doi: 10.1038/nature09654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Kang TH, Reardon JT, Kemp M, Sancar A. Circadian oscillation of nucleotide excision repair in mammalian brain. Proc Natl Acad Sci U S A. 2009;106:2864–2867. doi: 10.1073/pnas.0812638106. Regulation of one of the DNA repair systems through the control of transcription by the circadian clock is demonstrated. These data can help us to understand mechanism of circadian clock dependent regulation of ageing.
- 104.Kang TH, Sancar A. Circadian regulation of DNA excision repair: implications for chrono-chemotherapy. Cell Cycle. 2009;8:1665–1667. doi: 10.4161/cc.8.11.8707. [DOI] [PubMed] [Google Scholar]
- 105.Friguet B, Bulteau AL, Chondrogianni N, Conconi M, Petropoulos I. Protein degradation by the proteasome and its implications in aging. Ann N Y Acad Sci. 2000;908:143–154. doi: 10.1111/j.1749-6632.2000.tb06643.x. [DOI] [PubMed] [Google Scholar]
- 106.Cheung ZH, Ip NY. Autophagy deregulation in neurodegenerative diseases - recent advances and future perspectives. J Neurochem. 2011;118:317–325. doi: 10.1111/j.1471-4159.2011.07314.x. [DOI] [PubMed] [Google Scholar]
- 107.Sachdeva UM, Thompson CB. Diurnal rhythms of autophagy: implications for cell biology and human disease. Autophagy. 2008;4:581–589. doi: 10.4161/auto.6141. [DOI] [PubMed] [Google Scholar]
- 108.Pfeifer U, Strauss P. Autophagic vacuoles in heart muscle and liver. A comparative morphometric study including circadian variations in meal-fed rats. J Mol Cell Cardiol. 1981;13:37–49. doi: 10.1016/0022-2828(81)90227-3. [DOI] [PubMed] [Google Scholar]
- 109.Pfeifer U, Scheller H. A morphometric study of cellular autophagy including diurnal variations in kidney tubules of normal rats. J Cell Biol. 1975;64:608–621. doi: 10.1083/jcb.64.3.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Reme CE, Sulser M. Diurnal variation of autophagy in rod visual cells in the rat. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1977;203:261–270. doi: 10.1007/BF00409832. [DOI] [PubMed] [Google Scholar]
- 111.Ma D, Panda S, Lin JD. Temporal orchestration of circadian autophagy rhythm by C/EBPbeta. EMBO J. 2011;30:4642–4651. doi: 10.1038/emboj.2011.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cardinali DP, Furio AM, Brusco LI. Clinical aspects of melatonin intervention in Alzheimer's disease progression. Curr Neuropharmacol. 2010;8:218–227. doi: 10.2174/157015910792246209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Srinivasan V, et al. Role of melatonin in neurodegenerative diseases. Neurotox Res. 2005;7:293–318. doi: 10.1007/BF03033887. [DOI] [PubMed] [Google Scholar]
- 114.Olakowska E, Marcol W, Kotulska K, Lewin-Kowalik J. The role of melatonin in the neurodegenerative diseases. Bratisl Lek Listy. 2005;106:171–174. [PubMed] [Google Scholar]
- 115.Srinivasan V, et al. Melatonin in mood disorders. World J Biol Psychiatry. 2006;7:138–151. doi: 10.1080/15622970600571822. [DOI] [PubMed] [Google Scholar]
- 116.Mishima K, et al. Melatonin secretion rhythm disorders in patients with senile dementia of Alzheimer's type with disturbed sleep-waking. Biol Psychiatry. 1999;45:417–421. doi: 10.1016/s0006-3223(97)00510-6. [DOI] [PubMed] [Google Scholar]
- 117.Aziz NA, et al. Delayed onset of the diurnal melatonin rise in patients with Huntington's disease. J Neurol. 2009;256:1961–1965. doi: 10.1007/s00415-009-5196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gibbs JE, et al. The nuclear receptor REV-ERBalpha mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc Natl Acad Sci U S A. 2012;109:582–587. doi: 10.1073/pnas.1106750109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Castanon-Cervantes O, et al. Dysregulation of inflammatory responses by chronic circadian disruption. J Immunol. 2010;185:5796–5805. doi: 10.4049/jimmunol.1001026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Most EI, Scheltens P, Van Someren EJ. Prevention of depression and sleep disturbances in elderly with memory-problems by activation of the biological clock with light--a randomized clinical trial. Trials. 2010;11:19. doi: 10.1186/1745-6215-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Singer C, et al. A multicenter, placebo-controlled trial of melatonin for sleep disturbance in Alzheimer's disease. Sleep. 2003;26:893–901. doi: 10.1093/sleep/26.7.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gehrman PR, et al. Melatonin fails to improve sleep or agitation in double-blind randomized placebo-controlled trial of institutionalized patients with Alzheimer disease. Am J Geriatr Psychiatry. 2009;17:166–169. doi: 10.1097/JGP.0b013e318187de18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Serfaty M, Kennell-Webb S, Warner J, Blizard R, Raven P. Double blind randomised placebo controlled trial of low dose melatonin for sleep disorders in dementia. Int J Geriatr Psychiatry. 2002;17:1120–1127. doi: 10.1002/gps.760. [DOI] [PubMed] [Google Scholar]
- 124.Mishima K, Okawa M, Hozumi S, Hishikawa Y. Supplementary administration of artificial bright light and melatonin as potent treatment for disorganized circadian rest-activity and dysfunctional autonomic and neuroendocrine systems in institutionalized demented elderly persons. Chronobiol Int. 2000;17:419–432. doi: 10.1081/cbi-100101055. [DOI] [PubMed] [Google Scholar]
- 125.Dowling GA, et al. Melatonin and bright-light treatment for rest-activity disruption in institutionalized patients with Alzheimer's disease. J Am Geriatr Soc. 2008;56:239–246. doi: 10.1111/j.1532-5415.2007.01543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Feng Z, et al. Melatonin alleviates behavioral deficits associated with apoptosis and cholinergic system dysfunction in the APP 695 transgenic mouse model of Alzheimer's disease. J Pineal Res. 2004;37:129–136. doi: 10.1111/j.1600-079X.2004.00144.x. [DOI] [PubMed] [Google Scholar]
- 127.Matsubara E, et al. Melatonin increases survival and inhibits oxidative and amyloid pathology in a transgenic model of Alzheimer's disease. J Neurochem. 2003;85:1101–1108. doi: 10.1046/j.1471-4159.2003.01654.x. [DOI] [PubMed] [Google Scholar]
- 128.Pappolla M, et al. Inhibition of Alzheimer beta-fibrillogenesis by melatonin. J Biol Chem. 1998;273:7185–7188. doi: 10.1074/jbc.273.13.7185. [DOI] [PubMed] [Google Scholar]
- 129.Singhal NK, Srivastava G, Patel DK, Jain SK, Singh MP. Melatonin or silymarin reduces maneb- and paraquat-induced Parkinson's disease phenotype in the mouse. J Pineal Res. 50:97–109. doi: 10.1111/j.1600-079X.2010.00819.x. [DOI] [PubMed] [Google Scholar]
- 130.Medeiros CA, et al. Effect of exogenous melatonin on sleep and motor dysfunction in Parkinson's disease. A randomized, double blind, placebo-controlled study. J Neurol. 2007;254:459–464. doi: 10.1007/s00415-006-0390-x. [DOI] [PubMed] [Google Scholar]
- 131.Dowling GA, et al. Melatonin for sleep disturbances in Parkinson's disease. Sleep Med. 2005;6:459–466. doi: 10.1016/j.sleep.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 132.Willis GL. The role of ML-23 and other melatonin analogues in the treatment and management of Parkinson's disease. Drug News Perspect. 2005;18:437–444. doi: 10.1358/dnp.2005.18.7.939349. [DOI] [PubMed] [Google Scholar]
- 133.Willis GL. Parkinson's disease as a neuroendocrine disorder of circadian function: dopamine-melatonin imbalance and the visual system in the genesis and progression of the degenerative process. Rev Neurosci. 2008;19:245–316. doi: 10.1515/revneuro.2008.19.4-5.245. [DOI] [PubMed] [Google Scholar]
- 134.Pallier PN, et al. Pharmacological imposition of sleep slows cognitive decline and reverses dysregulation of circadian gene expression in a transgenic mouse model of Huntington's disease. J Neurosci. 2007;27:7869–7878. doi: 10.1523/JNEUROSCI.0649-07.2007. See below. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Pallier PN, Morton AJ. Management of sleep/wake cycles improves cognitive function in a transgenic mouse model of Huntington's disease. Brain Res. 2009;1279:1290–1298. doi: 10.1016/j.brainres.2009.03.072. This and previous reports demonstrate that restoration of circadian clock function can delay development of HD. These data may help to develop new strategies for treatment neurodegenerative diseases.
- 136.Maywood ES, et al. Disruption of peripheral circadian timekeeping in a mouse model of Huntington's disease and its restoration by temporally scheduled feeding. J Neurosci. 2010;30:10199–10204. doi: 10.1523/JNEUROSCI.1694-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bertram L, Tanzi RE. Thirty years of Alzheimer's disease genetics: the implications of systematic meta-analyses. Nat Rev Neurosci. 2008;9:768–778. doi: 10.1038/nrn2494. [DOI] [PubMed] [Google Scholar]
- 138.Hofman MA, Swaab DF. Alterations in circadian rhythmicity of the vasopressin-producing neurons of the human suprachiasmatic nucleus (SCN) with aging. Brain Res. 1994;651:134–142. doi: 10.1016/0006-8993(94)90689-0. [DOI] [PubMed] [Google Scholar]
- 139.Thomas B, Beal MF. Parkinson's disease. Hum Mol Genet. 2007;16(Spec No 2):R183–R194. doi: 10.1093/hmg/ddm159. [DOI] [PubMed] [Google Scholar]
- 140.Wakabayashi K, Tanji K, Mori F, Takahashi H. The Lewy body in Parkinson's disease: molecules implicated in the formation and degradation of alpha-synuclein aggregates. Neuropathology. 2007;27:494–506. doi: 10.1111/j.1440-1789.2007.00803.x. [DOI] [PubMed] [Google Scholar]
- 141.Willis GL, Kelly AM, Kennedy GA. Compromised circadian function in Parkinson's disease: enucleation augments disease severity in the unilateral model. Behav Brain Res. 2008;193:37–47. doi: 10.1016/j.bbr.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 142.Browne SE, Beal MF. Oxidative damage in Huntington's disease pathogenesis. Antioxid Redox Signal. 2006;8:2061–2073. doi: 10.1089/ars.2006.8.2061. [DOI] [PubMed] [Google Scholar]
- 143.Morton AJ, et al. Disintegration of the sleep-wake cycle and circadian timing in Huntington's disease. J Neurosci. 2005;25:157–163. doi: 10.1523/JNEUROSCI.3842-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]