Abstract
Zebrafish (Danio rerio) remains a versatile model organism for the investigation of early development and organogenesis, and has emerged as a valuable platform for drug discovery and toxicity evaluation [1–6]. Harnessing the genetic power and experimental accessibility of this system, three decades of research have identified key genes and pathways that control the development of multiple organ systems and tissues, including the heart, kidney, and craniofacial cartilage, as well as the hematopoietic, vascular, and central and peripheral nervous systems [7–31]. In addition to their application in large mutagenic screens, zebrafish has been used to model a variety of diseases such as diabetes, polycystic kidney disease, muscular dystrophy and cancer [32–36]. As this work continues to intersect with cellular pathways and processes such as lipid metabolism, glycosylation and vesicle trafficking, investigators are often faced with the challenge of determining the degree to which these pathways are functionally conserved in zebrafish. While they share a high degree of genetic homology with mouse and human, the manner in which cellular pathways are regulated in zebrafish during early development, and the differences in the organ physiology, warrant consideration before functional studies can be effectively interpreted and compared with other vertebrate systems. This point is particularly relevant for glycosylation since an understanding of the glycan diversity and the mechanisms that control glycan biosynthesis during zebrafish embryogenesis (as in many organisms) is still developing.
Nonetheless, a growing number of studies in zebrafish have begun to cast light on the functional roles of specific classes of glycans during organ and tissue development. While many of the initial efforts involved characterizing identified mutants in a number of glycosylation pathways, the use of reverse genetic approaches to directly model glycosylation-related disorders is now increasingly popular. In this review, the glycomics of zebrafish and the developmental expression of their glycans will be briefly summarized along with recent chemical biology approaches to visualize certain classes of glycans within developing embryos. Work regarding the role of protein-bound glycans and glycosaminoglycans (GAG) in zebrafish development and organogenesis will also be highlighted. Lastly, future opportunities and challenges in the expanding field of zebrafish glycobiology are discussed.
Keywords: Zebrafish, Glycosylation, Development, Sialylation, Glycosaminoglycans, N-glycans, Mucins, Cartilage
Structural diversity and developmental expression of glycans in zebrafish
While the earliest functional studies focused on the role of individual enzymes and structures, more recent MS-based analyses of zebrafish glycoconjugates have yielded a better appreciation of the glycan diversity in the organism. Leading the way, Khoo and colleagues described the major protein- and lipid-bound zebrafish glycans present in fertilized eggs and early (<48 h post-fertilization; hpf) embryos [37]. These analyses demonstrated that zebrafish embryos exhibit surprising structural diversity, particularly with regard to sialylation profiles. Complex and oligomannose N-glycans, which are abundant in embryos at all developmental stages, bear a wide range of monosialylated and oligosialylated termini that include Neu5Ac and Neu5Gc. This high degree of sialylation in zebrafish embryos was exemplified by the fact that the only non-sialylated structures detected were high mannose type N-glycans. The most common complex N-glycans were those terminating in the unusual motif, Galß1–4(Neu5Ac/Gc)Galß1–4(Fucα1–3) GlcNAc (Fig. 1). Along with core 1 and core 2 O-glycan structures common to mammalian tissues, abundant and unusual fucosylated mucin-type O-glycans were also observed (Fig. 1). Oligosialylation of this unusual core structure was noted but appeared to be restricted to the earliest development stages (<24hpf). Analyses of glycolipids in this study also led to the identification of a heterogeneous family of oligosialylated lactosylceramide compounds that appear only at later development stages (>24hpf). Together, these studies suggest a complex pattern of sialylation of both proteins and lipids during zebrafish embryogenesis and provided a starting point to explore the activity and expression of specific sialyltransferase families [38–42]. The recent glycomic profiling of a zebrafish liver cell line revealed a relatively simple panel of glycans, highlighted by the presence of multisialylated N- and O-glycans with the same unusual epitopes described above as well as sialylated gangliosides [43]. Characterization of the glycosyltransferases in this cell system showed that these glycan profiles correlated well with the expression patterns of all putative sialyl- and fucosyltransferases. This cell system will provide a valuable tool to study the regulation of glycosylation and the function of glycans in zebrafish.
Fig. 1.
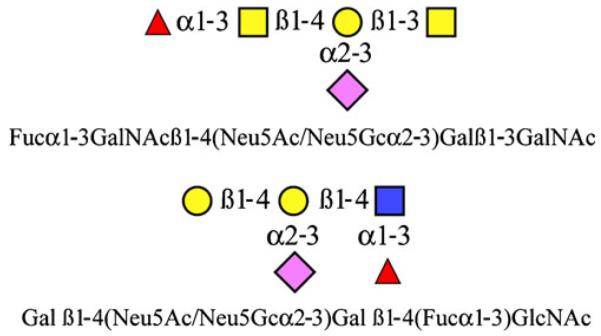
The structures of the abundant and unusual O-glycans (top) and N-glycans (bottom) isolated from zebrafish embryos
Phylogenetic and expression studies aimed at defining the sialyltransferase families have now been reported, lending some insight into the origin of the unique sialylation profile in zebrafish. All six ST8Sia, which are temporally regulated, exhibit distinct and overlapping patterns of expression in the embryonic central nervous system, suggesting an important role for the α2–8-sialylated compounds in its development [39]. Glycoproteins and glycolipids also differ by the extent and the nature of their substituting oligosialylated sequences, demonstrating that α2–8-linked sialylation was differentially regulated in these glycan classes during development [38]. The duplicated paralogs, ncam1a and ncam1b, appear to be major carriers of polysialic acid in zebrafish. Moreover, the distinct expression patterns of these paralogs within the embryo suggest diverse functions within the central and peripheral nervous system [44]. Surprisingly, two functional CMP-sialic acid synthetases (cmas1 and cmas2) have also been identified in zebrafish, one of which is exclusively localized to the cytosol [45]. These enzymes exhibit different substrate specificities as cmas1 binds to Neu5Ac with high affinity and cmas2 favors 5-deamino-neuraminic acid (Kdn). Further investigation will no doubt identify other examples of subfunctionalization within the glycosylation machinery of zebrafish.
The regulation of fucosylation in zebrafish embryos has also been characterized. This modification appears to correspond with the increase in the formation of complex type glycans by 12 hpf [46]. Seminal work by Hase and colleagues demonstrated activity of two α1,3-fucosyltransferases in zebrafish capable of generating Lewis × fucosylation [47]. The expression of these enzymes is tightly regulated and is restricted to the segmentation period of embryogenesis. Interestingly, the Lewis × epitope produced by zebrafish fucosyltransferases was found in abundance on free oligosaccharides, generated by the hydrolysis of glycoproteins by endogenous glycosidases [48].
Mass spectrometric studies have begun to elucidate the structural diversity of GAGs in zebrafish. Despite early reports suggesting that zebrafish lack heparan sulfate-containing proteoglycans [49], subsequent analysis demonstrated that developing embryos contain both chondroitin and heparan sulfate, and that expression of these GAGs is temporally and spatially regulated [50]. Chitin oligosaccharides have also been detected in zebrafish. The synthesis of these Nod-like structures was shown to be restricted to very early stages of development and was tied to the presence of a zebrafish homolog of the Xenopus developmental gene DG42 [51]. These oligosaccharides appear to have roles in early development as disruption of their synthesis leads to gastrulation defects [52, 53]. At least some of these effects appear to be due to the ability of the chitin oligosaccharides to activate ERK pathways [54].
Visualizing glycans in zebrafish
The use of lectins, such as Maclura pomifera, peanut agglutinin, and wheat germ agglutinin, to mark specific developmental stages or processes ranging from oogenesis to chondrogenesis and neurogenesis is well established and provided the earliest indications that glycan expression is dynamic during zebrafish embryogenesis [55–57]. More recently, advances in chemical biology methods have enabled specific classes of glycans to be visualized in living zebrafish embryos. Utilizing a powerful combination of metabolic labeling, click chemistry and confocal microscopy, Bertozzi and colleagues demonstrated that the biosynthesis of GalNAc-containing glycans, primarily mucin-type O-glycans, during zebrafish development is a highly dynamic and tissue-specific process [58]. Increases in de novo glycan biosynthesis at discrete stages were detected in the craniofacial region, pectoral fins, and olfactory organs. Furthermore, differential rates of glycan endocytosis were observed within certain embryonic tissues, providing novel insights into the tissue-specific expression and trafficking of glycans. Subsequent work showed that mucin-type O-glycans could be detected as early as 7hpf, during the gastrula stage of development. Live analysis of glycan trafficking revealed dramatic reorganization of glycans, including their rapid migration toward the cleavage furrow of mitotic cells [59]. Related studies have employed analogous approaches with different azide-modified monosaccharides to visualize fucosylated, sialylated and polylactosamine glycans in zebrafish [60–64]. We envision that this methodology will find highly useful applications in the context of the glycosylation-related zebrafish mutants and models discussed below.
Role of glycans in zebrafish development
A role for specific protein and lipid-bound glycans during zebrafish development has been uncovered by work using transient morpholino (MO)-driven knockdown and stable genetic mutants of the glycosyltransferases and the nucleotide-sugar transporters involved in their synthesis (Table 1). Among these, the slytherin (srn) mutant, which bears a missense mutation in the rate-limiting enzyme of GDP-fucose biosynthesis (GDP-mannose 4,6-dehydratase or gmds), demonstrated that defective protein fucosylation causes several neuronal phenotypes [65]. These phenotypes included alterations in neurogenesis and gliogenesis, defects in axonal path finding and arborization, and abnormal formation of both neuromuscular and central nervous system synapses [66]. Analogous to Drosophila mutants within the same pathway, several of these phenotypes were shown to be dependent on decreased Notch signaling. Importantly, the Notch receptor bears both N- and O-linked fucosylated glycans, and manipulation of these glycans has been shown to alter propagation of downstream signals [67–70]. Some of the srn phenotypes, in particular those affecting retinotectal connectivity, appear to be Notch-independent. Analysis of another mutant allele of gmds, towhead (twd), showed that fucosylated glycans expressed in neuroepithelial cells are required to guide the migration of vagus motor neuron progenitors [71]. As with the srn retinotectal defects, these phenotypes were also independent of changes in Notch signaling. In an effort to assign twd phenotypes to the generation of a particular type of fucosylated glycan, the authors also used MOs to knockdown expression of several fucosyltransferases, including pofut1, pofut2, ft1, ft2, fut7–9, and fut10; among these only fut10 (which itself did not fully phenocopy twd) affected vagus motor neuron development. A role for core fucosylation during midline patterning and retinal and motor neuron development has also been demonstrated. Knockdown of fut8, as well as one of the principal fut8 substrates, apolipoprotein B (ApoB), phenocopies defects noted in mutants of Sonic Hedgehog (Shh) signaling (smu, syu, yot) [72]. As had previously been demonstrated in Drosophila, these results indicate that altered core fucosylation of ApoB impacts Shh signaling, possibly by affecting its transport.
Table 1.
Known zebrafish glycosylation mutants
| Glycan Type/Modification | Mutant Name | Gene | Primary Phenotypes |
|---|---|---|---|
| Fucosylation | slytherin (srn) | gmds | Defects in neurogenesis, gliogenesis, axonal pathfinding, and synapse formation [ref 65, 66] |
| towhead (twd) | gmds | Altered migration of vagus motor neuron progenitors [ref 71] | |
| GAG biosynthesis | dackel (dak) | ext2 | Altered cartilage development, reduced osteoblast differentiation, optic tract missorting [ref 93, 94] |
| boxer (box) | extl3 | Optic tract missorting [93] | |
| xylt1 | xylt1 | Altered craniofacial chondrogenesis and accelerated osteogenesis [ref 92] | |
| Nucleotide-sugar biosynthesis | jekell (jek) | udgh | Defects in craniofacial and cardiac valve formation [ref 91] |
| perplexed (per) | cad | Abnormal craniofacial formation [ref 82] | |
| man 'o war (mow) | uxs1 | Defects in craniofacial and cardiac valve formation, decreased osteogenesis [ref 90] | |
| GAG modifying enzymes | fam20b | fam20b | Altered chondrogenesis and accelerated osteogenesis [ref 92] |
| pinscher (pic) | papst1 | Altered chondrogenesis and osteogenesis [ref 94] |
Depletion of other glycosyltransferases, including two ß1,4-galactosyltransferases (ß4Galt1, ß4Galt5) has provided insight into the functional relevance of galactosylation during early aspects of zebrafish development. Morpholino knockdown of ß4Galt1 resulted in aberrant convergent extension movements during gastrulation [73], and similar reductions in ß4GalT5 affected dorsoventral patterning of embryos by a Bmp2-dependent mechanism [74]. In the latter case, the authors suggest that aberrant galactosylation of proteoglycans, and not N-glycans or glycolipids, is responsible for the altered Bmp2-dependent signaling. Work by Vasta and colleagues have highlighted a role for galactose-binding galectins during zebrafish embryogenesis. The expression of this family of lectins is developmentally regulated and functional studies indicate important functions for galectins during gliogenesis, skeletal muscle development and the regeneration of rod photoreceptors [75–79]. The presence and abundance of the unusual terminal galactose-bearing N-glycans in zebrafish embryos may therefore be important with regard to the function of these galectins.
Substantial work has also been done to study the expression and function of polysialylation in zebrafish. Differential expression of multiple polysialyltransferases (stx/St8sia2 and pst/St8sia4) has been linked to neuronal migration and plasticity in the brains of developing and adult zebrafish, respectively [42]. Further, enzymatic removal of polysialylation adversely affects pathfinding of a subset of commissural axons within the developing midbrain and hindbrain [80]. Interestingly, morpholino knockdown of the single St8sia3 gene, which was heavily expressed in somitic musculature, led to anomalous myotomal morphologies, including defects in the architecture of the segment boundaries and integrity of the myotendonous-junction [41]. These myotomal defects were accompanied by altered projection of innervating motor axons. Although St8sia3 is clearly expressed in the musculature and the phenotypic data provide compelling evidence of a novel non-neuronal role for St8Sia3, it is currently unclear whether the somitic phenotypes are independent of the axonal defects.
Cytosolic glycosyltransferases, such as O-GlcNAc transferase (ogt), have also been studied in zebrafish [81]. Overexpression of Ogt or O-GlcNAcase (oga) resulted in embryos with shortened body axes, reduced brain size and increased rates of cell death. Ogt/Oga overexpression also delayed epiboly and caused disorganization of the cytoskeleton within the yolk syncytial layer. Lastly, enzymes that indirectly affect glycosylation have also been characterized in zebrafish mutants. The perplexed mutant harbors a mutation in the metabolic enzyme carbamoyl-phosphate synthetase2-asparate transcarbamylase-dihydroorotase (cad) gene [82]. This enzyme is required for de novo synthesis of pyrimidines used for UDP-dependent protein glycosylation. Although overall pyrimidine metabolism is likely affected in the perplexed mutant, the craniofacial phenotypes noted were highly similar to the UDP-glucuronic acid/UDP-N-acetylgalactosamine dual transporter mutant (hi3378), suggesting that deficient glycosylation is a contributing factor in the onset of these phenotypes.
Role of GAGs in zebrafish development
Arguably the largest body of work to date on the role of glycoconjugates during zebrafish development encompasses defects in the synthesis and modification of GAGs. The vast number of identified zebrafish mutants affecting synthesis of heparan sulfate (HS), chondroitin sulfate (CS) and hyaluronic acid (HA) underscores the importance of these carbohydrates during development [83]. The role of this class of glycans in embryogenesis was initially revealed following enzymatic removal of specific GAGs in developing embryos [84, 85]. Functional roles for several proteoglycan core proteins in zebrafish development have also been described but will not be covered in detail here [86–89]. The repertoire of existing mutations affect all aspects of GAG synthesis, including the production the sugar nucleotide precursors (uxs1, udgh/jek) [90, 91], the glycosyltransferases responsible for GAG synthesis (ext2/dak, extl3/box, xylt1) [92–94], and the proteins involved in their sulfation or phosphorylation (papst1/pic, C4ST-1, fam20b) [92, 94, 95]. Loss of these enzymes results in defects affecting a number of processes including craniofacial chondrogenesis, skeletogenesis, cardiac valve formation, and multiple aspects of axon guidance. In several cases, including uxs1, udgh/jek, xylt1, fam20b, ext2/dak, and pic, altered synthesis or modification of GAGs has been shown to affect morphogenesis and/or maturation of the craniofacial chondrocytes. In addition to altered chondrogenesis, several of these mutants also exhibit defects in bone formation, as evidenced by changes in the expression of bone markers, such as runx2b and osterix, or Alizarin red staining. In the cases of uxs1, dak, and pic, loss of GAG expression decreased osteogenic processes. In contrast, chondrocyte maturation, osteoblast differentiation and bone formation were actually accelerated in the xytl1 and fam20b mutants [92]. Reduced GAG synthesis also adversely affects cardiac valve formation. In the udgh mutant jek (defective in CS, HS and HA production), cells at the border of the atrial and ventricular chambers do not differentiate from their endocardial neighbors, and as such cardiac cushion and ultimately valve formation fail. Similarly, Peal et al. demonstrated that chemical or genetic inhibition of CS biosynthesis also perturbed these processes [83]. In this case, the authors demonstrate that in zebrafish the gelatinous matrix, or cardiac jelly, required for atrioventricular valve formation is rich in chondroitin sulfate GAGs, and their depletion impairs valve cell migration and cushion formation. Decreased expression of an accessory component, the dfna5 deafness gene, which was shown to regulate the expression of udgh, also resulted in craniofacial and ear defects that phenocopy the jek mutant [96].
Importantly, most of the discussed GAG-dependent embryonic defects occur at times when cells secrete an abundance of extracellular matrix (ECM). For some of these mutants, individual phenotypes have been linked to alterations in growth factor signaling; in particular loss of ext2/dak reduced Fgf and Wnt signaling as assessed by expression of downstream targets [97]. In addition, a recent study by Yost and co-workers, showed that reducing 2-O sulfation of HS GAGs following depletion of zebrafish 2-O sulfotransferase diminished Wnt signaling and Wnt-dependent initiation of epiboly. Reactivation of ß-catenin, an intracellular component of canonical Wnt-signaling, rescued this defect [98]. In other mutants, including those affecting heart valve development, it is unclear to what degree the phenotypes are due to downstream effects on signal transduction or alterations in the physiochemical properties of the ECM that result when the GAGs or the protein cores are depleted.
Modeling glycosylation disorders in zebrafish
Several groups have also now employed reverse genetic approaches both to directly ascertain the functional roles of glycans in zebrafish and to model glycosylation-related disorders. Congenital muscular dystrophies (CMDs) that arise due to defective glycosylation of α-dystroglycan are attractive targets for study since their primary phenotypes in muscle and brain can be readily investigated using the zebrafish system. Genomic studies demonstrated that the genes required for functional glycosylation of dystroglycan are conserved in zebrafish [99, 100]. Furthermore, morpholino-based reduction of the Large2 protein in embryos results in a loss of IIH6 reactivity, suggesting that the O-mannosylation of dystroglycan is altered [100]. Similar results were noted in fukutin-knockdown embryos. Fkrp knockdown results in muscle defects as well as neuronal and eye abnormalities, which are accompanied by a reduction in DG glycosylation and laminin binding [101, 102]. Interestingly, involvement of the unfolded protein response was also reported in fkrp morphants [103]. Injection of antisense morpholino oligonucleotides of pomt1 and pomt2 resulted in several severe phenotypes-including bent body, edematous pericaridium and abnormal eye pigmentation [104].
In addition to CMDs, the human disorder, mucolipidosis II (ML-II), which results from impaired mannose 6-phosphate (Man-6-P) biosynthesis, has also been studied in zebrafish. While zebrafish express all the components of this carbohydrate-based lysosomal targeting pathway, some intriguing differences with regard to the substrate specificity of the initiating enzyme in Man-6-P biosynthesis, GlcNAc-1-phosphotransferase (gnptab), have been noted. In particular, some of the acid hydrolases that bear Man-6-P modified glycans in mammals (ex. acid-α-glucosidase) do not appear to be Man-6-P modified in zebrafish [105]. Morpholino-based depletion of GlcNAc-1-phosphotransferase resulted in several phenotypes consistent with the human disorder including cardiac and craniofacial defects [106]. These phenotypes were associated with altered TGF-ß signaling and excessive deposition of type II collagen. Altered TGF-beta signaling has also been noted in zebrafish embryos with reduced expression of the lysosomal hydrolase, iduronate 2-sulfatase [107]. More recently, sustained and increased activity of one Man-6-P modified hydrolase, cathepsin K, was shown to play a central role in the cartilage phenotypes of ML-II morphant embryos [108]. Zebrafish models of lysosomal diseases and other inherited disorders such as the congenital disorders of glycosylation (CDG) will represent a valuable new tool to explore their pathogenesis.
Conclusion
The function of glycans during embryogenesis will continue to be revealed as more zebrafish mutants are identified and characterized, and researchers in the areas of glycobiology and zebrafish development find common ground. We also anticipate an increased use of zebrafish to model glycosylation-related disorders in the years to come. This effort will likely be aided by the isolation of additional mutants, generated by TILLING-based screens and other comprehensive forward and reverse mutagenic efforts. Caution in interpreting initially negative results or cases where phenotypes do not correlate with loss of enzymatic function is warranted since the genome wide duplications that have occurred in zebrafish may have created significant functional redundancy of many glycosylation-related genes. Moreover, because many glycosylation disorders arise due to point mutations that alter the function (but not expression) of enzymes, methods that reduce or eliminate wild type enzymes, such as morpholino knockdown, may not be effective at faithfully recapitulating the disease phenotypes noted in patients or “knock-in” mouse models. In these cases, transgenic reintroduction of altered gene sequences may prove more useful. Parallel efforts to understand how substrate biosynthesis and glycan expression is regulated in the zebrafish are also needed. This is particularly true in light of the dynamic nature of monosaccharide and lipid metabolism during early embryonic development and the influence of yolk-derived metabolites. As the range of glycan structures in zebrafish become more clearly elucidated, this system will represent an ideal platform to study the functional relevance of rare and unusual glycans.
References
- 1.Mullins MC, Hammerschmidt M, Haffter P, Nusslein-Volhard C. Large-scale mutagenesis in the zebrafish: in search of genes controlling development in a vertebrate. Curr. Biol. 1994;4(3):189–202. doi: 10.1016/s0960-9822(00)00048-8. [DOI] [PubMed] [Google Scholar]
- 2.Haffter P, et al. The identification of genes with unique and essential functions in the development of the zebrafish, Danio rerio. Development. 1996;123:1–36. doi: 10.1242/dev.123.1.1. [DOI] [PubMed] [Google Scholar]
- 3.Granato M, Nusslein-Volhard C. Fishing for genes controlling development. Curr. Opin. Genet. Dev. 1996;6(4):461–468. doi: 10.1016/s0959-437x(96)80068-2. [DOI] [PubMed] [Google Scholar]
- 4.Peterson RT, Macrae CA. Systematic approaches to toxicology in the zebrafish. Annu. Rev. Pharmacol. Toxicol. 2012;52:433–453. doi: 10.1146/annurev-pharmtox-010611-134751. [DOI] [PubMed] [Google Scholar]
- 5.Zon LI, Peterson RT. In vivo drug discovery in the zebrafish. Nat Rev Drug Discov. 2005;4(1):35–44. doi: 10.1038/nrd1606. [DOI] [PubMed] [Google Scholar]
- 6.MacRae CA, Peterson RT. Zebrafish-based small molecule discovery. Chem. Biol. 2003;10(10):901–908. doi: 10.1016/j.chembiol.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Baier H, et al. Genetic dissection of the retinotectal projection. Development. 1996;123:415–425. doi: 10.1242/dev.123.1.415. [DOI] [PubMed] [Google Scholar]
- 8.Brand M, et al. Mutations in zebrafish genes affecting the formation of the boundary between midbrain and hindbrain. Development. 1996;123:179–190. doi: 10.1242/dev.123.1.179. [DOI] [PubMed] [Google Scholar]
- 9.Brand M, et al. Mutations affecting development of the midline and general body shape during zebrafish embryogenesis. Development. 1996;123:129–142. doi: 10.1242/dev.123.1.129. [DOI] [PubMed] [Google Scholar]
- 10.Chen JN, et al. Mutations affecting the cardiovascular system and other internal organs in zebrafish. Development. 1996;123:293–302. doi: 10.1242/dev.123.1.293. [DOI] [PubMed] [Google Scholar]
- 11.Furutani-Seiki M, et al. Neural degeneration mutants in the zebrafish, Danio rerio. Development. 1996;123:229–239. doi: 10.1242/dev.123.1.229. [DOI] [PubMed] [Google Scholar]
- 12.Granato M, et al. Genes controlling and mediating locomotion behavior of the zebrafish embryo and larva. Development. 1996;123:399–413. doi: 10.1242/dev.123.1.399. [DOI] [PubMed] [Google Scholar]
- 13.Hammerschmidt M, et al. Mutations affecting morphogenesis during gastrulation and tail formation in the zebrafish, Danio rerio. Development. 1996;123:143–151. doi: 10.1242/dev.123.1.143. [DOI] [PubMed] [Google Scholar]
- 14.Heisenberg CP, et al. Genes involved in forebrain development in the zebrafish, Danio rerio. Development. 1996;123:191–203. doi: 10.1242/dev.123.1.191. [DOI] [PubMed] [Google Scholar]
- 15.Jiang YJ, et al. Mutations affecting neurogenesis and brain morphology in the zebrafish, Danio rerio. Development. 1996;123:205–216. doi: 10.1242/dev.123.1.205. [DOI] [PubMed] [Google Scholar]
- 16.Kane DA, et al. The zebrafish epiboly mutants. Development. 1996;123:47–55. doi: 10.1242/dev.123.1.47. [DOI] [PubMed] [Google Scholar]
- 17.Kane DA, et al. The zebrafish early arrest mutants. Development. 1996;123:57–66. doi: 10.1242/dev.123.1.57. [DOI] [PubMed] [Google Scholar]
- 18.Karlstrom RO, et al. Zebrafish mutations affecting retinotectal axon pathfinding. Development. 1996;123:427–438. doi: 10.1242/dev.123.1.427. [DOI] [PubMed] [Google Scholar]
- 19.Kelsh RN, et al. Zebrafish pigmentation mutations and the processes of neural crest development. Development. 1996;123:369–389. doi: 10.1242/dev.123.1.369. [DOI] [PubMed] [Google Scholar]
- 20.Mullins MC, et al. Genes establishing dorsoventral pattern formation in the zebrafish embryo: the ventral specifying genes. Development. 1996;123:81–93. doi: 10.1242/dev.123.1.81. [DOI] [PubMed] [Google Scholar]
- 21.Odenthal J, et al. Mutations affecting the formation of the notochord in the zebrafish, Danio rerio. Development. 1996;123:103–115. doi: 10.1242/dev.123.1.103. [DOI] [PubMed] [Google Scholar]
- 22.Piotrowski T, et al. Jaw and branchial arch mutants in zebrafish II: anterior arches and cartilage differentiation. Development. 1996;123:345–356. doi: 10.1242/dev.123.1.345. [DOI] [PubMed] [Google Scholar]
- 23.Ransom DG, et al. Characterization of zebrafish mutants with defects in embryonic hematopoiesis. Development. 1996;123:311–319. doi: 10.1242/dev.123.1.311. [DOI] [PubMed] [Google Scholar]
- 24.Schilling TF, et al. Jaw and branchial arch mutants in zebrafish I: branchial arches. Development. 1996;123:329–344. doi: 10.1242/dev.123.1.329. [DOI] [PubMed] [Google Scholar]
- 25.Trowe T, et al. Mutations disrupting the ordering and topographic mapping of axons in the retinotectal projection of the zebrafish, Danio rerio. Development. 1996;123:439–450. doi: 10.1242/dev.123.1.439. [DOI] [PubMed] [Google Scholar]
- 26.van Eeden FJ, et al. Mutations affecting somite formation and patterning in the zebrafish, Danio rerio. Development. 1996;123:153–164. doi: 10.1242/dev.123.1.153. [DOI] [PubMed] [Google Scholar]
- 27.van Eeden FJ, et al. Genetic analysis of fin formation in the zebrafish, Danio rerio. Development. 1996;123:255–262. doi: 10.1242/dev.123.1.255. [DOI] [PubMed] [Google Scholar]
- 28.Whitfield TT, et al. Mutations affecting development of the zebrafish inner ear and lateral line. Development. 1996;123:241–254. doi: 10.1242/dev.123.1.241. [DOI] [PubMed] [Google Scholar]
- 29.Amsterdam A, et al. A large-scale insertional mutagenesis screen in zebrafish. Genes Dev. 1999;13(20):2713–2724. doi: 10.1101/gad.13.20.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stainier DY, et al. Mutations affecting the formation and function of the cardiovascular system in the zebrafish embryo. Development. 1996;123:285–292. doi: 10.1242/dev.123.1.285. [DOI] [PubMed] [Google Scholar]
- 31.Pack M, et al. Mutations affecting development of zebrafish digestive organs. Development. 1996;123:321–328. doi: 10.1242/dev.123.1.321. [DOI] [PubMed] [Google Scholar]
- 32.Feitsma H, Cuppen E. Zebrafish as a cancer model. Mol Cancer Res. 2008;6(5):685–694. doi: 10.1158/1541-7786.MCR-07-2167. [DOI] [PubMed] [Google Scholar]
- 33.Feitsma H, Kuiper RV, Korving J, Nijman IJ, Cuppen E. Zebrafish with mutations in mismatch repair genes develop neurofibromas and other tumors. Cancer Res. 2008;68(13):5059–5066. doi: 10.1158/0008-5472.CAN-08-0019. [DOI] [PubMed] [Google Scholar]
- 34.Drummond IA. Kidney development and disease in the zebrafish. J. Am. Soc. Nephrol. 2005;16(2):299–304. doi: 10.1681/ASN.2004090754. [DOI] [PubMed] [Google Scholar]
- 35.Rubinstein AL. Zebrafish: from disease modeling to drug discovery. Curr Opin Drug Discov Devel. 2003;6(2):218–223. [PubMed] [Google Scholar]
- 36.Lieschke GJ, Currie PD. Animal models of human disease: zebrafish swim into view. Nat. Rev. Genet. 2007;8(5):353–367. doi: 10.1038/nrg2091. [DOI] [PubMed] [Google Scholar]
- 37.Guerardel Y, Chang LY, Maes E, Huang CJ, Khoo KH. Glycomic survey mapping of zebrafish identifies unique sialylation pattern. Glycobiology. 2006;16(3):244–257. doi: 10.1093/glycob/cwj062. [DOI] [PubMed] [Google Scholar]
- 38.Chang LY, et al. Developmental regulation of oligosialylation in zebrafish. Glycoconj. J. 2009;26(3):247–261. doi: 10.1007/s10719-008-9161-5. [DOI] [PubMed] [Google Scholar]
- 39.Chang LY, et al. Molecular cloning and characterization of the expression pattern of the zebrafish alpha2, 8-sialyltransferases (ST8Sia) in the developing nervous system. Glycoconj. J. 2009;26(3):263–275. doi: 10.1007/s10719-008-9165-1. [DOI] [PubMed] [Google Scholar]
- 40.Harduin-Lepers A, et al. Evolutionary history of the alpha2,8-sialyltransferase (ST8Sia) gene family: tandem duplications in early deuterostomes explain most of the diversity found in the vertebrate ST8Sia genes. BMC Evol. Biol. 2008;8:258. doi: 10.1186/1471-2148-8-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bentrop J, Marx M, Schattschneider S, Rivera-Milla E, Bastmeyer M. Molecular evolution and expression of zebrafish St8SiaIII, an alpha-2,8-sialyltransferase involved in myotome development. Dev. Dyn. 2008;237(3):808–818. doi: 10.1002/dvdy.21451. [DOI] [PubMed] [Google Scholar]
- 42.Marx M, Rivera-Milla E, Stummeyer K, Gerardy-Schahn R, Bastmeyer M. Divergent evolution of the vertebrate polysialyltransferase Stx and Pst genes revealed by fish-to-mammal comparison. Dev. Biol. 2007;306(2):560–571. doi: 10.1016/j.ydbio.2007.03.032. [DOI] [PubMed] [Google Scholar]
- 43.Vanbeselaere J, et al. Mapping the expressed glycome and glycosyltransferases of zebrafish liver cells as a relevant model system for glycosylation studies. J Proteome Res. 2012;11(4):2164–2177. doi: 10.1021/pr200948j. [DOI] [PubMed] [Google Scholar]
- 44.Langhauser M, et al. Ncam1a and Ncam1b: two carriers of polysialic acid with different functions in the developing zebrafish nervous system. Glycobiology. 2012;22(2):196–209. doi: 10.1093/glycob/cwr129. [DOI] [PubMed] [Google Scholar]
- 45.Schaper W, et al. Identification and Biochemical Characterization of Two Functional CMP-Sialic Acid Synthetases in Danio rerio. J. Biol. Chem. 2012;287(16):13239–13248. doi: 10.1074/jbc.M111.327544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takemoto T, Natsuka S, Nakakita S, Hase S. Expression of complex-type N-glycans in developmental periods of zebrafish embryo. Glycoconj. J. 2005;22(1–2):21–26. doi: 10.1007/s10719-005-0189-5. [DOI] [PubMed] [Google Scholar]
- 47.Kageyama N, Natsuka S, Hase S. Molecular cloning and characterization of two zebrafish alpha(1,3)fucosyltransferase genes developmentally regulated in embryogenesis. J. Biochem. 1999;125(4):838–845. doi: 10.1093/oxfordjournals.jbchem.a022357. [DOI] [PubMed] [Google Scholar]
- 48.Moriguchi K, et al. Free oligosaccharides with Lewis × structure expressed in the segmentation period of zebrafish embryo. J. Biochem. 2007;142(2):213–227. doi: 10.1093/jb/mvm128. [DOI] [PubMed] [Google Scholar]
- 49.Souza AR, et al. Chondroitin sulfate and keratan sulfate are the major glycosaminoglycans present in the adult zebrafish Danio rerio (Chordata-Cyprinidae) Glycoconj. J. 2007;24(9):521–530. doi: 10.1007/s10719-007-9046-z. [DOI] [PubMed] [Google Scholar]
- 50.Zhang F, et al. Structural characterization of glycosaminoglycans from zebrafish in different ages. Glycoconj. J. 2009;26(2):211–218. doi: 10.1007/s10719-008-9177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Semino CE, Specht CA, Raimondi A, Robbins PW. Homologs of the Xenopus developmental gene DG42 are present in zebrafish and mouse and are involved in the synthesis of Nod-like chitin oligosaccharides during early embryogenesis. Proc. Natl. Acad. Sci. U. S. A. 1996;93(10):4548–4553. doi: 10.1073/pnas.93.10.4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bakkers J, et al. An important developmental role for oligosaccharides during early embryogenesis of cyprinid fish. Proc. Natl. Acad. Sci. U. S. A. 1997;94(15):7982–7986. doi: 10.1073/pnas.94.15.7982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Semino CE, Allende ML. Chitin oligosaccharides as candidate patterning agents in zebrafish embryogenesis. Int. J. Dev. Biol. 2000;44(2):183–193. [PubMed] [Google Scholar]
- 54.Snaar-Jagalska BE, Krens SF, Robina I, Wang LX, Spaink HP. Specific activation of ERK pathways by chitin oligosaccharides in embryonic zebrafish cell lines. Glycobiology. 2003;13(10):725–732. doi: 10.1093/glycob/cwg103. [DOI] [PubMed] [Google Scholar]
- 55.van Asselt E, de Graaf F, Smit-Onel MJ, van Raamsdonk W. Spinal neurons in the zebrafish labeled with fluoro-gold and wheat-germ agglutinin. Neuroscience. 1991;43(2–3):611–622. doi: 10.1016/0306-4522(91)90320-n. [DOI] [PubMed] [Google Scholar]
- 56.Becker KA, Hart NH. Reorganization of filamentous actin and myosin-II in zebrafish eggs correlates temporally and spatially with cortical granule exocytosis. J Cell Sci. 1999;112(Pt 1):97–110. doi: 10.1242/jcs.112.1.97. [DOI] [PubMed] [Google Scholar]
- 57.Lugo-Villarino G, et al. Identification of dendritic antigen-presenting cells in the zebrafish. Proc. Natl. Acad. Sci. U. S. A. 2010;107(36):15850–15855. doi: 10.1073/pnas.1000494107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Laughlin ST, Baskin JM, Amacher SL, Bertozzi CR. In vivo imaging of membrane-associated glycans in developing zebrafish. Science. 2008;320(5876):664–667. doi: 10.1126/science.1155106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baskin JM, Dehnert KW, Laughlin ST, Amacher SL, Bertozzi CR. Visualizing enveloping layer glycans during zebrafish early embryogenesis. Proc. Natl. Acad. Sci. U. S. A. 2010;107(23):10360–10365. doi: 10.1073/pnas.0912081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dehnert KW, et al. Imaging the Sialome during Zebrafish Development with Copper-Free Click Chemistry. ChemBioChem. 2012;13(3):353–357. doi: 10.1002/cbic.201100649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dehnert KW, et al. Metabolic labeling of fucosylated glycans in developing zebrafish. ACS Chem. Biol. 2011;6(6):547–552. doi: 10.1021/cb100284d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zheng T, et al. Tracking N-acetyllactosamine on cell-surface glycans in vivo. Angew Chem Int Ed Engl. 2011;50(18):4113–4118. doi: 10.1002/anie.201100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jiang H, et al. Imaging glycans in zebrafish embryos by metabolic labeling and bioorthogonal click chemistry. J Vis Exp. 2011;52:2686. doi: 10.3791/2686. doi:10.3791/2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Soriano Del Amo D, et al. Biocompatible copper(I) catalysts for in vivo imaging of glycans. J. Am. Chem. Soc. 2010;132(47):16893–16899. doi: 10.1021/ja106553e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Panzer JA, et al. Neuromuscular synaptogenesis in wild-type and mutant zebrafish. Dev. Biol. 2005;285(2):340–357. doi: 10.1016/j.ydbio.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 66.Song Y, et al. Neural and synaptic defects in slytherin, a zebrafish model for human congenital disorders of glycosylation. PLoS One. 2010;5(10):e13743. doi: 10.1371/journal.pone.0013743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stanley P, Okajima T. Roles of glycosylation in Notch signaling. Curr. Top. Dev. Biol. 2010;92:131–164. doi: 10.1016/S0070-2153(10)92004-8. [DOI] [PubMed] [Google Scholar]
- 68.Jafar-Nejad H, Leonardi J, Fernandez-Valdivia R. Role of glycans and glycosyltransferases in the regulation of Notch signaling. Glycobiology. 2010;20(8):931–949. doi: 10.1093/glycob/cwq053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Haines N, Irvine KD. Glycosylation regulates Notch signalling. Nat. Rev. Mol. Cell Biol. 2003;4(10):786–797. doi: 10.1038/nrm1228. [DOI] [PubMed] [Google Scholar]
- 70.Haltiwanger RS. Regulation of signal transduction pathways in development by glycosylation. Curr. Opin. Struct. Biol. 2002;12(5):593–598. doi: 10.1016/s0959-440x(02)00371-8. [DOI] [PubMed] [Google Scholar]
- 71.Ohata S, et al. Neuroepithelial cells require fucosylated glycans to guide the migration of vagus motor neuron progenitors in the developing zebrafish hindbrain. Development. 2009;136(10):1653–1663. doi: 10.1242/dev.033290. [DOI] [PubMed] [Google Scholar]
- 72.Seth A, Machingo QJ, Fritz A, Shur BD. Core fucosylation is required for midline patterning during zebrafish development. Dev. Dyn. 2010;239(12):3380–3390. doi: 10.1002/dvdy.22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Machingo QJ, Fritz A, Shur BD. A beta1,4-galactosyltransferase is required for convergent extension movements in zebrafish. Dev. Biol. 2006;297(2):471–482. doi: 10.1016/j.ydbio.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 74.Machingo QJ, Fritz A, Shur BD. A beta1,4-galactosyltransferase is required for Bmp2-dependent patterning of the dorsoventral axis during zebrafish embryogenesis. Development. 2006;133(11):2233–2241. doi: 10.1242/dev.02378. [DOI] [PubMed] [Google Scholar]
- 75.Craig SE, et al. The zebrafish galectin Drgal1-l2 is expressed by proliferating Muller glia and photoreceptor progenitors and regulates the regeneration of rod photoreceptors. Invest Ophthalmol Vis Sci. 2010;51(6):3244–3252. doi: 10.1167/iovs.09-4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ahmed H, Du SJ, Vasta GR. Knockdown of a galectin-1-like protein in zebrafish (Danio rerio) causes defects in skeletal muscle development. Glycoconj. J. 2009;26(3):277–283. doi: 10.1007/s10719-008-9178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ahmed H, Vasta GR. Unlike mammalian GRIFIN, the zebrafish homologue (DrGRIFIN) represents a functional carbohydrate-binding galectin. Biochem. Biophys. Res. Commun. 2008;371(3):350–355. doi: 10.1016/j.bbrc.2008.04.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vasta GR, Ahmed H, Du S, Henrikson D. Galectins in teleost fish: Zebrafish (Danio rerio) as a model species to address their biological roles in development and innate immunity. Glycoconj. J. 2004;21(8–9):503–521. doi: 10.1007/s10719-004-5541-7. [DOI] [PubMed] [Google Scholar]
- 79.Ahmed H, Du SJ, O'Leary N, Vasta GR. Biochemical and molecular characterization of galectins from zebrafish (Danio rerio): notochord-specific expression of a prototype galectin during early embryogenesis. Glycobiology. 2004;14(3):219–232. doi: 10.1093/glycob/cwh032. [DOI] [PubMed] [Google Scholar]
- 80.Marx M, Rutishauser U, Bastmeyer M. Dual function of polysialic acid during zebrafish central nervous system development. Development. 2001;128(24):4949–4958. doi: 10.1242/dev.128.24.4949. [DOI] [PubMed] [Google Scholar]
- 81.Webster DM, et al. O-GlcNAc modifications regulate cell survival and epiboly during zebrafish development. BMC Dev. Biol. 2009;9:28. doi: 10.1186/1471-213X-9-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Willer GB, Lee VM, Gregg RG, Link BA. Analysis of the Zebrafish perplexed mutation reveals tissue-specific roles for de novo pyrimidine synthesis during development. Genetics. 2005;170(4):1827–1837. doi: 10.1534/genetics.105.041608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Peal DS, Burns CG, Macrae CA, Milan D. Chondroitin sulfate expression is required for cardiac atrioventricular canal formation. Dev. Dyn. 2009;238(12):3103–3110. doi: 10.1002/dvdy.22154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Becker CG, Becker T. Repellent guidance of regenerating optic axons by chondroitin sulfate glycosaminoglycans in zebrafish. J. Neurosci. 2002;22(3):842–853. doi: 10.1523/JNEUROSCI.22-03-00842.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bernhardt RR, Schachner M. Chondroitin sulfates affect the formation of the segmental motor nerves in zebrafish embryos. Dev. Biol. 2000;221(1):206–219. doi: 10.1006/dbio.2000.9673. [DOI] [PubMed] [Google Scholar]
- 86.Zoeller JJ, et al. A central role for decorin during vertebrate convergent extension. J. Biol. Chem. 2009;284(17):11728–11737. doi: 10.1074/jbc.M808991200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.De Cat B, et al. Processing by proprotein convertases is required for glypican-3 modulation of cell survival, Wnt signaling, and gastrulation movements. J Cell Biol. 2003;163(3):625–635. doi: 10.1083/jcb.200302152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Arrington CB, Yost HJ. Extra-embryonic syndecan 2 regulates organ primordia migration and fibrillogenesis throughout the zebrafish embryo. Development. 2009;136(18):3143–3152. doi: 10.1242/dev.031492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kramer KL, Yost HJ. Ectodermal syndecan-2 mediates left-right axis formation in migrating mesoderm as a cell-nonautonomous Vg1 cofactor. Dev Cell. 2002;2(1):115–124. doi: 10.1016/s1534-5807(01)00107-1. [DOI] [PubMed] [Google Scholar]
- 90.Eames BF, et al. UDP xylose synthase 1 is required for morphogenesis and histogenesis of the craniofacial skeleton. Dev. Biol. 2010;341(2):400–415. doi: 10.1016/j.ydbio.2010.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Walsh EC, Stainier DY. UDP-glucose dehydrogenase required for cardiac valve formation in zebrafish. Science. 2001;293(5535):1670–1673. doi: 10.1126/science.293.5535.1670. [DOI] [PubMed] [Google Scholar]
- 92.Eames BF, et al. Mutations in fam20b and xylt1 reveal that cartilage matrix controls timing of endochondral ossification by inhibiting chondrocyte maturation. PLoS Genet. 2011;7(8):e1002246. doi: 10.1371/journal.pgen.1002246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee JS, et al. Axon sorting in the optic tract requires HSPG synthesis by ext2 (dackel) and extl3 (boxer) Neuron. 2004;44(6):947–960. doi: 10.1016/j.neuron.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 94.Clement A, et al. Regulation of zebrafish skeletogenesis by ext2/dackel and papst1/pinscher. PLoS Genet. 2008;4(7):e1000136. doi: 10.1371/journal.pgen.1000136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mizumoto S, et al. Chondroitin 4-O-sulfotransferase-1 is required for somitic muscle development and motor axon guidance in zebrafish. Biochem. J. 2009;419(2):387–399. doi: 10.1042/BJ20081639. [DOI] [PubMed] [Google Scholar]
- 96.Busch-Nentwich E, Sollner C, Roehl H, Nicolson T. The deafness gene dfna5 is crucial for ugdh expression and HA production in the developing ear in zebrafish. Development. 2004;131(4):943–951. doi: 10.1242/dev.00961. [DOI] [PubMed] [Google Scholar]
- 97.Norton WH, Ledin J, Grandel H, Neumann CJ. HSPG synthesis by zebrafish Ext2 and Extl3 is required for Fgf10 signalling during limb development. Development. 2005;132(22):4963–4973. doi: 10.1242/dev.02084. [DOI] [PubMed] [Google Scholar]
- 98.Cadwalader EL, Condic ML, Yost HJ. 2-O-sulfotransferase regulates Wnt signaling, cell adhesion and cell cycle during zebrafish epiboly. Development. 2012 doi: 10.1242/dev.078238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Grewal PK, McLaughlan JM, Moore CJ, Browning CA, Hewitt JE. Characterization of the LARGE family of putative glycosyltransferases associated with dystroglycanopathies. Glycobiology. 2005;15(10):912–923. doi: 10.1093/glycob/cwi094. [DOI] [PubMed] [Google Scholar]
- 100.Moore CJ, Goh HT, Hewitt JE. Genes required for functional glycosylation of dystroglycan are conserved in zebrafish. Genomics. 2008;92(3):159–167. doi: 10.1016/j.ygeno.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 101.Thornhill P, Bassett D, Lochmuller H, Bushby K, Straub V. Developmental defects in a zebrafish model for muscular dystrophies associated with the loss of fukutin-related protein (FKRP) Brain. 2008;131(Pt 6):1551–1561. doi: 10.1093/brain/awn078. [DOI] [PubMed] [Google Scholar]
- 102.Wood AJ, et al. Abnormal vascular development in zebrafish models for fukutin and FKRP deficiency. Hum. Mol. Genet. 2011;20(24):4879–4890. doi: 10.1093/hmg/ddr426. [DOI] [PubMed] [Google Scholar]
- 103.Lin YY, et al. Zebrafish Fukutin family proteins link the unfolded protein response with dystroglycanopathies. Hum. Mol. Genet. 2011;20(9):1763–1775. doi: 10.1093/hmg/ddr059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Avsar-Ban E, et al. Protein O-mannosylation is necessary for normal embryonic development in zebrafish. Glycobiology. 2010;20(9):1089–1102. doi: 10.1093/glycob/cwq069. [DOI] [PubMed] [Google Scholar]
- 105.Fan X, Klein M, Flanagan-Steet HR, Steet R. Selective yolk deposition and mannose phosphorylation of lysosomal glycosidases in zebrafish. J. Biol. Chem. 2010;285(43):32946–32953. doi: 10.1074/jbc.M110.158295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Flanagan-Steet H, Sias C, Steet R. Altered chondrocyte differentiation and extracellular matrix homeostasis in a zebrafish model for mucolipidosis II. Am. J. Pathol. 2009;175(5):2063–2075. doi: 10.2353/ajpath.2009.090210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Moro E, et al. A novel functional role of iduronate-2-sulfatase in zebrafish early development. Matrix Biol. 2010;29(1):43–50. doi: 10.1016/j.matbio.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 108.Petrey AC, et al. Excessive activity of cathepsin K is associated with cartilage defects in a zebrafish model of mucolipidosis II. Dis Model Mech. 2012;5(2):177–190. doi: 10.1242/dmm.008219. [DOI] [PMC free article] [PubMed] [Google Scholar]


