Abstract
Background and Purpose
Cognitive decline after cardiac surgery remains common and diminishes patients’ quality of life. Based on experimental and clinical evidence, this study assessed the potential of intravenously administered lidocaine to reduce postoperative cognitive dysfunction following cardiac surgery employing cardiopulmonary bypass.
Methods
Following IRB approval, 277 patients undergoing cardiac surgery were enrolled into this prospective, randomized, double-blinded placebo controlled clinical trial. Subjects were randomized to receive: 1) Lidocaine as a 1 mg/kg bolus followed by a continuous infusion through 48 hours postoperatively or 2) Placebo bolus and infusion. Cognitive function was assessed preoperatively and again at 6 weeks and 1 year postoperatively. The effect of lidocaine on postoperative cognition was tested using multivariable regression modeling; p <0.05 was considered significant.
Results
Among the 241 allocated subjects (Lidocaine: N=114; Placebo: N=127), the incidence of cognitive deficit in the lidocaine group was 45.5% versus 45.7% in the placebo group (p=0.97). Multivariable analysis revealed a significant interaction between treatment group and diabetes, such that diabetic subjects receiving lidocaine were more likely to suffer cognitive decline (p=0.004). Secondary analysis identified total lidocaine dose (mg/kg) as a significant predictor of cognitive decline and also revealed a protective effect of lower dose lidocaine in nondiabetic subjects.
Conclusions
Lidocaine administered during and after cardiac surgery does not reduce the high rate of postoperative cognitive dysfunction. Higher doses of lidocaine and diabetic status were independent predictors of cognitive decline. Protective effects of lower dose lidocaine in nondiabetic subjects need to be further evaluated.
Keywords: lidocaine, cognition, cardiopulmonary bypass
Introduction
Although mortality and cardiac morbidity for patients undergoing cardiac surgery has declined significantly in the last decade, the incidence of postoperative neurocognitive decline remains high, detected in over 50% of patients at hospital discharge and persisting in 30% after 6 months.1 Quality of life is also diminished for these patients who anticipate that postoperative improvements in physical status will generally improve their lives.2 Initial reports suggested that impairment at hospital discharge was associated with long term decline but more recently, it has been reported that the late cognitive decline may not be related to surgery.1, 3-5 Potential mechanisms for this injury following cardiac surgery with cardiopulmonary bypass (CPB) include cerebral hypoperfusion, air and particulate embolism, ischemia-reperfusion injury, hemodilution, genetic predisposition, and an exaggerated inflammatory response.6-8 Attempts to ameliorate the injury have only yielded marginal effects.9-12
Lidocaine is a class IB antiarrhythmic that readily crosses the blood-brain barrier and may confer cerebral protection by modulation of inflammatory mediators, preservation of cerebral blood flow (CBF), reduction in cerebral metabolism, and deceleration of ischemic ion fluxes.13 Cerebral protection by lidocaine was initially demonstrated in a feline model of cerebral arterial gas embolism14 and much of the initial in vivo neuroprotective experiments assessed lidocaine as a treatment for decompression illness. Early clinical data were also limited to case reports describing benefit in divers with neurologic signs of decompression illness. In 1999, Mitchell et al,15 reported significantly less neurocognitive deficit in 55 lidocaine treated valve surgery patients. On the basis of these preliminary data, we hypothesized that intravenous lidocaine administered from induction of anesthesia to 48 hours after cardiac surgery would reduce postoperative neurocognitive decline and attenuate the inflammatory response associated with CPB.
Materials and Methods
Study Population
Subsequent to approval by the Duke University Health System Institutional Review Board and informed consent, 277 patients scheduled to undergo coronary artery bypass grafting and/or an open chamber procedure with CPB were enrolled into this prospective, randomized, double-blinded, placebo controlled clinical trial. Patients were excluded if they were undergoing circulatory arrest or had a history of symptomatic cerebrovascular disease (e.g. stroke with a residual deficit), psychiatric illness (any clinical diagnoses requiring therapy), renal failure (serum creatinine > 2 mg/dl), liver disease (liver function tests > 1.5 times the upper limit of normal), higher alcohol consumption (> 2 drinks/day), or were unable to read or had less than a seventh grade education. Subjects were randomized to two treatment groups: 1) lidocaine group - bolus of 1 mg/kg of lidocaine administered after induction of anesthesia and followed immediately by a continuous infusion at 4 mg/min for the first hour, 2 mg/min for the second hour, and 1 mg/min for the next 46 hours or 2) placebo group - normal saline administered as a bolus and an infusion for 48 hours with identical volume and rate changes as the treatment group such that blinding was preserved. A group assignment schedule was prepared using a randomization function in SAS® version 8.2 (SAS, Cary, NC, USA) and stored in consecutively numbered sealed envelopes until allocation.
Patient Management
Anesthesia was induced and maintained with midazolam, fentanyl, and isoflurane. All patients underwent nonpulsatile hypothermic (30°-32°C) CPB with a membrane oxygenator and an arterial line filter. The pump was primed with crystalloid and serial hematocrit levels were kept at ≥ 0.21. Perfusion was maintained at pump flow rates of 2-2.4 L • min−1 • m2 throughout CPB to maintain mean arterial pressure at 50-80 mmHg. Arterial blood gases were measured every 15-30 minutes to maintain arterial carbon dioxide partial pressures of 35 to 40 mmHg, unadjusted for temperature (α-stat), and oxygen partial pressures of 150 to 250 mmHg.
Measurement of Neurocognitive Function
Experienced psychometricians blinded to the treatment group examined subjects with a well-validated battery of 5 cognitive tests (producing 10 scores) on the day before surgery and again at 6 weeks and 1 year after surgery. In accordance with the Consensus Statement on Assessment of Neurobehavioral Outcomes after Cardiac Surgery,16 we used a cognitive test battery comprised of the following five instruments that yielded 10 scores:
The Short Story module of the Randt Memory Test17 requires subjects to recall the details of a short story immediately after it has been read to them and after a 30-minute delay. Verbatim and gist recall are both evaluated; (4 scores)
The Digit Span subtest of the Wechsler Adult Intelligence Scale-Revised (WAIS-R) Test18 requires subjects to repeat a series of digits that have been orally presented to them both forward and, in an independent test, in reverse order; (2 scores)
Modified Visual Reproduction Test from the Wechsler Memory Scale18 measures short- and long-term figural memory and requires subjects to reproduce from memory several geometric shapes both immediately and after a 30-minutes delay; (2 scores)
The Digit Symbol subtest of the WAIS-R18 is a paper and pencil task that requires subjects to reproduce, within 90 seconds, as many coded symbols as possible in blank boxes beneath randomly generated digits according to a coding scheme for pairing digits with symbols; (1 score)
The Trail Making Test (part B)19 requires subjects to connect, by drawing a line, a series of numbers, and letters in sequence (i.e., 1-A-2-B) as quickly as possible. (1 score)
Laboratory Assessments
Blood was sampled at baseline, end of CPB and 24 hours after CPB for measurement of lidocaine levels. Plasma was also sampled at baseline, end of CPB, and 4.5 hours and 24 hours after cross-clamp removal and immediately frozen at −70° C for subsequent assessment of the cytokine response. Caspase-3, C-reactive protein (CRP), interleukin-8 (IL-8), matrix metalloproteinase-9 (MMP-9), vascular endothelial growth factor (VEGF), and S-100β (S100β) levels were performed by Biosite Diagnostics (San Diego, CA) using forward immunometric assays in 384-well microtiter plates and a Tecan Genesis RSP 200/8 Workstation (Tecan, Research Triangle Park, NC). Finally, whole blood was obtained from each subject prior to surgery and genomic DNA was prepared using the Puregene™ kit according to manufacturer protocols (Gentra Systems, Minneapolis, MN). A sample of this DNA was used to determine APOE genotype as previously described.20 All study personnel were blinded to the results of the laboratory analyses.
Statistical Analysis
To characterize cognitive function over time while minimizing potential redundancy in the cognitive measures, a factor analysis was performed on the ten cognitive test scores from baseline. The ten scores were incorporated into a principal components analysis with orthogonal rotation (a linear transformation of the data) to produce uncorrelated factors. The factor analysis was conducted on the enrolled subjects in this study, and scoring coefficients for all time points were determined using this sample’s baseline rotated factor scores; thus, cognitive domains remained consistent over time. We chose a four-factor solution, which accounts for 84% of the variability in the original 10 test scores and represents four cognitive domains: 1) verbal memory 2) abstraction and visuo-spatial orientation (executive function) 3) visual memory and 4) attention and concentration. (Table 1 – available online only) Two summary measures were calculated to represent cognitive function: 1) Cognitive deficit (the binary outcome) was defined as a decline of 1 standard deviation or more in at least 1 of the 4 domains. 2) To quantify overall cognitive function and the degree of learning (i.e., practice effect from repeated exposure to the testing procedures), a baseline cognitive index was first calculated as the mean of the 4 preoperative domain scores. A continuous change score (the continuous outcome) was then calculated by subtracting the baseline from the follow-up cognitive index.
Table 1.
Rotated factor pattern loadings.
| Test | Factor 1 (Verbal Memory) |
Factor 2 (Abstraction, Visuo-spatial orientation) |
Factor 3 (Visual Memory) |
Factor 4 (Attention and Concentration) |
|---|---|---|---|---|
| RANDT-IV | 0.89564 | 0.09074 | 0.18828 | 0.21279 |
| RANDT-IG | 0.91578 | 0.08445 | 0.09134 | 0.07255 |
| RANDT-DV | 0.86515 | 0.27823 | 0.22667 | 0.14295 |
| RANDT-DG | 0.86883 | 0.22907 | 0.19082 | 0.11991 |
| FIGM-D | 0.25786 | 0.28410 | 0.84594 | 0.18972 |
| FIGM-I | 0.20781 | 0.22121 | 0.88340 | 0.18791 |
| REPFOR | 0.07552 | 0.06744 | 0.25875 | 0.87666 |
| REPBACK | 0.29858 | 0.32424 | 0.07672 | 0.76200 |
| DIGTSYM | 0.21749 | 0.87397 | 0.22395 | 0.10587 |
| TRAILSB | 0.18069 | 0.82802 | 0.26162 | 0.24790 |
RANDT = Randt Short Story Memory Test (suffixes I, V, D, and G refer to Immediate, Verbatim, Delayed, and Gist); FIGM = Figural memory from Modified Visual Reproduction Test in the Wechsler Memory Scale (suffixes I and D refer to Immediate and Delayed); REPFOR = Forward order repetition in the Digit Span Test; REPBACK = Reverse order repetition in the Digit Span Test; DIGITSYM = Digit Symbol Test; TRAILSB = Trail Making Test, Part B.
Categorical and continuous demographic characteristics were compared between treatment groups with Pearson Chi-Square, Fisher Exact, and t-tests. The effect of lidocaine treatment on the cognitive change score was tested using multivariable linear regression modeling accounting for age, sex, weight, years of education, baseline cognition, diabetes, ApoE genotype, maximum intraoperative glucose, CPB time, and cross-clamp time; interactions between age and diabetes and treatment group were also examined. Variables with a p-value > 0.10 were eliminated from the final model. Similarly, the effect of lidocaine upon cognitive deficit was tested using multivariable logistic regression accounting for age, years of education, baseline cognition, and diabetes. Due to the number of outcomes, not all potential predictors could be tested together in the logistic regression model; these variables were selected based on their importance in the linear regression model.
Differences in serum biomarkers were assessed using a repeated measures analysis of variance based on log transformation with unstructured covariance and the Tukey adjustment for post hoc pairwise comparisons. All analyses were performed with SAS® version 9.13 (SAS, Cary, NC, USA); p < 0.05 was considered significant.
Based on preliminary data, we expected that the incidence of cognitive deficit in patients undergoing cardiac valvular surgery would be approximately 45%. We hypothesized that lidocaine treatment would decrease this incidence by forty percent to 27%, and a sample size of 112 per group would yield power of 80% at a significance level of 0.05 to detect this difference. To allow for a 10% loss to follow-up, we intended to recruit a total of 250 patients.
Results
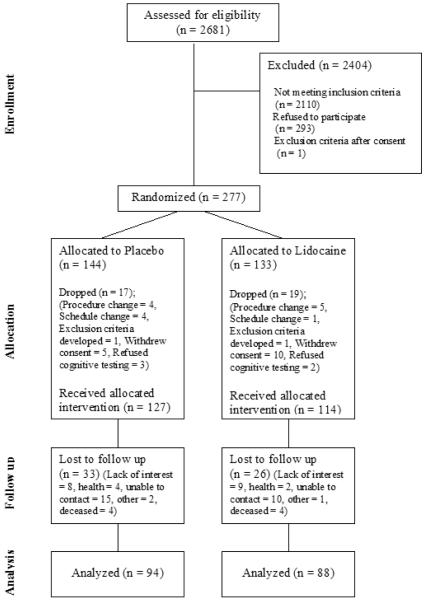
From March 1, 1999 to April 21, 2003, a total of 277 patients were consented to participate in the study and randomized (Figure 1). Thirty six of these subjects were not subsequently treated (refused neurologic testing = 5, withdrew consent = 15, exclusion criteria developed = 2, procedure change = 9, change in surgical schedule = 5), leaving 114 subjects who were allocated to the lidocaine group and 127 to the placebo group (N = 241).
Figure 1.
CONSORT diagram showing the flow of participants.
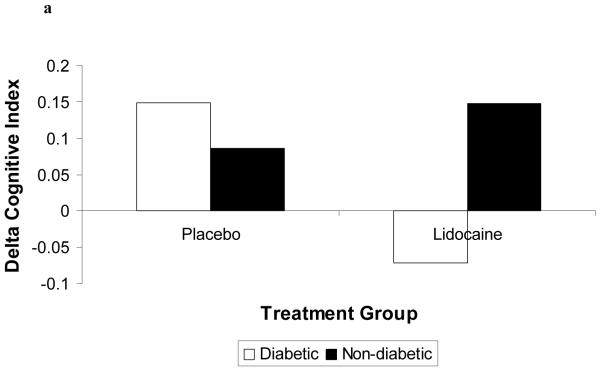
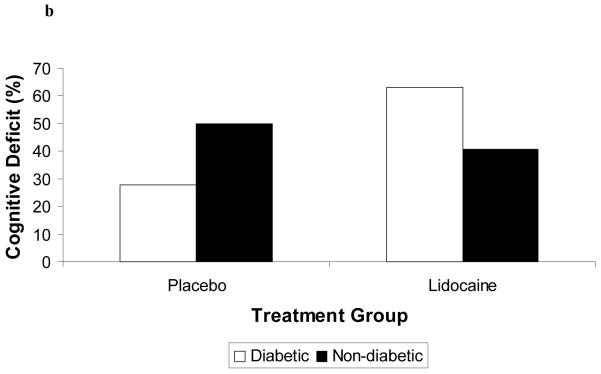
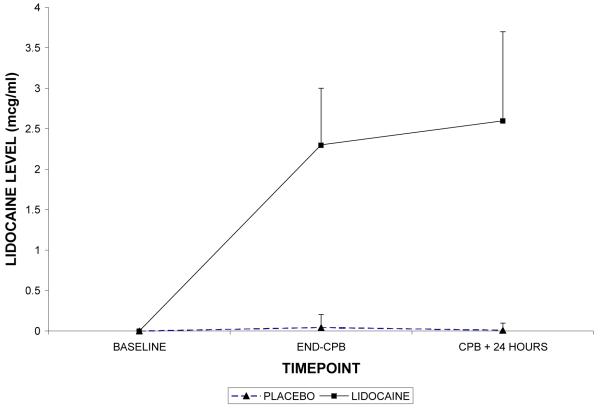
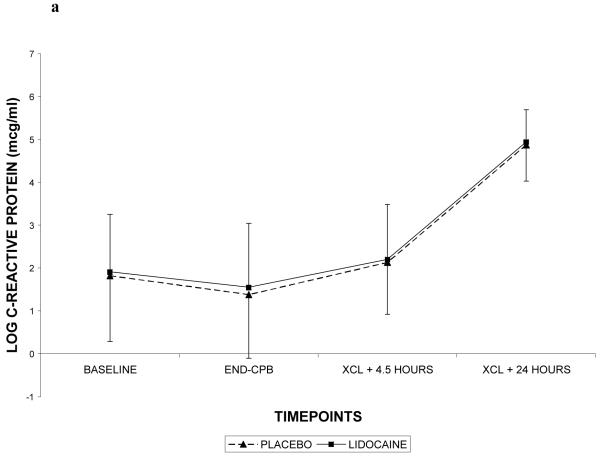
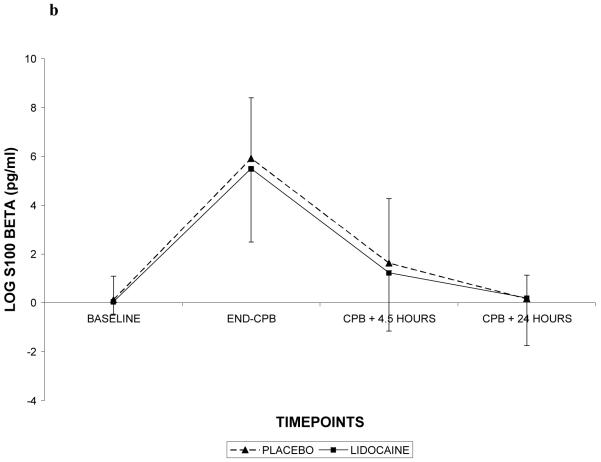
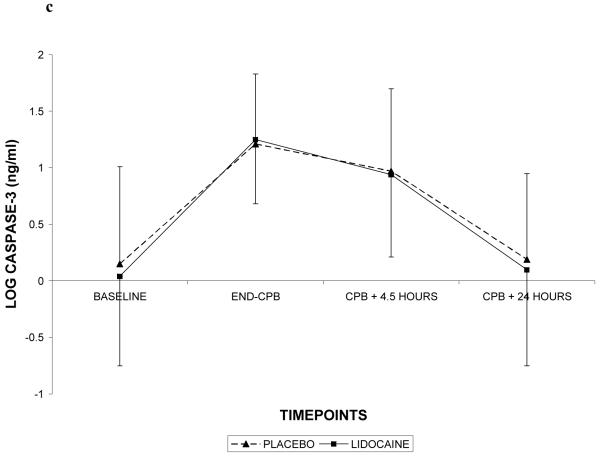
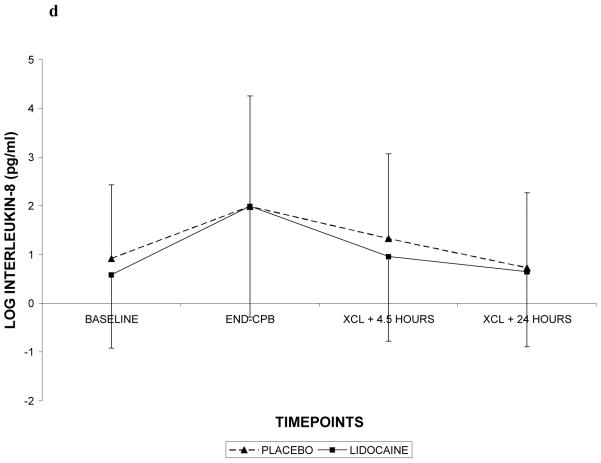
Demographic characteristics of the randomized subjects are listed in Table 2. Despite randomization, subjects in the lidocaine group tended to weigh more (p=0.06) and were more likely to be Caucasian (p=0.07); all other characteristics were similar between treatment groups. Among the 182 subjects who retuned for follow-up testing (Figure 1), cognitive deficits at 6 weeks after surgery were present in 45.5% of subjects randomized to lidocaine and in 45.7% of subjects randomized to placebo (p=0.97). The continuous cognitive score was also not significantly different between the treatment groups (lidocaine: 0.10 + 0.30 vs placebo: 0.10 + 0.30; p=0.97). Multivariable analysis accounting for the covariable effects of age, years of education, weight, baseline cognition level, and diabetes, however, revealed a significant interaction between the treatment group and diabetes, such that diabetic subjects in the lidocaine group were more likely to suffer cognitive decline (continuous outcome: p=0.004, Table 3, Figure 2a – available online only); binary outcome: p=0.012, Figure 2b – available online only). As expected, lidocaine levels were significantly higher in the lidocaine group (Figure 3); however, there was no difference between the treatment groups in any of the measured serum biomarkers (Figure 4a, b, c, d, e, f – available online only).
Table 2.
Demographic characteristics of the subjects allocated to treatment.
| Variable |
Lidocaine (n=114) |
Placebo (n= 127) |
P value |
|---|---|---|---|
| Age in years (SD) | 61.7 (11.9) | 61.4 (13.9) | 0.86 |
| Gender (% female) | 27.2 | 33.1 | 0.32 |
| Race (% Caucasian) | 91.2 | 83.5 | 0.07 |
| Weight in kg (SD) | 86.1 (18.7) | 81.6 (18.0) | 0.06 |
| History of hypertension (%) | 59.7 | 55.9 | 0.56 |
| Diabetes (%) | 23.7 | 20.5 | 0.55 |
| Previous MI (%) | 29.8 | 24.4 | 0.34 |
| Ejection fraction (SD) | 50 (13) | 52 (11) | 0.16 |
| Years of education (SD) | 13.3 (3.4) | 12.7 (3.3) | 0.15 |
| Preoperative cognitive index (SD) | 0.03 (0.51) | −0.01 (0.50) | 0.45 |
| Surgical procedure (%) | 0.46 | ||
| CABG | 44.7 | 40.9 | |
| CABG + Valve | 19.3 | 18.1 | |
| Valve | 35.1 | 37.0 | |
| Other | 0.9 | 3.9 | |
| Redo surgery (%) | 14.0 | 18.1 | 0.39 |
| Number of grafts (SD) | 1.9 (1.7) | 1.6 (1.5) | 0.10 |
| Cross-clamp time in minutes (SD) | 96 (48) | 96 (53) | 0.93 |
| CPB time in minutes (SD) | 168 (76) | 161 (73) | 0.49 |
CPB = cardiopulmonary bypass; MI = myocardial infarction; CABG = coronary artery bypass grafting; SD = standard deviation.
Table 3.
Multivariable linear regression model predicting cognitive change (continuous outcome) at 6-week follow-up.
| Variable | DF | Parameter Estimate (95% confidence limits) |
P value |
|---|---|---|---|
| Age | 1 | −0.009 [−0.012 - (−0.005)] |
<0.001 |
| Years of Education | 1 | 0.012 [−0.002 - 0.027] |
0.098 |
| Weight | 1 | 0.002 [−0.0001 - 0.004] |
0.070 |
| Preoperative cognitive index | 1 | −0.264 [−0.372 - (−0.156)] |
< 0.001 |
| Lidocaine treatment | 1 | 0.074 [−0.016 - 0.164] |
0.108 |
| Diabetes | 1 | 0.031 [−0.111 - 0.172] |
0.671 |
| Diabetes• Lidocaine | 1 | −0.291 [−0.489- (−0.093)] |
0.004 |
DF = degrees of freedom.
Figure 2 (Online only).
Diabetic patients receiving lidocaine were more likely to experience cognitive decline (2a) or cognitive deficit (2b). Delta Cognitive Index = (6 week – preoperative cognitive index); Cognitive Deficit = dichotomous outcome.
Figure 3.
Lidocaine levels with standard deviations in patients receiving lidocaine or placebo. CPB = cardiopulmonary bypass.
Figure 4 (Online Only).
C-reactive protein (4a), S-100β (4b), caspase-3 (4c), interleukin-8 (4d), matrix metalloproteinase-9 (4e), and vascular endothelial growth factor (4f) levels with standard deviations in patients receiving lidocaine or placebo. Values have been log-transformed to achieve normality. CPB = cardiopulmonary bypass; XCL = aortic cross clamp.
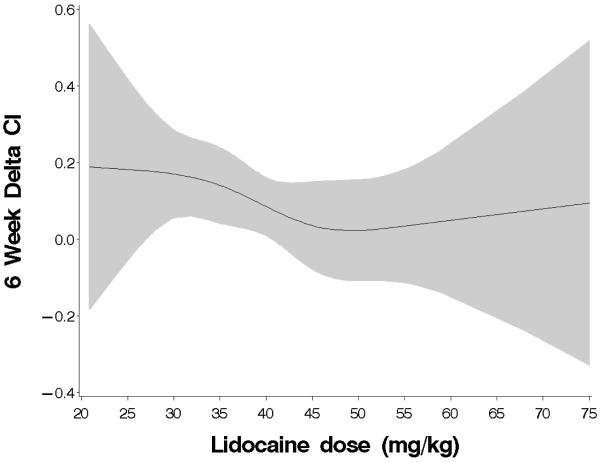
In order to assess the significance of lower weight (Table 3) as a predictor of cognitive change, post hoc multivariable linear regression analysis was conducted in the lidocaine group alone (N = 114). As the lidocaine infusion was at a fixed rate regardless of patient weight, the lower weight patients would have received a larger total dose of lidocaine. This analysis revealed that the total lidocaine dose over the 48 hour treatment period (mg/kg) was a significant predictor of cognitive decline independent of diabetes (continuous outcome: p=0.029, Table 4; binary outcome: p=0.116), suggesting that an increased exposure to lidocaine may be detrimental. To investigate the possibility of a non-linear association between lidocaine dose and cognitive change, an analysis using restricted cubic splines was performed in the same subset of subjects who received lidocaine. Restricted cubic splines, which are smooth at the joint points, or knots (slope is allowed to vary at these points) and which are constrained to be linear in the tails, can greatly improve the fit of the model.21 Based on the size of the dataset, 4 knots were placed at 25, 40, 45, and 55 mg/kg of lidocaine (approximating the 5th, 25th, 75th, and 95th percentiles). Figure 5 (online only) shows the resulting fitted line with 95% confidence intervals indicating that cognitive decline was relatively unchanged until the dose of lidocaine exceeded approximately 35 mg/kg. Finally, exploratory multivariable analyses, conducted without adjustment for multiple comparisons, on the nondiabetic subjects (N=166) comparing those who received a total dose of lidocaine < 42.6 mg/kg (75th percentile) to those not receiving lidocaine revealed a primary protective effect of lidocaine (continuous outcome: p=0.030; binary outcome: Odds Ratio 0.41 [0.19-0.89], p=0.024). There were no differences between diabetics and nondiabetics with regard to lidocaine levels or cytokine expression.
Table 4.
Multivariable linear regression model predicting cognitive change (continuous outcome) at 6-week follow-up in the lidocaine group only.
| Variable | DF | Parameter Estimate (95% confidence limits) |
P value |
|---|---|---|---|
| Age | 1 | −0.006 [−0.011 – (−0.008)] |
0.024 |
| Preoperative cognitive index | 1 | −0.208 [−0.336 – (−0.079)] |
0.002 |
| Diabetes | 1 | −0.283 [−0.425 – (−0.142)] |
<0.001 |
| Lidocaine dose (mg/kg) | 1 | −0.008 [−0.015- (−0.001)] |
0.029 |
DF = degrees of freedom.
Figure 5.
Decline in cognition with increasing total dose of lidocaine. The shaded area represents 95% confidence intervals. The threshold for cognitive decline appears to be at an approximately 35 mg/kg of lidocaine. Delta CI = (6 week – preoperative cognitive index).
At one year after surgery, 141 subjects (58.5%) underwent cognitive testing. Cognitive deficit (binary outcome) was present in 51.7% of placebo subjects compared to 40% of lidocaine subjects (p=0.185). The continuous cognitive score was 0.09 + 0.30 in the placebo group versus 0.19 + 0.25 in the lidocaine group (p=0.082). Multivariable linear regression adjusting for age, years of education, and baseline level of cognition revealed a marginally protective effect of lidocaine treatment (p=0.049). Similarly, logistic regression analyses of the binary outcome revealed a trend toward a protective effect (Odds Ratio: 0.60[0.30-1.22], p=0.156). No interaction between treatment and diabetes was detected in this smaller sample (N = 25 diabetics) evaluated at 1 year.
Adverse events were not significantly different between treatment groups; serious adverse events were recorded in 12.3% of lidocaine and 10.2% of placebo subjects (p=0.68). The length of hospital stay was 9 [IQR: 7-13] in the lidocaine group and 9 [IQR: 7-16] in the placebo group (p=0.15). In-hospital, 6 week and 1 year mortality rates were not different between lidocaine and placebo groups (2.63% vs 2.36%, 3.51% vs 3.15%, and 6.30% vs 6.14% respectively, p=NS).
Discussion
In this prospective, placebo-controlled, randomized study of lidocaine during adult cardiac surgery with CPB, no neuroprotective effect of lidocaine was found. In addition, we found that diabetic subjects receiving lidocaine were more likely to suffer cognitive decline at 6 weeks. In secondary analyses, an association between higher total dose of lidocaine and increased neurocognitive decline was detected in the lidocaine treated group; a total dose of 35 mg/kg approximated the threshold for this cognitive decline. Furthermore, when the study sample was restricted to only nondiabetic subjects who received a dose of lidocaine < 42.6 mg/kg (75th percentile), a protective effect of lidocaine upon cognition was seen. A marginally significant improvement in cognition was also detected at 1 year after surgery in subjects receiving lidocaine, although this finding is limited by substantial loss to follow-up. There was no diminution of the perioperative cytokine response in lidocaine treated subjects.
Lidocaine is a cationic amide that blocks the sodium channel and has achieved widespread use as a local anesthetic and antiarrhythmic. The use of lidocaine as cerebral protectant largely arose from its assessment as a treatment for decompression illness. Evans et al14 were the first to demonstrate that cerebral somatosensory evoked response was preserved to a greater degree in anesthetized cats pretreated with lidocaine before a single bolus of air in the vertebral artery. Since then, numerous studies have attempted to define the neuroprotective mechanisms of lidocaine; these include reduction in activation and residual cerebral metabolism, deceleration of ischemic ion fluxes, preservation of CBF, and modulation of inflammatory mediators.13 During neuronal ischemia, Astrup et al22 in a canine model of global ischemia demonstrated that large doses of lidocaine (100-160 mg/kg) reduced the metabolic rate by 15-20% beyond that achieved by barbiturates, thus preserving cellular energy stores. Of note, the effects of lidocaine and hypothermia were additive. These investigators attributed their findings to lidocaine’s ability to block anoxic sodium influx and potassium efflux that lead to cellular edema and loss of cell function. Similarly, in rat hippocampal slices exposed to varying concentrations of lidocaine, anoxic depolarization was less frequent and delayed when it did occur.23 With more conventional dosing of lidocaine (0.2 mg/kg/min) administered to rabbits undergoing global ischemia, anoxic depolarization was again delayed.24 As a consequence, secondary neurotoxic events such as intracellular edema,25 cytosolic calcium accumulation,26 and glutamate27 and aspartate28 release are attenuated.
In conventional doses, lidocaine also has been reported to preserve CBF29 and reduce intracranial pressure30 but the existing data are conflicting. Depending on the dose of lidocaine and the vascular bed of interest, both vasodilatory and vasoconstrictive responses have been described.31-33 Furthermore, Lei et al34 in a rat model of focal cerebral ischemia demonstrated that an antiarrhythmic dose of lidocaine reduced infarct size 24 hours after ischemia but had no significant effect on CBF in the penumbra or the core during ischemia and reperfusion. The infarct-reducing effect of lidocaine was thought to be related to the inhibition of apoptotic cell death in the penumbra. Finally, neuroprotection with lidocaine may be a consequence of inflammatory modulation, largely manifested as a reduction in neutrophil adherence to injured endothelium, transmigration into the ischemic zone, and release of proinflammatory cytokines.35, 36
On the basis of the animal data presented above and case reports37 suggesting a beneficial effect of lidocaine in the treatment of cerebral arterial gas embolism, Mitchell and colleagues13 assessed the effect of lidocaine in 55 evaluable patients undergoing left heart valve surgery. Lidocaine was administered intravenously to 28 patients for a total duration of 48 hours. Significantly fewer lidocaine patients had a deficit in at least one neuropsychological test at 10 days and 10 weeks (p<0.025) and lidocaine patients achieved superior percentage change scores in 6 of the 11 tests (p<0.05). Of note, only 3 of the 55 patients (5.5%) in this study were diabetic and body mass index was lower in the lidocaine group. In a second study, Wang et al38 administered lidocaine until the end of surgery in 88 patients undergoing coronary revascularization. Patients treated with lidocaine again had a lower incidence of cognitive deficit (18.6% vs 40%, p=0.028); body weight was not different between treatment groups but only 9 subjects in each group were diabetic.
In the largest lidocaine study to date, our primary analysis revealed only a treatment by diabetes interaction such that diabetic subjects treated with lidocaine were more likely to experience cognitive decline. Post hoc analyses, however, revealed not only a detrimental effect of higher total dose of lidocaine but also a potential protective effect of lidocaine when administered to nondiabetics at lower doses. The plasma concentration of lidocaine has been related to therapeutic and side effects; an accepted therapeutic range is 2-5 mcg/ml, with central nervous system toxicity manifested as visual disturbances, confusion, impaired concentration, tinnitus, tremors, dysarthria, or even seizures, psychosis, and coma at levels above 6-10 mcg/ml.39, 40 The distribution half life of lidocaine is typically short (6.8-9.3 minutes) and hepatic metabolism is the primary route of elimination, being highly dependent on hepatic blood flow with 60-70% of the drug extracted in the first pass. The pharmacokinetics of lidocaine, however, appear to change with prolonged infusions of lidocaine. When lidocaine is given as a constant infusion for more than 24 hours, the elimination rate constant and clearance decrease by approximately one half while the hepatic extraction rate decreases by almost two-thirds.41-43 Lidocaine disposition is also altered by disease states common to cardiac surgical patients such as congestive heart failure.44 In such patients, the volume of distribution of the central compartment is smaller so that the same dose will achieve a higher plasma concentration while a diminished cardiac index results in a decrease in clearance. Finally, lidocaine has active metabolites whose pharmacokinetics should be considered. Lidocaine is de-ethylated to monoethyglycinexylidide (MEGX), with further de-ethylation to glycinexylidide (GX). MEGX and GX have 83% and 10-26%, respectively of the antiarrhythmic potency of lidocaine and both may contribute to central nervous system toxicity.39, 43-45 The ratio of serum levels of MEGX to lidocaine and of GX to lidocaine averaged 0.36 + 0.26 and 0.11 + 0.11 in 33 cardiac patients receiving lidocaine for more than 24 hours.46 In the same study, MEGX levels were reported to be higher in patients with manifestations of lidocaine toxicity compared to the nontoxic patients. The importance of metabolites in toxicity is also highlighted by a study in normal volunteers where side effects were most common when lidocaine and MEGX were administered in combination.47 Lidocaine clearance also appeared to be inhibited by MEGX, a finding that may explain the delayed elimination seen with prolonged infusion.47 Therefore, despite normal plasma lidocaine levels, accumulation of metabolites may account for the development of toxicity.
The detrimental effect of lidocaine in diabetic subjects may also be related to its metabolism. Gawronska-Szklarz et al48 evaluated the effect of streptozotocin-induced diabetes on the elimination kinetics of lidocaine in male Wistar rats and found that experimental diabetes enhanced lidocaine elimination. In contrast, MEGX elimination was impaired with the MEGX half-life increasing from 0.34 hours in the control group to 0.89 hours in rats with diabetes. In an isolated perfused liver model (removing variations in hepatic flow), however, these same investigators reported that diabetes reduces lidocaine elimination possibly due to an impairment in the de-ethylation pathway.49 Unfortunately, pharmacokinetic data during extended lidocaine infusion and corroborating human data are lacking. Aside from differences in lidocaine pharmacokinetics, it has been reported that ATP-sensitive potassium channels, which are activated during cerebral hypoxia or ischemia to produce arteriolar dilation, demonstrate diminished responsiveness in diabetics50 and may be further impaired by lidocaine.51 However, the net effect of these findings are uncertain as channel function has not been compared with and without lidocaine in diabetic and nondiabetic subjects.
Methodological limitations to neuropsychological testing in the setting of cardiac surgery include the difficulty in obtaining corroborating brain imaging studies, the lack of control groups, and the often arbitrary nature of the definition of postoperative cognitive decline. While we also lack imaging measures, our placebo group serves as a control group and to partially overcome the arbitrary nature of our dichotomous outcome variable, we have also examined the continuous change scores. The principal limitation to our study is the loss to follow-up, particularly at the 1 year timepoint. Twenty-five percent of our subjects allocated to treatment did not return for follow-up testing at 6 weeks, rising to 42.5% at 1 year. At 6 weeks non-returning subjects had lower baseline cognitive scores and lower levels of education than returnees while at 1 year they had lower levels of education and were more likely to be diabetic. However, the other demographic characteristics listed in Table 2 were not different and importantly, the rate of loss to follow-up was not different between the 2 treatment groups. The loss of diabetic subjects to follow-up at 1 year likely accounts for the marginally protective effect of lidocaine seen at 1 year. Another limitation to our study is the lack of lidocaine levels after 24 hours or lidocaine metabolite data in any of the enrolled subjects; thus, we are left to speculate that impaired elimination with prolonged infusion and accumulation of metabolites may be responsible for the observed detrimental effects. Finally, all posthoc analyses were considered exploratory and are unadjusted for multiple comparisons.
In summary, our study is the largest trial to date to evaluate the effect of lidocaine on neurocognitive outcomes following adult cardiac surgery in a prospective randomized manner. Lidocaine administration to the entire study population had no neuroprotective benefit and in fact was detrimental when given to diabetic subjects and at higher doses. Lidocaine also did not diminish the inflammatory response associated with CPB. When given to nondiabetics at reduced doses, a protective effect of lidocaine was observed, suggesting that further study in nondiabetic subjects is warranted.
Supplementary Material
Acknowledgements and Funding
Supported in part by grants #9970128N (Dr. Newman) from the American Heart Association, Dallas, TX, USA, #M01-RR-30 from the National Institutes of Health, Washington, D.C., USA, and by the Division of Cardiothoracic Anesthesiology and Critical Care Medicine, Department of Anesthesiology, Duke University Medical Center, Durham, NC, USA.
Footnotes
Conflict Of Interest: Dr. Laskowitz is a consultant for Biosite Diagnostics.
References
- 1.Newman MF, Kirchner JL, Phillips-Bute B, Gaver V, Grocott H, Jones RH, Mark DB, Reves JG, Blumenthal JA. Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N Engl J Med. 2001;344:395–402. doi: 10.1056/NEJM200102083440601. [DOI] [PubMed] [Google Scholar]
- 2.Phillips-Bute B, Mathew JP, Blumenthal JA, Grocott HP, Laskowitz DT, Jones RH, Mark DB, Newman MF. Association of neurocognitive function and quality of life 1 year after coronary artery bypass graft (CABG) surgery. Psychosom Med. 2006;68:369–375. doi: 10.1097/01.psy.0000221272.77984.e2. [DOI] [PubMed] [Google Scholar]
- 3.Zimpfer D, Czerny M, Vogt F, Schuch P, Kramer L, Wolner E, Grimm M. Neurocognitive deficit following coronary artery bypass grafting: A prospective study of surgical patients and nonsurgical controls. Ann Thorac Surg. 2004;78:513–518. doi: 10.1016/j.athoracsur.2004.03.006. discussion 518-519. [DOI] [PubMed] [Google Scholar]
- 4.Stygall J, Newman SP, Fitzgerald G, Steed L, Mulligan K, Arrowsmith JE, Pugsley W, Humphries S, Harrison MJ. Cognitive change 5 years after coronary artery bypass surgery. Health Psychol. 2003;22:579–586. doi: 10.1037/0278-6133.22.6.579. [DOI] [PubMed] [Google Scholar]
- 5.Selnes OA, Grega MA, Bailey MM, Pham LD, Zeger SL, Baumgartner WA, McKhann GM. Cognition 6 years after surgical or medical therapy for coronary artery disease. Ann Neurol. 2008;63:581–590. doi: 10.1002/ana.21382. [DOI] [PubMed] [Google Scholar]
- 6.Moody DM, Bell MA, Challa VR, Johnston WE, Prough DS. Brain microemboli during cardiac surgery or aortography. Ann Neurol. 1990;28:477–486. doi: 10.1002/ana.410280403. [DOI] [PubMed] [Google Scholar]
- 7.Mathew JP, Mackensen GB, Phillips-Bute B, Stafford-Smith M, Podgoreanu MV, Grocott HP, Hill SE, Smith PK, Blumenthal JA, Reves JG, Newman MF. Effects of extreme hemodilution during cardiac surgery on cognitive function in the elderly. Anesthesiology. 2007;107:577–584. doi: 10.1097/01.anes.0000281896.07256.71. [DOI] [PubMed] [Google Scholar]
- 8.Mathew JP, Podgoreanu MV, Grocott HP, White WD, Morris RW, Stafford-Smith M, Mackensen GB, Rinder CS, Blumenthal JA, Schwinn DA, Newman MF. Genetic variants in P-selectin and C-reactive protein influence susceptibility to cognitive decline after cardiac surgery. J Am Coll Cardiol. 2007;49:1934–1942. doi: 10.1016/j.jacc.2007.01.080. [DOI] [PubMed] [Google Scholar]
- 9.Arrowsmith JE, Harrison MJ, Newman SP, Stygall J, Timberlake N, Pugsley WB. Neuroprotection of the brain during cardiopulmonary bypass: A randomized trial of remacemide during coronary artery bypass in 171 patients. Stroke. 1998;29:2357–2362. doi: 10.1161/01.str.29.11.2357. [DOI] [PubMed] [Google Scholar]
- 10.Mathew JP, Shernan SK, White WD, Fitch JC, Chen JC, Bell L, Newman MF. Preliminary report of the effects of complement suppression with pexelizumab on neurocognitive decline after coronary artery bypass graft surgery. Stroke. 2004;35:2335–2339. doi: 10.1161/01.STR.0000141938.00524.83. [DOI] [PubMed] [Google Scholar]
- 11.Doraiswamy PM, Babyak MA, Hennig T, Trivedi R, White WD, Mathew JP, Newman MF, Blumenthal JA. Donepezil for cognitive decline following coronary artery bypass surgery: A pilot randomized controlled trial. Psychopharmacol Bull. 2007;40:54–62. [PubMed] [Google Scholar]
- 12.Grigore AM, Mathew J, Grocott HP, Reves JG, Blumenthal JA, White WD, Smith PK, Jones RH, Kirchner JL, Mark DB, Newman MF. Prospective randomized trial of normothermic versus hypothermic cardiopulmonary bypass on cognitive function after coronary artery bypass graft surgery. Anesthesiology. 2001;95:1110–1119. doi: 10.1097/00000542-200111000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell SJ. Lidocaine in the treatment of decompression illness: A review of the literature. Undersea Hyperb Med. 2001;28:165–174. [PubMed] [Google Scholar]
- 14.Evans DE, Kobrine AI, LeGrys DC, Bradley ME. Protective effect of lidocaine in acute cerebral ischemia induced by air embolism. J Neurosurg. 1984;60:257–263. doi: 10.3171/jns.1984.60.2.0257. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell SJ, Pellett O, Gorman DF. Cerebral protection by lidocaine during cardiac operations. Ann Thorac Surg. 1999;67:1117–1124. doi: 10.1016/s0003-4975(99)00057-0. [DOI] [PubMed] [Google Scholar]
- 16.Murkin JM, Newman SP, Stump DA, Blumenthal JA. Statement of consensus on assessment of neurobehavioral outcomes after cardiac surgery. Ann Thorac Surg. 1995;59:1289–1295. doi: 10.1016/0003-4975(95)00106-u. [DOI] [PubMed] [Google Scholar]
- 17.Randt C, Brown E. Administration manual: Randt memory test. Life Sciences Associates; New York: 1983. [Google Scholar]
- 18.Wechsler D. The Wechsler Adult Intelligence Scale-Revised (manual) Psychological Corporation; 1981. [Google Scholar]
- 19.Reitan RM. Validity of the Trail making test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- 20.Saunders AM, Strittmatter WJ, Schmechel D, George-Hyslop PH, Pericak-Vance MA, Joo SH, Rosi BL, Gusella JF, Crapper-MacLachlan DR, Alberts MJ, et al. Association of apolipoprotein e allele epsilon 4 with late-onset familial and sporadic Alzheimer’s disease. Neurology. 1993;43:1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- 21.Harrell FE. Regression modeling strategies: With applications to linear models, logistic regression, and survival analysis. Springer; New York: 2001. [Google Scholar]
- 22.Astrup J, Sorensen PM, Sorensen HR. Inhibition of cerebral oxygen and glucose consumption in the dog by hypothermia, pentobarbital, and lidocaine. Anesthesiology. 1981;55:263–268. doi: 10.1097/00000542-198109000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Weber ML, Taylor CP. Damage from oxygen and glucose deprivation in hippocampal slices is prevented by tetrodotoxin, lidocaine and phenytoin without blockade of action potentials. Brain Res. 1994;664:167–177. doi: 10.1016/0006-8993(94)91967-4. [DOI] [PubMed] [Google Scholar]
- 24.Ayad M, Verity MA, Rubinstein EH. Lidocaine delays cortical ischemic depolarization: Relationship to electrophysiologic recovery and neuropathology. J Neurosurg Anesthesiol. 1994;6:98–110. doi: 10.1097/00008506-199404000-00005. [DOI] [PubMed] [Google Scholar]
- 25.LoPachin RM, Gaughan CL, Lehning EJ, Weber ML, Taylor CP. Effects of ion channel blockade on the distribution of Na, K, Ca and other elements in oxygen-glucose deprived ca1 hippocampal neurons. Neuroscience. 2001;103:971–983. doi: 10.1016/s0306-4522(01)00035-5. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Lipton P. Cytosolic ca2+ changes during in vitro ischemia in rat hippocampal slices: Major roles for glutamate and Na+-dependent Ca2+ release from mitochondria. J Neurosci. 1999;19:3307–3315. doi: 10.1523/JNEUROSCI.19-09-03307.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fujitani T, Adachi N, Miyazaki H, Liu K, Nakamura Y, Kataoka K, Arai T. Lidocaine protects hippocampal neurons against ischemic damage by preventing increase of extracellular excitatory amino acids: A microdialysis study in Mongolian gerbils. Neurosci Lett. 1994;179:91–94. doi: 10.1016/0304-3940(94)90942-3. [DOI] [PubMed] [Google Scholar]
- 28.Diaz L, Gomez A, Bustos G. Lidocaine reduces the hypoxia-induced release of an excitatory amino acid analog from rat striatal slices in superfusion. Prog Neuropsychopharmacol Biol Psychiatry. 1995;19:943–953. doi: 10.1016/0278-5846(95)00122-c. [DOI] [PubMed] [Google Scholar]
- 29.Dutka AJ, Mink R, McDermott J, Clark JB, Hallenbeck JM. Effect of lidocaine on somatosensory evoked response and cerebral blood flow after canine cerebral air embolism. Stroke. 1992;23:1515–1520. doi: 10.1161/01.str.23.10.1515. discussion 1520-1511. [DOI] [PubMed] [Google Scholar]
- 30.Evans DE, Kobrine AI. Reduction of experimental intracranial hypertension by lidocaine. Neurosurgery. 1987;20:542–547. doi: 10.1227/00006123-198704000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Shokunbi MT, Gelb AW, Peerless SJ, Mervart M, Floyd P. An evaluation of the effect of lidocaine in experimental focal cerebral ischemia. Stroke. 1986;17:962–966. doi: 10.1161/01.str.17.5.962. [DOI] [PubMed] [Google Scholar]
- 32.Sakabe T, Maekawa T, Ishikawa T, Takeshita H. The effects of lidocaine on canine cerebral metabolism and circulation related to the electroencephalogram. Anesthesiology. 1974;40:433–441. doi: 10.1097/00000542-197405000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Johns RA, DiFazio CA, Longnecker DE. Lidocaine constricts or dilates rat arterioles in a dose-dependent manner. Anesthesiology. 1985;62:141–144. doi: 10.1097/00000542-198502000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Lei B, Popp S, Capuano-Waters C, Cottrell JE, Kass IS. Lidocaine attenuates apoptosis in the ischemic penumbra and reduces infarct size after transient focal cerebral ischemia in rats. Neuroscience. 2004;125:691–701. doi: 10.1016/j.neuroscience.2004.02.034. [DOI] [PubMed] [Google Scholar]
- 35.MacGregor RR, Thorner RE, Wright DM. Lidocaine inhibits granulocyte adherence and prevents granulocyte delivery to inflammatory sites. Blood. 1980;56:203–209. [PubMed] [Google Scholar]
- 36.Schmidt W, Schmidt H, Bauer H, Gebhard MM, Martin E. Influence of lidocaine on endotoxin-induced leukocyte-endothelial cell adhesion and macromolecular leakage in vivo. Anesthesiology. 1997;87:617–624. doi: 10.1097/00000542-199709000-00023. [DOI] [PubMed] [Google Scholar]
- 37.Mitchell SJ, Benson M, Vadlamudi L, Miller P. Cerebral arterial gas embolism by helium: An unusual case successfully treated with hyperbaric oxygen and lidocaine. Ann Emerg Med. 2000;35:300–303. doi: 10.1016/s0196-0644(00)70086-2. [DOI] [PubMed] [Google Scholar]
- 38.Wang D, Wu X, Li J, Xiao F, Liu X, Meng M. The effect of lidocaine on early postoperative cognitive dysfunction after coronary artery bypass surgery. Anesth Analg. 2002;95:1134–1141. doi: 10.1097/00000539-200211000-00002. table of contents. [DOI] [PubMed] [Google Scholar]
- 39.Waller ES. Pharmacokinetic principles of lidocaine dosing in relation to disease state. J Clin Pharmacol. 1981;21:181–194. doi: 10.1002/j.1552-4604.1981.tb05698.x. [DOI] [PubMed] [Google Scholar]
- 40.Benowitz NL, Meister W. Clinical pharmacokinetics of lignocaine. Clin Pharmacokinet. 1978;3:177–201. doi: 10.2165/00003088-197803030-00001. [DOI] [PubMed] [Google Scholar]
- 41.LeLorier J, Grenon D, Latour Y, Caille G, Dumont G, Brosseau A, Solignac A. Pharmacokinetics of lidocaine after prolonged intravenous infusions in uncomplicated myocardial infarction. Ann Intern Med. 1977;87:700–706. doi: 10.7326/0003-4819-87-6-700. [DOI] [PubMed] [Google Scholar]
- 42.LeLorier J, Moisan R, Gagne J, Caille G. Effect of the duration of infusion on the disposition of lidocaine in dogs. J Pharmacol Exp Ther. 1977;203:507–511. [PubMed] [Google Scholar]
- 43.Fredrick DS, Boersma RB. Lidocaine infusions: Effect of duration and method of discontinuation on recurrence of arrhythmias and pharmacokinetic variables. Am J Hosp Pharm. 1979;36:778–781. [PubMed] [Google Scholar]
- 44.Halkin H, Meffin P, Melmon KL, Rowland M. Influence of congestive heart failure on blood vessels of lidocaine and its active monodeethylated metabolite. Clin Pharmacol Ther. 1975;17:669–676. doi: 10.1002/cpt1975176669. [DOI] [PubMed] [Google Scholar]
- 45.Blumer J, Strong JM, Atkinson AJ., Jr. The convulsant potency of lidocaine and its n-dealkylated metabolites. J Pharmacol Exp Ther. 1973;186:31–36. [PubMed] [Google Scholar]
- 46.Drayer DE, Lorenzo B, Werns S, Reidenberg MM. Plasma levels, protein binding, and elimination data of lidocaine and active metabolites in cardiac patients of various ages. Clin Pharmacol Ther. 1983;34:14–22. doi: 10.1038/clpt.1983.122. [DOI] [PubMed] [Google Scholar]
- 47.Thomson AH, Elliott HL, Kelman AW, Meredith PA, Whiting B. The pharmacokinetics and pharmacodynamics of lignocaine and MEGX in healthy subjects. J Pharmacokinet Biopharm. 1987;15:101–115. doi: 10.1007/BF01062338. [DOI] [PubMed] [Google Scholar]
- 48.Gawronska-Szklarz B, Musial DH, Pawlik A, Paprota B. Effect of experimental diabetes on pharmacokinetic parameters of lidocaine and MEGX in rats. Pol J Pharmacol. 2003;55:619–624. [PubMed] [Google Scholar]
- 49.Gawronska-Szklarz B, Musial HD, Loniewski I, Paprota B, Drozdzik M. Lidocaine metabolism in isolated perfused liver from streptozotocin-induced diabetic rats. J Pharm Pharmacol. 2006;58:1073–1077. doi: 10.1211/jpp.58.8.0008. [DOI] [PubMed] [Google Scholar]
- 50.Zimmermann PA, Knot HJ, Stevenson AS, Nelson MT. Increased myogenic tone and diminished responsiveness to ATP-sensitive k+ channel openers in cerebral arteries from diabetic rats. Circ Res. 1997;81:996–1004. doi: 10.1161/01.res.81.6.996. [DOI] [PubMed] [Google Scholar]
- 51.Kinoshita H, Nakahata K, Dojo M, Kimoto Y, Hating Y. Lidocaine impairs vasodilatation mediated by adenosine troposphere-sensitive K+ channels but not by inward rectifier K+ channels in rat cerebral microvessels. Anesth Analg. 2004;99:904–909. doi: 10.1213/01.ANE.0000133912.54318.0F. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.