Abstract
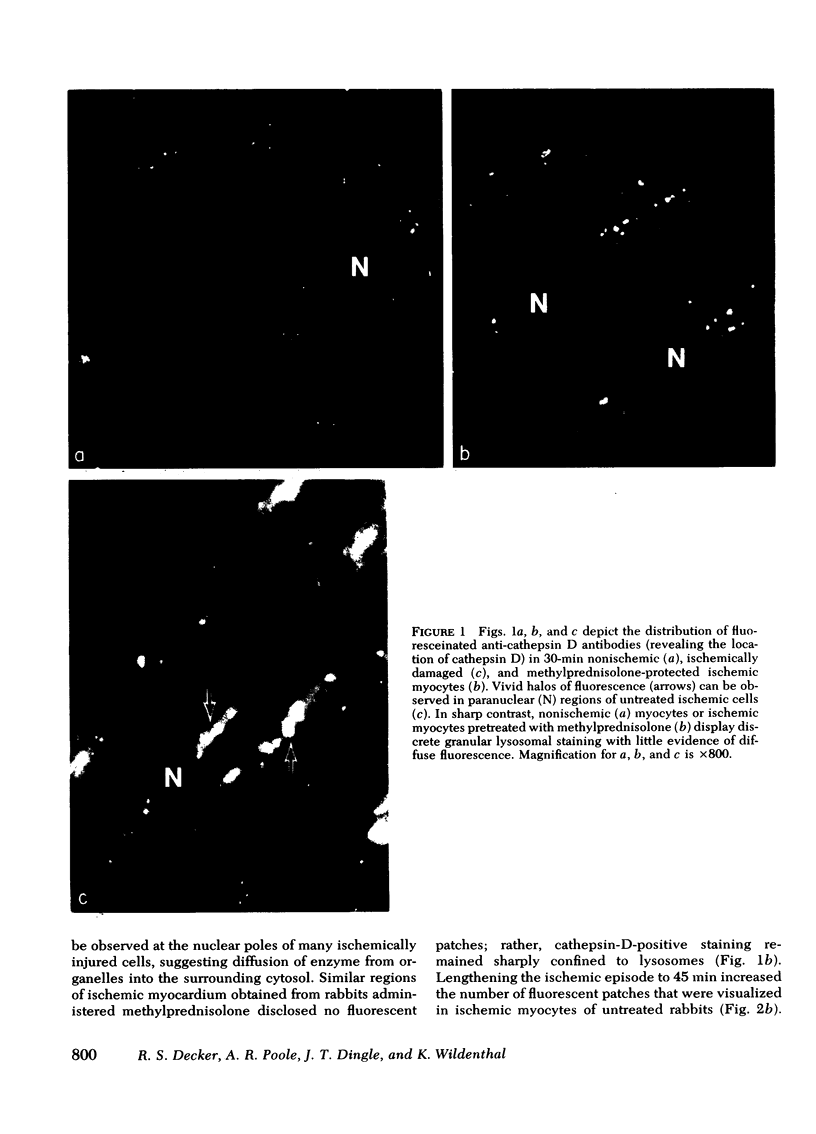
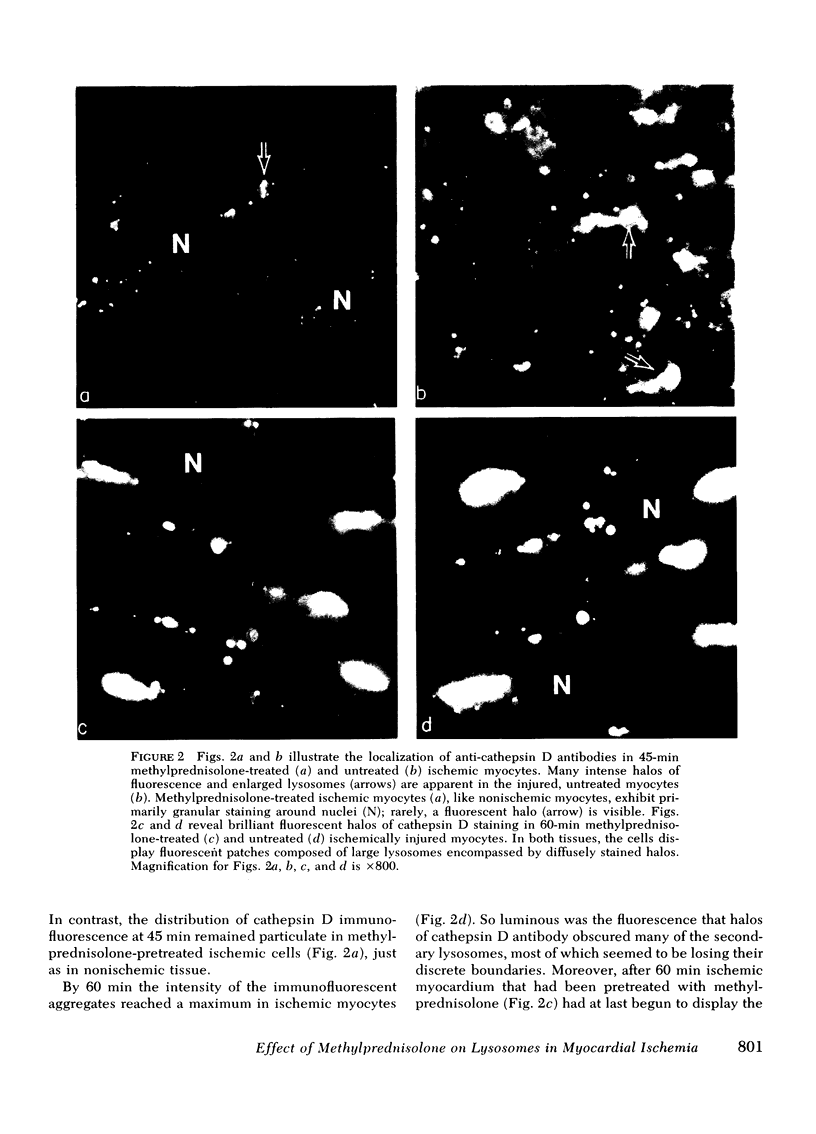
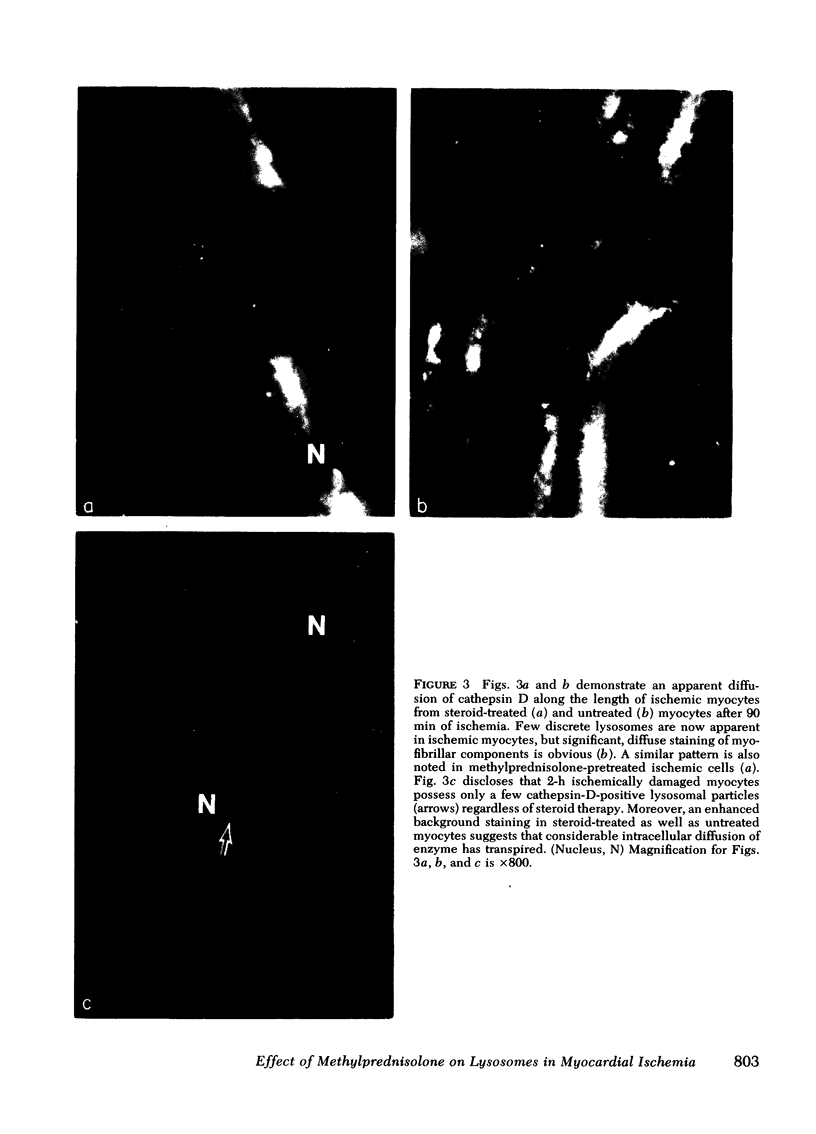
Occlusion of the circumflex coronary artery induced a profound redistribution in ischemic rabbit myocardium of several lysosomal acid hydrolases, including cathepsin D, B-acetylglycosaminidase, and acid phosphatase. 30-45 min after ligation non-sedimentable cathepsin D activity rose from 36% of the total activity to 42-48%, and in immunohistochemical preparations cathepsin D appeared to have diffused from lysosomes into the cytosol of injured cells. A pharmacologic dose of methylprednisolone (50mg/kg) significantly delayed the subcellular redistribution of cathepsin D and the other hydrolases in ischemic heart. Thus, in treated hearts the nonsedimentable activity of cathepsin D rose to only 38% after 30 min of ischemia and 42% after 45 min (P is less than 0.05 compared to untreated ischemia at each time). Similarly, unlike untreated hearts, noevidence of enzyme diffusion from lysosomes could be demonstrated immunohistochemically in corticosteroid-treated ischemic hearts for over 45 min. After 1-2 h of ischemia, however, steroid-protected myocytes deteriorated and the biochemical activity and anatomical distribution of cathepsin D were indistinguishable from untreated ischemic hearts. This study demonstrates that corticosteroid pretreatment does not prevent alterations in cardiac lysosomes during severe ischemia indefinitely, but does delay their development significantly.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali M., Ellis A., Glick G. Effects of methylprednisolone on cardiac lymph in acute myocardial ischemia in dogs. Am J Physiol. 1977 Jun;232(6):H602–H607. doi: 10.1152/ajpheart.1977.232.6.H602. [DOI] [PubMed] [Google Scholar]
- Brachfeld N. Metabolic evaluation of agents designed to protect the ischemic myocardium and to reduce infarct size. Am J Cardiol. 1976 Mar 31;37(4):528–532. doi: 10.1016/0002-9149(76)90392-1. [DOI] [PubMed] [Google Scholar]
- Busuttil R. W., George W. J., Hewitt R. L. Protective effect of methylprednisolone on the heart during ischemic arrest. J Thorac Cardiovasc Surg. 1975 Dec;70(6):955–965. [PubMed] [Google Scholar]
- Decker R. S., Poole A. R., Griffin E. E., Dingle J. T., Wildenthal K. Altered distribution of lysosomal cathepsin D in ischemic myocardium. J Clin Invest. 1977 May;59(5):911–921. doi: 10.1172/JCI108713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker R. S., Wildenthal K. Influence of methylprednisolone on ultrastructural and cytochemical changes during myocardial ischemia. Selective effects on various cell inclusions and organelles including lysosomes. Am J Pathol. 1978 Jul;92(1):1–22. [PMC free article] [PubMed] [Google Scholar]
- Decker R. S., Wildenthal K. Sequential lysosomal alterations during cardiac ischemia. II. Ultrastructural and cytochemical changes. Lab Invest. 1978 Jun;38(6):662–673. [PubMed] [Google Scholar]
- Feola M., Rovetto M., Soriano R., Cho S. Y., Wiener L. Glucocorticoid protection of the myocardial cell membrane and the reduction of edema in experimental acute myocardial ischemia. J Thorac Cardiovasc Surg. 1976 Oct;72(4):631–643. [PubMed] [Google Scholar]
- Goldstein S. Letter: Pacing the WPW patient. Circulation. 1976 Jan;53(1):204–205. doi: 10.1161/01.cir.53.1.204. [DOI] [PubMed] [Google Scholar]
- Hearse D. J., Humphrey S. M. Enzyme release during myocardial anoxia: a study of metabolic protection. J Mol Cell Cardiol. 1975 Jul;7(7):463–482. doi: 10.1016/0022-2828(75)90164-9. [DOI] [PubMed] [Google Scholar]
- Ingwall J. S., DeLuca M., Sybers H. D., Wildenthal K. Fetal mouse hearts: a model for studying ischemia. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2809–2813. doi: 10.1073/pnas.72.7.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings R. B., Ganote C. E. Structural changes in myocardium during acute ischemia. Circ Res. 1974 Sep;35 (Suppl 3):156–172. [PubMed] [Google Scholar]
- Kloner R. A., Fishbein M. C., Lew H., Maroko P. R., Braunwald E. Mummification of the infarcted myocardium by high dose corticosteroids. Circulation. 1978 Jan;57(1):56–63. doi: 10.1161/01.cir.57.1.56. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Libby P., Maroko P. R., Bloor C. M., Sobel B. E., Braunwald E. Reduction of experimental myocardial infarct size by corticosteroid administration. J Clin Invest. 1973 Mar;52(3):599–607. doi: 10.1172/JCI107221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller E. A., Griffin W. S., Wildenthal K. Isoproterenol-induced cardiomyopathy: changes in cardiac enzymes and protection by methylprednisolone. J Mol Cell Cardiol. 1977 Jul;9(7):565–578. doi: 10.1016/s0022-2828(77)80371-4. [DOI] [PubMed] [Google Scholar]
- Nayler W. G., Seabra-Gomes R. Effect of methylprednisolone sodium succinate on hypoxic heart muscle. Cardiovasc Res. 1976 May;10(3):349–358. doi: 10.1093/cvr/10.3.349. [DOI] [PubMed] [Google Scholar]
- RAGAN C., HOWES E. L. Effect of cortisone on production of granulation tissue in the rabbit. Proc Soc Exp Biol Med. 1949 Dec;72(3):718-21, illust. doi: 10.3181/00379727-72-17555. [DOI] [PubMed] [Google Scholar]
- Rovetto M. J. Effect of hyaluronidase and methylprednisolone on myocardial function, glucose metabolism, and coronary flow in the isolated ischemic rat heart. Circ Res. 1977 Sep;41(3):373–379. doi: 10.1161/01.res.41.3.373. [DOI] [PubMed] [Google Scholar]
- Spath J. A., Jr, Lane D. L., Lefer A. M. Protective action of methylprednisolone on the myocardium during experimental myocardial ischemia in the cat. Circ Res. 1974 Jul;35(1):44–51. doi: 10.1161/01.res.35.1.44. [DOI] [PubMed] [Google Scholar]
- Spath J. A., Lefer A. M. Effects of dexamethasone on myocardial cells in the early phase of acute myocardial infarction. Am Heart J. 1975 Jul;90(1):50–55. doi: 10.1016/0002-8703(75)90256-2. [DOI] [PubMed] [Google Scholar]
- Vogel W. M., Zannoni V. G., Abrams G. D., Lucchesi B. R. Inability of methylprednisolone sodium succinate to decrease infarct size or preserve enzyme activity measured 24 hours after coronary occlusion in the dog. Circulation. 1977 Apr;55(4):588–595. doi: 10.1161/01.cir.55.4.588. [DOI] [PubMed] [Google Scholar]
- Welman E., Peters T. J. Prevention of lysosome disruption in anoxic myocardium by chloroquine and methyl prednisolone. Pharmacol Res Commun. 1977 Jan;9(1):29–38. doi: 10.1016/s0031-6989(77)80051-9. [DOI] [PubMed] [Google Scholar]
- Wildenthal K., Decker R. S., Poole A. R., Dingle J. T. Age-related alterations in cardiac lysosomes. J Mol Cell Cardiol. 1977 Oct;9(10):859–866. doi: 10.1016/s0022-2828(77)80062-x. [DOI] [PubMed] [Google Scholar]
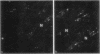
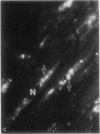
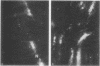
- Wildenthal K., Decker R. S., Poole A. R., Griffin E. E., Dingle J. T. Sequential lysosomal alterations during cardiac ischemia. I. Biochemical and immunohistochemical changes. Lab Invest. 1978 Jun;38(6):656–661. [PubMed] [Google Scholar]
- Wildenthal K., Poole A. R., Dingle J. T. Influence of starvation on the activities and localization of cathepsin D and other lysosomal enzymes in hearts of rabbits and mice. J Mol Cell Cardiol. 1975 Nov;7(11):841–855. doi: 10.1016/0022-2828(75)90135-2. [DOI] [PubMed] [Google Scholar]
- da Luz P. L., Forrester J. S., Wyatt H. L., Diamond G. A., Chag M., Swan H. J. Myocardial reperfusion in acute experimental ischemia. Beneficial effects of prior treatment with steroids. Circulation. 1976 May;53(5):847–852. doi: 10.1161/01.cir.53.5.847. [DOI] [PubMed] [Google Scholar]