Abstract
Aims
While chronic alterations in cardiac triacylglycerol (TAG) metabolism and accumulation are associated with cardiomyopathy, it is unclear whether TAG catabolizing enzymes such as adipose triglyceride lipase (ATGL) play a role in acquired cardiomyopathies. Importantly, germline deletion of ATGL leads to marked cardiac steatosis and heart failure in part through reducing peroxisome proliferator-activated receptor α (PPARα) activity and subsequent fatty acid oxidation (FAO). However, whether ATGL deficiency specifically in adult cardiomyocytes contributes to impaired PPARα activity, cardiac function, and metabolism is not known.
Methods and results
To study the effects of acquired cardiac ATGL deficiency on cardiac PPARα activity, function, and metabolism, we generated adult mice with tamoxifen-inducible cardiomyocyte-specific ATGL deficiency (icAtglKO). Within 4–6 weeks following ATGL ablation, icAtglKO mice had markedly increased myocardial TAG accumulation, fibrotic remodelling, and pathological hypertrophy. Echocardiographic analysis of hearts in vivo revealed that contractile function was moderately reduced in icAtglKO mice. Analysis of energy metabolism in ex vivo perfused working hearts showed diminished FAO rates which was not paralleled by markedly impaired PPARα target gene expression.
Conclusions
This study shows that acquired cardiomyocyte-specific ATGL deficiency in adult mice is sufficient to promote fibrotic and hypertrophic cardiomyopathy and impair myocardial FAO in the absence of markedly reduced PPARα signalling.
Keywords: Cardiomyopathy, Lipid metabolism, Lipid signalling, Remodelling, ATGL
1. Introduction
Under physiological conditions, the heart relies primarily on mitochondrial fatty acid (FA) oxidation (FAO) for ATP production.1 Upon entry into the cardiomyocyte, FAs that are not immediately oxidized are esterified to triacylglycerol (TAG) and stored in cytosolic lipid droplets.2 However, chronically increased myocardial TAG content (i.e. myocardial steatosis) observed in certain diseases is associated with cardiomyopathy in both rodents and humans.3–9 Having said that, it is unlikely that intramyocardial TAGs per se directly cause cardiomyocyte dysfunction.10–15 Instead, it is more probable that changes in TAG metabolism indirectly contribute to cardiac dysfunction by influencing myocardial accumulation of lipotoxic FA metabolites, such as ceramides, diacylglycerols, long-chain acyl-CoAs, or acylcarnitines. Previous work has identified adipose triglyceride lipase (ATGL) as rate limiting for cytosolic TAG hydrolysis in many tissues.16 The importance of ATGL action in the heart is supported by the observation that patients with mutations in the ATGL gene (PNPLA2) exhibit myocardial steatosis and cardiomyopathy.17–21 Increased myocardial TAG accumulation is associated with cardiomyopathy in humans during conditions such as obesity, insulin resistance, diabetes mellitus, and ageing,3,9,22–27 suggesting that alterations in myocardial TAG metabolism and specifically ATGL may also play a role in acquired human cardiomyopathies.
Similar to humans with (inactivating) ATGL mutations, mice with constitutive whole body ATGL deficiency (totalAtglKO) showed markedly increased myocardial TAG accumulation, which was associated with reduced peroxisome proliferator-activated receptor α (PPARα) activity, leading to mitochondrial dysfunction and lethal cardiomyopathy.28 These data suggest that myocardial ATGL is crucial for PPARα activation and mitochondrial function.28 In addition to these metabolic derangements, totalAtglKO mice also presented with cardiac fibrosis and increased ventricular wall dimensions.16,28 However, since whole body ATGL deficiency results in potentially confounding effects such as reduced serum lipid levels, altered concentrations of circulating adipokines, and impaired insulin secretion,16,29 as well as developmental adaptations due to germline ATGL deficiency, it is not clear whether acquired cardiac ATGL deficiency in the adult cardiomyocyte would recapitulate the cardiac metabolic phenotype observed in the totalAtglKO mice. In addition, it is unknown whether cardiac fibrosis in totalAtglKO mice was a direct effect of ATGL deficiency in cardiac fibroblasts.
The aim of this study was to determine whether short term, acquired ATGL deficiency in adult cardiomyocytes contributes to impaired cardiac function, metabolism, and remodelling.
2. Methods
An expanded Methods section is available in the Supplementary material online.
2.1. Generation of the Atgl/Pnpla2-loxP targeting construct and icAtglKO mice
Mice carrying a LoxP-modified Atgl allele (B6.129-Pnpla2tm1Eek mice; herein designated as Atgl-flox mice) were generated in the laboratory of Erin Kershaw using BAC recombineering. Atgl-flox mice [backcrossed onto the C57BL/6NTac (Taconic) background for more than four generations] were interbred with B6.Cg-Tg(Myh6-cre/Esr1)1Jmk/J mice (#005657, The Jackson Laboratory, backcrossed onto the C57BL/6J background for >10 generations), which express a tamoxifen-inducible Cre recombinase driven by the cardiomyocyte-specific α-myosin heavy chain promoter (offspring are estimated to have >96.9% C57BL/6 background). The C57BL/6J, but not C57BL/6NTac subline harbours a mutation in the gene encoding nicotinamide nucleotide transhydrogenase, which promotes antioxidant capacity in mitochondria and is expressed in the heart.30 Control and icAtglKO mice were derived from the same breeder pairs to avoid confounding effects due to differences in genetic background. Tamoxifen (#T5648, Sigma) dissolved in corn oil was administered orally to adult control (homozygous Atgl-flox without Cre, Atglflox/flox −/−) and inducible cardiomyocyte-specific ATGL knockout (icAtglKO; homozygous Atgl-flox and hemizygous for Cre, Atglflox/flox Cre/−) mice at a dose of 80 mg/kg/day for six consecutive days. Mice were housed on a 12 h light:12 h dark cycle with ad libitum access to chow diet (#5001 from Lab Diet with 13.5% kcal from fat) and water. Unless otherwise stated, non-fasted mice were used and euthanasia was performed by decapitation. All protocols involving mice were approved by the University of Alberta Institutional Animal Care and Use Committee and conform with the Guide for the Care and Use of Laboratory Animals published by the United States National Institutes of Health.
2.2. Analysis of serum insulin, lipids, and lactate
Serum insulin was determined using the mouse insulin enzyme-linked immunosorbent assay kit (#90080, Crystal Chem). Serum free fatty acids (FFA) and TAGs were determined using colorimetric kit assays [#HR Series NEFA-HR(2), Wako and #2780–400H Infinity TAG reagent, Thermo Electron]. Serum lactate was determined using a fluorometric kit assay (#700510, Cayman).
2.3. Echocardiography
Mice were mildly anaesthetized using 0.75% isoflurane, and transthoracic echocardiography was performed as described previously.31
2.4. Heart perfusions
Hearts were perfused aerobically in working mode with Krebs–Henseleit buffer containing 0.8 mmol/L oleate prebound to 3% delipidated bovine serum albumin, 5 mmol/L glucose, and 50 μU/mL insulin for 60 min as described previously.31
2.5. Cardiomyocyte isolation
Ventricular calcium-tolerant myocytes were isolated from Langendorff perfused hearts similar to a previously described procedure.32
2.6. Tissue homogenization and lipid analysis
Tissues were homogenized as described previously.31 Lipids were isolated from homogenates, followed by the colorimetric determination of TAG concentrations. To examine oleate incorporation into TAGs from perfused hearts, TAGs were isolated by thin layer chromatography, and labelled oleate was measured by liquid scintillation counting.
2.7. Immunoblot analysis
Tissue lysates were resolved by SDS–PAGE and immunoblot analysis was performed as described previously.31
2.8. Mitochondrial enzyme activities and ATP production
Citrate synthase and β-hydroxyacyl-CoA dehydrogenase (HADH) activity in ventricular homogenates was assessed spectrophotometrically according to Boudina et al.33 with modifications. ATP synthesis rates in freshly isolated mitochondria were measured using a bioluminescence-based method employing firefly luciferase.34
2.9. Gene expression
Gene expression analysis was performed by quantitative reverse transcriptase PCR as previously described35 (see Supplementary material online, Table S1).
2.10. Microarray analysis
Total RNA was isolated from frozen ventricular tissue using TRIzol reagent (Invitrogen), and microarray analysis was performed as described previously36 with modifications. Differentially expressed genes were identified using the moderated t-test with the R/Bioconductor package limma. Multiple hypothesis testing was considered according to the method of Benjamini and Hochberg.37
2.11. Histology
Haematoxylin and eosin (H&E) and Masson's trichrome (M.T.) stains of paraffin-embedded sections were visualized as described previously.31
2.12. Electron microscopy and mitochondrial morphometry
Electron microscopy was performed as previously described.38 Intermyofibrillar mitochondria, which were entirely included in the field of view, were quantified from images at 7100-fold magnification. Mitochondrial cross-sectional area and minimum and maximum Feret's diameter were determined using ImageJ software (National Institutes of Health).
2.13. Statistical analysis
Results are expressed as means ± SEM. Comparisons between groups were made by the unpaired two-tailed Student t test using the GraphPad Prism software, unless otherwise stated. P-values of <0.05 were considered statistically significant.
3. Results
3.1. Inducible cardiomyocyte-specific ATGL deficiency results in myocardial steatosis and fibrotic remodelling
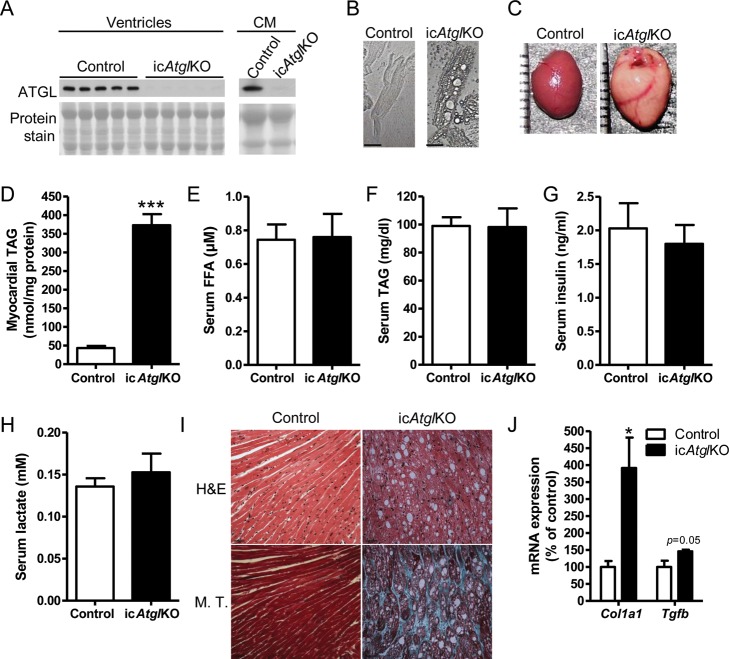
ATGL protein expression was diminished in ventricles and isolated cardiomyocytes from icAtglKO mice compared with control mice (Figure 1A), confirming ablation of ATGL protein. Cardiomyocytes isolated from icAtglKO mice also exhibited a drastically altered morphology with large vacuoles (Figure 1B). Similar to previous observations in totalAtglKO and striated muscle-specific ATGL knockout (muscleAtglKO) mice,28 the cardiac TAG content was markedly increased in icAtglKO mice (Figures 1C, D and 5D). Serum concentrations of FFA, TAGs, insulin, and lactate were unchanged between genotypes (Figure 1E–H). These findings show that ATGL specifically in cardiomyocytes is rate limiting for myocardial TAG catabolism.
Figure 1.
Cardiac morphology, myocardial TAG content, and serum analysis. (A) Cardiac ATGL protein expression (4 weeks post-TAM, 6–7-month-old females) and isolated cardiomyocytes (CM) (4 weeks post-TAM, 11.5-month-old males). (B) Representative light microscopy images of isolated cardiomyocytes at ×400 magnification (6 weeks post-TAM, 7.5-month-old males). Scale bars denote 30 µm. (C) Representative images of isolated hearts (5 weeks post-TAM, 5–6-month-old males). (D) Myocardial TAG content (5 weeks post-TAM, 5–6-month-old males, n = 5, ***P < 0.0001). (E) Serum FFA (5 weeks post-TAM, 5–6-month-old males, n = 5), (F) TAGs (5 weeks post-TAM, 5–6-month-old males, n = 5), (G) insulin (5 weeks post-TAM, 5–6-month-old males, n = 5), and (H) lactate (4 week post-TAM, 4–7-month-old females, n = 6). (I) Representative histological images of apical heart sections (5 weeks post-TAM, 5–6-month-old males) stained with H&E and M.T. stain at ×400 magnification. Scale bars denote 30 µm. (J) Cardiac mRNA expression of Col1a1 and Tgfb (5 weeks post-TAM, 5–6-month-old males, n = 4–5, *P < 0.05).
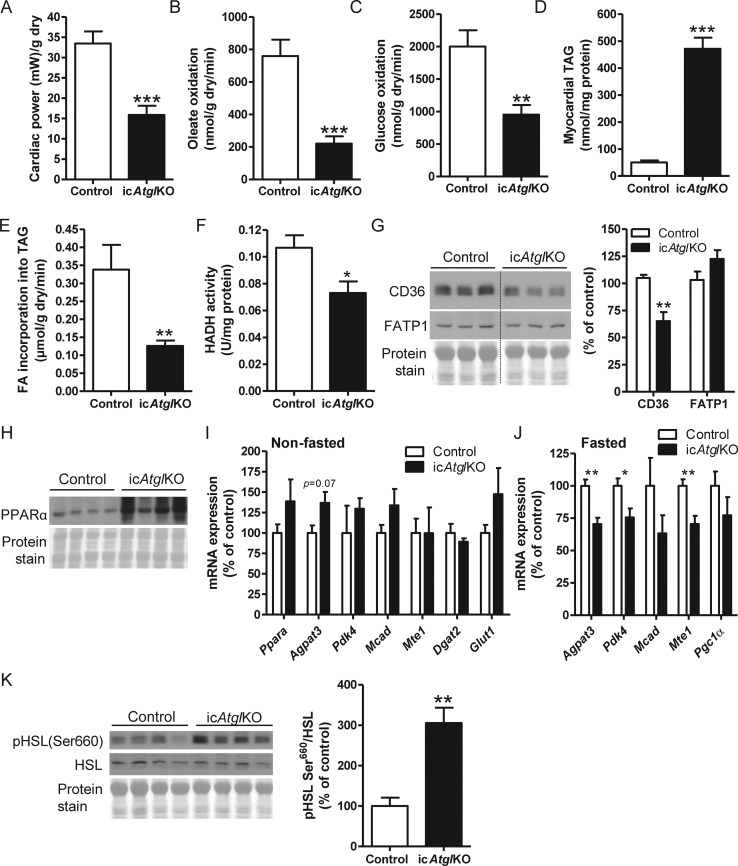
Figure 5.
Myocardial energy metabolism. (A) Cardiac power (n = 9–10), (B) oleate oxidation rates (n = 9–10), (C) glucose oxidation rates (n = 9–10), (D) myocardial TAG content (n = 5–6), and (E) oleate incorporation into TAG (n = 5–6) in ex vivo perfused working hearts (4–5 weeks post-TAM, 6–8-month-old females, **P < 0.01, ***P < 0.001). (F) Cardiac HADH activity (4–5 weeks post-TAM, 4–5.5-month-old females, n = 6, *P < 0.05). (G) Cardiac CD36 and FATP1 protein expression (4 weeks post-TAM, 6-month-old females, n = 4–6, **P < 0.01). (H) Cardiac PPARα protein expression (5 weeks post-TAM, 4–5-month-old females). (I and J) mRNA expression of genes involved in FA and glucose metabolism in hearts from (I) non-fasted (5 weeks post-TAM, 5–6-month-old, n = 4–5) and (J) 12 h-fasted (4 weeks post-TAM, 6.5–7.5-month-old, n = 5–7) male mice (*P < 0.05, **P < 0.01). (K) Cardiac HSL phosphorylation at Ser660 (4 weeks post-TAM, 6–7-month-old females, n = 5–6, **P < 0.01).
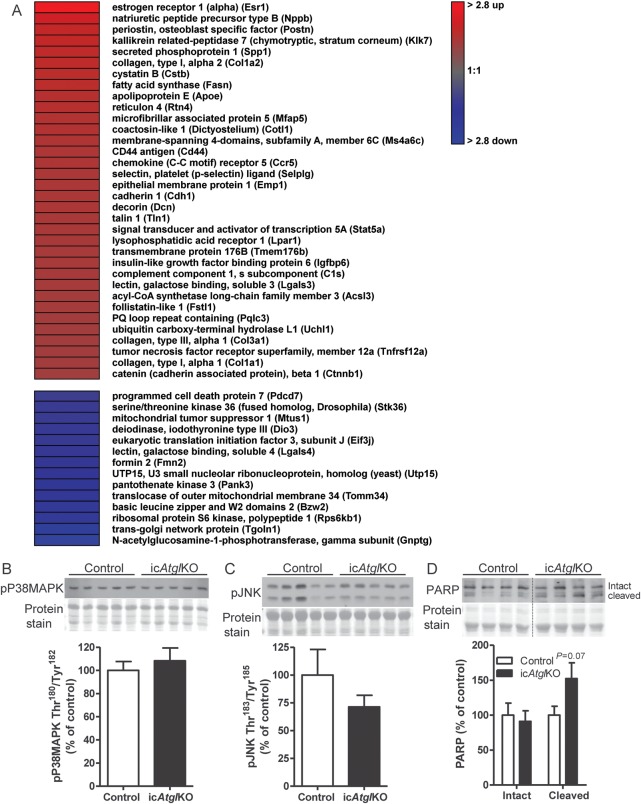
Histological analysis showed significantly altered tissue morphology in hearts from icAtglKO mice, which was characterized by accumulation of vacuoles as well as interstitial fibrosis (Figure 1I) (15.53 ± 0.51% fibrosis area in icAtglKO compared with none in control hearts, averages of two to three images per heart×3 hearts per group). Fibrotic remodelling in hearts from icAtglKO mice also corresponded with increased collagen (Col1a1) and transforming growth factor β (Tgfb) mRNA expression (Figure 1J). In addition, microarray analysis showed up-regulation of numerous genes involved in extracellular matrix remodelling in hearts from icAtglKO mice, including periostin (Postn), kallikrein-related peptidase 7 (Klk7), collagen type I (Col1a1 and Col1a2) and III (Col3a1), reticulon 4 (Nogo, Rtn4), CD44 antigen (CD44), chemokine (C-C motif) receptor 2/5 (Ccr2/Ccr5), decorin (Dcn), and galectin-3 (Lgals3) (Figure 2A). Together, these findings suggest that icAtglKO mice develop rapid fibrotic myocardial remodelling in parallel with cardiac steatosis.
Figure 2.
Microarray analysis and markers of apoptosis. (A) Heat map of differentially regulated genes (fold change ≥ 1.5, P < 0.05) comparing hearts from 5 h-fasted icAtglKO vs. control mice (5.5 weeks post-TAM, 5–8-month-old females, n = 3) as assessed by microarray analysis. (B) Cardiac protein expression of P38MAPK phosphorylated at Thr180/Tyr182 and (C) JNK phosphorylated at Thr183/Tyr185 (4 weeks post-TAM, 6-month-old females, n = 5–6). (D) Cardiac protein expression of PARP (4 weeks post-TAM, 5–6-month-old females, n = 4–5).
Protein expression of the apoptosis markers, phospho-P38 mitogen-activated protein kinase (MAPK) (Thr180/Tyr182), and phospho-c-Jun-amino-terminal kinase (JNK) (Thr183/Tyr185) was comparable between hearts from icAtglKO and control mice (Figure 2B and C), although expression of cleaved Poly(ADP-Ribose) Polymerase (PARP) trended to increase in hearts from icAtglKO mice (Figure 2D). These data indicate the absence of marked induction of apoptosis following short-term ATGL ablation and are consistent with low levels of apoptosis in hearts from totalAtglKO mice.16
3.2. Inducible cardiomyocyte-specific ATGL deficiency leads to moderate in vivo cardiac dysfunction
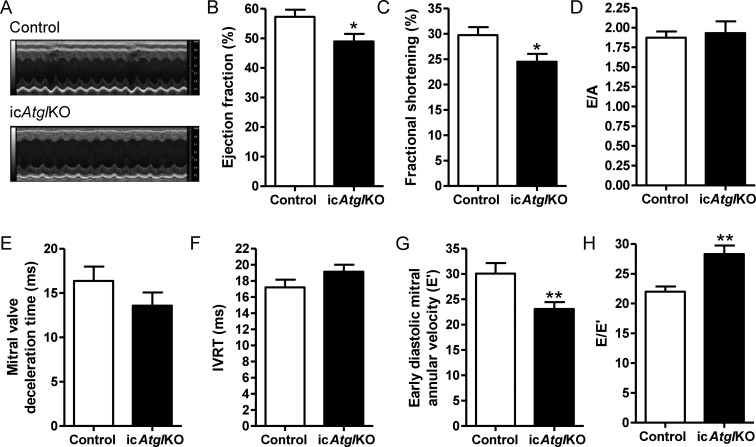
Examination of in vivo cardiac function using transthoracic echocardiography revealed that ejection fraction and fractional shortening were reduced by 15 and 18%, respectively, in hearts from icAtglKO mice (Figure 3A–C), which also corresponded with reduced peak systolic mitral annular velocity (S′) (Table 1). Despite unchanged diastolic parameters such as the ratio of early (E) to late (A) transmitral filling velocity (Figure 3D), mitral valve deceleration time (Figure 3E), and isovolumic relaxation time (Figure 3F), early and late diastolic mitral annular velocity (E′ and A′, respectively) (Figure 3G, Table 1) were decreased in hearts from icAtglKO mice with concomitantly increased ratio of E to E′ (Figure 3H), indicating elevated left ventricular filling pressure in icAtglKO mice. Together, these data suggest that icAtglKO mice exhibit modest cardiac dysfunction in vivo.
Figure 3.
In vivo cardiac function. (A–H) Functional parameters obtained by transthoracic echocardiography (4 weeks post-TAM, 3–6-month-old females, n = 8–13, *P < 0.05, **P < 0.01). Cardiac function was similar within this age group. (A) Representative M-mode images. (B) Ejection fraction. (C) Fractional shortening. (D) Ratio of E to A. (E) Mitral valve deceleration time. (F) Isovolumic relaxation time. (G) E’. (H) Ratio of E to E’.
Table 1.
Body weight and in vivo heart function of anaesthetized control and icAtglKO mice
| Control | icAtglKO | |
|---|---|---|
| Body weight (g) | 24.4 ± 0.9 | 22.9 ± 0.7 |
| Heart rate (b.p.m.) | 426 ± 12 | 416 ± 11 |
| Left ventricular mass (mg) | 69.2 ± 2.8 | 90.2 ± 3.6‡ |
| LVIDd (mm) | 3.85 ± 0.04 | 3.80 ± 0.05 |
| LVIDs (mm) | 2.71 ± 0.07 | 2.88 ± 0.09 |
| IVSs (mm) | 0.97 ± 0.04 | 1.1 ± 0.02† |
| LVPWs (mm) | 0.97 ± 0.03 | 1.08 ± 0.02† |
| Ejection time (ms) | 49.8 ± 1.0 | 48.4 ± 1.2 |
| IVCT (ms) | 19.0 ± 0.9 | 19.0 ± 1.2 |
| Mitral E (mm/s) | 654 ± 41 | 634 ± 21 |
| E′/A′ | 1.13 ± 0.08 | 1.17 ± 0.04 |
| A′ (cm/s) | 26.8 ± 0.9 | 20.1 ± 1.4† |
| S′ (cm/s) | 21.5 ± 0.9 | 17.2 ± 1.0† |
4 weeks post-TAM, 3–6 month-old females, n = 8–13.
†P < 0.01.
‡P < 0.001.
3.3. Inducible cardiomyocyte-specific ATGL deficiency leads to hypertrophy
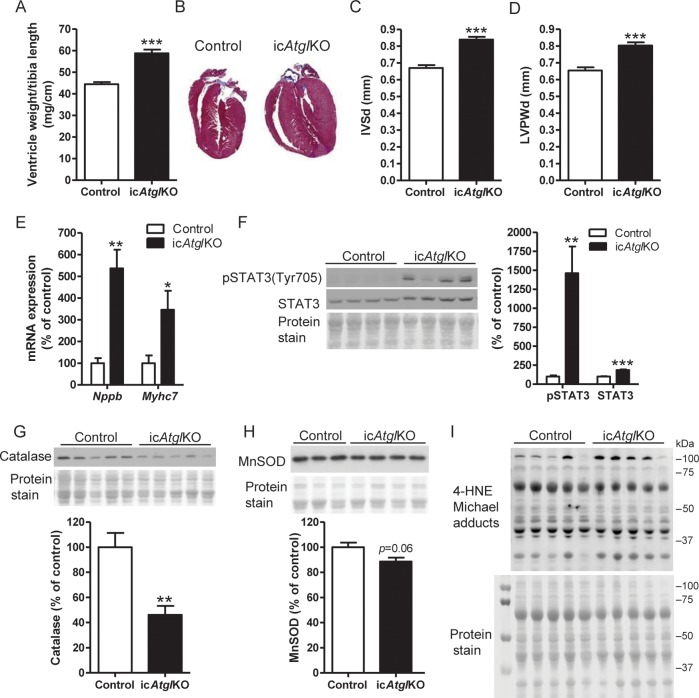
Since hearts from icAtglKO mice are so lipid laden, we tested whether true hypertrophic remodelling contributes to the enlarged appearance of cardiomyocytes and hearts from icAtglKO mice (Figure 1B and C). The ventricle weight-to-tibia length ratio (Figure 4A) and the left ventricular mass as assessed by echocardiography (Table 1) were significantly increased in icAtglKO mice compared with controls. Histological images of long-axis heart sections also showed increased ventricle wall thickness in icAtglKO mice (Figure 4B). Assessment of ventricular wall dimensions and left ventricular internal diameters in diastole and systole (LVIDd and LVIDs, respectively) using echocardiography confirmed increased thickness of the interventricular septum and left ventricular posterior wall in icAtglKO mice in the absence of ventricular dilatation (Figure 4C and D, Table 1). Most importantly, transcript levels of brain natriuretic peptide (Nppb) and beta myosin heavy chain (Myh7), which are marker genes for pathological hypertrophy, were correspondingly up-regulated in hearts from icAtglKO mice (Figure 4E). In accordance with this finding, microarray analysis showed up-regulation of several genes involved in pathological hypertrophic growth, such as signal transducer and activator of transcription 5A (Stat5a), follistatin-like 1 (Fstl1), and β-catenin (Ctnnb1) (Figure 2A). In addition, phosphorylation and protein expression of STAT3, which is also an indicator of cardiac hypertrophy, was increased in hearts from icAtglKO mice (Figure 4F). In contrast, protein expression of catalase (Figure 4G), manganese superoxide dismutase (MnSOD) (Figure 4H), and 4-hydroxy-2-nonenal (4-HNE) Michael adducts (Figure 4I; n = 5–6, P = 0.96) was not increased in hearts from icAtglKO mice, suggesting that hypertrophic and fibrotic remodelling of the myocardium is not due to increased oxidative stress in icAtglKO mice. Taken together, these findings suggest that icAtglKO mice develop overt hypertrophic remodelling in the absence of increased oxidative stress.
Figure 4.
Analysis of cardiac hypertrophy. (A) Ratio of ventricle weight to tibia length (4.5–5.5 weeks post-TAM, 4–6-month-old females, n = 7–13, ***P < 0.001). (B) Representative M.T.-stained long-axis heart sections (6 weeks post-TAM, 8–9-month-old females). (C) Echocardiographic assessment of IVSd and (D) LVPWd (4 weeks post-TAM, 3–6-month-old females, n = 8–13, ***P < 0.001). (E) Cardiac mRNA expression of Nppb and Myh7 (5 weeks post-TAM, 5–6-month-old males, n = 4–5, *P < 0.05, **P < 0.01). (F) Cardiac STAT3 phosphorylation at Tyr705 (ratio of phosphorylated to total STAT3) and protein expression (5 weeks post-TAM, 4–5-month-old females, n = 5, **P < 0.01, ***P < 0.001). (G) Cardiac protein expression of catalase (5 h-fasted, 5.5 weeks post-TAM, 8.5-month-old males, n = 6, **P < 0.01). (H) Cardiac protein expression of MnSOD (4 weeks post-TAM, 5–6-month-old females, n = 3–4). (I) Cardiac protein expression of 4-HNE Michael adducts (4 weeks post-TAM, 6–7-month-old females).
3.4. Inducible cardiomyocyte-specific ATGL deficiency results in diminished myocardial FAO
We determined FA and glucose oxidation rates in ex vivo perfused working hearts using radiolabelled substrates. Ex vivo cardiac power was comparable between genotypes (see Supplementary material online, Figure S2A), but was reduced in hearts from icAtglKO mice when normalized to cardiac dry weight (Figure 5A). Oleate oxidation rates were decreased in hearts from icAtglKO mice compared with controls (Figure 5B, see Supplementary material online, Figure S2B), which was not associated with a compensatory increase in glucose oxidation rates (Figure 5C, see Supplementary material online, Figure S2C). Incorporation of oleate into the TAG pool was decreased in hearts from icAtglKO mice (Figure 5E), suggesting that enhanced sequestration of labelled oleate in TAGs does not confound oleate oxidation rates measured in these hearts. Interestingly, reduced FAO and FA incorporation into TAGs were also associated with decreased protein expression of the FA translocase, CD36, but not FATP1 (Figure 5G). In addition, the activity of the FAO enzyme, HADH, was also decreased in hearts from icAtglKO mice (Figure 5F). Together, these data suggest that reduced myocardial FAO likely contributes to the cardiac contractile dysfunction in vivo in icAtglKO mice.
3.5. Inducible cardiomyocyte-specific ATGL deficiency does not dramatically impair PPARα activity
Contrary to our expectations, we found that PPARα protein expression was increased in hearts from icAtglKO mice (Figure 5H) and that mRNA expression of PPARα (Ppara) and the PPARα target genes, 1-acylglycerol-3-phosphate O-acyltransferase 3 (Agpat3), pyruvate dehydrogenase kinase isozyme 4 (Pdk4), medium-chain acyl-CoA dehydrogenase (Mcad), and mitochondrial acyl-CoA thioesterase 1 (Mte1) was unimpaired in hearts from non-fasted icAtglKO mice (Figure 5I). In addition, mRNA expression of diacylglycerol O-acyltransferase 2 (Dgat2) and glucose transporter type 1 (Glut1) was also unchanged in hearts from icAtglKO mice (Figure 5I). Microarray analysis likewise showed no significant changes in the expression of PPARα, PPARα target genes, and other genes involved in FA utilization (data not shown). However, a relatively moderate reduction in the mRNA expression of some PPARα target genes was observed in hearts from icAtglKO mice following prolonged (12 h) fasting (Figure 5J). These findings suggest that PPARα target gene expression is not significantly impaired in hearts from icAtglKO mice in the non-fasted and short-term fasted state, but may become more physiologically relevant during prolonged fasting. Interestingly, hormone-sensitive lipase (HSL) phosphorylation at Ser660 was increased in hearts from icAtglKO mice (Figure 5K), suggestive of enhanced HSL activation that may contribute to sustained PPARα target gene expression.
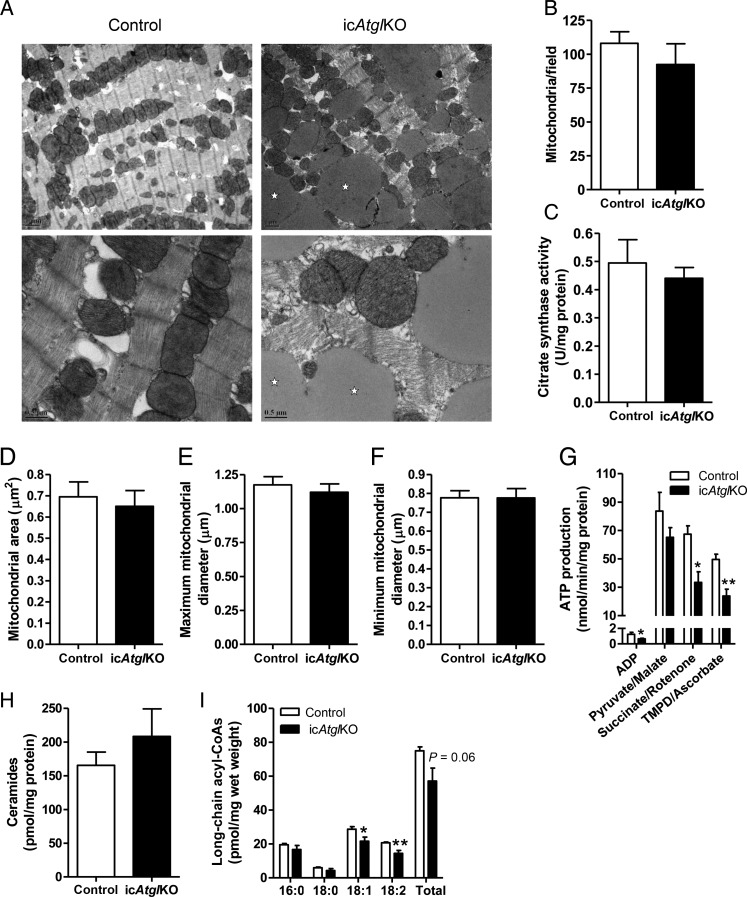
To determine the impact of inducible cardiomyocyte-specific ATGL deficiency on mitochondria directly, we assessed multiple mitochondrial parameters. Inspection of myocardial tissue morphology by transmission electron microscopy revealed dramatically enlarged lipid droplets in the intermyofibrillar space in proximity to mitochondria (Figure 6A). However, no clear differences were noted between genotypes for mitochondria shape or ultrastructural characteristics (Figure 6A). More objective quantification of mitochondrial number (Figure 6B) as well as individual mitochondrial area (Figure 6D) and diameter (Figure 6E and F) revealed that these parameters did not differ between genotypes. In addition, citrate synthase activity (Figure 6C), a surrogate measure of both mitochondrial mass and Krebs cycle activity, was similar between genotypes. ATP production rates via complex I (pyruvate/malate) were not significantly different between genotypes, but were reduced via complex II (succinate/rotenone) and IV (TMPD/ascorbate) as well as in the absence of exogenous substrate (ADP only) in icAtglKO mitochondria (Figure 6G). Together, these data suggest that inducible cardiomyocyte-specific ATGL deficiency does not significantly impact mitochondrial morphology or mass, but impairs mitochondrial function at the level of ATP synthesis. Moreover, hearts from icAtglKO mice displayed areas of myofibrillar disarray (Figure 6A), which may contribute to hypertrophic remodelling.
Figure 6.
Mitochondrial analysis. (A) Representative electron micrographs of heart sections (4 weeks post-TAM, 5.5-month-old females) at ×7100 (upper panel, scale bars denote 1 µm) and ×22000 (lower panel, scale bars denote 0.5 µm) magnification. Stars indicate lipid droplets. (B) Mitochondrial number quantified from electron micrographs (n = 3 hearts, 5–7 images per heart). (C) Cardiac citrate synthase activity (4–5 weeks post-TAM, 4–5.5-month-old females, n = 6). (D) Mitochondrial cross-sectional area, (E) maximum and (F) minimum mitochondrial diameter (n = 3 hearts, 3 sections per heart, 33–46 mitochondria per section). (G) Mitochondrial ATP production (6 weeks post-TAM, 9-month-old females, n = 4–5, *P < 0.05, **P < 0.01). (H) Cardiac concentrations of ceramides and (I) long-chain acyl-CoAs (5 weeks post-TAM, 5–6-month-old males, n = 5, *P < 0.05, **P < 0.01).
Lastly, to examine whether increased accumulation of lipotoxic FA metabolites could underlie mitochondrial dysfunction in hearts from icAtglKO mice, we measured the cardiac content of ceramides and long-chain acyl-CoAs (Figure 6H and I). Interestingly, cardiac levels of ceramides and long-chain acyl-CoA species were unchanged or decreased in hearts from icAtglKO mice, suggesting that mitochondrial dysfunction in these hearts was not secondary to increased accumulation of these lipotoxic FA metabolites.
4. Discussion
In this study, we tested whether acquired impairment of myocardial TAG catabolism via induced cardiomyocyte-specific ATGL deficiency influences mitochondrial substrate metabolism as well as cardiac structure and function and investigated whether this was associated with impaired PPARα signalling.
As expected, hearts from icAtglKO mice exhibited a substantial increase in myocardial TAG content that is comparable with that observed in constitutive totalAtglKO and muscleAtglKO mice.28 In addition, hearts from icAtglKO mice exhibited extensive interstitial fibrosis that was caused by the up-regulation of several genes related to extracellular matrix remodelling. Although increased fibrosis was also observed in hearts from totalAtglKO mice,16 it was unclear whether these effects were a result of ATGL deficiency in cardiomyocytes or other cardiac cell types, such as collagen-producing fibroblasts. Our findings suggest that fibrotic remodelling following ATGL ablation is the result of intercellular signalling from cardiomyocytes to fibroblasts and ensuing up-regulation of profibrotic genes. Since fibrosis can cause increased systolic and diastolic stiffness, our data support the conclusion that increased fibrotic remodelling contributes to cardiac dysfunction in icAtglKO mice.
Interestingly, hearts from icAtglKO mice also develop overt pathological hypertrophy. Although the precise mechanisms of hypertrophic remodelling in hearts from icAtglKO mice require further investigation, it is possible that excessive TAG deposition in cardiomyocytes leads to intracellular mechanical stretch that perturbs the myofibrillar organization, thereby triggering a pro-hypertrophic response to compensate for these adverse changes in myofibril arrangement and function. This notion is supported by the finding that myofibrillar disarray and dysfunction secondary to mutations in sarcomeric proteins are causative for the development of compensatory hypertrophic remodelling in familial hypertrophic cardiomyopathy.39
Similar to findings using crude cardiac homogenates from totalAtglKO mice,28 FAO was reduced in working hearts from icAtglKO mice, which was not compensated by increased glucose oxidation. However, it is possible that the rate of glucose oxidation via TCA cycle anaplerosis is augmented in hearts from icAtglKO mice, which could not be detected using the perfusion system employed in this study. Indeed, previous studies have demonstrated that hypertrophied hearts exhibit decreased FAO, unchanged glucose oxidation, but increased diversion of glucose-derived pyruvate towards anaplerosis.40
Interestingly, mRNA expression of PPARα target genes was maintained in hearts from icAtglKO mice in the non-fasted and short-term (5 h) fasted state and only mildly reduced with prolonged (12 h) fasting. Possible reasons for this milder effect on myocardial PPARα signalling in icAtglKO mice include differences in background strain (>96.9% C57BL/6 for icAtglKO vs. pure, >99.91% C57BL/6 for totalAtglKO mice, and 93.8–96.9% C57BL/6 for muscleAtglKO mice), the duration of ATGL deficiency (4–6 weeks in icAtglKO vs. at least 8 weeks and 15–16 weeks, not including time in utero, in totalAtglKO mice and muscleAtglKO mice, respectively), and the age at the onset of ATGL deficiency (ablation in adult icAtglKO mice vs. congenital deficiency in totalAtglKO and muscleAtglKO mice). Although we cannot exclude the possibility that sufficient residual ATGL activity is still present in hearts from icAtglKO mice to maintain PPARα signalling at baseline, this is not sufficient to prevent marked cardiac steatosis. However, it is conceivable that up-regulation of HSL activity via increased phosphorylation at Ser660 could partially rescue the effect of ATGL ablation on cardiac TAG accumulation and PPARα signalling. It should be noted that cardiac-specific HSL over-expression protected from fasting- and diabetes-induced cardiac steatosis,41,42 indicating that up-regulation of HSL in hearts from icAtglKO mice could compensate to some extent for the lack of ATGL. Interestingly, Reilich et al.19 also suggested that β-adrenergic agonist-mediated HSL activation in cells from patients with inactivating ATGL/PNPLA2 mutations was able to bypass the lipolytic blockade.
PPARα agonist treatment of totalAtglKO mice protected from cardiac steatosis, mitochondrial dysfunction, and heart failure.28 Nonetheless, a beneficial effect of PPARα agonist treatment on cardiac function in humans with ATGL/PNPLA2 mutations has yet to be demonstrated.20 Although we have not tested this in the present study, it is likely that over-activation of PPARα via PPARα agonist treatment improves cardiac steatosis and function in icAtglKO mice by increasing oxidation of FAs taken up into cardiomyocytes in the absence of enhanced TAG catabolism, as has been previously demonstrated using totalAtglKO mice.28
Regardless of the differences in PPARα signalling between mouse models, the findings of this study suggest that mechanisms other than changes in PPARα signalling also contribute to impaired FAO in hearts from icAtglKO mice. Consistent with the concept that a significant proportion of FAs in the heart are shuttled through the TAG pool prior to being oxidized in the mitochondria,1,43 it is likely that a decrease in FAs liberated by TAG hydrolysis may contribute to the reduction in myocardial FAO in icAtglKO mice. In addition, CD36 protein expression and FA incorporation into the TAG and FFA pool (data not shown) were decreased in hearts from icAtglKO mice, suggesting that decreased cardiomyocyte FA uptake via CD36 may also contribute to diminished FAO in the absence of marked changes in PPARα signalling.
In summary, acquired cardiomyocyte-specific ATGL deficiency leads to marked myocardial steatosis, pathological hypertrophy, as well as fibrotic remodelling, which are associated with moderate cardiac dysfunction in vivo. Furthermore, mitochondrial FAO is impaired in hearts from icAtglKO mice, even in the absence of marked changes in cardiac PPARα signalling.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: none declared.
Funding
This work was supported by grants from the Canadian Institute of Health Research and the Heart and Stroke Foundation of Canada to J.R.B.D., post-doctoral fellowships from the Heart and Stroke Foundation of Canada and the Canadian Diabetes Association to P.C.K., and Alberta Innovates-Health Solutions post-doctoral fellowships to P.C.K. and T.P., as well as NIH grant R01DK090166 and HHMI ECA grant to E.E.K.
Supplementary Material
References
- 1.Banke NH, Wende AR, Leone TC, O'Donnell JM, Abel ED, Kelly DP, et al. Preferential oxidation of triacylglyceride-derived fatty acids in heart is augmented by the nuclear receptor PPARalpha. Circ Res. 2010;107:233–241. doi: 10.1161/CIRCRESAHA.110.221713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kienesberger PC, Pulinilkunnil T, Nagendran J, Dyck JR. Myocardial triacylglycerol metabolism. J Mol Cell Cardiol. 2013;55:101–110. doi: 10.1016/j.yjmcc.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 3.McGavock JM, Lingvay I, Zib I, Tillery T, Salas N, Unger R, et al. Cardiac steatosis in diabetes mellitus: a 1H-magnetic resonance spectroscopy study. Circulation. 2007;116:1170–1175. doi: 10.1161/CIRCULATIONAHA.106.645614. [DOI] [PubMed] [Google Scholar]
- 4.McGavock JM, Victor RG, Unger RH, Szczepaniak LS. Adiposity of the heart, revisited. Ann Intern Med. 2006;144:517–524. doi: 10.7326/0003-4819-144-7-200604040-00011. [DOI] [PubMed] [Google Scholar]
- 5.Marfella R, Di Filippo C, Portoghese M, Barbieri M, Ferraraccio F, Siniscalchi M, et al. Myocardial lipid accumulation in patients with pressure-overloaded heart and metabolic syndrome. J Lipid Res. 2009;50:2314–2323. doi: 10.1194/jlr.P900032-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan RS, Drosatos K, Goldberg IJ. Creating and curing fatty hearts. Curr Opin Clin Nutr Metab Care. 2010;13:145–149. doi: 10.1097/MCO.0b013e3283357272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma S, Adrogue JV, Golfman L, Uray I, Lemm J, Youker K, et al. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. Faseb J. 2004;18:1692–1700. doi: 10.1096/fj.04-2263com. [DOI] [PubMed] [Google Scholar]
- 8.Christoffersen C, Bollano E, Lindegaard ML, Bartels ED, Goetze JP, Andersen CB, et al. Cardiac lipid accumulation associated with diastolic dysfunction in obese mice. Endocrinology. 2003;144:3483–3490. doi: 10.1210/en.2003-0242. [DOI] [PubMed] [Google Scholar]
- 9.Korosoglou G, Humpert PM, Ahrens J, Oikonomou D, Osman NF, Gitsioudis G, et al. Left ventricular diastolic function in type 2 diabetes mellitus is associated with myocardial triglyceride content but not with impaired myocardial perfusion reserve. J Magn Reson Imaging. 2012;35:804–811. doi: 10.1002/jmri.22879. [DOI] [PubMed] [Google Scholar]
- 10.Goldberg IJ, Trent CM, Schulze PC. Lipid metabolism and toxicity in the heart. Cell Metab. 2012;15:805–812. doi: 10.1016/j.cmet.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGavock J, Szczepaniak LS, Ayers CR, Abdullah SM, See R, Gore MO, et al. The effects of rosiglitazone on myocardial triglyceride content in patients with type 2 diabetes: a randomised, placebo-controlled trial. Diab Vasc Dis Res. 2012;9:131–137. doi: 10.1177/1479164111428628. [DOI] [PubMed] [Google Scholar]
- 12.Son NH, Yu S, Tuinei J, Arai K, Hamai H, Homma S, et al. PPARgamma-induced cardiolipotoxicity in mice is ameliorated by PPARalpha deficiency despite increases in fatty acid oxidation. J Clin Invest. 2010;120:3443–3454. doi: 10.1172/JCI40905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chokshi A, Drosatos K, Cheema FH, Ji R, Khawaja T, Yu S, et al. Ventricular assist device implantation corrects myocardial lipotoxicity, reverses insulin resistance, and normalizes cardiac metabolism in patients with advanced heart failure. Circulation. 2012;125:2844–2853. doi: 10.1161/CIRCULATIONAHA.111.060889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu L, Shi X, Bharadwaj KG, Ikeda S, Yamashita H, Yagyu H, et al. DGAT1 expression increases heart triglyceride content but ameliorates lipotoxicity. J Biol Chem. 2009;284:36312–36323. doi: 10.1074/jbc.M109.049817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu L, Yu S, Khan RS, Homma S, Schulze PC, Blaner WS, et al. Diacylglycerol acyl transferase 1 overexpression detoxifies cardiac lipids in PPARgamma transgenic mice. J Lipid Res. 2012;53:1482–1492. doi: 10.1194/jlr.M024208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haemmerle G, Lass A, Zimmermann R, Gorkiewicz G, Meyer C, Rozman J, et al. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 2006;312:734–737. doi: 10.1126/science.1123965. [DOI] [PubMed] [Google Scholar]
- 17.Hirano K, Ikeda Y, Zaima N, Sakata Y, Matsumiya G. Triglyceride deposit cardiomyovasculopathy. N Engl J Med. 2008;359:2396–2398. doi: 10.1056/NEJMc0805305. [DOI] [PubMed] [Google Scholar]
- 18.Schweiger M, Lass A, Zimmermann R, Eichmann TO, Zechner R. Neutral lipid storage disease: genetic disorders caused by mutations in adipose triglyceride lipase/PNPLA2 or CGI-58/ABHD5. Am J Physiol Endocrinol Metab. 2009;297:E289–E296. doi: 10.1152/ajpendo.00099.2009. [DOI] [PubMed] [Google Scholar]
- 19.Reilich P, Horvath R, Krause S, Schramm N, Turnbull DM, Trenell M, et al. The phenotypic spectrum of neutral lipid storage myopathy due to mutations in the PNPLA2 gene. J Neurol. 2011;258:1987–1997. doi: 10.1007/s00415-011-6055-4. [DOI] [PubMed] [Google Scholar]
- 20.van de Weijer T, Havekes B, Bilet L, Hoeks J, Sparks L, Bosma M, et al. Effects of bezafibrate treatment in a patient and a carrier with mutations in the PNPLA2 gene, causing neutral lipid storage disease with myopathy. Circ Res. 2013;112:e51–e54. doi: 10.1161/CIRCRESAHA.113.300944. [DOI] [PubMed] [Google Scholar]
- 21.Fischer J, Lefevre C, Morava E, Mussini JM, Laforet P, Negre-Salvayre A, et al. The gene encoding adipose triglyceride lipase (PNPLA2) is mutated in neutral lipid storage disease with myopathy. Nat Genet. 2007;39:28–30. doi: 10.1038/ng1951. [DOI] [PubMed] [Google Scholar]
- 22.Utz W, Engeli S, Haufe S, Kast P, Hermsdorf M, Wiesner S, et al. Myocardial steatosis, cardiac remodelling and fitness in insulin-sensitive and insulin-resistant obese women. Heart. 2011;97:1585–1589. doi: 10.1136/hrt.2011.224451. [DOI] [PubMed] [Google Scholar]
- 23.Rijzewijk LJ, van der Meer RW, Smit JW, Diamant M, Bax JJ, Hammer S, et al. Myocardial steatosis is an independent predictor of diastolic dysfunction in type 2 diabetes mellitus. J Am Coll Cardiol. 2008;52:1793–1799. doi: 10.1016/j.jacc.2008.07.062. [DOI] [PubMed] [Google Scholar]
- 24.Ng AC, Delgado V, Bertini M, van der Meer RW, Rijzewijk LJ, Hooi Ewe S, et al. Myocardial steatosis and biventricular strain and strain rate imaging in patients with type 2 diabetes mellitus. Circulation. 2010;122:2538–2544. doi: 10.1161/CIRCULATIONAHA.110.955542. [DOI] [PubMed] [Google Scholar]
- 25.Szczepaniak LS, Victor RG, Orci L, Unger RH. Forgotten but not gone: the rediscovery of fatty heart, the most common unrecognized disease in America. Circ Res. 2007;101:759–767. doi: 10.1161/CIRCRESAHA.107.160457. [DOI] [PubMed] [Google Scholar]
- 26.Szczepaniak LS, Dobbins RL, Metzger GJ, Sartoni-D'Ambrosia G, Arbique D, Vongpatanasin W, et al. Myocardial triglycerides and systolic function in humans: in vivo evaluation by localized proton spectroscopy and cardiac imaging. Magn Reson Med. 2003;49:417–423. doi: 10.1002/mrm.10372. [DOI] [PubMed] [Google Scholar]
- 27.van der Meer RW, Rijzewijk LJ, Diamant M, Hammer S, Schar M, Bax JJ, et al. The ageing male heart: myocardial triglyceride content as independent predictor of diastolic function. Eur Heart J. 2008;29:1516–1522. doi: 10.1093/eurheartj/ehn207. [DOI] [PubMed] [Google Scholar]
- 28.Haemmerle G, Moustafa T, Woelkart G, Buttner S, Schmidt A, van de Weijer T, et al. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-alpha and PGC-1. Nat Med. 2011;17:1076–1085. doi: 10.1038/nm.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kienesberger PC, Lee D, Pulinilkunnil T, Brenner DS, Cai L, Magnes C, et al. Adipose triglyceride lipase deficiency causes tissue-specific changes in insulin signaling. J Biol Chem. 2009;284:30218–30229. doi: 10.1074/jbc.M109.047787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang TT, Naeemuddin M, Elchuri S, Yamaguchi M, Kozy HM, Carlson EJ, et al. Genetic modifiers of the phenotype of mice deficient in mitochondrial superoxide dismutase. Hum Mol Genet. 2006;15:1187–1194. doi: 10.1093/hmg/ddl034. [DOI] [PubMed] [Google Scholar]
- 31.Kienesberger PC, Pulinilkunnil T, Sung MM, Nagendran J, Haemmerle G, Kershaw EE, et al. Myocardial ATGL overexpression decreases the reliance on fatty acid oxidation and protects against pressure overload-induced cardiac dysfunction. Mol Cell Biol. 2012;32:740–750. doi: 10.1128/MCB.06470-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Connell TD, Rodrigo MC, Simpson PC. Isolation and culture of adult mouse cardiac myocytes. Methods Mol Biol. 2007;357:271–296. doi: 10.1385/1-59745-214-9:271. [DOI] [PubMed] [Google Scholar]
- 33.Boudina S, Sena S, O'Neill BT, Tathireddy P, Young ME, Abel ED. Reduced mitochondrial oxidative capacity and increased mitochondrial uncoupling impair myocardial energetics in obesity. Circulation. 2005;112:2686–2695. doi: 10.1161/CIRCULATIONAHA.105.554360. [DOI] [PubMed] [Google Scholar]
- 34.Tasseva G, Bai HD, Davidescu M, Haromy A, Michelakis E, Vance JE. Phosphatidylethanolamine deficiency in Mammalian mitochondria impairs oxidative phosphorylation and alters mitochondrial morphology. J Biol Chem. 2013;288:4158–4173. doi: 10.1074/jbc.M112.434183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsai JY, Kienesberger PC, Pulinilkunnil T, Sailors MH, Durgan DJ, Villegas-Montoya C, et al. Direct regulation of myocardial triglyceride metabolism by the cardiomyocyte circadian clock. J Biol Chem. 2010;285:2918–2929. doi: 10.1074/jbc.M109.077800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pinent M, Hackl H, Burkard TR, Prokesch A, Papak C, Scheideler M, et al. Differential transcriptional modulation of biological processes in adipocyte triglyceride lipase and hormone-sensitive lipase-deficient mice. Genomics. 2008;92:26–32. doi: 10.1016/j.ygeno.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 37.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J R Statist Soc B. 1995;57:289–300. [Google Scholar]
- 38.Pulinilkunnil T, Kienesberger PC, Nagendran J, Waller TJ, Young ME, Kershaw EE, et al. Myocardial adipose triglyceride lipase overexpression protects diabetic mice from the development of lipotoxic cardiomyopathy. Diabetes. 2013;62:1464–177. doi: 10.2337/db12-0927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bashyam MD, Savithri GR, Kumar MS, Narasimhan C, Nallari P. Molecular genetics of familial hypertrophic cardiomyopathy (FHC) J Hum Genet. 2003;48:55–64. doi: 10.1007/s100380300007. [DOI] [PubMed] [Google Scholar]
- 40.Sorokina N, O'Donnell JM, McKinney RD, Pound KM, Woldegiorgis G, LaNoue KF, et al. Recruitment of compensatory pathways to sustain oxidative flux with reduced carnitine palmitoyltransferase I activity characterizes inefficiency in energy metabolism in hypertrophied hearts. Circulation. 2007;115:2033–2041. doi: 10.1161/CIRCULATIONAHA.106.668665. [DOI] [PubMed] [Google Scholar]
- 41.Ueno M, Suzuki J, Zenimaru Y, Takahashi S, Koizumi T, Noriki S, et al. Cardiac overexpression of hormone-sensitive lipase inhibits myocardial steatosis and fibrosis in streptozotocin diabetic mice. Am J Physiol Endocrinol Metab. 2008;294:E1109–E1118. doi: 10.1152/ajpendo.00016.2008. [DOI] [PubMed] [Google Scholar]
- 42.Suzuki J, Shen WJ, Nelson BD, Patel S, Veerkamp JH, Selwood SP, et al. Absence of cardiac lipid accumulation in transgenic mice with heart-specific HSL overexpression. Am J Physiol Endocrinol Metab. 2001;281:E857–E866. doi: 10.1152/ajpendo.2001.281.4.E857. [DOI] [PubMed] [Google Scholar]
- 43.Saddik M, Lopaschuk GD. Myocardial triglyceride turnover and contribution to energy substrate utilization in isolated working rat hearts. J Biol Chem. 1991;266:8162–8170. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.