Abstract
Background
While evidence supports using sustained release morphine for chronic refractory breathlessness, little is known about the longitudinal pattern of breathlessness intensity as people achieve symptomatic benefit. The aim of this study is to describe this pattern.
Methods
This secondary analysis used breathlessness intensity scores (100 mm visual analogue scale (VAS)) from a prospective, dose increment study of once daily (morning) sustained release morphine for chronic refractory breathlessness. Participants who achieved <10% improvement over their own baseline at one week (10 mg) were titrated to 20 mg and if no response, another week later to 30 mg for one week. Time was standardized at the first day of the week in which participants responded generating twice daily data one week either side of symptomatic benefit. Analysis used random effect mixed modeling.
Results
Of the 83 participants, 17/52 responders required >10 mg: 13 participants (20 mg) and 4 (30 mg), contributing 634 VAS observations. In the week leading to a response, average VAS scores worsened by 0.3 mm/day (p=0.16); the average improvement in the first 24 hours of response was 10.9 mm (7.0 to 14.7; p<0.0001), with continued improvement of 2.2 mm/day (p<0.001) for six more days.
Conclusion
When treating chronic refractory breathlessness with once daily sustained release morphine, titrate to effect, since inadequate dose may generate no response; and following an initial response, further dose increases should not occur for at least one week. Whether further benefit would be derived beyond day six on the dose to which people respond, and what net effect a further dose increase would have are questions yet to be answered.
Introduction
Many diseases are associated with chronic breathlessness that no longer respond to disease-modifying therapies (chronic refractory breathlessness).1 Internationally, there are no medications registered with regulatory agencies for reducing symptomatic breathlessness, despite the burden seen across the community.2,3 Across the population, the prevalence and intensity of breathlessness both increase as death approaches.4,5
Level I evidence supports the use of morphine for reducing the subjective sensation of breathlessness.1,6 More recently, these studies have been complemented by a phase IV study confirming that the net benefits are sustained over time without further dose increases7 and identifying people whose breathlessness is most likely to respond to opioids.8 Recent laboratory studies are also helping in defining the role of opioids in modulating breathlessness.9,10 Recommendations from the American Thoracic Society and the American College of Physicians for the clinical management of refractory breathlessness now include the use of opioids.11,12
Phase II dose-ranging data can help to understand how people's breathlessness intensity responds to morphine, thus informing how best to titrate morphine in this population. Possibilities include a progressive dose/response relationship or a cumulative dose theshold at which people respond.
In a recently published study, sustained release morphine administered orally once daily (morning) to people with chronic refractory breathlessness was titrated to effect using a set algorithm.7 The starting dose was 10 mg and each week an additional 10 mg/day was administered if participants did not achieve at least a 10% reduction over their own baseline breathlessness intensity and did not experience unacceptable side effects. The study demonstrated that over 60% of participants (52/83) with chronic refractory breathlessness responded symptomatically to sustained release morphine, requiring either 10 mg/day (n=35), 20 mg/day (n=13), or 30mg/day (n=4). This current substudy used data from the latter two groups where data for a full week before and after responses were available when measured by morning and evening 100 mm visual analogue scales (VAS). The aim of this analysis was to describe the pattern of response for breathlessness intensity in order to inform dose titration in people who have responded in routine clinical care.
Material and Methods
Study design
The primary study in chronic refractory breathlessness was a prospective, open-label phase II/IV study of once daily (morning) sustained release morphine (KapanolR, KadianR), together with sodium docusate with sennosides to help treat morphine-induced constipation.7 Morning and evening scores for breathlessness intensity “right now” were averaged for the last three days of each week of treatment. The starting dose was 10 mg sustained release morphine each morning; participants whose breathlessness intensity had not reduced by at least 10% over their own baseline at one week were titrated up by 10 mg each week to a maximum dose of 30 mg/day.
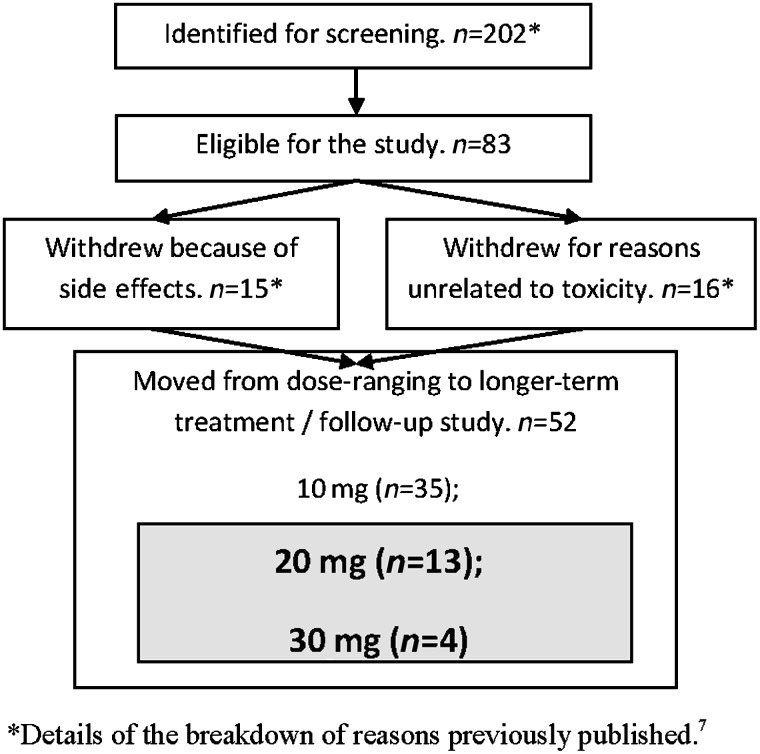
In this analysis we created a standardized point in time by using the first day of the week in which the 17 people who required a dose greater than 10 mg daily achieved their >10% reduction in breathlessness intensity (see Figure 1). This corresponded to day 8 for those who gained symptomatic benefit with 20 mg (n=13) of sustained release morphine and day 15 for those who required 30 mg (n=4).
FIG. 1.
Patient flow through the dose-ranging/long-term therapy study for sustained release morphine for the reduction in chronic refractory breathlessness. Participants who are the subject of this sub study.
Study participants
Participants were opioid-naïve outpatients aged ≥18 years with ongoing breathlessness scored at ≥3 on the modified Medical Research Council (mMRC) scale (see Table 1).13–15 To be eligible, all underlying reversible causes of the breathlessness must have been maximally treated as judged by the participant's relevant physician (e.g., a pulmonologist for a participant with chronic obstructive pulmonary disease, a cardiologist for someone with heart failure). All medications including oxygen must have been stable for the seven days before commencing the study, and clinician-estimated life expectancy must have been greater than one month.
Table 1.
Modified Medication Research Council Scale (mMRC)15
| Grade | Description of breathlessness |
|---|---|
| 0 | I only get breathless with strenuous exercise. |
| 1 | I get short of breath when hurrying on level ground or walking up a slight hill. |
| 2 | On level ground, I walk slower than people of the same age because of breathlessness, or have to stop for breath when walking at my own pace. |
| 3 | I stop for breath after walking about 100 yards or after a few minutes on level ground. |
| 4 | I am too breathless to leave the house or I am breathless when dressing. |
Exclusion criteria included regular use of any opioid medication or monoamine oxidase inhibitor in the two weeks before screening; a true hypersensitivity reaction to morphine; a history of substance misuse; performance status ≤40 on the Australian-modified Karnofsky Performance scale (AKPS) at baseline;16 severe renal impairment (a calculated creatinine clearance of ≤15 ml/min/1.73m2 using the Modification of Diet in Renal Disease (MDRD) formula);17,18 pregnancy; or poor cognition (less than 24/30 on a Mini-Mental State Examination (MMSE)).19
Setting
Participants were recruited from four university teaching hospitals in two Australian states between July 2007 and October 2009, with follow-up data collection ceasing in January 2010.
Measurements
Intensity of breathlessness was recorded morning and evening in participant diaries measured on 100 mm VAS anchored at 0 mm (“no breathlessness”) and at 100 mm (“worst imaginable breathlessness”). The question referred to breathlessness “right now.” Breathlessness was measured twice daily (morning and evening) with at least 17 days of data (34 observations) per participant in this subgroup of 17. Other measures included demographic and clinical data, the McGill Quality of Life Questionnaire, and functional status.20
Statistical methods
Differences between the groups at the two dose levels were compared using χ2 or t-tests as appropriate. Changes in VAS were analyzed using random effect mixed modeling to account for correlated readings within an individual. The covariates were day of trial; response; the interaction term “day*response;” age; gender; chronic obstructive pulmonary disease (COPD; yes/no); and AKPS. For each participant, the day was synchronized so that day zero corresponded to the first day of the week with the highest incremental dose received. Residuals at each level were inspected for normality and linearity. There was no evidence of model violation. A p-value of 0.05 (two-tailed) was considered statistically significant. All analyses were performed using Stata statistical software version 12.0 (StataCorp., College Station, TX).
Ethics, consent, and trial registration
The research was approved by all participating institutions' research and ethics committees. All participants provided written informed consent. The study received clinical trials notification approval to use KapanolR for an unregistered indication. The trial was registered with the Australian and New Zealand Clinical Trials Registry (ACTRNO-12606000269538).
Results
Participant flow and study population
Eighty-three people participated in the original study for a mean of 142 days (median 29; range 2–665). Thirty-five derived benefit at 10 mg/day sustained release morphine; 17 participants responded at higher dose (this substudy); and 31 derived no net benefit. Reasons for no net benefit included no benefit at the maximum dose of 30 mg (n=8), lacked benefit at lower doses and withdrew (n=3), unacceptable side effects (n=15), death unrelated to the intervention (n=4), or clinician request (n=1).
Of the 17 participants requiring a higher dose for response, eight (47%) were male and nine (53%) had COPD as the underlying cause of breathlessness (see Table 2). Age ranged from 63 to 88 (median 82; interquartile range 73–83); and median AKPS was 60 (range 50–80). The four participants who responded at doses of 30 mg were significantly older (mean age 80.2 years) than the 13 participants who responded at a dose of 20 mg (mean age 71.2 years; p=0.042). There were no other differences between the groups.
Table 2.
Baseline Mean (SD) or N (%) Demographic Data by Morphine Dose
| 20 mg (n=13) | 30 mg (n=4) | P-value (Difference) | |
|---|---|---|---|
| Age in years (mean) | 80.2 (6.5) | 71.5 (8.2) | 0.04 |
| Male sex | 5 (38.5) | 3 (75.0) | 0.29 |
| Performance status (AKPS) | 1.0 | ||
| 50 | 2 | 1 | |
| 60 | 6 | 2 | |
| 70 | 4 | 1 | |
| 80 | 1 | 0 | |
| COPD | 7 (53.9) | 2 (50.0) | 1.0 |
AKPS, Australian–modified Karnofsky Performance Status scale; COPD, Chronic Obstructive Pulmonary Disease.
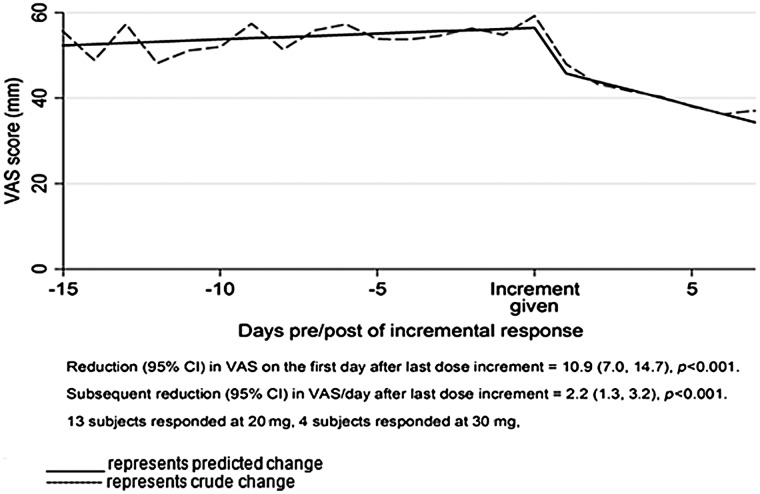
Individuals responding to 20 mg and 30 mg were combined to evaluate the curve of response before and after the dose increment (see Figure 2). Using all of the variables outlined, three distinct phases were seen: (1) a plateau phase in the week preceding response; (2) an immediate and significant reduction in breathlessness scores at initial response (mean absolute reduction 10.9 mm (95% confidence interval (CI) 7.0 to 14.7 (i<0.001; relative reduction 21.0%) in the first 24 hours of response after dose increment compared to the minimum VAS reading on the previous incremental dose; and (3) a phase of continued improvement of breathlessness daily over the following six days of 2.2 mm per day (95% CI 1.3 to 3.2; i<0.001).
FIG. 2.
Crude vs. predicted change in VAS per day.
By the end of the week after the initial response, the magnitude of response had doubled compared with the response seen in the first 24 hours (see Figure 2).
Performance status as a potential confounder on the longitudinal trends was explored. The interaction between breathlessness and performance status demonstrated that as performance status worsened, intensity of breathlessness increased. For every 10 unit decrease in AKPS, VAS increased by 16 mm. No other confounders were identified.
Discussion
This is the first study to document breathlessness intensity on a daily basis in people who responded to sustained release morphine for chronic refractory breathlessness. The temporal course of the reduction in breathlessness in response to treatment is important to inform both clinical management and future research to refine the place of morphine for the management of breathlessness. These data suggest that when people's refractory breathlessness responds to sustained release morphine, there is a rapid and marked decline in the intensity of breathlessness. There is further reduction in intensity in the ensuing week that doubles the response of the first 24 hours of response and provides guidance for the interval before further dose increases.
In chronic refractory breathlessness, the reduction in the intensity required to be clinically meaningful is approximately 10 mm on a VAS.21–28 In this study, those who respond have, on average, twice that reduction (19.8 mm) after a further week at the same dose.
Response in the whole study population and in this substudy was independent of underlying etiology of breathlessness (COPD or not), providing additional support to the notion of a ‘final common pathway’ generating the perception of breathlessness.8 The concept that benefit from morphine in reducing the intensity of breathlessness may be seen in a range of underlying etiologies is currently being explored in phase III double-blind, randomized trials.
The finding of a relationship between performance status and breathlessness is consistent with other data that suggest that breathlessness worsens as death approaches.4,5 It will be important in future work to define whether the relationship between breathlessness and disease trajectory is linear or whether there is an accelerated late phase.
Limitations of the study
These results may underestimate the net clinical benefit, since the minimum VAS in the week preceding the successful dose increment was the anchor point for the calculations and hence was the most conservative model. Using either the average or highest VAS in that week would demonstrate a more dramatic response. The longitudinal period explored after the dose increment was only a week, and continued decline in breathlessness intensity was observed on all days. Understanding whether there was continued reduction in breathlessness intensity beyond that point is crucial.
Data from this dose titration and pharmacovigilance study do not inform how quickly upward titration of sustained release morphine can occur until a symptomatic response, although this is likely to be much less than one week. The data only refer to the symptomatic improvement after initial response, suggesting that further titration should be limited to the time when benefit is maximized. If there are parallels between pain and breathlessness, it is of note that the one study in pain that has looked at rapid titration found that initiating therapy with immediate release oral morphine solution reached steady doses no more quickly than controlled release morphine.29 The current study suggests that the continuing increase in symptom control after a regular dose of morphine has been established should be further studied in both pain and breathlessness.
Another important potential confounder in studies of the management of symptoms such as breathlessness or pain as they relate to the level of function is that people may increase activity as the symptom is better controlled. If the exertional threshold at which breathlessness becomes troublesome can be raised, people's exercise tolerance may increase while they still report troublesome breathlessness as that (higher) threshold is reached. Supporting this theory, a study of people with COPD appears to show that exercise tolerance improved with fentanyl, when compared with placebo, but breathlessness intensity scores were unchanged.30 As such, future work in this area needs to have a more objective level of understanding of clinically meaningful day-to-day improvements in function over the person's own baseline either through more frequent use of validated tools (e.g., Lifespace) or direct measurement using actigraphs.31–33 Given the interplay between exercise, muscle function, and breathlessness intensity, function should be measured in future studies of the symptomatic measurement of breathlessness.34
Although the number of participants included in this substudy is relatively small (n=17), participants provided many observations (634 observations in total). As a result, these are statistically significant results that are clinically relevant. The McGill quality of life questionnaire and AKPS were only sought weekly.16,20 When recorded more frequently in future studies (daily for AKPS, second daily for McGill) it will be important to see if and when they shift in relation to the documented reduction in breathlessness.
Generalizability
The findings usefully inform clinicians how to safely titrate sustained release morphine. This multisite study reports people whose unifying feature is the intensity of their breathlessness. The level of breathlessness seen at baseline in this current study is of the same order of magnitude as several recent studies in refractory breathlessness.6,35 This supports the generalizability of these findings to a range of clinical settings.
Implications for clinicians and policymakers
Current practice in the use of morphine for breathlessness does not recommend titration to benefit or toxicity, although this approach is familiar in pain management. This study, as one aspect of the information needed in a full pharmacodynamic profile of morphine for chronic refractory breathlessness, adds a new dimension to the titration of sustained release morphine. After a dramatic reduction in breathlessness, there appears to be continued improvement in levels of breathlessness for at least one week. Titration within that time would increase the likelihood of toxicity, as the maximal benefit may not have been achieved.
Future research
The magnitude of improvement beyond the first seven days is unknown, as the data are not available. The question of whether further reductions in breathlessness could be achieved with further dose increases and without unacceptable toxicities has not been answered. Blinding this increment in an enriched cohort design would improve the level of evidence generated by the findings. As noted above, the McGill Quality of Life questionnaire, objective measures of activity, self-reported activities of daily living need to be measured more frequently in any subsequent study. An optimally designed study would include measurement of both intensity and unpleasantness of breathlessness, given recent evidence that these dimensions of breathlessness have different trajectories.36 For safety, noninvasive measurement of the partial pressures of oxygen and carbon dioxide is necessary.
Conclusion
Continued effort to understand how to optimize prescribing of sustained release morphine for chronic refractory breathlessness is a clinical priority. This analysis focuses on the increasing benefits seen days after an initial response, informing directly the timing of any subsequent upward titration.
Acknowledgments
Thanks go to all of the participants who gave their time. Dr. Matthew Doogue and Professor Felix Bochner provided helpful comments on the manuscript, and their reviews have helped to shape this paper. Thanks also go to the clinical research staff and to Ms. Debbie Marriott for her expertise in manuscript formatting and submission and to Ms. Heather Grigg for her help with data.
This study was generously supported by National Health and Medical Research Council grant #480459.
Author Disclosure Statement
The authors declare they have no competing interests.
References
- 1.Abernethy AP. Currow DC. Frith P. Fazekas BS. McHugh A. Bui C. Randomised double-blind placebo-controlled crossover trial of sustained-release morphine for the management of refractory dyspnoea. Br Med J. 2003;7414(327):523–525. doi: 10.1136/bmj.327.7414.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Currow DC. Plummer J. Crockett A. Abernethy AP. A community population survey of prevalence and severity of dyspnoea in adults. J Pain Symptom Manage. 2009;38:533–545. doi: 10.1016/j.jpainsymman.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Hammond E. Some preliminary findings on physical complaints from a prospective study of 1,064,004 men and women. Am J Pub Health. 1964;54:11–23. doi: 10.2105/ajph.54.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Currow DC. Smith J. Davidson PM. Newton PJ. Agar MR. Abernethy AP. Do the trajectories of dyspnoea differ in prevalence and intensity by diagnosis at the end of life? A consecutive cohort study. J Pain Symptom Manage. 2010;39:680–690. doi: 10.1016/j.jpainsymman.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 5.Seow H. Barbera L. Sutradhar R. Howell D. Dudgeon D. Atzema C. Liu Y. Husain A. Sussman J. Earle C. Trajectory of performance status and symptom scores for patients with cancer during the last six months of life. J Clin Oncol. 2011;29:1151–1158. doi: 10.1200/JCO.2010.30.7173. [DOI] [PubMed] [Google Scholar]
- 6.Jennings AL. Davies AN. Higgins JP. Gibbs JR. Broadley KE. A systematic review of the use of opioids in the management of dyspnea. Thorax. 2002;57:939–944. doi: 10.1136/thorax.57.11.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Currow DC. McDonald C. Oaten S. Kenny B. Allcroft P. Frith P. Briffa M. Johnson MJ. Abernethy AP. Once-daily opioids for chronic dyspnoea: A dose increment and pharmacovigilance study. J Pain Symptom Manage. 2011;42:388–399. doi: 10.1016/j.jpainsymman.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 8.Johnson MJ. Bland MJ. Oxberry SG. Abernethy AP. Currow DC. Opioids for chronic refractory breathlessness: Patient predictors of beneficial response. Eur Respir J. 2012 2012 Dec. doi: 10.1183/09031936.00139812. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 9.Mahler DA. Murray JA. Waterman LA, et al. Endogenous opioids modify dyspnoea during treadmill exercise in patients with COPD. Eur Respir J. 2009;33:771–777. doi: 10.1183/09031936.00145208. [DOI] [PubMed] [Google Scholar]
- 10.Gifford AH. Mahler DA. Waterman LA. Ward J. Kraemer WJ. Kupchak BR. Baird JC. Neuromodulatory effect of endogenous opioids on the intensity and unpleasantness of breathlessness during resistive load breathing in COPD. COPD. 2011;8:160–166. doi: 10.3109/15412555.2011.560132. [DOI] [PubMed] [Google Scholar]
- 11.Mahler DA. Selecky PA. Harrod CG. Benditt JO. Carrieri-Kohlman V. Curtis JR. Manning HLm Mularski RA. Varkey B. Campbell M. Carter ER. Chiong JR. Ely EW. Hansen-Flaschen J. O'Donnell DE. Waller A. American College of Chest Physicians consensus statement on the management of dyspnea in patients with advanced lung or heart disease. Chest. 2010;137:674–691. doi: 10.1378/chest.09-1543. [DOI] [PubMed] [Google Scholar]
- 12.Parshall MB. Schwartzstein RM. Adams L. Banzett RB. Manning HL. Bourbeau J. Calverley PM. Gift AG. Harver A. Lareau SC. Mahler DA. Meek PM. O'Donnell DE American Thoracic Society Committee on Dyspnea. An official American Thoracic Society statement: Update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185:435–452. doi: 10.1164/rccm.201111-2042ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fletcher CM. Elmes PC. Fairbairn MB, et al. The significance of respiratory symptoms and the diagnosis of chronic bronchitis in a working population. Br Med J. 1959;2:257–266. doi: 10.1136/bmj.2.5147.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Medical Research Council Committee on Aetiology of Chronic Bronchitis: Standardized questionnaires on respiratory symptoms. Br Med J. 1960;II:1665. [Google Scholar]
- 15.Bestall JC. Paul EA. Garrod R. Garnham R. Jones PW. Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54:581–586. doi: 10.1136/thx.54.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abernethy AP. Shelby-James TM. Fazekas BS. Woods D. Currow DC. The Australian-modified Karnofsky Performance Status (AKPS) scale: A revised scale for contemporary palliative care clinical practice. BMC Pall Care. 2005;4:7. doi: 10.1186/1472-684X-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levey AS. Bosch JP. Lewis JB. Greene T. Rogers N. Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 18.Rossi S. Australian Medicines Handbook. Adelaide: Australian Medicines Handbook; 2012. [Google Scholar]
- 19.Folstein MF. Folstein SE. McHugh PR. “Mini-mental state:” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 20.Cohen SR. Mount BM. Strobel MG. Bui F. The McGill Quality of Life Questionnaire, a measure of quality of life appropriate for people with advanced disease: A preliminary study of validity and acceptability. Palliat Med. 1995;9:207–219. doi: 10.1177/026921639500900306. [DOI] [PubMed] [Google Scholar]
- 21.Ries AL. Minimally clinically important difference for the UCSD Shortness of Breath Questionnaine, Borg Scale, and Visual Analogue Scale. COPD. 2005;2:105–110. doi: 10.1081/copd-200050655. [DOI] [PubMed] [Google Scholar]
- 22.Mahler DA. Mechanisms and measurement of dyspnea in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3:234–238. doi: 10.1513/pats.200509-103SF. [DOI] [PubMed] [Google Scholar]
- 23.Oxberry SG. Bland JM. Clark AL. Cleland JGF. Johnson MJ. Minimally Clinically Important Difference (MCID) in chronic breathlessness: Every little helps. J Am Heart Assoc. 2012;164:229–235. doi: 10.1016/j.ahj.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 24.Sloan JA. Assessing the minimally clinically significant difference: Scientific considerations, challenges and solutions. COPD. 2005;2:57–62. doi: 10.1081/copd-200053374. [DOI] [PubMed] [Google Scholar]
- 25.Wyrwich KW. Tierney WM. Wolinsky FD. Further evidence supporting an SEM-based criterion for identifying meaningful intra-individual changes in health-related quality of life. J Clin Epidemiol. 1999;52:861–873. doi: 10.1016/s0895-4356(99)00071-2. [DOI] [PubMed] [Google Scholar]
- 26.Mahler DA. Witek TJ., Jr. The MCID of the transition dyspnea index is a total score of one unit. COPD. 2005;2:99–103. doi: 10.1081/copd-200050666. [DOI] [PubMed] [Google Scholar]
- 27.Norman GR. Sloan JA. Wyrwich KW. Interpretation of changes in health-related quality of life: The remarkable universality of half a standard deviation. Med Care. 2003;41:582–592. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 28.Johnson MJ. Bland JM. Oxberry SG. Abernethy AP. Currow DC. Clinically important differences in the intensity of chronic fractory breathlessness. J Pain Symptom Manage. 2013 Apr 19; doi: 10.1016/j.jpainsymman.2013.01.011. Epub a head of print. [DOI] [PubMed] [Google Scholar]
- 29.Klepstad P. Kaasa S. Jystad A, et al. Immediate- or sustained-release morphine for dose finding during start of morphine to cancer patients: A randomized, double-blind trial. Pain. 2003;101(1):193–198. doi: 10.1016/s0304-3959(02)00328-7. [DOI] [PubMed] [Google Scholar]
- 30.Jensen D. Alsuhail A. Viola R. Dudgeon DJ. Webb KA. O'Donnell DE. Inhaled fentanyl citrate improves exercise endurance during high-intensity constant work rate cycle exercise in chronic obstructive pulmonary disease. J Pain Symptom Manage. 2012;43:706–719. doi: 10.1016/j.jpainsymman.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 31.Baker PS. Bodner EV. Allman RM. Measuring life-space mobility in community-dwelling older adults. J Am Geriatr Soc. 2003;51:1610–1614. doi: 10.1046/j.1532-5415.2003.51512.x. [DOI] [PubMed] [Google Scholar]
- 32.Maddocks M. Wilcock A. Exploring physical activity level in patients with thoracic cancer: Implications for use as an outcome measure. Support Care Cancer. 2012;20:1113–1116. doi: 10.1007/s00520-012-1393-z. [DOI] [PubMed] [Google Scholar]
- 33.Currow DC. Abernethy AP. Johnson MJ. Activity as a measure of symptom control. J Pain Symptom Manage. 2012;44:e1–e2. doi: 10.1016/j.jpainsymman.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 34.Currow DC. Abernethy AP. Johnson MJ. Activity as a measure of symptom control. J Pain Symptom Manage. 2012 Sep 24; doi: 10.1016/j.jpainsymman.2012.07.005. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 35.Abernethy AP. McDonald CF. Frith PA. Clark K. Herndon JE. Marcello J. Young IH. Bull J. Wilcock A. Booth S. Wheeler JL. Tulsky JA. Crockett AJ. Currow DC. Effect of palliative oxygen versus room air in relieving breathlessness in patients with refractory dyspnea: A double-blind randomized controlled trial ( NCT00327873) Lancet. 2010;376:784–793. doi: 10.1016/S0140-6736(10)61115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.von Leupoldt A. Ambruzsova R. Nordmeyer S. Jeske N. Dahme B. Sensory and affective aspects of dyspnea contribute differentially to the Borg scale's measurement of dyspnea. Respiration. 2006;73:762–768. doi: 10.1159/000095910. [DOI] [PubMed] [Google Scholar]