Abstract
Context:
The effectiveness of a new continuous diathermy unit, ReBound, as a heating modality is unknown.
Objective:
To compare the effects of ReBound diathermy with silicate-gel moist hot packs on tissue temperature in the human triceps surae muscle.
Design:
Crossover study.
Setting:
University research laboratory.
Patients or Other Participants:
A total of 12 healthy, college-aged volunteers (4 men, 8 women; age = 22.2 ± 2.25 years, calf subcutaneous fat thickness = 7.2 ± 1.9 mm).
Intervention(s):
On 2 different days, 1 of 2 modalities (ReBound diathermy, silicate-gel moist hot pack) was applied to the triceps surae muscle of each participant for 30 minutes. After 30 minutes, the modality was removed, and temperature decay was recorded for 20 minutes.
Main Outcome Measure(s):
Medial triceps surae intramuscular tissue temperature at a depth of 1 cm was measured using an implantable thermocouple inserted horizontally into the muscle. Measurements were taken every 5 minutes during the 30-minute treatment and every minute during the 20-minute temperature decay, for a total of 50 minutes. Treatment was analyzed through a 2 × 7 mixed-model analysis of variance with repeated measures. Temperature decay was analyzed through a 2 × 21 mixed-model analysis of variance with repeated measures.
Results:
During the 30-minute application, tissue temperatures at a depth of 1 cm increased more with the ReBound diathermy than with the moist hot pack (F6,66 = 7.14, P < .001). ReBound diathermy and moist hot packs increased tissue temperatures 3.69°C ± 1.50°C and 2.82°C ± 0.90°C, respectively, from baseline. Throughout the temperature decay, ReBound diathermy produced a greater rate of heat dissipation than the moist hot pack (F20,222 = 4.42, P < .001).
Conclusions:
During a 30-minute treatment at a superficial depth, the ReBound diathermy increased tissue temperature to moderate levels, which were greater than the levels reached with moist hot packs.
Key Words: continuous diathermy, intramuscular temperature, heat
Key Points.
Superficial tissue temperatures were greater after a 30-minute treatment with ReBound diathermy than with moist hot packs.
ReBound diathermy produced moderate heating of superficial tissues.
Further research is needed to better understand the place of ReBound diathermy as a therapeutic modality in physical medicine and rehabilitation settings.
Heat has been used as a therapeutic modality for several years and has been shown to increase circulation,1,2 increase metabolism,1 decrease pain and relax muscle spasms,3–6 and decrease tissue stiffness.4,6–8 Heat as a therapeutic modality usually is described by 2 categories: superficial- and deep-heating agents. Examples of superficial-heating modalities are dry and moist hot packs, paraffin baths, hot whirlpools, and hot tubs. Therapeutic ultrasound and shortwave diathermy have been shown to produce vigorous heating temperatures at deeper tissue depths.9,10
The effectiveness of superficial- and deep-heating agents can be described by the level of increased temperature they create in the tissues. Researchers have suggested that tissue temperature must increase by at least 1.0°C for mild heating, 2°C to 3°C for moderate heating, and 4°C or more for vigorous heating, with an increase in physiologic effects as tissue temperature increases.9,11
Moist hot packs are perhaps the most widely used form of superficial heating in the clinical setting, and they effectively increase superficial tissue temperatures. At a depth of 1 cm, moist hot packs increased tissue temperatures by 2.2°C to 3.8°C from baseline.11–13 With this increase in tissue temperature, moist hot packs increased tissue extensibility6,7 and range of motion of a joint7 and decreased muscle spasm.7
A newer continuous diathermy device, ReBound (ReGear Life Sciences, Inc, Pittsburgh, PA), recently has been introduced into the clinical setting. It uses an induction helical-coil sleeve to heat the surrounding tissues at the variables of 35 W and 13.56 MHz.14 In another study,15 we found ReBound diathermy was not as effective as pulsed shortwave diathermy, the Megapulse II, at creating vigorous heating temperatures at a depth of 3 cm in the triceps surae muscle. Its capacity as a heating device at more superficial depths has not been determined.15 Therefore, the purpose of our study was to assess the effectiveness of ReBound diathermy and silicate-gel moist hot packs by measuring tissue temperature changes of the triceps surae muscle group at a depth of 1 cm during and after a 30-minute treatment.
METHODS
We used a 2 × 27 repeated-measures crossover design for temperature heating and decay. The dependent variable was tissue temperature of the triceps surae muscle group at a depth of 1 cm measured to the nearest 0.1°C. The independent variables were 2 levels of treatment groups (ReBound diathermy, moist hot packs) and time. Time was measured pretreatment as baseline, then with 6 measurements that were 5 minutes apart over a 30-minute treatment. Temperature decay was measured after the treatment ended over 20 time points that were 1 minute apart.
Participants
Four men and 8 women who were healthy, college-aged volunteers (age = 22.2 ± 2.25 years, calf subcutaneous fat = 7.2 ± 1.9 mm) participated in our study. Each participant was screened for disqualifying conditions that included pregnancy, fever, peripheral vascular disease, infection, injury to the triceps surae muscle in the 2 months before the study, compromised circulation, compromised sensation to the area being treated, and subcutaneous fat thickness of the calf greater than 15 mm.
During the study, all participants were fully compliant, and we did not need to terminate a treatment with either modality throughout the study. All participants provided written informed consent, and the study was approved by Brigham Young University's institutional review board.
Instruments
Implantable IT-21 thermocouples (Physitemp Instruments Inc, Clifton, NJ) were plugged into an electrothermometer (Iso-Thermex; Columbus Instruments, Columbus, OH) to record tissue temperatures. The reliability and validity of the IT-21 thermocouples and Iso-Thermex electrothermometer have been described.16 We used an imaging ultrasound (LOGIQ 5e; General Electric Company, Fairfield, CT) to measure the subcutaneous fat thickness of our participants and to verify the depth of the inserted thermocouples.
We used standard (10 × 12-in [25.4 × 30.48-cm]) hydrocollator moist hot packs (model 1064; Chattanooga Corporation, City of Industry, CA). An insulated terry cloth cover (Chattanooga Corporation) folded over the moist hot pack and 1 large towel were used as a barrier between the moist hot pack and the skin. Throughout the study, the hydrocollator (model E-1; Chattanooga Corporation) was checked to ensure the water level was correct and the water temperature was consistent with the manufacturer's recommended guidelines of 71°C to 74°C.
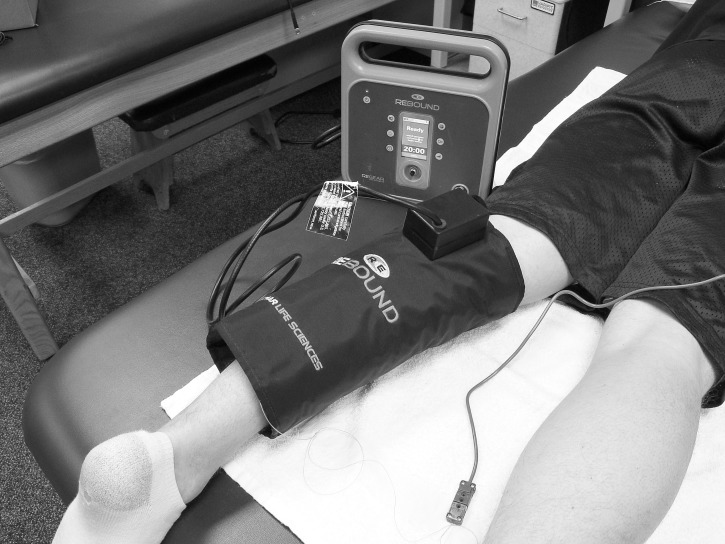
The diathermy device used in our study was the ReBound. The diathermy met the recommended calibrated guidelines. We used a size 18 therapy garment sleeve (ReGear Life Sciences, Inc) to provide the ReBound diathermy treatment (Figure 1).
Figure 1.
ReBound (ReGear Life Sciences, Inc, Pittsburgh, PA) diathermy sleeve with thermocouple insertion.
Procedures
Participants were instructed to refrain from exercise for at least 2 hours before testing. Each participant wore shorts for the treatments and reported to the modalities laboratory at Brigham Young University. We used an orthogonal Latin square to randomize treatment order for the participants. Successive treatments were administered with at least a 48-hour recovery period between sessions. Participants lay prone on the treatment table, and ultrasound gel was applied to the right posterior calf. We used the imaging ultrasound to produce an image of the posterior triceps surae muscle and observed the thickest portion of the muscle or area of the muscle with the largest girth, marking a line there on the posterior skin. Next, we measured and recorded subcutaneous fat thickness using the imaging ultrasound at the marked site and confirmed that each participant had less than 15-mm subcutaneous fat thickness over the treatment area. A small carpenter square was used to measure perpendicularly from the already marked line on the posterior skin surface to a 1-cm, posterior-to-anterior distance on the medial side of the calf. A dot was marked on the skin on the medial side of the triceps surae at a 1-cm measured distance.
The skin was prepared using an iodine swab and wiped clean using an isopropyl alcohol preparation pad. A 20-gauge, 1-in (2.54-cm) catheter (BD Medical, Franklin Lakes, NJ) was inserted at a depth of 1 cm into the medial aspect of the posterior triceps surae. The depth of the catheter insertion was verified to be within 0.2 cm of the desired depth using the imaging ultrasound. Next, we inserted 1 IT-21 thermocouple via the catheter at a depth of 1 cm to the end of the catheter. We slowly removed the catheter, leaving the thermocouple intact. The catheter was removed to expose the end of the thermocouple so that a proper intramuscular tissue temperature could be obtained. If not removed, the catheter would have covered the thermocouple and interfered with its ability to properly measure the tissue temperature. The thermocouple was secured to the skin with clear medical tape; it was attached to an Iso-Thermex electrothermometer and set to measure and record tissue temperature every 5 seconds. Baseline temperature was recorded and considered reached when the temperature did not change more than 0.5°C for 6 measurements during a 30-second period.
ReBound Treatment
After the thermocouples were inserted and baseline temperature was measured, the diathermy sleeve was pulled over the triceps surae muscle of the participant, and the ReBound diathermy was turned on, set for a 30-minute treatment, and tuned (Figure 1). The ReBound diathermy does not have different variables that can be adjusted. We used a 30-minute treatment duration to ensure that we observed the peak temperature increase. The ReBound diathermy also interfered with any other computer or electrical device in close proximity. Therefore, this diathermy needed to be paused to allow for an accurate tissue temperature reading. As reported by other authors,1 the diathermy was paused after 5, 10, 15, 20, 25, and 30 minutes of treatment, and we measured temperatures at a depth of 1 cm and recorded the average of 3 measurements using the Iso-Thermex electrothermometer. The ReBound diathermy was paused for an average of 20 seconds during each recording session.
Moist Hot Pack Treatment
After baseline temperature was measured and recorded, a moist hot pack covered by a terry cloth sleeve was laid on the triceps surae muscle of the participant. One 24 × 48-in (60.96 × 121.92-cm) towel folded in half was placed between the moist hot pack and the skin. After 5, 10, 15, 20, 25, and 30 minutes of treatment, we measured the tissue temperatures at a depth of 1 cm and recorded the average of 3 measurements using the Iso-Thermex electrothermometer. After treatment, the moist hot pack was returned to the hydrocollator unit, where it remained for at least 24 hours to allow reheating to occur.
After the treatment had concluded, we removed the hot pack and towel or turned off the ReBound diathermy and removed the sleeve, and we left the treated triceps surae exposed to room air. Room temperature was maintained at 23°C throughout the study.
Tissue temperature decay was measured with the participant still lying in a prone position. The thermocouple was left in the tissue for 20 minutes posttreatment, and tissue temperature was measured and recorded every minute using the Iso-Thermex electrothermometer.
After measuring temperature decay posttreatment for the ReBound diathermy and moist hot pack, we carefully removed the thermocouple and applied bacitracin ointment and an elastic bandage over the insertion site. Participants were instructed about appropriate care for the insertion site. The thermocouple was sterilized in a 2% CidexPlus (Johnson & Johnson Medical, Arlington, TX) solution for 24 hours, and needles and catheters were disposed in a sharps biohazard container.
Statistical Analysis
We analyzed the data in 2 parts: treatment and temperature decay. First, to analyze the treatment, a 2 × 7 mixed-model analysis of variance with repeated-measures blocking by participant was used to assess the effects of treatments and time and their interaction. We accounted for the differences in individual baseline temperatures by adding a baseline temperature variable in the model.
Second, a 2 × 21 mixed-model analysis of variance with repeated measures was used to analyze the temperature decay between the 2 treatments. We accounted for the different treatment temperatures produced by the treatments during the analysis by adding a final treatment temperature variable to the model. Statistical analyses were performed with JMP (version 9.0; SAS Inc, Cary, NC). The α level was set a priori ≤.05.
RESULTS
Treatment
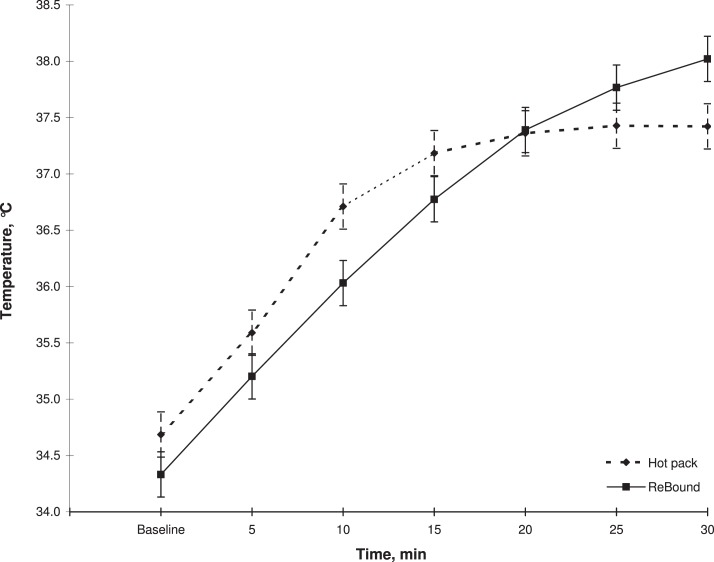
We noted a treatment-by-time interaction (F6,66 = 7.14, P < .001). We did not find a main effect of treatment (F1,11 = 0.32, P = .58) but did find a main effect of time (F1,17 = 12.96, P < .001). Over the 30-minute treatment, ReBound diathermy created a higher tissue temperature at a depth of 1 cm (Figure 2). The Table depicts the mean peak absolute tissue temperatures and the mean peak residual, or change from baseline, tissue temperatures for each modality.
Figure 2.
Mean temperature increased at a depth of 1 cm in the triceps surae muscle during a 30-minute application of the ReBound diathermy system (ReGear Life Sciences, Inc, Pittsburgh, PA) and hot pack.
Table.
Absolute and Residual, or Change from Baseline, Tissue Temperatures After a 30-Minute Application of ReBound or Hot Pack (Mean ± SD)
| Treatment |
Absolute Temperature, °C |
Residual Temperature, °C |
| ReBound diathermya | 37.94 ± 0.85 | 3.69 ± 1.50 |
| Hot pack | 37.60 ± 0.39 | 2.82 ± 0.90 |
ReGear Life Sciences, Inc, Pittsburgh, PA.
Temperature Decay
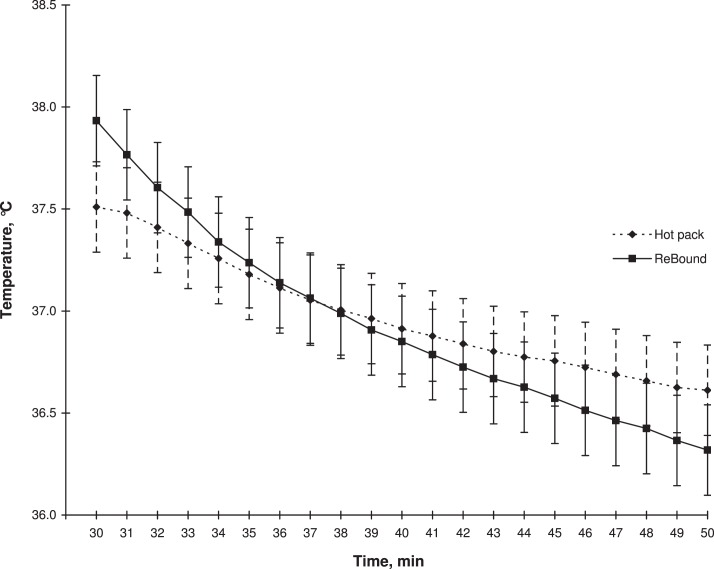
We observed a treatment-by-time interaction (F20,222 = 4.42, P < .001). We found a main effect of time (F1,420 = 96.45, P < .001) but did not find a main effect of treatment (F1,11 = 0.39, P = .54). During the temperature decay, the tissue temperatures at a depth of 1 cm decreased at a faster rate with ReBound diathermy than with the moist hot packs (Figure 3).
Figure 3.
Mean temperatures during the 20-minute temperature decay at a depth of 1 cm for the ReBound diathermy system (ReGear Life Sciences, Inc, Pittsburgh, PA) and hot pack.
DISCUSSION
The primary purpose of our study was to examine the temperature increases of 2 modalities: ReBound continuous shortwave diathermy and moist hot packs. Given the absence of research specifically on the ReBound diathermy, we wanted to better understand its role as a superficial heating modality. To do this, we examined its ability to increase tissue temperature.
To our knowledge, we are the first to evaluate superficial mean temperature increases with ReBound diathermy. Over the 30-minute treatment, tissue temperature increased to a greater level with ReBound diathermy than with moist hot packs, but this greater heating level did not occur until 20 minutes into the treatment. Therefore, if ReBound diathermy is to be used instead of moist hot packs as a superficial-heating agent, an application time of more than 20 minutes is needed.
At a depth of 1 cm, the peak temperature we observed with ReBound diathermy occurred at the 30-minute treatment mark. This finding suggests that at least a 30-minute treatment duration should be recommended for ReBound diathermy treatments when peak temperature is desired. We stopped the treatments at 30 minutes, so we do not know whether a greater temperature increase could have been observed after the 30-minute mark or when the body might attempt to cool the tissue.
The temperature increase from baseline we observed with moist hot packs at 1 cm (2.82°C ± 0.90°C) is similar to the temperature increase shown in previous studies.11,13 Our results are similar to those reported in other studies because we recorded moist hot pack tissue temperatures peaking around 15 minutes.12 Our results confirm that 15 minutes is the ideal application time for moist hot packs. When compared with the ReBound diathermy at the 15-minute treatment time, the moist hot packs produced a similar heating rate. Thus, no difference in modality effectiveness may exist between the 2 when a shorter-duration treatment is used.
Results are conflicting when assessing the abilities of superficial heat to increase tissue extensibility.6,7 One factor that may have a role in the ability of a heating agent to increase tissue extensibility is the characteristic of temperature decay after the modality is applied.4,17 Our results indicated that ReBound diathermy increased the tissues to a higher temperature, but the temperature also dissipated at a higher rate. These results may have 2 explanations. First, the greater increase in temperature may have resulted in an increase in blood flow that was greater with the ReBound diathermy application than with the moist hot pack application. Given the increase in blood flow, the body's mechanism to dissipate heat is enhanced and results in a faster decline in tissue temperature.18 The differences in temperature decay also may be explained by the different methods of heat transfer between the 2 modalities. ReBound diathermy uses electromagnetic waves that are transferred into heat through conversion. Moist hot packs transfer heat through conduction. We do not know whether ReBound diathermy introduced electromagnetic waves that created a uniform area of heating in the tissues and consequently produced heat that might have been conducted to cooler surrounding tissues at a faster rate. Given that moist hot packs transfer heat through conduction and work through a temperature gradient, the surrounding tissues are more likely to be the same temperature or even a greater temperature at the measured site. This may allow the tissues to maintain the greater tissue temperatures for longer periods.12
In addition to being an effective superficial heating agent, ReBound diathermy has some other advantages over moist hot packs. ReBound diathermy is lighter and more mobile, so it is easier to transport. Hydrocollators tend to be bulky and heavy, making them hard to transport. ReBound diathermy possibly heats a greater surface area than hot packs because the sleeve wraps around the whole body part. However, researchers need to study whether ReBound diathermy actually heats throughout the whole surface area the sleeve covers. Another advantage is that ReBound diathermy does not require a reheating period as moist hot packs do. The advantages and disadvantages of ReBound diathermy and moist hot packs should be considered when trying to choose the modality best suited for an individual.
Our study had limitations. Our results can be applied directly only to the 2 specific modality units that we used: standard hydrocollator silicate-gel moist hot packs and ReBound diathermy. We also studied healthy, college-aged students with a subcutaneous fat thickness measurement of less than 15 mm over the treatment area, and we do not know how the results transfer to injured individuals. Our results also can be inferred only with regard to treatments lasting 30 minutes or less.
The effectiveness of ReBound diathermy at creating the desired heating effects and when used in an injured population should be researched because it is a relatively new device. The size of the heating area produced by ReBound diathermy should be established.
CONCLUSIONS
The results of our study indicate that ReBound diathermy produced higher superficial tissue temperatures than moist hot packs after a 30-minute treatment. ReBound diathermy produced moderate heating of superficial tissues. Given that we are the first to evaluate the heating characteristics of ReBound diathermy, further research should be performed to understand its place as a therapeutic modality in physical medicine and rehabilitation settings.
REFERENCES
- 1.Hill J, Lewis M, Mills P, Kielty C. Pulsed short-wave diathermy effects on human fibroblast proliferation. Arch Phys Med Rehabil. 2002;83(6):832–836. doi: 10.1053/apmr.2002.32823. [DOI] [PubMed] [Google Scholar]
- 2.Sekins KM, Lehmann JF, Esselman P, et al. Local muscle blood flow and temperature responses to 915MHz diathermy as simultaneously measured and numerically predicted. Arch Phys Med Rehabil. 1984;65(1):1–7. [PubMed] [Google Scholar]
- 3.Cetin N, Aytar A, Atalay A, Akman MN. Comparing hot pack, short-wave diathermy, ultrasound, and TENS on isokinetic strength, pain, and functional status of women with osteoarthritic knees: a single-blind, randomized, controlled trial. Am J Phys Med Rehabil. 2008;87(6):443–451. doi: 10.1097/PHM.0b013e318174e467. [DOI] [PubMed] [Google Scholar]
- 4.Draper DO, Castro JL, Feland B, Schulthies S, Eggett D. Shortwave diathermy and prolonged stretching increase hamstring flexibility more than prolonged stretching alone. J Orthop Sports Phys Ther. 2004;34(1):13–20. doi: 10.2519/jospt.2004.34.1.13. [DOI] [PubMed] [Google Scholar]
- 5.Jan MH, Chai HM, Wang CL, Lin YF, Tsai LY. Effects of repetitive shortwave diathermy for reducing synovitis in patients with knee osteoarthritis: an ultrasonographic study. Phys Ther. 2006;86(2):236–244. [PubMed] [Google Scholar]
- 6.Robertson VJ, Ward AR, Jung P. The effect of heat on tissue extensibility: a comparison of deep and superficial heating. Arch Phys Med Rehabil. 2005;86(4):819–825. doi: 10.1016/j.apmr.2004.07.353. [DOI] [PubMed] [Google Scholar]
- 7.Knight CA, Rutledge CR, Cox ME, Acosta M, Hall SJ. Effect of superficial heat, deep heat, and active exercise warm-up on the extensibility of the plantar flexors. Phys Ther. 2001;81(6):1206–1214. [PubMed] [Google Scholar]
- 8.Peres SE, Draper DO, Knight KL, Ricard MD. Pulsed shortwave diathermy and prolonged long-duration stretching increase dorsiflexion range of motion more than identical stretching without diathermy. J Athl Train. 2002;37(1):43–50. [PMC free article] [PubMed] [Google Scholar]
- 9.Draper DO, Castel JC, Castel D. Rate of temperature increase in human muscle during 1 MHz and 3 MHz continuous ultrasound. J Orthop Sports Phys Ther. 1995;22(4):142–150. doi: 10.2519/jospt.1995.22.4.142. [DOI] [PubMed] [Google Scholar]
- 10.Draper DO, Knight K, Fujiwara T, Castel JC. Temperature change in human muscle during and after pulsed short-wave diathermy. J Orthop Sports Phys Ther. 1999;29(1):13–18. doi: 10.2519/jospt.1999.29.1.13. [DOI] [PubMed] [Google Scholar]
- 11.Halvorson GA. Therapeutic heat and cold for athletic injuries. Physician Sportsmed. 1990;18(5):87–92. doi: 10.1080/00913847.1990.11710045. 94. [DOI] [PubMed] [Google Scholar]
- 12.Draper DO, Harris ST, Schulthies S, Durrant E, Knight KL, Hot-pack Ricard M. and 1-MHz ultrasound treatments have an additive effect on muscle temperature increase. J Athl Train. 1998;33(1):21–24. [PMC free article] [PubMed] [Google Scholar]
- 13.Lehmann JF, Stonebridge JB, deLateur BJ, Warren CG, Halar E. Temperatures in human thighs after hot pack treatment followed by ultrasound. Arch Phys Med Rehabil. 1978;59(10):472–475. [PubMed] [Google Scholar]
- 14.ReGear Life Sciences, Inc. ReBound diathermy system: technical specifications. 2010 http://regearlife.com/downloads/rebound-specification-sheet.pdf. Accessed September 18. [Google Scholar]
- 15.Draper DO, Hawkes AR, Johnson AW, Diede MT, Rigby JH. Muscle heating with Megapulse II shortwave diathermy and ReBound diathermy. J Athl Train. 2013;48(4):477–482. doi: 10.4085/1062-6050-48.3.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Long BC, Jutte LS, Knight KL. Response of thermocouples interfaced to electrothermometers when immersed in 5 water bath temperatures. J Athl Train. 2010;45(4):338–343. doi: 10.4085/1062-6050-45.4.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Draper DO, Ricard MD. Rate of temperature decay in human muscle following 3 MHz ultrasound: the stretching window revealed. J Athl Train. 1995;30(4):304–307. [PMC free article] [PubMed] [Google Scholar]
- 18.Ducharme MB, Tikuisis P. Role of blood as heat source or sink in human limbs during local cooling and heating. J Appl Physiol. 1994;76(5):2084–2094. doi: 10.1152/jappl.1994.76.5.2084. [DOI] [PubMed] [Google Scholar]