Abstract
The ventromedial prefrontal cortex (vmPFC) plays a critical role in processing appetitive stimuli. Recent investigations have shown that reward value signals in the vmPFC can be altered by emotion regulation processes; however, to what extent the processing of positive emotion relies on neural regions implicated in reward processing is unclear. Here, we investigated the effects of emotion regulation on the valuation of emotionally evocative images. Two independent experimental samples of human participants performed a cognitive reappraisal task while undergoing fMRI. The experience of positive emotions activated the vmPFC, whereas the regulation of positive emotions led to relative decreases in vmPFC activation. During the experience of positive emotions, vmPFC activation tracked participants' own subjective ratings of the valence of stimuli. Furthermore, vmPFC activation also tracked normative valence ratings of the stimuli when participants were asked to experience their emotions, but not when asked to regulate them. A separate analysis of the predictive power of vmPFC on behavior indicated that even after accounting for normative stimulus ratings and condition, increased signal in the vmPFC was associated with more positive valence ratings. These results suggest that the vmPFC encodes a domain-general value signal that tracks the value of not only external rewards, but also emotional stimuli.
Introduction
Characterizing how the brain evaluates rewarding stimuli has been one of the central goals of the field of neuroeconomics. Single-unit recording studies in animals (Wallis, 2011; Monosov and Hikosaka, 2012) and functional neuroimaging studies in humans (Smith and Huettel, 2010) support the idea that reward-related computations occur in the ventromedial prefrontal cortex (vmPFC), and that activity in this region does not reflect objective properties of rewards (but see Padoa-Schioppa and Assad, 2008), but rather subjective value (Kable and Glimcher, 2007). Several investigations have suggested that the vmPFC encodes the subjective exchange rate between different reward modalities (e.g., food versus social rewards; Chib et al., 2009; Rangel and Hare, 2010; Levy and Glimcher, 2011; Levy and Glimcher, 2012). It thereby creates a “common neural currency” for reward (Montague and Berns, 2002), allowing disparate rewards to be compared. This signal also facilitates judgments of value to be extended beyond canonical rewards. For example, the vmPFC has been shown to respond to emotional stimuli that generate positive affect (Goel and Dolan, 2001; Cunningham, Johnsen, and Waggoner, 2011; Vrtička et al., 2011), which suggests a more general role for the vmPFC in signaling the presence of motivationally attractive stimuli (Schoenbaum et al., 2009).
How emotion regulation alters such value processes in the vmPFC remains unclear. A recent meta-analysis indicated that, across several studies using cognitive reappraisal, fear extinction, or placebo manipulations, regulating negative emotions showed converging activation within a subregion of the vmPFC (Diekhof et al., 2011). This investigation and other studies on the neural mechanisms of emotion regulation (Urry et al., 2006; Johnstone et al., 2007; Delgado et al., 2008b) have suggested that vmPFC activations observed during regulation are consistent with a role in cognitive control. Because this meta-analysis and most existing studies of reappraisal focused only on the regulation of negative affect, an alternative explanation remains: the increased signal in the vmPFC reflects the change in emotional value after emotion regulation. fMRI data have indicated that the regulation of neural responses to monetary rewards engages aspects of the prefrontal cortex similar to those engaged by the regulation of negative emotion (Delgado et al., 2008a; Staudinger et al., 2009; Staudinger et al., 2011). Although numerous studies have investigated behavioral effects of the regulation of positive emotion (Nezlek and Kuppens, 2008; Quoidbach et al., 2010; Beblo et al., 2012; Meyer et al., 2012; for review, see Tugade and Fredrickson, 2006), substantially fewer studies have examined the relationship between the regulation and experience of positive stimuli using neuroscience methods (Heller et al., 2009; Kanske et al., 2011; Winecoff et al., 2011). Examining the encoding of emotional value in the vmPFC during the experience and regulation of both positive and negative emotional stimuli would reconcile disparate research conclusions from neuroeconomics and social neuroscience about vmPFC function.
To understand the role of the vmPFC during emotion experience and regulation, we scanned two groups of participants while they used cognitive reappraisal to regulate their emotional responses to positive and negative emotional images. We used typical neuroeconomic approaches such as modeling affect using subjective valuation to determine whether the vmPFC was involved in valuation (i.e., consistent with a common-currency account) or in reappraisal (i.e., consistent with an emotional-control account). Our results indicate that the vmPFC encodes positive emotion with a value signal similar to that for reward and that emotion regulation modulates this signal.
Materials and Methods
We analyzed data from two experimental samples collected with similar experimental tasks and imaging parameters. Our experimental sample (Exp1) was newly collected for this study and we validated our results in a replication sample (Exp2) drawn from a previously published study (Winecoff et al., 2011). Experimental parameters were nearly identical between the two experiments. Where differences are present, we indicate the parameters for Exp1 in the main text and then list the parameters for Exp2 in parentheses following.
Participants.
Participants in Exp1 were 31 younger adults between the ages of 19 and 40 years (mean 25; 10 males). Participants reported no history of psychiatric or neurological problems or contraindications to fMRI scanning. Exp2 comprised 20 older adults between the ages of 59 and 73 years (mean 69; 10 males) and 22 younger adults between the ages of 19 and 33 years (mean 23; 11 males). In addition to meeting all the exclusion criteria for Exp1, all participants in Exp2 also scored at 27 or higher on the Folstein Mini-Mental State Examination (Folstein et al., 1975). The vast majority of fMRI studies have constrained their samples to healthy younger adults, so interpretation of these results cannot necessarily be extended to a more general demographic. Including both younger and older adults in the Exp2 dataset ensures that the results hold not only for a typical fMRI sample, but are also valid for inferences on a broader population in which there is more variance.
In both experiments, participants were paid $20/h for time in the scanner and $10/h for time outside the scanner. Each participant provided written consent for a protocol approved by the institutional review board of Duke University Medical Center.
Emotion regulation paradigm.
While undergoing scanning, participants completed a cognitive reappraisal task. Before beginning the fMRI task, participants were extensively trained to perform emotion regulation using a cognitive reappraisal strategy. Participants were instructed to imagine themselves as an objective observer to the situation depicted or to imagine the event as having no personal relevance to them. These instructions are consistent with prior research using a “self-focused” or “detachment” reappraisal strategy (Ochsner et al., 2004; Kalisch et al., 2005; Goldin et al., 2008; Shiota and Levenson, 2009). For the Experience condition, participants were instructed to experience their emotions naturally. During training, participants verbally reported their responses to the experimenter to signal that they understood the task. After this training, but before undergoing scanning, each participant completed practice trials with the same timing as the fMRI task.
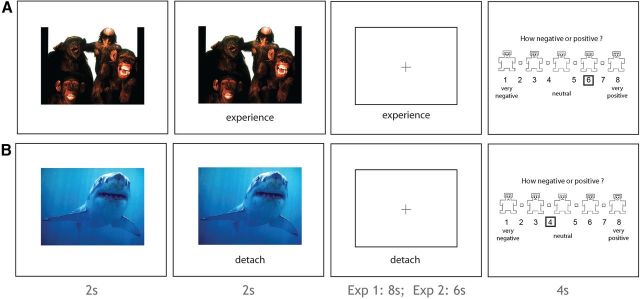
In the fMRI task (Fig. 1), participants were presented with images from the International Affective Picture System (IAPS; Lang et al., 2008) and were asked to either regulate their emotions using cognitive reappraisal (Regulate Condition) or to experience their emotions naturally (Experience Condition). Pictures were categorized as positive, negative, or neutral based on the normative IAPS ratings (valence: positive mean = 6.8; negative mean = 2.4; neutral mean = 5; Exp2: 7.3, 2.5, 5.1). Positive and negative images were approximately matched for arousal (positive mean = 5.8; negative mean = 5.8; Exp2: 5.6, 5.4), and each condition had equal numbers of images with and without people.
Figure 1.
Reappraisal task. A, During Experience trials, participants viewed the image and then saw a cue to experience their emotional reaction to the image naturally. Participants were asked to continue to experience their emotions even after the image disappeared. After each trial, participants rated the valence of the image. B, During Regulate trials, participants were asked to emotionally detach themselves from the image.
At the beginning of each trial, a picture appeared on screen for 2 s. A cue (“Experience” or “Detach”; Exp2: “Experience” or “Decrease”) would then appear below the image for 2 s to indicate which strategy to use. The picture would then disappear and be replaced by a fixation cross and the cue would remain on screen for an additional 8 s (Exp2: 6 s). At the end of each trial, participants were asked to rate how positive or negative they felt after having implemented the strategy. Intertrial intervals were a minimum of 4 s (range: 4–13 s) and were exponentially distributed (Exp2: 0–8 s in 2 s intervals). Participants viewed 125 total images evenly distributed across the 5 trial types (25 Positive-Experience, 25 Negative-Experience, 25 Positive-Reappraise, 25 Negative-Reappraise, 25 Neutral-Experience) over the course of 5 runs (Exp2: 150 total trials, 30 trials per trial type, 6 runs). Image presentation was pseudorandomized such that within each run, participants saw equal numbers of pictures in each of the five trial types, but the order of presentation was randomized within each run. Whether each image was assigned to the reappraisal or experience condition was counterbalanced across participants. Stimuli were presented using the Psychophysics Toolbox 3 in MATLAB (Brainard, 1997).
fMRI acquisition.
We acquired functional data on a General Electric 3T scanner using a gradient-echo inverse-spiral pulse sequence using standard scanning parameters (TR = 2000 ms; TE = 30 ms; FOV = 256 mm; flip angle = 60°; 30 axial slices parallel to the AC–PC plane; voxel size = 4 × 4 × 4 mm; Exp2: 4T scanner, TE = 31 ms; flip angle = 90°, 34 slices, voxel size = 3.75 × 3.75 × 3.8 mm). Each run contained 288 (Exp2: 238) volumes of data (first six volumes discarded). A high-resolution inversion-recovery prepared SPGR anatomical image was used for normalization and coregistration of the functional data (TR = 7.48 ms; TE = 2.98 ms; whole brain coverage with 1 × 1 × 1 mm voxels; Exp2: TR = 12.3; TE = 5.5 ms).
fMRI preprocessing.
fMRI data were analyzed using FSL Version 4.1.8 FEAT Version 5.98 (Smith et al., 2004). Preprocessing included motion correction using MCFLIRT, brain extraction, spatial smoothing using an isotropic Gaussian kernel of 6 mm (FWHM), and high-pass filtering (>100 s). Functional images were normalized using transforms estimated from each participant's own high-resolution anatomical image and FSL's MNI template using FLIRT (six degrees of freedom for registration to participant's main structural image, 12 degrees of freedom for registration to standard space). All reported results survived full whole-brain correction (individual voxel threshold z > 2.3; cluster-corrected significance threshold: p < 0.05). All coordinates are reported in MNI space and brain figures were created using MRIcron (Rorden et al., 2007).
fMRI and behavioral analysis.
FSL's general linear model (GLM) was used to assess the influence of our behavioral manipulation on brain activation. First-level models corrected for local autocorrelation (Woolrich et al., 2001) and assessed brain responses to all trials within an explanatory variable within a single run. At the second level, we used a fixed-effects model to combine the data across all runs within a single participant. At the third level, we used a mixed-effects analysis (FLAME 1) to model effects across all participants (Beckmann et al., 2003; Woolrich et al., 2004). For all imaging data, runs with >5 volumes with movement of >1 mm in any direction were discarded to ameliorate any contributions of head motion (6.5% of data discarded; Exp2: 14.3%). For all analyses not performed within FSL, we used SAS Version 9.3.
In our first analysis (“Effect of emotion experience and regulation”), we investigated the basic behavioral and neural effects of emotion experience and regulation. For our behavioral analysis, we implemented a repeated measures multilevel model in which we modeled valence and condition as fixed effects and each participant as a random effect. We used a restricted maximum likelihood estimation method and a variance components covariance structure. For the fMRI analysis, we modeled valence and condition. We created one regressor of interest for each trial type (Positive-Experience, Negative-Experience, Positive-Regulate, Negative-Regulate, Neutral-Experience), which modeled the 8 s (Exp2: 6 s) implementation period. Nuisance regressors were included in the model for the initial presentation of the picture and for the response period. All regressors were convolved with a standard double-gamma hemodynamic response function.
In our second model of the effects of emotion regulation and experience, we sought to characterize participants' subjective emotional responses using a parametric model including participants' own trial-by-trial ratings of the valence of stimuli. We collapsed across valence (e.g., positive and negative) and examined the effect of trial-by-trial ratings within each regulation condition (e.g., Experience vs Reappraise). The amplitude of the event-related response was modulated by participants' ratings for each image. The parametric regressor was orthogonalized to the main effect to examine activations that specifically scaled with valence ratings. We also included a quadratic regressor constructed by squaring the parametric regressor to control for the effect of any nonlinear results.
We also tested the possibility that neural responses may differ across conditions based on the normative IAPS ratings for the stimuli. In this analysis (“Neural response to normative valence by condition”), we used a repeated multilevel model using mean-centered IAPS normative ratings and regulation condition as fixed effects and participant as a random effect. We then tested whether IAPS ratings, condition, and their interaction predicted activation in the vmPFC. A region of interest (ROI) in the posterior vmPFC (pvmPFC) was defined based on coordinates (x = 6, y = 26, z = −14) drawn from a previous study of the subjective exchange rate between monetary rewards and attractive faces (Smith et al., 2010). Using the methods for single trial analysis described by Mumford et al. (2012), a first-level model was created for each trial for each participant using the 8 s implementation period (Exp2: 6 s). In each first-level model, nuisance regressors were included to model all other trials, the initial picture presentation, and the response phase. FSL's motion outlier function was used to identify bad time points. Trials corresponding to these time points were excluded from this analysis. Signal was then extracted from the pvmPFC ROI.
In our final analysis (“pvmPFC activation is associated with differences in behavioral ratings”), we examined whether activation in the pvmPFC would predict trial-by-trial valence ratings even after accounting for IAPS normative ratings, regulation condition, and the interaction of ratings and condition. We constructed a GLM including experiment number, IAPS normative ratings, condition, and the trial-by-trial pvmPFC βs used in the previous analysis as independent variables and trial-by-trial valence ratings as the dependent variable.
Results
Effects of emotion experience and regulation
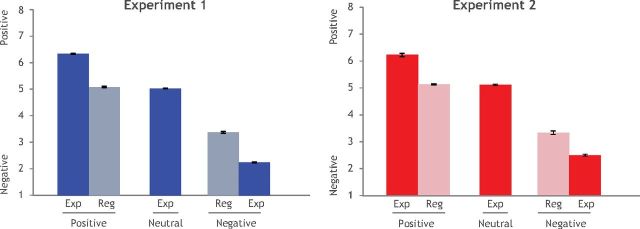
We first tested whether participants' ratings differed as a function of valence (Positive vs Negative) and condition (Regulate vs Experience). In both experiments, there was a main effect of valence (Exp1: F(2,2981) = 2217.36, p < 0.0001; Exp2: F(2,4995) = 3266.72, p < 0.0001). In Exp2, there was an effect of condition (F(1,4995) = 9.83, p = 0.0017) and, in both experiments, there was a valence by condition interaction (Exp1: F(1,116) = 27.05, p < 0.0001; Exp2: F(1,160) = 4.496, p = 0.04), such that regulation led to more positive ratings (e.g., more neutral) for negative stimuli, but less positive (e.g., more neutral) ratings for positive stimuli (Exp 1: Positive-Experience, mean = 6.328, SE = 0.057; Positive-Reappraise, mean = 5.068, SE = 0.035; Neutral-Experience, mean = 5.044, SE = 0.046; Negative-Reappraise, mean = 3.360, SE = 0.0375, Negative-Experience mean = 2.211, SE = 0.0375; Exp 2: Positive-Experience, mean = 6.232, SE = 0.042; Positive-Reappraise, mean = 5.151, 0.042; Neutral-Experience, mean = 5.145, SE = 0.046; Negative-Reappraise, mean = 3.331, SE = 0.042; Negative-Experience, mean = 2.464, SE = 0.044).
We then tested whether ratings differed between valenced stimuli (Positive vs Negative) that had been regulated and neutral images that had been experienced. In both experiments, there was a significant overall effect of valence category (Exp1: F(2,87) = 5.71, p = 0.005; Exp2: F(2,120) = 43.21, p < 0.0001). Follow-up tests indicated that regulated negative versus regulated positive stimuli were rated significantly different from each other (two-sided paired t tests: Exp1: t(30) = 54.47, p < 0.0001; Exp2: t(41) = 22.88, p < 0.0001) and that both regulated positive as well as regulated negative stimuli were rated differently from neutral stimuli (Exp1: neg: t(41) = −20.42, p < 0.0001; pos: t(41) = 5.65, p < 0.0001; Exp2: neg: t(41) = −71, p < 0.0001; pos: t(41) = 4.71, p < 0.0001). These results indicate that, for both positive and negative stimuli, regulation was successful in changing the subjective experience of valence but did not completely neutralize emotional responses (Fig. 2).
Figure 2.
Average valence ratings by task condition. In both Exp 1 and Exp 2, stimuli in the Regulate (Reg) condition were rated as less emotionally evocative than images in the Experience (Exp) condition. Error bars represent SEM within subjects.
In our imaging data, negative regulation (Negative-Regulate > Negative-Experience) activated regions of the prefrontal cortex including the inferior frontal gyrus, the middle frontal gyrus, and the superior frontal gyrus (see Table 1 for activation coordinates). In addition, regulating negative emotion led to increased activation in the posterior cingulate and the angular gyrus. In contrast, negative experience (Negative-Experience > Negative-Regulate) revealed no significant overlapping activations between the two experiments.
Table 1.
Cluster peaks for emotional experience or regulation
| Experiment 1 |
Experiment 2 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | Max Z | Voxels | x | y | z | Max Z | Voxels | ||
| Positive Experience > Positive Regulate | |||||||||||
| Subcallosal gyrus | 4 | 22 | −2 | 5.24 | 16004 | Frontal pole | −8 | 56 | 2 | 5.35 | 9507 |
| Frontal pole | −6 | 62 | 6 | 5.11 | |||||||
| Paracingulate | −8 | 52 | 10 | 5.21 | |||||||
| Paracingulate | −4 | 42 | −6 | 4.81 | |||||||
| Occipital pole | 24 | −98 | 8 | 4.81 | 5876 | ||||||
| Lingual gyrus | −4 | −88 | −10 | 3.97 | |||||||
| Postcentral gyrus | −46 | −22 | 28 | 5.09 | 5482 | ||||||
| SMA | −8 | −8 | 46 | 4.83 | |||||||
| Postcentral gyrus | −44 | −26 | 34 | 4.58 | |||||||
| Cingulate | −4 | −2 | 36 | 4.33 | |||||||
| Parietal operculum | −48 | −28 | 24 | 4.15 | |||||||
| Increasingly Positive Valence | |||||||||||
| Medial frontal gyrus | −6 | 34 | −16 | 4.69 | 1554 | Paracingulate | 2 | 42 | −8 | 4.79 | 2351 |
| Subcallosal gyrus | −2 | 20 | −16 | 4.47 | Paracingulate | 4 | 46 | −6 | 4.71 | ||
| Subcallosal gyrus | −6 | 28 | −14 | 4.39 | Frontal pole | −6 | 70 | 6 | 4.2 | ||
| Precuneus | −2 | −62 | 20 | 4.16 | 1389 | Precuneus | 0 | −66 | 22 | 3.58 | |
| Precuneus | −8 | −54 | 6 | 4.01 | Precuneus | 22 | −54 | 6 | 4.1 | 861 | |
| Precuneus | −16 | −50 | 4 | 3.78 | Precuneus | 10 | −62 | 18 | 3.23 | ||
| Posterior cingulate | 14 | −48 | 4 | 3.59 | |||||||
| Lingual gyrus | 18 | −50 | 0 | 3.55 | |||||||
| Supracalcarine cortex | −18 | −64 | 12 | 3.4 | |||||||
| Positive Regulate > Positive Experience | |||||||||||
| Frontal pole | 34 | 58 | −8 | 4.25 | 1616 | Precuneus | 0 | −74 | 42 | 5.02 | 985 |
| Frontal pole | −44 | 50 | −10 | 4.51 | 1976 | Angular gyrus | 60 | −52 | 34 | 5.66 | 3102 |
| Inferior frontal gyrus | −52 | 28 | −4 | 3.59 | L47 | Angular gyrus | 64 | −48 | 24 | 4.14 | |
| Angular gyrus | 52 | −48 | 38 | 4.8 | 2550 | Angular gyrus | 48 | −52 | 32 | 5.85 | |
| Angular gyrus | 48 | −48 | 34 | 4.57 | Lateral occipital cortex | 50 | −62 | 42 | 4.78 | ||
| Angular gyrus | 62 | −54 | 32 | 4.45 | Inferior frontal gyrus | −38 | 24 | 20 | 5.41 | 4410 | |
| Lateral occipital cortex | 48 | −60 | 52 | 4.39 | Lateral occipital cortex | −38 | −62 | 38 | 5.9 | ||
| Angular gyrus | −50 | −60 | 30 | 4.27 | Middle temporal gyrus | −62 | −44 | −4 | 4.42 | ||
| Angular gyrus | −48 | −52 | 44 | 4.93 | Middle frontal gyrus | 42 | 22 | 36 | 5.71 | 16530 | |
| Lateral occipital cortex | −42 | −64 | 46 | 5.32 | 3927 | Middle frontal gyrus | −46 | 14 | 44 | 5.59 | |
| Frontal pole | 6 | 42 | 54 | 4.09 | Middle frontal gyrus | −44 | 16 | 30 | 5.32 | ||
| Lingual gyrus | 46 | 24 | 36 | 5.49 | 6285 | Superior frontal gyrus | 0 | 24 | 50 | 5.7 | |
| Middle frontal gyrus | −42 | 18 | 34 | 4.5 | |||||||
| Middle frontal gyrus | −38 | 20 | 40 | 4.19 | |||||||
| Middle frontal gyrus | −36 | 12 | 52 | 4.04 | |||||||
| Negative Experience > Negative Regulate | |||||||||||
| None | Occipital pole | 28 | −100 | 8 | 4.15 | 3189 | |||||
| Parietal operculum | 48 | −22 | 26 | 4.3 | 1795 | ||||||
| Hippocampus | −20 | −2 | −12 | 4.34 | 1754 | ||||||
| Postcentral gyrus | −64 | −20 | 20 | 4.32 | 1370 | ||||||
| Negative Regulate > Negative Experience | |||||||||||
| Superior frontal gyrus | −2 | 26 | 50 | 5.29 | 18161 | Inferior frontal gyrus | −54 | 20 | 2 | 5.45 | 7331 |
| Lateral occipital cortex | −48 | −68 | 48 | 4.89 | 3571 | Lateral occipital cortex | −40 | −66 | 42 | 4.45 | 2200 |
| Posterior cingulate | −2 | −26 | 24 | 5.29 | 3343 | Insular cortex | 40 | 20 | −4 | 3.91 | 1288 |
| Angular gyrus | 44 | −56 | 30 | 5.72 | 1830 | Middle frontal cortex | 36 | 22 | 38 | 4.07 | 1141 |
| Middle temporal gyrus | −62 | −38 | −10 | 4.88 | 1167 | ||||||
x, y, z indicate coordinates of peak voxel shown in MNI space. Max Z = z-statistic of peak voxel. Voxels indicates cluster size. For each new cluster, the number of voxels per cluster is indicated next to the region label.
Positive regulation (Positive-Regulate > Positive-Experience) was associated with increased activation in regions of the prefrontal and parietal cortex including the middle frontal gyrus, the inferior frontal gyrus, and the angular gyrus. Conversely, positive emotion experience (Positive Experience > Positive-Regulate) was associated with activation in the bilateral amygdala and the vmPFC in both experiments. Therefore, emotion regulation reduces vmPFC activation compared with that observed during emotion experience (Fig. 3A; vmPFC peak activations within overlap, Exp1: max Z = 4.88, x = 4, y = 22, z = −4; Exp2: max Z = 4.61, x = −4, y = 44, z = −6).
Figure 3.
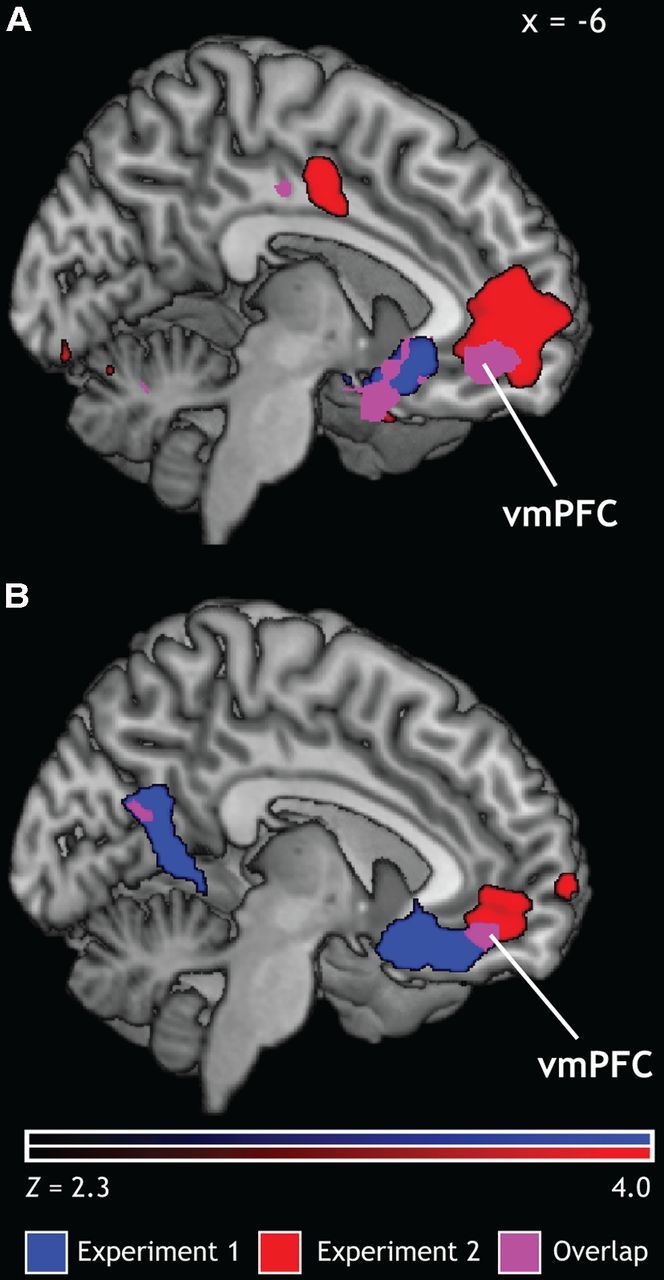
Neural mechanisms of positive emotion. A, Contrast of Positive-Experience > Positive-Regulate for both Exp 1 and Exp 2 revealed an overlapping activation in the vmPFC. B, A parametric model using participants' trial-by-trial valence ratings showed that increasingly positive valence ratings were associated with increased activation in the vmPFC in the Experience condition.
To assess the relationship between vmPFC activation and subjective experiences of positive emotion, we also interrogated the effects of participants' own trial-by-trial ratings of stimuli. This model tested the possibility that, like reward, emotional value is encoded in the vmPFC as a continuous, graded signal. For stimuli in the Experience condition, activation in the vmPFC increased with increasing ratings of positivity (Fig. 3B). In the same model for regulated stimuli, however, there were no significant activations. One potential explanation for this result is that, after regulation, stimuli in both the positive and negative regulation condition clustered around neutral valence ratings. Therefore, if the vmPFC does track final value, we would not expect its activation to vary in conditions in which the subjective experience of emotion is essentially constant. Alternatively, it is possible that the encoding of emotional value during emotion regulation shifts to another area of the brain. Given the reduced variability in ratings in the regulation condition, it is not possible to distinguish between these two explanations.
Neural response to normative valence by condition
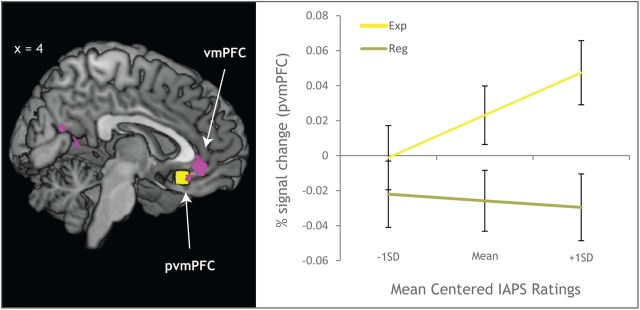
We next investigated whether normative IAPS ratings and condition would predict trial-by-trial estimates of activation in the pvmPFC. Given that the IAPS picture set has been rated by thousands of participants (Lang et al., 2008) and that normative ratings for each stimulus were highly predictive of our participants' ratings of each stimulus (Experience Condition: r = 0.785; Regulate Condition: r = 0.707), we took normative valence ratings to represent a typical response to these stimuli. We then investigated whether those normative ratings had differential effects upon activation in pvmPFC contingent upon the regulation condition. There was a marginally significant main effect of IAPS normative rating (F(1,7982) = 3.64, p = 0.057) and a main effect of condition (F(1,7982) = 19.89, p < 0.0001). These main effects were qualified by an IAPS normative rating by condition interaction (F(1,7982) = 6.8, p = 0.0091). Follow-up tests indicated that the effect of IAPS rating was significant in the experience condition (t(72) = 3.2, p = 0.0014, b = 0.011), but not in the regulation condition (t(72) = −0.49, p = 0.622, b = −0.0017; Fig. 4). These data indicate that pvmPFC activation tracks a typical emotional value during the basic experience of emotion, but not during emotion regulation.
Figure 4.
pvmPFC activation by normative IAPS rating. Left: Overlap between the vmPFC ROI from the analysis in Fig. 3B and the pvmPFC ROI used to extract parameter estimates. Right: Interaction plot for IAPS normative rating and condition on pvmPFC. Error bars reflect SEs of the model-predicted pvmPFC estimates. Within the Experience condition, IAPS normative rating predicted pvmPFC activation; however, there was no association with IAPS normative rating or pvmPFC activation during emotion regulation.
pvmPFC activation is associated with differences in behavioral ratings
As a strong test for the role of pvmPFC in subjective emotional value, we next investigated whether pvmPFC activation predicted participants' trial-by-trial subjective ratings of each image. A GLM including experiment number, IAPS normative ratings, condition, the interaction of IAPS normative ratings and condition, and trial-by-trial estimates of pvmPFC activation significantly predicted behavioral ratings (full model: F(5,8052) = 2370.75, p < 0.0001). The model revealed a main effect of normative IAPS rating (F(1,8052) = 13,449.78, p < 0.0001, b = 0.37) and a main effect of condition (F(1,8052) = 190.44, p < 0.0001, b = 0.026); however, the main effects of normative IAPS ratings and condition were qualified by a significant interaction (F(1,8052) = 1841.71, p < 0.0001, b = 0.433). In addition, there was a main effect of pvmPFC activation (F(1,8052) = 4.0, p = 0.046, b = 0.051). These data indicate that, even after accounting for the strong behavioral effects of our manipulation, pvmPFC activation still predicts the subjective experience of emotional value (Table 2).
Table 2.
Effect of reappraisal, IAPS rating, and pvmPFC activation on behavior
| Variable | Parameter estimate (SE) |
|---|---|
| Intercept | 4.18 (0.047)* |
| Experiment | 0.04 (0.026) |
| IAPS rating | 0.37 (0.008)* |
| Condition | 0.31 (0.026)* |
| IAPS*Condition | 0.43 (0.012)* |
| pvmPFC | 0.05 (0.026)* |
| Overall model R2 | 0.6* |
GLM revealed that IAPS normative rating, condition, and an IAPS normative-rating-by-condition interaction predicted valence ratings. In addition, there was also a significant relationship between pvmPFC activation and behavior.
*Significant.
Discussion
The vast majority of studies on the neural mechanisms of emotion regulation have focused on emotional reactions to negative stimuli. Although positive affect is beneficial for mental and physical health (Tugade et al., 2004; Richman et al., 2005), not all of the consequences of positive affect are adaptive. When purchasing a new home, for example, the positive emotion induced by visualizing a blooming garden in springtime could interfere with the negotiation of a better price. Unregulated positive affect increases distractibility in tasks that require cognitive control (Dreisbach and Goschke, 2004), inflates evaluations of the probability of winning in monetary gambling tasks (Nygren et al., 1996), and increases the likelihood of impulsive purchasing decisions (Weinberg and Gottwald, 1982). In each case, a failure to regulate positive affect interferes with the attainment of goals.
Using stimuli falling along a continuous scale of emotional valence from positive to negative and replication between two experiments, we show that a region within vmPFC tracks the subjective value of emotional stimuli. We also examined which sorts of affective processes are encoded by the vmPFC: normative stimulus valence (Lebreton et al., 2009), the engagement of goal-directed regulatory processes (Hare et al., 2009), and/or trial-to-trial variation in subjective value across stimuli (Kable and Glimcher, 2007; Smith et al., 2010; Levy and Glimcher, 2011). Using an independently defined ROI, we show that trial-to-trial variation in pvmPFC predicts the emotional value of affective images during the experience of emotion. These analyses converge on one common conclusion: in the context of emotion regulation, the vmPFC encodes stimulus-specific subjective emotional value.
vmPFC: common economic and emotional value
Studies of reward processing have implicated the vmPFC in the valuation of rewards from various modalities: juice (Kim et al., 2006), faces (Smith et al., 2010; Lin et al., 2012), and non-monetary goods such as snack foods and CDs (Chib et al., 2009). This has led to the hypothesis that the vmPFC encodes a standardized value signal (Montague and Berns, 2002; Rangel and Hare, 2010). This neuroeconomic concept has not been used previously to understand the neural effects of typical emotional manipulations (i.e., affective images or videos), yet it can account for findings from such studies. The vmPFC is engaged by a range of positive stimuli not typically characterized as rewards, including faces displaying positive emotion (Lin et al., 2012), pleasant imagined stimuli (Cunningham et al., 2011), and happy memories (Lane et al., 1997). These studies support the idea that vmPFC computes a domain-general appetitive value signal (Roy et al., 2012) manifest in our own data as emotional reward value. In other words, our results indicate that two sorts of value, responses to rewards and to the emotional content of images, are tracked similarly in vmPFC along continuous scales.
vmPFC: cognitive control or affective integration?
Several lines of research have implicated the vmPFC as being central to executing cognitive control. Studies of fear conditioning link vmPFC to the extinction of fear (Hartley and Phelps, 2010). Similarly, the reappraisal of negative emotion leads to activation in the vmPFC (Urry et al., 2006; Johnstone et al., 2007; Delgado et al., 2008b). One investigation of reappraisal of positive emotion demonstrated that increasing positive emotion also recruits the vmPFC (Kim and Hamann, 2007). These findings suggest that the vmPFC, like regions of the dlPFC also activated during emotion regulation (Ochsner and Gross, 2005), serves as a locus of affective control.
Another interpretation is equally plausible, however: changes in vmPFC activation during regulation could reflect changes in the final integrated affective value of emotional stimuli. Our own analyses are consistent with this conclusion. Similarly, our results complement a prior study demonstrating a striatal mediation of the relationship between activation in the prefrontal cortex and decreased negative affect during emotion regulation (Wager et al., 2008).
We investigated whether vmPFC activation would be predicted by normative IAPS ratings (i.e., what typical subjects experience before the implementation of any regulatory processes). Activation in vmPFC was differentially predicted by IAPS ratings in the experience condition, which suggests that the vmPFC does not encode what could be deemed a natural emotional response, but rather a signal that represents the current subjective experience of emotion. We note that our participants were largely successful at emotion regulation, and thus there was little dynamic range in subjects' ratings within the regulation condition. This lack of variance did not allow for a detailed analysis of subjective emotional value encoding during emotion regulation. There was, however, a clear reduction in vmPFC activation during the regulation of positive emotion compared with the experience of positive emotion, consistent with the conclusion that value signals in vmPFC are present both during experience and regulation conditions.
Our results predict that regions other than vmPFC will track the process of regulation rather than its success. In one recent investigation (Hutcherson et al., 2012), food-deprived participants were prompted by a cue to downregulate, experience, or upregulate their affective responses to images of food items. Participants then indicated how much money they would be willing to pay for an opportunity to consume that item. During regulation compared with experience there was a reduction in the association between vmPFC activation and monetary bids and a concurrent increase in the association between the dlPFC and monetary bids. Consistent with this result, we found greater signal in the dlPFC during the reappraisal of stimuli than during the experience of images even when they were matched in subjective emotional value, and this relationship held for both positive and negative images. Conversely, for the experience of neutral images compared with the regulation of positive images, we found activation in regions previously linked to the emotional content of experienced stimuli.
The dlPFC signal in the regulation condition might reflect computations that are ultimately integrated into the final encoding of value (e.g., context). Participants in our regulation condition attempted to emotionally detach themselves from the images (i.e., to neutralize emotional responses). Therefore, achieving the goal of regulation might contribute to an integrated neural signal for value. This interpretation is consistent with findings from a study in which dieters rated both the tastiness and healthiness of food items in advance of consumption decisions (Hare et al., 2009). Subjects who successfully implemented self-control evinced increased activation in the dlPFC (BA 9) and decreased activation in the vmPFC when deciding not to consume a tasty but unhealthy food, a result interpreted as evidence that the vmPFC integrates goal value as well as hedonic value. For both experimental samples, we observed regulation-related activation in the same dlPFC region that interacted with vmPFC in the connectivity analysis of Hare et al. (2009). Therefore, activation in the dlPFC during regulation could reflect the parametric contribution of regulation to the ultimate value of the stimulus, which is manifest in our data as decreased activation in the vmPFC. Our results extend these findings by indicating that processing of emotional stimuli has similar characteristics to processing of canonical reward stimuli.
In summary, our results indicate that the vmPFC does not act as a control region during emotion regulation, but rather encodes the affective value of emotional stimuli along a continuous scale. Our results point to an adaptive, flexible computation of value: conditional factors such as emotion regulation affect how emotional value signals are encoded in the vmPFC.
Footnotes
This work was supported by the National Institutes of Health–National Institute of Aging (Grant #R21-30771 to S.A.H.) and the National Institutes of Health–National Institute of Mental Health (Grant #P50-60451 to S.A.H.). We thank Yizheng He for assistance with data collection, Chris Coutlee, David Smith, Lawrence Ngo, and Kevin LaBar for comments on the manuscript, and Madeline Carrig for guidance on data analysis.
References
- Beblo T, Fernando S, Klocke S, Griepenstroh J, Aschenbrenner S, Driessen M. Increased suppression of negative and positive emotions in major depression. J Affect Disord. 2012;141:474–479. doi: 10.1016/j.jad.2012.03.019. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in fMRI. Neuroimage. 2003;20:1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spat Vis. 1997;10:433–436. doi: 10.1163/156856897X00357. [DOI] [PubMed] [Google Scholar]
- Chib VS, Rangel A, Shimojo S, O'Doherty JP. Evidence for a common representation of decision values for dissimilar goods in human ventromedial prefrontal cortex. J Neurosci. 2009;29:12315–12320. doi: 10.1523/JNEUROSCI.2575-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham WA, Johnsen IR, Waggoner AS. Orbitofrontal cortex provides cross-modal valuation of self-generated stimuli. Soc Cogn Affect Neurosci. 2011;6:286–293. doi: 10.1093/scan/nsq038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Gillis MM, Phelps EA. Regulating the expectation of reward via cognitive strategies. Nat Neurosci. 2008a;11:880–881. doi: 10.1038/nn.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Nearing KI, Ledoux JE, Phelps EA. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron. 2008b;59:829–838. doi: 10.1016/j.neuron.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekhof EK, Geier K, Falkai P, Gruber O. Fear is only as deep as the mind allows: A coordinate-based meta-analysis of neuroimaging studies on the regulation of negative affect. Neuroimage. 2011;58:275–285. doi: 10.1016/j.neuroimage.2011.05.073. [DOI] [PubMed] [Google Scholar]
- Dreisbach G, Goschke T. How positive affect modulates cognitive control: reduced perseveration at the cost of increased distractibility. J Exp Psychol Learn Mem Cogn. 2004;30:343–353. doi: 10.1037/0278-7393.30.2.343. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J Psychiatry Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Goel V, Dolan RJ. The functional anatomy of humor: segregating cognitive and affective components. Nat Neurosci. 2001;4:237–238. doi: 10.1038/85076. [DOI] [PubMed] [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biol Psychiatry. 2008;63:577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324:646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- Hartley CA, Phelps EA. Changing fear: the neurocircuitry of emotion regulation. Neuropsychopharmacology. 2010;35:136–146. doi: 10.1038/npp.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller AS, Johnstone T, Shackman AJ, Light SN, Peterson MJ, Kolden GG, Kalin NH, Davidson RJ. Reduced capacity to sustain positive emotion in major depression reflects diminished maintenance of fronto-striatal brain activation. Proc Natl Acad Sci U S A. 2009;106:22445–22450. doi: 10.1073/pnas.0910651106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcherson CA, Plassmann H, Gross JJ, Rangel A. Cognitive regulation during decision making shifts behavioral control between ventromedial and dorsolateral prefrontal value systems. J Neurosci. 2012;32:13543–13554. doi: 10.1523/JNEUROSCI.6387-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci. 2007;27:8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nat Neurosci. 2007;10:1625–1633. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisch R, Wiech K, Critchley HD, Seymour B, O'Doherty JP, Oakley DA, Allen P, Dolan RJ. Anxiety reduction through detachment: subjective, physiological, and neural effects. J Cogn Neurosci. 2005;17:874–883. doi: 10.1162/0898929054021184. [DOI] [PubMed] [Google Scholar]
- Kanske P, Heissler J, Schönfelder S, Bongers A, Wessa M. How to regulate emotion? Neural networks for reappraisal and distraction. Cereb Cortex. 2011;21:1379–1388. doi: 10.1093/cercor/bhq216. [DOI] [PubMed] [Google Scholar]
- Kim H, Shimojo S, O'Doherty JP. Is avoiding an aversive outcome rewarding? Neural substrates of avoidance learning in the human brain. PLoS Biol. 2006;4:e233. doi: 10.1371/journal.pbio.0040233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Hamann S. Neural correlates of positive and negative emotion regulation. J Cogn Neurosci. 2007;19:776–798. doi: 10.1162/jocn.2007.19.5.776. [DOI] [PubMed] [Google Scholar]
- Lane RD, Reiman EM, Ahern GL, Schwartz GE, Davidson RJ. Neuroanatomical correlates of happiness, sadness, and disgust. Am J Psychiatr. 1997;154:926–933. doi: 10.1176/ajp.154.7.926. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Gainesville, FL: University of Florida; 2008. International affective picture system (IAPS): affective ratings of pictures and instruction manual. Technical report A-8. [Google Scholar]
- Lebreton M, Jorge S, Michel V, Thirion B, Pessiglione M. An automatic valuation system in the human brain: evidence from functional neuroimaging. Neuron. 2009;64:431–439. doi: 10.1016/j.neuron.2009.09.040. [DOI] [PubMed] [Google Scholar]
- Levy DJ, Glimcher PW. Comparing apples and oranges: using reward-specific and reward-general subjective value representation in the brain. J Neurosci. 2011;31:14693–14707. doi: 10.1523/JNEUROSCI.2218-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DJ, Glimcher PW. The root of all value: a neural common currency for choice. Curr Opin Neurobiol. 2012;22:1027–1038. doi: 10.1016/j.conb.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A, Adolphs R, Rangel A. Social and monetary reward learning engage overlapping neural substrates. Soc Cogn Affect Neurosci. 2012;7:274–281. doi: 10.1093/scan/nsr006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T, Smeets T, Giesbrecht T, Merckelbach H. The efficiency of reappraisal and expressive suppression in regulating everyday affective experiences. Psychiatry Res. 2012;200:964–969. doi: 10.1016/j.psychres.2012.05.034. [DOI] [PubMed] [Google Scholar]
- Monosov IE, Hikosaka O. Regionally distinct processing of rewards and punishments by the primate ventromedial prefrontal cortex. J Neurosci. 2012;32:10318–10330. doi: 10.1523/JNEUROSCI.1801-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague PR, Berns GS. Neural economics and the biological substrates of valuation. Neuron. 2002;36:265–284. doi: 10.1016/S0896-6273(02)00974-1. [DOI] [PubMed] [Google Scholar]
- Mumford JA, Turner BO, Ashby FG, Poldrack RA. Deconvolving BOLD activation in event-related designs for multivoxel pattern classification analyses. Neuroimage. 2012;59:2636–2643. doi: 10.1016/j.neuroimage.2011.08.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nezlek JB, Kuppens P. Regulating positive and negative emotions in daily life. J Personality. 2008;76:561–580. doi: 10.1111/j.1467-6494.2008.00496.x. [DOI] [PubMed] [Google Scholar]
- Nygren TE, Isen AM, Taylor PJ, Dulin J. The influence of positive affect on the decision rule in risk situations: focus on outcome (and especially avoidance of loss) rather than probability. Organizational Behavior and Human Decision Processes. 1996;66:59–72. doi: 10.1006/obhd.1996.0038. [DOI] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, Gross JJ. For better or for worse: neural systems supporting the cognitive down-and up-regulation of negative emotion. Neuroimage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Padoa-Schioppa C, Assad JA. The representation of economic value in the orbitofrontal cortex is invariant for changes of menu. Nat Neurosci. 2008;11:95–102. doi: 10.1038/nn2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quoidbach J, Berry EV, Hansenne M, Mikolajczak M. Positive emotion regulation and well-being: Comparing the impact of eight savoring and dampening strategies. Personality and Individual Differences. 2010;49:368–373. doi: 10.1016/j.paid.2010.03.048. [DOI] [Google Scholar]
- Rangel A, Hare T. Neural computations associated with goal-directed choice. Curr Opin Neurobiol. 2010;20:262–270. doi: 10.1016/j.conb.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Richman LS, Kubzansky L, Maselko J, Kawachi I, Choo P, Bauer M. Positive emotion and health: going beyond the negative. Health Psychol. 2005;24:422–429. doi: 10.1037/0278-6133.24.4.422. [DOI] [PubMed] [Google Scholar]
- Rorden C, Karnath HO, Bonilha L. Improving lesion-symptom mapping. J Cogn Neurosci. 2007;19:1081–1088. doi: 10.1162/jocn.2007.19.7.1081. [DOI] [PubMed] [Google Scholar]
- Roy M, Shohamy D, Wager TD. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends Cogn Sci. 2012;16:147–156. doi: 10.1016/j.tics.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch MR, Stalnaker TA, Takahashi YK. A new perspective on the role of the orbitofrontal cortex in adaptive behaviour. Nat Rev Neurosci. 2009;10:885–892. doi: 10.1038/nrn2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiota MN, Levenson RW. Effects of aging on experimentally instructed detached reappraisal, positive reappraisal, and emotional behavior suppression. Psychol Aging. 2009;24:890–900. doi: 10.1037/a0017896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith DV, Hayden BY, Truong TK, Song AW, Platt ML, Huettel SA. Distinct value signals in anterior and posterior ventromedial prefrontal cortex. J Neurosci. 2010;30:2490–2495. doi: 10.1523/JNEUROSCI.3319-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DV, Huettel SA. Decision neuroscience: neuroeconomics. Wiley Interdiscip Rev Cogn Sci. 2010;1:854–871. doi: 10.1002/wcs.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudinger MR, Erk S, Abler B, Walter H. Cognitive reappraisal modulates expected value and prediction error encoding in the ventral striatum. Neuroimage. 2009;47:713–721. doi: 10.1016/j.neuroimage.2009.04.095. [DOI] [PubMed] [Google Scholar]
- Staudinger MR, Erk S, Walter H. Dorsolateral prefrontal cortex modulates striatal reward encoding during reappraisal of reward anticipation. Cereb Cortex. 2011;21:2578–2588. doi: 10.1093/cercor/bhr041. [DOI] [PubMed] [Google Scholar]
- Tugade MM, Fredrickson BL. Regulation of positive emotions: emotion regulation strategies that promote resilience. Journal of Happiness Studies. 2006;8:311–333. [Google Scholar]
- Tugade MM, Fredrickson BL, Barrett LF. Psychological resilience and positive emotional granularity: examining the benefits of positive emotions on coping and health. J Personality. 2004;72:1161–1190. doi: 10.1111/j.1467-6494.2004.00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Jackson CA, Frye CJ, Greischar LL, Alexander AL, Davidson RJ. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J Neurosci. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrtička P, Sander D, Vuilleumier P. Effects of emotion regulation strategy on brain responses to the valence and social content of visual scenes. Neuropsychologia. 2011;49:1067–1082. doi: 10.1016/j.neuropsychologia.2011.02.020. [DOI] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis JD. Cross-species studies of orbitofrontal cortex and value-based decision-making. Nat Neurosci. 2011;15:13–19. doi: 10.1038/nn.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg P, Gottwald W. Impulsive consumer buying as a result of emotions. J Bus Res. 1982;10:43–57. [Google Scholar]
- Winecoff A, Labar KS, Madden DJ, Cabeza R, Huettel SA. Cognitive and neural contributors to emotion regulation in aging. Soc Cogn Affect Neurosci. 2011;6:165–176. doi: 10.1093/scan/nsq030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of fMRI data. Neuroimage. 2001;14:1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TE, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modelling for fMRI group analysis using Bayesian inference. Neuroimage. 2004;21:1732–1747. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]