Abstract
Objective
To translate, culturally adapt and validate the "Knee Society Score"(KSS) for the Portuguese language and determine its measurement properties, reproducibility and validity.
Methods
We analyzed 70 patients of both sexes, aged between 55 and 85 years, in a cross-sectional clinical trial, with diagnosis of primary osteoarthritis ,undergoing total knee arthroplasty surgery. We assessed the patients with the English version of the KSS questionnaire and after 30 minutes with the Portuguese version of the KSS questionnaire, done by a different evaluator. All the patients were assessed preoperatively, and again at three, and six months postoperatively.
Results
There was no statistical difference, using Cronbach's alpha index and the Bland-Altman graphical analysis, for the knees core during the preoperative period (p =1), and at three months (p =0.991) and six months postoperatively (p =0.985). There was no statistical difference for knee function score for all three periods (p =1.0).
Conclusion
The Brazilian version of the Knee Society Score is easy to apply, as well providing as a valid and reliable instrument for measuring the knee score and function of Brazilian patients undergoing TKA. Level of Evidence: Level I - Diagnostic Studies- Investigating a Diagnostic Test- Testing of previously developed diagnostic criteria on consecutive patients (with universally applied 'gold' reference standard).
Keywords: Knee; Arthroplasty, Replacement, Knee; Scale; Questionnaires
INTRODUCTION
There is a consensus in the literature that a scale for evaluation of treatment results is necessary for individuals with knee lesions in order to have a standardized method that can be reproduced consistently and that reports the results of established treatments.1
The use of questionnaires as evaluation parameters is recommended as they allow the standardization, uniformity and reproducibility of the proposed measures.2 However, the choice of an evaluation segment should take into account whether its components are clear, whether it is simple, easy to understand and apply, and whether its administration time is appropriate.3 When a questionnaire is prepared, its measurement properties need to be tested and validated first in a group of patients, to allow them to be used afterwards in population groups.4
With the development of translation and cultural adaptation methods it is quite possible that an instrument developed for use in a particular language and culture can also be used, after translation, validation and adaptation, in another language and in another cultural context.5
The advent of total arthroplasty has revolutionized the treatment of osteoarthritis and of rheumatoid arthritis, changing the patient's functional state, quality of life and incapacities such as pain, stiffness and deformities caused by these joint disorders.6,7 The joint replacement procedure has become very frequent due to the increase in the incidence of OA, which is responsible for the labor incapacity of about 15% of the world's adult population.6 Insall et al.8 developed the Knee Society Score, which combines subjective and objective information and separates the knee score (pain, stability, range of motion etc.) from the functional score of the patient (ability to walk, go up and down stairs).
The Knee Society Score - KSS8,9 evaluates the clinical profile with regards to pain intensity, range of motion and stability in the anteroposterior and mediolateral planes, flexion deformities, contractures and poor alignment.
The translation and validation of the KSS scale for the Portuguese language, for use in Brazil, considering sociocultural adaptations, are of crucial importance to allow us to take advantage of all the questions of the instrument, aiming at the analysis of the total knee arthroplasty procedure, and to use the scale as an aid in postsurgical follow-up and treatment.
OBJECTIVE
The aim of this study was to translate, culturally adapt and validate the Knee Society Score (KSS) for the Portuguese language and to determine its measurement properties, reproducibility and validity, in order to allow its use as a specific instrument for evaluation of the postoperative period of total knee arthroplasty.
CASUISTRY AND METHOD
We analyzed 70 patients of both sexes (49 women and 21 men), aged between 55 and 85 years (mean age of 66.43 years), in a cross-sectional clinical trial, with diagnosis of primary osteoarthritis, undergoing total knee arthroplasty surgery with condylar prosthesis, fixed platform and without posterior stabilization in treatment in the Knee Group of the Institute of Orthopedics and Traumatology of HC/FMUSP.
After the patients were selected according to the inclusion criteria, all the individuals received and signed an Informed Consent Form.
The individuals were evaluated with the KSS questionnaire by evaluator 1 (evaluation in English) and after 30 minutes by evaluator 2 (evaluation translated into the Portuguese) in the preoperative period, and this procedure was repeated three and six months after the surgical procedure in the same manner by the same evaluators. The evaluators used the method described by Insall et al.8 to perform the evaluations.
The criteria used for the patients' inclusion were: a) age between 55 and 85 years; b) both sexes; c) diagnosis of primary osteoarthritis as indication of total knee arthroplasty; d) patients not suffering from other types of associated diseases affecting the lower limbs (e.g.: ankylosing spondylitis, rheumatoid arthritis, degenerative diseases, neurological diseases, diabetes mellitus, fractures of the ankle and foot, Parkinson's disease, cerebral palsy); e) patients who had not undergone hip arthroplasty in the studied or contralateral limb; f) patients without any neurological disorder that promotes cognitive alterations; g) patients without any type of metal implant and/or pacemaker; h) patients without previous muscle or nerve lesions and/or fractures in the lower limbs; i) patients who had signed the informed consent form.
The exclusion criteria were as follows: a) postoperative complications (e.g.: infection or deep venous thrombosis); b) individuals who refused to answer the questionnaire.
Scale translation procedure
Authorization for the translation and cross-cultural adaptation was obtained from Lawrence Dorr, author of the original scale, via e-mail. (Attachment 1)
The KSS questionnaire was translated (Attachment 2) according to the translation, cultural adaptation and validation protocol proposed by Guillemin and collaborators10:
1. Translation: the items of the KSS version were initially translated from English into Portuguese by two independent sworn translators, Brazilian and aware of the objectives of the translation; conceptual translation was emphasized in addition to the literary translation. The two translations were compared by the translators, by the researchers and by the research advisor, arriving at a consensus of a Portuguese version.
2. Evaluation of the initial translation: the Portuguese version, called "Escore da Sociedade do Joelho", was back translated into the original language by an English native speaker teacher who had not participated previously, and the result was compared with the original instrument by the survey participants.
3. Revision: the translations were compared by a multidisciplinary team to resolve discrepancies; this process resulted in the final version in Portuguese.
4. Pre-test: the final version was applied to 10 patients, together with an interview with the evaluators questioning queries regarding application of the scale.
5. Sample calculation: this was carried out with the criteria of 95% of confidence, 80% of power in the tests and 40% of standard deviation to obtain the sample size. We used the one recommended by Kelinger, 1986, and applied the Kaiser-Meyer-Olkin Measure of Sampling Adequacy (KMO) test.11,12
6. Validation: the questionnaire was applied by two independent evaluators with an interval of approximately 30 minutes, and the data were analyzed to evaluate the inter- and intra-examiner reproducibility. The evaluations were performed in the preoperative period and at three and six months after surgery.
STATISTICAL ANALYSIS
The observed values of the variables considered in the study were summarized by means of the calculation of the descriptive statistics, namely, mean, standard deviation (SD), minimum, median and maximum.
Reliability was evaluated by internal consistency, estimated by Cronbach's alpha coefficient for each evaluation period.
The reproducibility and concordance among the scores assigned by the two evaluators were analyzed by the intraclass correlation coefficient and by Bland-Altman's plot of differences.13,14
The significance level of 0.05 was set in all the hypothesis tests conducted.
RESULTS
Factor analysis of sample size: Kaiser-Meyer-Olkin Measure of Sampling Adequacy (KMO) test resulted in 0.962.11,12
Table 1 presents Cronbach's alpha coefficient values in the knee scoring and knee function score according to the evaluation period.
Table 1.
Cronbach's alpha coefficient values in the knee score and knee function score according to the period: preoperative, 3 months and 6 months.
| Period | Cronbach's alpha coefficient | |
| Knee score | Pre | 1 |
| 3 months | 0.96 | |
| 6 months | 0.92 | |
| Knee function score | Pre | 1 |
| 3 months | 1 | |
| 6 months | 1 | |
Table 2 presents the mean, standard deviation, minimum, maximum and median values of the knee score during the preoperative period, and at three and 6 months, according to the evaluation in the different language. No difference was detected between the means of the two evaluations (between evaluators in different languages) in the preoperative period (p=1.000), three months (p=0.991) and six months (p=0.985) postoperatively.
Table 2.
Means, standard deviations (SD), minimums, medians and maximums of the knee score according to the period: preoperative, 3 months and 6 months according to the evaluation in the different language.
| Knee score | Period | Language | N | Mean | SD | Minimum | Median | Maximum |
| Pre | English | 70 | 65.53 | 18.84 | 23 | 64.5 | 97 | |
| Portuguese | 70 | 65.53 | 18.63 | 23 | 64.5 | 99 | ||
| 3 months | English | 70 | 92.23 | 5.73 | 63 | 94 | 99 | |
| Portuguese | 70 | 92.21 | 5.72 | 63 | 94 | 99 | ||
| 6 months | English | 70 | 93.06 | 4.35 | 76 | 94 | 99 | |
| Portuguese | 70 | 92.94 | 4.34 | 76 | 94 | 98 | ||
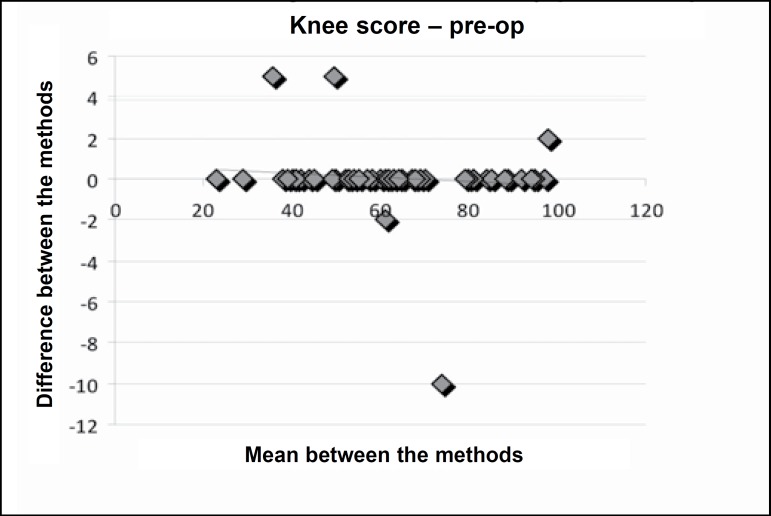
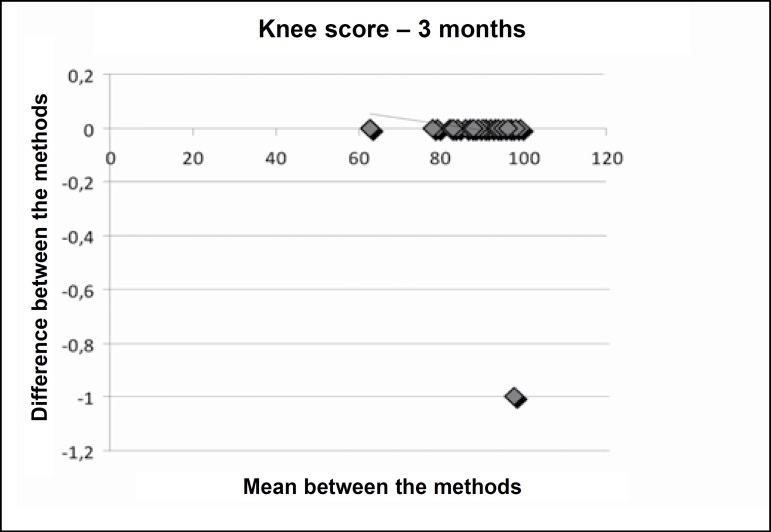
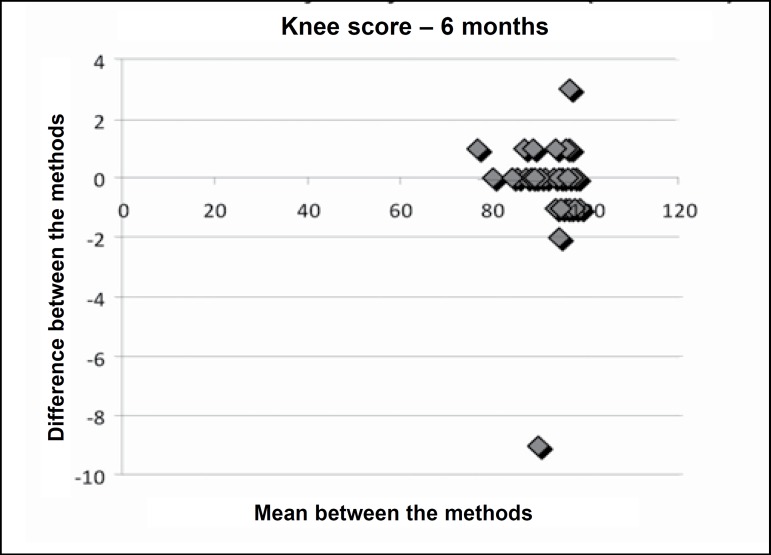
Figures 1, 2 and 3 present Bland-Altman's plot of differences between the mean of the methods (evaluations in the English and Portuguese languages) and the differences between the means in the knee score preoperatively, and at three and six months postoperatively.
Figure 1.
Bland-Altman’s plot of differences among means between the methods (evaluation in English and Portuguese and the differences between the means (Bland-Altman plot differences) in the knee score in the preoperative period.
Figure 2.
Bland-Altman’s plot of difference among means between the methods (evaluation in English and Portuguese and the differences between the means (Bland-Altman plot differences) in the knee score in the 3rd postoperative month.
Figure 3.
Bland-Altman’s plot of differences between the means of the methods (evaluation in the English and Portuguese languages and differences between the means in the knee score in the 6th postoperative month).
Table 3 presents the mean, standard deviation, minimum, maximum and median values of the knee function score in the preoperative period, and three months and six months postoperatively, according to the evaluation in the different language.
Table 3.
Means, standard deviations (SD), minimums, medians and maximums of the knee function score, according to the period: preoperative, 3 months and 6 months according to the evaluation in the.
| Knee function score | Period | Language | N | Mean | SD | Minimum | Median | Maximum |
| Pre | English | 70 | 21.43 | 11.65 | 0 | 20 | 40 | |
| Portuguese | 70 | 21.43 | 11.65 | 0 | 20 | 40 | ||
| 3 months | English | 70 | 59.93 | 6.17 | 35 | 60 | 70 | |
| Portuguese | 70 | 59.93 | 6.17 | 35 | 60 | 70 | ||
| 6 months | English | 70 | 87 | 9.98 | 60 | 90 | 100 | |
| Portuguese | 70 | 87 | 9.98 | 60 | 90 | 100 | ||
No difference was detected between the means of the two evaluations (between evaluators in different languages) in the preoperative period, and at 3 and 6 months postoperatively (p=1.000).
As the values are exactly the same in the knee function score, Bland-Altman's plot of differences proved unnecessary.
In the patient category item of the evaluation called "Knee Score", 17 patients were included in category A (unilateral or bilateral - arthroplasty on the opposite knee), 53 in category B (unilateral - other symptomatic knee) and none in category C (multiple arthritis or medical condition).
DISCUSSION
The Hospital for Special Surgery Knee Rating is an evaluation system introduced when the surgical procedure of total knee arthroplasty was in a very early stage and expectations regarding results were low. The disadvantage of this system is the fact that in incorporating a functional component, the score tends to decrease as the patient ages or presents non-orthopedic diseases, although the knee remains functionally unaltered. For this reason, Insall et al.8 developed the Knee Society Score to resolve the problem of the previous method: it combines subjective and objective information and separates the knee score (pain, stability, range of motion etc.) from the functional score of the patient (ability to walk, go up and down stairs).
The Knee Society Score is divided into three sessions: it consists of the Knee Score (100 points), the Knee Function (100 points) and the patient classification system. The classification system separates the patients into three categories depending on their medical conditions - A: unilateral or bilateral (contralateral knee operated successfully); B: unilateral -contralateral knee symptomatic; C: multiple arthritis. The two scores are initially marked at zero and points are assigned or deducted according to specific criteria.9
The Knee Society Score - KSS8,9 evaluates the clinical picture in terms of pain intensity, range of motion and stability in the anteroposterior and mediolateral planes, flexion deformities, contractures and poor alignment, and is widely used in our clinic and mentioned in orthopedic literature.6,7,15
A prerequisite for the therapeutic success of the cooperation between different specialists to occur is the use of a common language in the evaluation of the severity of the functional impairment of patients. In this context, the use of scales that measure the degree of functional impairment in the postoperative period of a surgical procedure gains importance.
The use of scales as an evaluation instrument has been intensified in scientific research in recent years. This is due to the fact that health researchers are showing more and more interest in accurate clinical evaluation methods.
There is a consensus in the literature that a scale for evaluation of treatment results is necessary for individuals with knee lesions in order to have a standardized method that can be reproduced consistently and that reports the results of established treatments.1
The use of questionnaires as evaluation parameters is recommended as they allow the standardization, uniformity and reproducibility of the proposed measures.2 However, the choice of an evaluation segment should take into account whether its components are clear, whether it is simple, easy to understand and to apply, and whether it has an appropriate administration time.3 When a questionnaire is prepared, its measurement properties need to be tested and validated first in a group of patients, to allow them to be used afterwards in population groups.4
These instruments, generally prepared in the English language, evaluate the impact of these dysfunctions on the quality of life of patients.16
With the development of the translation and cultural adaptation methods it is quite possible that an instrument developed for use in a particular language and culture can also be used, after translation, validation and adaptation, in another language and in another cultural context.5
For translations to attain a high level of quality, they should follow guidelines, since when translations are completed without the existence of criteria and necessary adaptations, it is not possible to achieve reproducibility and reliability.17 In order to adapt their use to other languages and cultures, it is necessary to submit them to international rules on translation and cultural adaptation for the target language.18
This study followed the guidelines recommended by Guillemin et al.10 thus minimizing the occurrences of biases and inclinational results. This methodology made the Brazilian version of the KSS fit for application in Brazilian patients, thus making it possible to measure clinical outcomes and treatments at the same time, or through a particular follow-up.
As regards semantic validity, the Brazilian adaptation of the Knee Evaluation Scale, translated and culturally adapted, demonstrated excellent semantic and conceptual equivalence, according to the results of the inter-evaluator analysis, whereas the entire process was based on the studies of Ciconelli,19 Duarte et al.17 and Guillemin et al.10
As we were able to observe in the studies that performed the validation, it is important to supplement the translation with the sociocultural adaptation of the version for the language, in this case, Portuguese, to allow the scale to be better evaluated in the country.
In the translation and validation of the original version of the KSS, only one alteration was made in the knee function score, in the item walking, in which the distance that is evaluated in Manhattan city blocks, in which a block is equal to 80 meters, was modified to distance in meters. This change is necessary as it corresponds more closely to the Brazilian situation, since blocks in Brazil are not standardized in all cities with the same measurements in meters.
The sample of 70 patients divided by the number of items of the scale (5 - deductions are not considered) results in 14 subjects per item. Kelinger20 recommends, as a general rule for the validation of instruments, the use of the largest possible sample and suggests 10 subjects per item of the instrument. In this study the participants conducted the Kaiser-Meyer-Olkin Measure of Sampling Adequacy (KMO) study, which measures the adequacy of data for the factor analysis. The KMO resulted in 0.962, which indicates that the data were optimal for the factor analysis, i.e., the sample size was adequate.11
Reliability was evaluated by internal consistency, estimated by Cronbach's alpha coefficient, for each evaluation period and in each score. We evaluated the contribution of each item to the reliability of the domains. This index can range from 0 to 1 and the higher this value, the greater the reliability of the scale.11 All the correlations between and among items in the periods were positive and significantly different from zero, which indicates that it makes sense to form a scale with these items, as they measure the same attribute: self-efficacy.
The inter-evaluator reliability can be observed in Tables 2 and 3, in which the applications of the Knee Evaluation Scale (Knee Score and Knee Function Score) performed by evaluator 1 (questionnaire in English) and evaluator 2 (questionnaire in Portuguese) were compared in the preoperative period and at three and six months postoperatively. There is clear indication that there was no difference between the two evaluators as regards the application of the questionnaire. This fact is confirmed in Figures 1, 2 and 3 in Bland-Altman's plot of differences.
In the knee function score we obtained equal results between the two evaluators, which can be explained by the fact that this score is based on the patient's answers regarding walking distance, use of stairs and use or non-use of a walking aids.
All the patients from our sample were in a stable condition, presenting excellent post-total knee arthroplasty improvement. This could justify the excellent intra-examiner concordance, as important changes in the patient's profile were not observed. We emphasize that this scale is easy to apply and to understand, as shown in the validation process.
CONCLUSION
The Brazilian version of the Knee Society Score proved to be an easily understandable and applicable instrument; valid and reliable to measure the knee score and function of Brazilian patients who have undergone total knee arthroplasty, providing more assistance in the monitoring and evolution of this surgical procedure.
Attachment 1.
Authorization for development of the translation and cultural adaptation of the KSS.
message from Dr. Larry Dorr
From: PJ Paul (patriciajpaul@yahoo.com)
Sent: Monday, July 21, 2008 19:59:08
To: adriana_pastore@hotmail.com
VIA EMAIL Adriana_pastore@hotmail.com
July 21, 2008
Adriana Pastore
Dear Adriana Pastore,
This EMAIL is to give you permission to translate the Knee Society Score to Portuguese. I can give you permission for that from myself as a spokesman for the Knee Society.
Sincerely,
Lawrence D. Dorr, M.D.
Anexo 2.
Escore da Sociedade do Joelho - Versão em Português.
| Nota | Esquerdo | Direito | |||||||
| Pré | 3 mês | 6 mês | 1 ano | Pré | 3 mês | 6 mês | 1 ano | ||
| Dor - Nenhuma | 50 | ||||||||
| Leve ou ocasional | 45 | ||||||||
| Apenas em escada | 40 | ||||||||
| Ao caminhar e em escada | 30 | ||||||||
| Moderada ocasional | 20 | ||||||||
| Contínua | 10 | ||||||||
| Forte | 0 | ||||||||
| Amplitude de movimento (5º = 1 ponto) | 25 | ||||||||
| Estabilidade (mov. max em qualquer posição) | |||||||||
| A/P < 5 | 10 | ||||||||
| 5 - 10 mm | 5 | ||||||||
| 10 mm | 0 | ||||||||
| M/L < 5º | 15 | ||||||||
| 6 - 9º | 10 | ||||||||
| 10 - 14º | 5 | ||||||||
| 15º | 0 | ||||||||
| Total | |||||||||
| Deduções (menos) | |||||||||
| Contratura em flexão | |||||||||
| Nenhum | 0 | ||||||||
| 5 - 10º | 2 | ||||||||
| 10 - 15º | 5 | ||||||||
| 16 - 20º | 10 | ||||||||
| > 20º | 15 | ||||||||
| Déficit de extensão | |||||||||
| Nenhum | 0 | ||||||||
| < 10º | 5 | ||||||||
| 10 - 20º | 10 | ||||||||
| > 20º | 15 | ||||||||
| Alinhamento | |||||||||
| 5 - 10º (nenhum) | 0 | ||||||||
| 0 - 4º (3 pontos por grau) | |||||||||
| 11 - 15º (3 pontos por grau) | |||||||||
| Outros | 20 | ||||||||
| Total de deduções | |||||||||
| Pontuação do joelho (se o total for negativo, a nota é zero) | |||||||||
| Função | |||||||||
| Caminhar: Sem limites | 50 | ||||||||
| > 800 metros | 40 | ||||||||
| 400 a 800 metros | 30 | ||||||||
| < 400 metros | 20 | ||||||||
| Anda dentro de casa | 10 | ||||||||
| Não anda | 0 | ||||||||
| Escada: Normal para subir e descer | 50 | ||||||||
| Normal para subir, usa corrimão para descer | 40 | ||||||||
| Uso de corrimão para subir e descer | 30 | ||||||||
| Corrimão para subir, não consegue descer | 15 | ||||||||
| Não consegue subir nem descer | 0 | ||||||||
| Total | |||||||||
| Deduções (menos) | |||||||||
| Bengala | 5 | ||||||||
| Duas bengalas | 10 | ||||||||
| Muleta / Andador | 20 | ||||||||
| Total de deduções | |||||||||
| Nota da função | |||||||||
Footnotes
All the authors declare that there is no potential conflict of interest referring to this article.
Study conducted at LIM 41 - Laboratory of Medical Investigation of the Musculoskeletal System of the Department of Orthopedics and Traumatology ofthe School of Medicine of Universidade de São Paulo.
REFERENCES
- 1.Demirdjian AM, Petrie SG, Guanche CA, Thomas KA. The outcomes of two knee scoring questionnaires in a normal population. Am J Sports Med. 1998;26:46–51. doi: 10.1177/03635465980260012401. [DOI] [PubMed] [Google Scholar]
- 2.Levine DW, Simmons BP, Koris MJ, Daltroy LH, Hohl GG, Fossel AH, et al. A self-administered questionnaire for the assessment of severity of symptoms and functional status in carpal tunnel syndrome. J Bone Joint Surg Am. 1993;75:1585–92. doi: 10.2106/00004623-199311000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Bell MJ, Bombardier C, Tugwell P. Measurement of functional status, quality of life, and utility in rheumatoid arthritis. Arthritis Rheum. 1990;33:591–601. doi: 10.1002/art.1780330420. [DOI] [PubMed] [Google Scholar]
- 4.de Campos CC, Manzano GM, de Andrade LB, Castelo Filho A, Nóbrega JA. [Translation and validation of an instrument for evaluation of severity of symptoms and the functional status in carpal tunnel syndrome] Arq Neuropsiquiatr. 2003;61:51–5. doi: 10.1590/s0004-282x2003000100009. [DOI] [PubMed] [Google Scholar]
- 5.Fernandes MI. Tradução e validação do questionário de qualidade de vida específico para osteoartrose WOMAC para a língua portuguesa [dissertação] São Paulo: Universidade Federal de São Paulo; 2003. [Google Scholar]
- 6.Heck DA, Robinson RL, Partridge CM, Lubitz RM, Freund DA. Patient outcomes after knee replacement. Clin Orthop Relat Res. 1998;(356):93–110. doi: 10.1097/00003086-199811000-00015. [DOI] [PubMed] [Google Scholar]
- 7.Fortin PR, Clarke AE, Joseph L, Liang MH, Tanzer M, Ferland D, et al. Outcomes of total hip and knee replacement: preoperative functional status predicts outcomes at six months after surgery. Arthritis Rheum. 1999;42:1722–8. doi: 10.1002/1529-0131(199908)42:8<1722::AID-ANR22>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 8.Insall JN, Dorr LD, Scott RD, Scott WN. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res. 1989;(248):13–4. [PubMed] [Google Scholar]
- 9.Alicea J. Scoring systems and their validation for the arthritic knee. In: Insall JN, Scott WN, editors. Surgery of the knee. New York: Churchill Livingstone; 2001. pp. 1507–15. [Google Scholar]
- 10.Guillemin F, Bombardier C, Beaton D. Cross-cultural adaptation of health-related quality of life measures: literature review and proposed guidelines. J Clin Epidemiol. 1993;46:1417–32. doi: 10.1016/0895-4356(93)90142-n. [DOI] [PubMed] [Google Scholar]
- 11.Pereira JC. Análise de dados qualitativos. Estratégias metodológicas para as ciências da saúde, humanas e sociais. EDUSP; São Paulo: 1999. [Google Scholar]
- 12.Salvetti MG, Pimenta CA. Validação da chronic pain self-efficacy scale para a lingual portuguesa. Rev Psiquiatr Clin. 2005;32:202–10. [Google Scholar]
- 13.Bland JM, Altman DG. Comparing methods of measurement: why plotting difference against standard method is misleading. Lancet. 1995;346:1085–7. doi: 10.1016/s0140-6736(95)91748-9. [DOI] [PubMed] [Google Scholar]
- 14.SILVA LM, SILVA LC. Validação do questionário de qualidade de vida em asma (Juniper) para o português brasileiro. Rev AMRINGS. 2007;51:31–7. [Google Scholar]
- 15.Anouchi YS, McShane M, Kelly F Jr, Elting J, Stiehl J. Range of motion in total knee replacement. Clin Orthop Relat Res. 1996;(331):87–92. doi: 10.1097/00003086-199610000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Tamanini JT, Dambros M, D' Ancona CA, Palma PC, Rodrigues Netto N Jr. [Validation of the "International Consultation on Incontinence Questionnaire - Short Form" (ICIQ-SF) for Portuguese] Rev Saude Publica. 2004;38:438–44. doi: 10.1590/s0034-89102004000300015. [DOI] [PubMed] [Google Scholar]
- 17.Duarte OS, Miyazaki MC, Ciconelli RM, Sesso R. Tradução e adaptação cultural do instrumento de avaliação de qualidade de vida para pacientes renais crônicos. Rev Assoc Med Bras. 2003;49:375–81. doi: 10.1590/s0104-42302003000400027. [DOI] [PubMed] [Google Scholar]
- 18.Nigri PZ, Peccin MS, Almeida GJ, Cohen M. Tradução, validação e adaptação cultural da escala de atividade de vida diária. Acta Ortop Bras. 2007;15:101–4. [Google Scholar]
- 19.Ciconelli RM. Medidas de avaliação de qualidade de vida. Rev Bras Reumatol. 2003;43:9–13. [Google Scholar]
- 20.Kelinger FN, Lee HB. Foundations of behavioral research. 3rd ed. New York: Holt Rinehart and Winston; 1986. [Google Scholar]