Abstract
Objective
Recently, electrocorticography-based brain-computer interfaces have been successfully used to translate cortical activity into control signals for external devices. However, the utility of such devices would be greatly enhanced by somatosensory feedback. Direct stimulation of somatosensory cortex evokes sensory perceptions, and is thus a promising option for closing the loop. Before this can be implemented in humans it is necessary to evaluate how changes in stimulus parameters are perceived and the extent to which they can be discriminated.
Approach
Electrical stimulation was delivered to the somatosensory cortex of human subjects implanted with electrocorticography grids. Subjects were asked to discriminate between stimuli of different frequency and amplitude as well as to report the qualitative sensations elicited by the stimulation.
Main Results
In this study we show that in humans implanted with electrocorticography grids, variations in the amplitude or frequency of cortical electrical stimulation produce graded variations in percepts. Subjects were able to reliably distinguish between different stimuli.
Significance
These results indicate that direct cortical stimulation is a feasible option for sensory feedback with brain-computer interface devices.
Keywords: direct cortical stimulation, somatosensory feedback, brain-computer interfaces
1. Introduction
Cortical surface stimulation became popular in the early 20th century for clinical use in the surgical treatment of epilepsy (Borchers et al., 2012). This technique was also used to reveal the cortical representation of sensory and motor function by mapping evoked responses to stimulation sites (Penfield and Boldrey, 1937). Since then surface stimulation has been widely adopted for clinical use but non-clinical research on cortical stimulation has, for practical and ethical reasons, been conducted primarily in animal models, typically using fine-wire intracortical electrodes.
With the advent of new technologies in brain-computer interfaces (BCIs) there has been an interest in using direct cortical stimulation to provide sensory feedback for these devices (Nicolelis and Lebedev, 2009). Most BCIs have relied entirely on visual feedback, but this is cognitively taxing and in some cases insufficient. This is especially the case for BCIs in which the user controls a robotic arm/hand to interact with the physical environment. Visual feedback is notoriously inadequate for judging the surface pressures applied to a grasped object. Tactile feedback is normally essential for achieving the appropriate level of grip force so that objects are neither crushed nor slip from the grasp (Johansson and Cole, 1994). Likewise proprioceptive feedback provides information about the limb joints when visual information is unavailable or when the visual system is otherwise engaged (Graziano, 1999).
It has been shown that monkeys can discriminate and interpret frequency-varying stimulation of somatosensory cortex (Romo et al., 1998; O’Doherty et al., 2009, 2011). However, it is not clear how these animals perceive electrical stimulation and thus it is unknown whether or how closely this form of feedback resembles natural somatosensory input or can substitute for it. Furthermore, these studies used fine wire intracortical electrodes. While some BCIs do use fine wire electrodes, the vast majority of human BCIs are based on surface recordings, either electroencephalography (EEG) or electrocorticography (ECoG). ECoG-based devices are receiving a great deal of attention because while the interface is less invasive and the signal is more stable than that provided by intracortical electrodes, the spatial resolution is much better than non-invasive scalp recordings and the information content is quite high (Moran, 2010). However, stimulation through the much larger ECoG electrodes excites a different volume of tissue compared to fine wire intracortical electrodes, and this may evoke different percepts. In this study humans implanted with ECoG electrodes were tested to determine how subjects perceived variations in stimulus frequency and amplitude and further, how discriminable changes in these parameters were. This experimental design provides a unique opportunity to combine quantitative results of frequency and amplitude discrimination with qualitative reports of perceived sensations and the characteristics of the evoked sensations that allow them to be discriminated.
2. Methods
2.1 Subjects
All subjects were patients undergoing long-term electrocorticography (ECoG) monitoring in preparation for surgical treatment of intractable epilepsy. Data were collected from two subjects (1 female, ages 19 and 36) with subdural platinum electrode arrays (Ad-Tech, Racine, WI). Decisions about electrode placement were based exclusively on clinical considerations. Electrodes on the grids had a 2.3mm exposed surface diameter and were spaced at 1cm. All subjects gave informed consent according to the protocol approved by the Institutional Review Boards of The University of Washington.
2.2 Electrode Localization
Electrode locations were determined based on post-operative x-ray images (figures 1 and 2). These images were co-registered with a pre-operative structural MRI scan and electrode locations were projected onto a rendering of the cortical surface (Hermes et al., 2010).
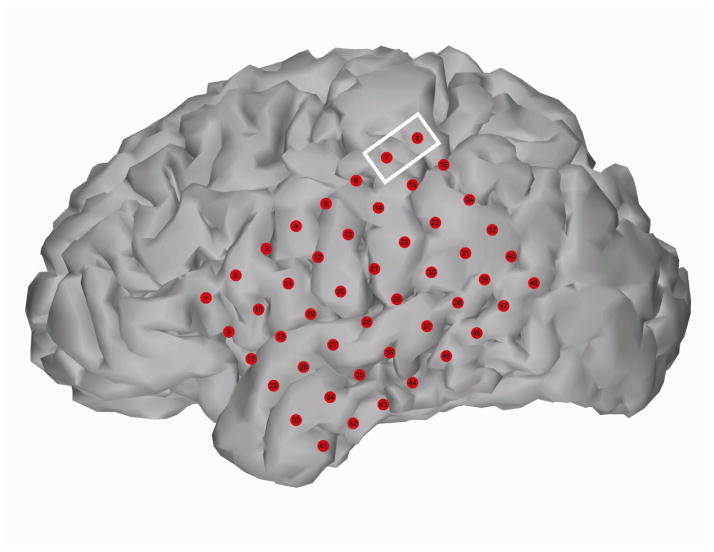
Figure 1.
Subject 1 cortical reconstruction with electrodes Pre-surgical MRI and post-surgical CT scans were co-registered to localize electrodes on the cortical surface. The white box surrounds the electrodes that were stimulated (7 and 8) during the experiment. According to clinical sensorimotor mapping this site corresponded to hand sensory area.
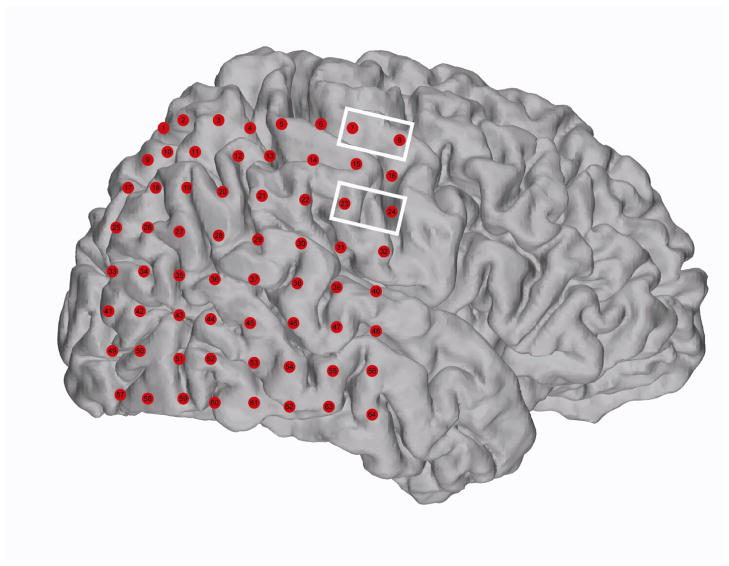
Figure 2.
Subject 2 cortical reconstruction with electrodes Pre-surgical MRI and post-surgical CT scans were co-registered to localize electrodes on the cortical surface. The white boxes surround the electrodes that were stimulated (7 and 8, 23 and 24) during the experiment. According to clinical sensorimotor mapping these sites corresponded to hand sensory area and mouth sensory area respectively.
2.3 Stimulation Protocol
The Ojemann Cortical Stimulator (Integra Neurosciences, Plainsboro, NJ) was used for direct constant-current stimulation of somatosensory cortex. Electrodes overlaying sensory areas were previously identified by clinical sensorimotor mapping either by cortical stimulation (subject 1) or somatosensory evoked potentials (subject 2). In subject 1 current was delivered between main grid electrodes 7 and 8 (identified by clinical mapping as hand sensory) (figure 1); in subject 2 current was delivered between main grid electrodes 23 and 24 (identified by clinical mapping as mouth sensory) and main grid electrodes 7 and 8 (identified by clinical mapping as hand sensory) (figure 2). The stimulation was performed using standard clinical variables in order to define sensory thresholds as part of a larger study of multi-electrode interactions. Current was delivered as a biphasic square wave with equal duration positive and negative phases. Each phase had a pulse-width of 50μsec. The amplitude and frequency were varied throughout the experiment. The stimulator unit does not accept an external trigger and must be manually operated, thus the stimulus duration could not be precisely controlled. The duration of each stimulus was approximately 1sec (for frequency variant stimuli, mean = 0.91sec, standard deviation = 0.16sec) with an inter-stimulus interval (within each pair) of about 3sec. At the start of the experiment the current amplitude at 60Hz corresponding to perceptual threshold was determined by slowly increasing the amplitude of successive stimulus trains until the subject reported sensation. Subjects were encouraged to report their qualitative experience of the percept (i.e. what it felt like) as well as the location and intensity of the sensation. The threshold amplitude was then used in frequency mapping experiments. Due to limited experimental time, only three different frequencies were presented. Stimuli were presented in pairs and the subject was asked to determine if the second stimulus was “stronger,” “weaker,” or “the same” as the first. One or both of the stimuli were presented again if the subject requested it. Instructions were given to the subject after they had reported their initial experience of the percept, but before any stimulus pairs were presented. The pairs of stimuli as well as the relative order within pairs were randomized to prevent an order effect. Again, subjects were asked to report their qualitative impressions. In subject 2, an amplitude discrimination experiment was run in a similar way. The frequency was held constant at 50Hz and different pairs of stimulation amplitudes were presented. The subject was asked to report the relative intensity of the stimuli and the qualitative perceived experience of the stimulation. In subject 1 a rudimentary amplitude discrimination experiment was performed in which stimuli of increasing amplitude were presented and the subject was asked if the stimulus felt stronger (more intense) than the previous stimulus. Both subjects were explicitly asked to report if their perception was different for variations in amplitude than it had been for variations in frequency.
3. Results
In the first subject electrical stimulation was applied to a cortical site which corresponded to somatic sensation of the right hand. The subject described the elicited sensation as being like a “wind running down the hand.” When presented with two paired stimuli of constant amplitude and different frequencies, subject 1 was able to correctly determine the relative frequencies of the stimuli in every case (N = 9, table 1). This discrimination was based on the perceived strength, or intensity of the stimulus. Thus, the subject was able to say that the 100Hz stimulus felt “stronger” than the 75Hz stimulus. Importantly, the subject was also able to identify when stimuli had the same frequency, indicating that the perceived intensity was the same for the same stimulus. Although a rigorous amplitude discrimination test was not performed in this subject, he was able to qualitatively say that increasing amplitude also increased the perceived intensity of the stimulus (table 2).
Table 1.
Subject 1 Frequency Discrimination at Hand Sensory Site, 7.1mA. Each row corresponds to a stimulus pair; the pairs were presented in the order shown from top to bottom. The amplitude was held constant at 7.1mA as the frequency was varied. The subject was asked to say whether the first frequency (column 1) was stronger than, weaker than or equivalent to the second frequency (column 3). The reported relationship and the correctness of the report are shown in column 2.
| Frequency 1 (Hz) | Reported Relative Intensity | Frequency 2 (Hz) | Comments |
|---|---|---|---|
| 50 | < correct |
75 | |
| 75 | = correct |
75 | |
| 100 | = correct |
100 | |
| 75 | < correct |
100 | |
| 100 | > correct |
75 | |
| 75 | > correct |
50 | |
| 50 | < correct |
100 | |
| 50 | = correct |
50 | |
| 100 | > correct |
50 |
The symbol “>” indicates that the first stimulus was stronger than the second, the symbol “<” indicates the second stimulus was stronger than the first, the symbol “≫” indicates that the first stimulus was much stronger than the second, and the symbol “=” indicates that the stimuli were the same strength. Any comments the subject made about the stimulus pair are recorded in the fourth column.
Table 2.
Subject 1 Amplitude Discrimination at Hand Sensory Site, 50Hz. The subject was presented with a series of stimuli at 50Hz and incrementally increasing amplitudes (ordered left to right). The subject was asked to indicate whether the strength of each stimulus was greater than, less than, or equal to the strength of the previous stimulus. The reported relationship between each stimulus and the subsequent stimulus and the correctness of the report are shown in the same column.
| 7 mA< correct |
7.6mA < correct |
8.2mA < correct |
8.6mA = incorrect |
9.2mA < correct |
9.8mA |
The subject reported that the qualitative experience of the stimulation was similar for all stimuli; different stimulation parameters altered only the intensity of the sensation. This subject reported no qualitative difference between changes due to frequency modulation and amplitude modulation.
In subject 2, stimulation was applied at two different cortical locations. Stimulation at the lateral site produced sensations on the lower lip, stimulation at the medial site produced sensations on the middle finger of the left hand. The subject described the elicited sensation on the lip as a “light rub or a light buzz” and the sensation elicited on the finger as “muffled” or as if “something was wrapped around” the finger. This subject was almost always able to correctly determine the relative frequencies of paired stimuli, with two exceptions (Nmouth=10, Nhand=9, tables 3 and 4 respectively). Regarding these misclassifications, in the first case, with two identical stimuli (75Hz) at the mouth site the subject felt the second was stronger. When these stimuli were presented again later, the subject made the correct discrimination. In the second case the subject twice reported that the second of two identical stimuli (100Hz) at the hand site was perceived to be larger.
Table 3.
Subject 2 Frequency Discrimination at Mouth Sensory Site, 3mA. The format is the same as for Table 1. The amplitude was held constant at 3mA while the frequency was varied.
| Frequency 1 (Hz) | Reported Relative Intensity | Frequency 2 (Hz) | Comments |
|---|---|---|---|
| 50 | < correct |
75 | |
| 75 | < incorrect |
75 | |
| 100 | = correct |
100 | “close” |
| 75 | < correct |
100 | “stronger” |
| 100 | > correct |
75 | |
| 75 | > correct |
50 | |
| 50 | < correct |
100 | |
| 50 | = correct |
50 | “so close” |
| 100 | > correct |
50 | |
| 75 | = correct |
75 | “very close, very strong” |
Table 4.
Subject 2 Frequency Discrimination at Hand Sensory Site, 2.8mA. The format is the same as for Table 1. The amplitude was held constant at 2.8mA while the frequency was varied.
| Frequency 1 (Hz) | Reported Relative Intensity | Frequency 2 (Hz) | Comments |
|---|---|---|---|
| 75 | = correct |
75 | “close” |
| 100 | < incorrect |
100 | |
| 100 | < incorrect |
100 | |
| 75 | < correct |
100 | |
| 100 | > correct |
75 | |
| 75 | > correct |
65 | |
| 65 | < correct |
100 | |
| 50 | = correct |
50 | |
| 100 | ≫ correct |
50 | |
| 75 | = correct |
75 |
The symbol “>” indicates that the first stimulus was stronger than the second, the symbol “<” indicates the second stimulus was stronger than the first, the symbol “≫” indicates that the first stimulus was much stronger than the second, and the symbol “=” indicates that the stimuli were the same strength. Any comments the subject made about the stimulus pair are recorded in the fourth column.
This subject also participated in an amplitude discrimination experiment. The subject was able to determine the relative amplitudes of paired stimuli at both stimulation sites, although not perfectly (Nmouth=5, Nhand=6, tables 5 and 6 respectively). At the mouth site the subject reported that stimulation at 3.0mA and 2.7mA felt the same. However, when the same stimulation pair was tried immediately afterwards, the subject reported the correct relationship. At the hand stimulation site the subject first reported that perceived stimulation at 3.4mA and 2.8mA was the same, but subsequently corrected the answer. The subjected also reported a series of two stimuli, both at 3.4mA, as different, but when the same pair of stimuli was presented moments later the subject reported that the two stimuli were the same.
Table 5.
Subject 2 Amplitude Discrimination at Mouth Sensory Site, 60Hz. Each row corresponds to a stimulus pair; the pairs were presented in the order shown from top to bottom. The frequency was held constant at 60Hz, the amplitude was varied. The subject was asked to say whether the first stimulus (column 1) was stronger than, weaker than or equivalent to the second stimulus (column 3). The reported relationship and the correctness of the report are shown in column 2.
| Amplitude 1 (mA) | Reported Relative Intensity | Amplitude 2 (mA) | Comments |
|---|---|---|---|
| 3.3 | > correct |
3.0 | |
| 3.0 | = incorrect |
2.7 | |
| 3.0 | > correct |
2.7 | |
| 3.6 | ≫ correct |
3.0 | |
| 3.5 | > correct |
3.3 |
Table 6.
Subject 2 Amplitude Discrimination at Hand Sensory Site, 50Hz. The frequency was held constant at 50Hz as the amplitude was varied. The format is the same as for Table 5.
| Amplitude 1 (mA) | Reported Relative Intensity | Amplitude 2 (mA) | Comments |
|---|---|---|---|
| 3.0 | < correct |
3.8 | |
| 3.8 | > correct |
3.4 | |
| 3.2 | = incorrect |
2.8 | |
| 3.4 | > correct |
2.8 | Initially said “=” but changed to “>” |
| 3.4 | > incorrect |
3.4 | |
| 3.4 | = correct |
3.4 |
Like subject 1, this subject reported that the qualitative experience of the stimulation was similar for all stimuli. Modulation of stimulation parameters changed the perceived strength or intensity of the sensation but the qualia of the sensation were not changed by either frequency or amplitude modulation.
4. Discussion
Our results demonstrate that human subjects are able to discriminate different intensities of stimulation as a function of either the stimulation frequency or the stimulation amplitude. To the authors’ knowledge, this is the first report that humans experience graded sensations in response to graded sensorimotor-cortical stimulation. These findings support previous studies in non-human primates with intracortical electrodes (Romo et al., 1998). The unique contributions of this study are first, that it was conducted in humans who are able to report the qualitative experience of the stimulation and second, that it employed ECoG electrodes rather than fine wire intracortical microstimulation electrodes. This presents opportunities for future investigation into the role of surface stimulation as a feedback modality in human BCI experiments.
In a recent study monkeys were trained to discriminate different presumed tactile “textures” with a brain-machine-brain-interface where the tactile feedback was provided as intracortical microstimulation (O’Doherty et al., 2011). In this case different textures were encoded by high-frequency pulse trains modulated by lower frequency carrier waves. Three different stimulus patterns were presented; 200Hz pulses at a 10Hz interval, 400Hz pulses at a 5Hz interval, and a null stimulus or the absence of any stimulation. While the animals were able to discriminate these different patterns, it is not possible to say whether any of the stimuli were experienced as textures, nor is it clear whether such different stimuli would even elicit similar sensations. In the current study subjects reported that, for the stimulation parameters that were tested, the qualitative experience of stimulation was the same, only the intensity of the sensation changed. The ability of subjects to discriminate relatively small changes in stimulus frequency (25Hz) as well as their perception of a graded sensation is encouraging with respect to feedback for BCIs as it means that small changes in stimulus parameters can be used to control small changes in perception.
It is not clear whether stimulation delivered through large ECoG electrodes on the surface of the cortex will produce percepts similar to fine-wire intracortical electrodes. ECoG is an intermediate technology in that it has better spatial resolution and sensitivity than EEG and is less invasive and provides broader coverage than fine wire electrodes. As such, it has received considerable attention as a potential interface for BCI (Moran, 2010). Standard size ECoG electrodes are relatively large and stimulation involves a correspondingly large volume of brain tissue. The evoked response is, therefore, a complex event reflecting the summation of activity in large neural populations. Even so, our results show that stimulation through these electrodes evokes a positive sensation (i.e. not numbness) whose intensity can be modulated in a predictable way.
Variations in amplitude and variations in frequency were reported as being qualitatively the same by both subjects. This supports the hypothesis that electrical stimulation parameters are not perceived independently but rather jointly contribute to a unified perception of intensity (Fridman et al., 2010). This would seem to conflict with the classic experiments of Romo et al. (Romo et al., 1998) who showed that periodic stimulation of the skin and electrical stimulation of quickly adapting neurons in area 3b were behaviourally indistinguishable (and therefore presumably perceptually equivalent) in non-human primates. They further reported that above a certain threshold, changes in the stimulus amplitude did not change the behavioural performance. These differences could be accounted for by a number differences in the methods employed in that experiment and in the experiments described here. First, Romo et al. used intracortical microelectrodes, second they used lower stimulation frequencies (5–50Hz) and finally, they targeted a specific population of neurons. Further investigation is necessary to resolve which of these factors, if any, explains the discrepancy in outcomes.
This study was limited by the approved experimental protocol which allowed for only a small number of supra-threshold stimulations. As a result, the data maps only a subset of the space of stimulation parameters. We could not determine the smallest perceivable change in frequency or amplitude, or test how the comodulation of these parameters affected perceived sensation or discrimination; this is left for future studies. In addition, it will be of interest to test the discriminability of stimulation on two different electrode pairs both serially and simultaneously.
Some caveats must be attached to the interpretation of these results. First, our experiments were conducted in subjects with known brain pathologies, although in neither case was the seizure focus located under the stimulated electrodes. Second, we did not evaluate any remote effects or test for negative (suppressed) behavioural responses. Additionally, because the electrodes were implanted for clinical reasons the electrode placement and design was not optimized for the experiment. Current FDA approved cortical stimulation devices require manual operation and thus do not allow for precise control of the duration of the stimulus train or for the operator to be blind to the frequency and amplitude of the stimulus. It is possible that longer stimulation trains could lead to stronger percepts and it should be noted that, while differences in stimulus duration should be random, the design of these experiments does not prevent bias on the part of the stimulator operator.
We could not measure the effective stimulation area or any changes to this area resulting from increases in stimulation amplitude. Using similar stimulation parameters and optical imaging in the monkey visual cortex Haglund et al. (Haglund et al., 1993) found that graded increases in stimulation amplitude corresponded to graded increases in the area of activation. Furthermore, they observed activation only around the stimulating electrode; no incongruent areas of activation were identified (Haglund et al., 1993). Estimates for the physical spread of surface stimulation in motor cortex for current magnitudes up to 3mA can be found in (Philips and Porter, 1964).
Finally, we did not do any long-term assessment of the reproducibility of the results or of how repeated stimulation (over the short- or long-term) impacts perception of subsequent stimuli. Previously reported studies in animal models suggest that these issues are worthy of further consideration. Repetitive stimulation is known to increase indirect activation by temporal summation of synaptic effects, although, this outcome (demonstrated in pyramidal tract cells) was much more pronounced for intracortical stimulation than for surface stimulation (Jankowska et al., 1975). Cortical micro-electrode array stimulation with frequencies of 20Hz and higher has been previously reported to elicit short periods of excitation followed by longer periods of inhibition (~100ms), independent of any further increases in stimulus intensity, leading to a picture of excitatory responses against a constant background of inhibition (Butovas and Schwarz, 2003). However, in rat somatosensory cortex, it has been shown that increasing the number of repetitive pulses in a given stimulus train incrementally decreases the threshold pulse intensity for stimulus detection (Butovas and Schwarz, 2007). This would be consistent with the misperceived increase in perceived strength with no increase in stimulus intensity (see tables 3 and 4). Otherwise, our data did not show any consistent effects of this sort. However, this effect may become more apparent over a longer duration of stimulation.
Studies of the effects of repetitive cortical stimulation in humans thus far are limited to non-invasive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS). rTMS and tDCS over sensorimotor cortex have been shown to cause changes in M1 neuronal excitability lasting minutes to hours with the direction of influence determined by the stimulation parameters. In addition to influencing M1 cortical excitability by itself, tDCS can also modulate the response of primary M1 cortex to subsequent rTMS- induced motor evoked potential facilitation (Cambieri et al., 2012). Theta-burst stimulation has been shown to affect the magnitude of sensory evoked potentials (Katayama and Rothwell, 2007; Katayama et al., 2010). Other studies have demonstrated transient changes in tactile perception and somatosensory evoked potentials with tDCS (Matsunaga et al., 2004; Rogalewski et al., 2004), and a generalized reduction in the experience of pain and temperature-associated pain perception in response to high frequency sensorimotor TMS (Summers et al., 2004; Oliviero et al., 2005; Johnson et al., 2006; Bachmann et al., 2010). In chronic pain treatment, cathodal tDCS has been shown to decrease acute and chronic pain perception and A-fiber mediated cold temperature and mechanical pain detection (Fregni et al., 2006; Antal et al., 2008; Boggio et al., 2008). However, fMRI and PET studies suggest that rTMS and tDCS may have effects at distant subcortical sites including the thalamus and corticothalamic projections (Bestmann et al., 2004; Pleger et al., 2004; Lang et al., 2005), which may play a role in clinical observations of altered pain and temperature sensation.
These cautionary notes notwithstanding, our results serve as a proof of concept that direct somatosensory cortical stimulation is a viable option for sensory feedback from a brain-controlled device. This kind of feedback will provide valuable information either as an alternative to visual feedback or as a supplement to visual feedback, removing the burden of constant visual attention and providing additional short-latency information in situations where visual input is insufficient.
Table 5. Subject 2 Amplitude Discrimination at Mouth Sensory Site, 60Hz. Each row corresponds to a stimulus pair; the pairs were presented in the order shown from top to bottom. The frequency was held constant at 60Hz, the amplitude was varied. The subject was asked to say whether the first stimulus (column 1) was stronger than, weaker than or equivalent to the second stimulus (column 3). The reported relationship and the correctness of the report are shown in column 2.
Acknowledgments
This research was supported by National Institutes of Health Grants R01 NS065186, T90 DA023436 and R25 NS079200, award number EEC-1028725 from the National Science Foundation and a grant from the W.M. Keck Foundation.
References
- Antal A, Brepohl N, Poreisz C, Boros K, Csifcsak G, Paulus W. Transcranial direct current stimulation over somatosensory cortex decreases experimentally induced acute pain perception. Clin J Pain. 2008;24:56–63. doi: 10.1097/AJP.0b013e318157233b. [DOI] [PubMed] [Google Scholar]
- Bachmann CG, Muschinsky S, Nitsche MA, Rolke R, Magerl W, Treede R-D, Paulus W, Happe S. Transcranial direct current stimulation of the motor cortex induces distinct changes in thermal and mechanical sensory percepts. Clin Neurophysiol. 2010;121:2083–2089. doi: 10.1016/j.clinph.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Bestmann S, Baudewig J, Siebner HR, Rothwell JC, Frahm J. Functional MRI of the immediate impact of transcranial magnetic stimulation on cortical and subcortical motor circuits. Eur J Neurosci. 2004;19:1950–1962. doi: 10.1111/j.1460-9568.2004.03277.x. [DOI] [PubMed] [Google Scholar]
- Boggio PS, Zaghi S, Lopes M, Fregni F. Modulatory effects of anodal transcranial direct current stimulation on perception and pain thresholds in healthy volunteers. Eur J Neurol. 2008;15:1124–1130. doi: 10.1111/j.1468-1331.2008.02270.x. [DOI] [PubMed] [Google Scholar]
- Borchers S, Himmelbach M, Logothetis N, Karnath H-O. Direct electrical stimulation of human cortex — the gold standard for mapping brain functions? Nat Rev Neurosci. 2012;13:63–70. doi: 10.1038/nrn3140. [DOI] [PubMed] [Google Scholar]
- Butovas S, Schwarz C. Spatiotemporal effects of microstimulation in rat neocortex: a parametric study using multielectrode recordings. J Neurophysiol. 2003;90:3024–3039. doi: 10.1152/jn.00245.2003. [DOI] [PubMed] [Google Scholar]
- Butovas S, Schwarz C. Detection psychophysics of intracortical microstimulation in rat primary somatosensory cortex. Eur J Neurosci. 2007;25:2161–2169. doi: 10.1111/j.1460-9568.2007.05449.x. [DOI] [PubMed] [Google Scholar]
- Cambieri C, Scelzo E, Li Voti P, Priori A, Accornero N, Inghilleri M. Transcranial direct current stimulation modulates motor responses evoked by repetitive transcranial magnetic stimulation. Neurosci Lett. 2012;522:167–171. doi: 10.1016/j.neulet.2012.06.033. [DOI] [PubMed] [Google Scholar]
- Fregni F, Gimenes R, Valle AC, Ferreira MJL, Rocha RR, Natalle L, Bravo R, Rigonatti SP, Freedman SD, Nitsche MA, Pascual-Leone A, Boggio PS. A randomized, sham-controlled, proof of principle study of transcranial direct current stimulation for the treatment of pain in fibromyalgia. Arthritis Rheum. 2006;54:3988–3998. doi: 10.1002/art.22195. [DOI] [PubMed] [Google Scholar]
- Fridman GY, Blair HT, Blaisdell AP, Judy JW. Perceived intensity of somatosensory cortical electrical stimulation. Exp Brain Res. 2010;203:499–515. doi: 10.1007/s00221-010-2254-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano MSA. Where is my arm? The relative role of vision and proprioception in the neuronal representation of limb position. Proc Natl Acad Sci U S A. 1999;96:10418–10421. doi: 10.1073/pnas.96.18.10418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haglund MM, Ojemann GA, Blasdel GG. Optical imaging of bipolar cortical stimulation. J Neurosurg. 1993;78:785–793. doi: 10.3171/jns.1993.78.5.0785. [DOI] [PubMed] [Google Scholar]
- Hermes D, Miller KJ, Noordmans HJ, Vansteensel MJ, Ramsey NF. Automated electrocorticographic electrode localization on individually rendered brain surfaces. J Neurosci Methods. 2010;185:293–298. doi: 10.1016/j.jneumeth.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Padel Y, Tanaka R. The mode of activation of pyramidal tract cells by intracortical stimuli. J Physiol. 1975;249:617–636. doi: 10.1113/jphysiol.1975.sp011034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson RS, Cole KJ. Grasp stability during manipulative actions. Can J Physiol Pharmacol. 1994;72:511–524. doi: 10.1139/y94-075. [DOI] [PubMed] [Google Scholar]
- Johnson S, Summers J, Pridmore S. Changes to somatosensory detection and pain thresholds following high frequency repetitive TMS of the motor cortex in individuals suffering from chronic pain. Pain. 2006;123:187–192. doi: 10.1016/j.pain.2006.02.030. [DOI] [PubMed] [Google Scholar]
- Katayama T, Rothwell JC. Modulation of somatosensory evoked potentials using transcranial magnetic intermittent theta burst stimulation. Clin Neurophysiol. 2007;118:2506–2511. doi: 10.1016/j.clinph.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Katayama T, Suppa A, Rothwell JC. Somatosensory evoked potentials and high frequency oscillations are differently modulated by theta burst stimulation over primary somatosensory cortex in humans. Clin Neurophysiol. 2010;121:2097–2103. doi: 10.1016/j.clinph.2010.05.014. [DOI] [PubMed] [Google Scholar]
- Lang N, Siebner HR, Ward NS, Lee L, Nitsche MA, Paulus W, Rothwell JC, Lemon RN, Frackowiak RS. How does transcranial DC stimulation of the primary motor cortex alter regional neuronal activity in the human brain? Eur J Neurosci. 2005;22:495–504. doi: 10.1111/j.1460-9568.2005.04233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga K, Nitsche MA, Tsuji S, Rothwell JC. Effect of transcranial DC sensorimotor cortex stimulation on somatosensory evoked potentials in humans. Clin Neurophysiol. 2004;115:456–460. doi: 10.1016/s1388-2457(03)00362-6. [DOI] [PubMed] [Google Scholar]
- Moran D. Evolution of brain–computer interface: action potentials, local field potentials and electrocorticograms. Current Opinion in Neurobiology. 2010;20:741–745. doi: 10.1016/j.conb.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolelis MAL, Lebedev MA. Principles of neural ensemble physiology underlying the operation of brain–machine interfaces. Nature Reviews Neuroscience. 2009;10:530–540. doi: 10.1038/nrn2653. [DOI] [PubMed] [Google Scholar]
- O’Doherty JE, Lebedev MA, Hanson TL, Fitzsimmons NA, Nicolelis MAL. A Brain-Machine Interface Instructed by Direct Intracortical Microstimulation. Front Integr Neurosci. 2009:3. doi: 10.3389/neuro.07.020.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty JE, Lebedev MA, Ifft PJ, Zhuang KZ, Shokur S, Bleuler H, Nicolelis MAL. Active tactile exploration enabled by a brain-machine-brain interface. Nature. 2011;479:228–231. doi: 10.1038/nature10489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliviero A, Esteban MR, De la Cruz FS, Cabredo LF, Di Lazzaro V. Short-lasting impairment of temperature perception by high frequency rTMS of the sensorimotor cortex. Clin Neurophysiol. 2005;116:1072–1076. doi: 10.1016/j.clinph.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Penfield W, Boldrey E. Somatic Motor and Sensory Representation in the Cerebral Cortex of Man as Studied by Electrical Stimulation. Brain. 1937;60:389–443. [Google Scholar]
- Philips CG, Porter R. The Pyramidal Projection to Motoneurones of Some Muscle Groups of the Baboon’s Forelimb. Prog Brain Res. 1964;12:222–245. doi: 10.1016/s0079-6123(08)60625-1. [DOI] [PubMed] [Google Scholar]
- Pleger B, Janssen F, Schwenkreis P, Völker B, Maier C, Tegenthoff M. Repetitive transcranial magnetic stimulation of the motor cortex attenuates pain perception in complex regional pain syndrome type I. Neurosci Lett. 2004;356:87–90. doi: 10.1016/j.neulet.2003.11.037. [DOI] [PubMed] [Google Scholar]
- Rogalewski A, Breitenstein C, Nitsche MA, Paulus W, Knecht S. Transcranial direct current stimulation disrupts tactile perception. Eur J Neurosci. 2004;20:313–316. doi: 10.1111/j.0953-816X.2004.03450.x. [DOI] [PubMed] [Google Scholar]
- Romo R, Hernández A, Zainos A, Salinas E. Somatosensory discrimination based on cortical microstimulation. Nature. 1998;392:387–390. doi: 10.1038/32891. [DOI] [PubMed] [Google Scholar]
- Summers J, Johnson S, Pridmore S, Oberoi G. Changes to cold detection and pain thresholds following low and high frequency transcranial magnetic stimulation of the motor cortex. Neurosci Lett. 2004;368:197–200. doi: 10.1016/j.neulet.2004.07.008. [DOI] [PubMed] [Google Scholar]