Abstract
Purpose
To provide our perspective on why the cornea is resistant to infection based on our research results with Pseudomonas aeruginosa.
Perspective
We focus on our current understanding of the interplay between bacteria, tear fluid and the corneal epithelium that determine health as the usual outcome, and propose a theoretical model for how contact lens wear might change those interactions to enable susceptibility to P. aeruginosa infection.
Methods
Use of “null-infection” in vivo models, cultured human corneal epithelial cells, contact lens-wearing animal models, and bacterial genetics help to elucidate mechanisms by which P. aeruginosa survive at the ocular surface, adheres, and traverses multilayered corneal epithelia. These models also help elucidate the molecular mechanisms of corneal epithelial innate defense.
Results and Discussion
Tear fluid and the corneal epithelium combine to make a formidable defense against P. aeruginosa infection of the cornea. Part of that defense involves the expression of antimicrobials such as β-defensins, the cathelicidin LL-37, cytokeratin-derived antimicrobial peptides, and RNase7. Immunomodulators such as SP-D and ST2 also contribute. Innate defenses of the cornea depend in part on MyD88, a key adaptor protein of TLR and IL-1R signaling, but the basal lamina represents the final barrier to bacterial penetration. Overcoming these defenses involves P. aeruginosa adaptation, expression of the type three secretion system, proteases, and P. aeruginosa biofilm formation on contact lenses.
Conclusion
After more than two decades of research focused on understanding how contact lens wear predisposes to P. aeruginosa infection, our working hypothesis places blame for microbial keratitis on bacterial adaptation to ocular surface defenses, combined with changes to the biochemistry of the corneal surface caused by trapping bacteria and tear fluid against the cornea under the lens.
Keywords: Pseudomonas aeruginosa, cornea, epithelium, tear fluid, innate immunity, antimicrobial peptides, bacterial pathogenesis, type three secretion, intracellular survival, bacterial cytotoxicity, virulence
Introduction
Pseudomonas aeruginosa is a leading cause of corneal infection associated with contact lens wear 1-3. During P. aeruginosa keratitis, both the infecting bacteria and host immune response contribute to the pathology observed. Thus, irreversible damage and vision loss can occur even after successful antimicrobial therapy. For this reason, host responses to P. aeruginosa keratitis that occur after disease is initiated, e.g. phagocyte infiltration and adaptive immunity, have been extensively investigated with the goal of developing new therapies to control the damage that they cause 4, 5. While host responses are important in the pathogenesis of P. aeruginosa corneal infections, and recovery from them, they are beyond the scope of this paper. Instead, we focus on the mechanisms behind the inherent resistance of a healthy cornea to P. aeruginosa, about which much less is known, and how factors that render the cornea susceptible to infection compromise that resistance. This perspective is based upon our own work, and not intended as a review of the literature to which many investigators have contributed.
Vulnerability of corneal epithelial cells to P. aeruginosa in vitro
Considering how resistant the healthy cornea is to P. aeruginosa, it is striking how vulnerable the epithelial cells that line the corneal surface become when grown in vitro. More than 50% of clinical and laboratory isolates of P. aeruginosa have the capacity to invade and then replicate within cultured corneal epithelial cells 6, 7. Once inside the cell, they induce the formation of, and then traffic to, plasma membrane blebs, which can detach and carry the bacteria swimming within them to distant locations 8, 9. This sequence of events requires ExoS, a toxin that P. aeruginosa can inject across host cell membranes (a type three secretion system). Cytotoxic strains of P. aeruginosa, that constitute about half of isolates that cause contact lens-related infection, lack ExoS and instead encode ExoU. While ExoU is also a type three-secreted toxin, it causes a much more rapid form of cell death than ExoS, and it exerts its pathogenic effects while the bacteria are outside of the target cell 10-12.
In vivo factors and corneal resistance to P. aeruginosa
P. aeruginosa is ubiquitous in nature. As such, we are often exposed to it as we go about our daily activities. The same is true for most pathogens that cause corneal infections. Thus, it is fortuitous that the healthy cornea, in contrast to cultured corneal epithelial cells, is exquisitely resistant to microbial attack. Indeed, the inoculation of extremely large inocula (a thick bacterial suspension) of either invasive or cytotoxic P. aeruginosa onto intact mouse or rat corneas in vivo results in rapid bacterial clearance from the ocular surface without pathology 13. Thus, defense mechanisms exist in the healthy eye that protect against corneal infection, which are absent from laboratory culture conditions. These defenses are likely to differ from the type of host immune responses that are activated when an infection occurs, since they are constantly present under conditions of health. Studying health, and factors involved in maintaining it, requires the use of completely different models and methods from those used to study disease, the latter being used for most research to date in this field. Importantly, studying parameters that maintain health is a significant challenge due to the lack of observable changes when disease is absent. To address this problem, our laboratory has developed multiple models to mimic the intrinsic resistance of the in vivo cornea in a research setting, and we have also begun to use these models to dissect apart the mechanisms involved in defense of the healthy cornea.
Tear fluid
One approach that we have used to determine which in vivo factors confer resistance to microbial attack is to consider what is missing in cell culture that makes cells vulnerable in vitro, but not in vivo. A very obvious factor missing in cell culture is the tear film. We have confirmed that tear fluid can protect corneal epithelial cells in culture against both invasive and cytotoxic P. aeruginosa 14. Importantly, we have found that human tears can protect the injured and healing mouse cornea from infection by P. aeruginosa in vivo 15.
How does tear fluid protect? It is well recognized that tear fluid and blinking can physically cleanse the ocular surface and wash away potential pathogens, and that tear fluid also contains molecules with direct antimicrobial activity against many microbes, e.g. lysozyme, lactoferrin 16,17 (also see Fig. 1 and Table 1). However, our data have shown that the capacity of tear fluid to protect cells against P. aeruginosa is independent of direct antimicrobial activity 14. In fact, we found that many P. aeruginosa strains, including clinical isolates from microbial keratitis, grow readily in undiluted human tear fluid, yet tear fluid can still protect corneal epithelial cells against them 14, 18.
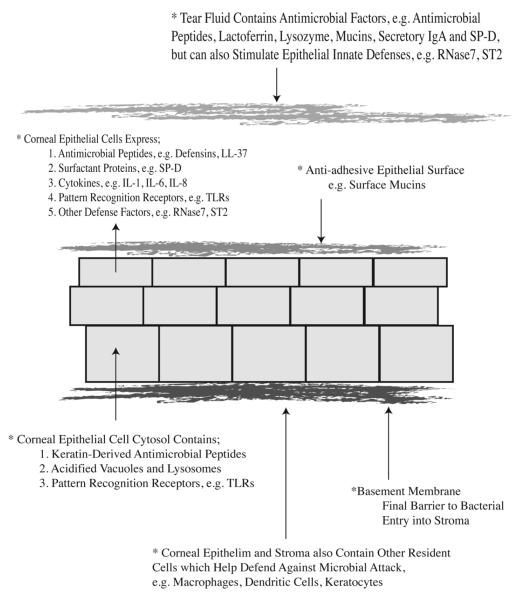
Figure 1.
Schematic overview of intrinsic corneal defenses against P. aeruginosa and other microbial pathogens during health. Several of these defenses are dependent upon MyD88, an adaptor protein associated with TLR and IL-1R signaling. MyD88-dependent defenses include anti-adhesive factors of the epithelial surface, which can be removed by tissue paper blotting, and they also include epithelial barrier function against P. aeruginosa traversal after bacterial adhesion is enabled 27 (also see Table 1).
Table.
Factors helping a healthy cornea resist P. aeruginosa (PA) and other microbes
| Factor | Location(s) | Mode of Action(s) | Reference(s) |
|---|---|---|---|
| α-Defensins β-Defensins (Antimicrobial Peptides) |
Tear Fluid Epithelium |
Inhibition of microbial growth/viability a β-defensin protects against PA colonization in vivo, and epithelial traversal in vitro |
30, 36, 37
37 |
| Cathelicidin LL-37 (Antimicrobial Peptide) |
Epithelium | Inhibition of microbial growth/viability b |
30 |
| Soluble Mucins Membrane-Bound Mucins |
Tear Fluid Epithelium |
PA binding and aggregation/ inhibition of PA adherence to corneal epithelium Inhibition of bacterial adherence c |
19
41 |
| Secretory IgA | Tear Fluid | PA binding/inhibition of PA adherence to corneal epithelium |
21 |
| Surfactant Protein-D | Tear Fluid and Epithelium |
PA binding and aggregation/ inhibits PA epithelial invasion (in vitro), and PA epithelial traversal (in vivo)/promotes PA ocular clearance/direct antimicrobial |
13, 20, 26, 28 |
| Lactoferrin and Lysozyme | Tear Fluid | Inhibition of microbial growth/viability d |
16, 17 |
| MyD88-Dependent Receptors, e.g. TLRs, IL-1R On Resident Corneal Cells (Detect PA antigens, IL-1) |
Epithelial cells Macrophages Dendritic cells Keratocytes (cell surface/ intracellular) |
Regulation of innate defenses including the expression of antimicrobial peptides and cytokines/chemokines e Prevents P. aeruginosa traversal of corneal epithelium (ex vivo) |
29 to 33, 38
27 |
| RNase7 and ST2 | Epithelium | Induced by tear fluid/inhibit PA invasion of epithelial cells f |
18 |
| KDAMPs (Antimicrobial Peptides From Cytokeratin 6A) |
Epithelium (Cytosol) |
Inhibition of microbial growth/viability a Inhibits PA corneal colonization |
39 |
| Basement Membrane | Blocks PA penetration to stroma (mechanism unknown, pore size?) |
45 | |
Other activities include immune cell chemotaxis, and promotion of wound healing
Also multifunctional, e.g. promotion epithelial wound healing, and cytokine expression
Shown for Staphylococcus aureus
Lactoferrin also exerts anti-inflammatory activity
Also links to phagocyte recruitment and adaptive immunity
RNase7 is an antimicrobial ribonuclease first discovered in skin (stratum corneum)
If tear fluid does not inhibit bacteria growth, how does it protect cells against P. aeruginosa? Our data show that at least part of the answer to that question is that tear fluid acts directly upon corneal epithelial cells to make them more resistant to P. aeruginosa virulence strategies. We showed this experimentally by pre-treating human corneal epithelial cells with human tear fluid, and then washing the tear fluid away before adding a bacterial inoculum. The results showed that human tear pre-treatment rendered human corneal epithelial cells more resistant to invasion by invasive P. aeruginosa strains and cell death caused by cytotoxic strains 18. Tear fluid-induced resistance was associated with an up-regulation of stress response transcription factors, NF-κB (Nuclear Factor-kappaB) and AP-1 (Activating Protein-1), and the up- and down-regulated expression of many epithelial genes. The latter included genes encoding cytokines, transcription factors, and junctional proteins. Importantly, that work also showed that tear fluid up-regulated the antimicrobial RNase7 (Ribonuclease 7), and the immunomodulator ST2 (a member of the Interleukin-1 Receptor [IL-1R] family), and that both factors contributed to tear fluid-induced corneal epithelial cell defense against P. aeruginosa 18. We have also shown that tear fluid increased trans-epithelial resistance (barrier function) of corneal epithelial cells in vitro15. That phenomenon is likely to help the multilayered corneal epithelium protect itself against microbial traversal, a key event for the pathogenesis of infection. Whether or not mucosal fluids elsewhere in the body also regulate the immunity of the epithelia that they bathe is yet to be determined. However, it is also possible that this protective function of tear fluid serves to replace the now well-established roles played by commensal microbes at other sites in modulating innate defense and homeostasis.
While tear fluid does not consistently inhibit P. aeruginosa viability, it remains possible that tears suppress bacterial virulence strategies, which would augment its effects on epithelial cell immunity. Tear fluid contains mucins, sIgA (Secretory Immunoglobulin A), and surfactant proteins, e.g. SP-D (Surfactant Protein-D), each of which can bind microbes and potentially alter their interactions with corneal epithelial cells 19-21. Other tear components may also help defend the corneal surface against infection including tear lipocalin, an endonuclease 22, and other, as yet unidentified, factors.
The corneal epithelium
For more than three decades, researchers who study the pathogenesis of corneal infection have used either corneal scarification or stromal injection as methods to enable susceptibility in animal infection models 23-25. The principle upon which this practice is based is that the corneal epithelium is a formidable barrier to infecting microbes, so it needs to be by-passed for infection to be initiated. However, models that bypass this layer do not enable study of the mechanisms for its resistance. To address this problem, we have experimented with more subtle manipulations of the corneal epithelium. Our goal has been to make the epithelium more susceptible to bacterial binding, both with and without susceptibility to bacterial penetration (traversal), so that we can study these events while also deciphering the defenses that protect against them 26, 27. To enable us to track bacteria as they penetrate, we have developed a suite of imaging technologies that allow accurate localization of live bacteria within living mouse eyeballs over time relative to the epithelial surface, individual epithelial cells, and the underlying basal lamina 27.
It is commonly thought that tight junctions, which reside within the superficial cell layer, are responsible for barrier function of the corneal epithelium against penetrating microbes. However, using the methods described above we have found that this is only part of the story. Using tissue paper blotting of the corneal surface, we have shown that subtle injury to the superficial epithelium resulting in loss of barrier function to fluorescein, allows P. aeruginosa to adhere to the cornea, but not penetrate beyond the epithelial surface 26. Thus, the tight junctions that exclude fluorescein are not needed for the corneal epithelium to stop adherent bacteria from penetrating. The fact that we are able to promote bacterial adhesion without bacterial penetration shows that defenses against these first two steps in corneal infection are separable, and that they are likely to involve different players.
Of course, it remains possible that some type of cell-to-cell junction(s) beyond the superficial surface are involved in stopping bacteria from penetrating the epithelium, and that the reason fluorescein, but not bacteria, go through is that they are less “tight” than the superficial tight junctions. In fact, treating the cornea with a calcium chelator, EGTA (Ethylene Glycol Tetra-acetic Acid) after tissue paper blotting, does allow bacteria to penetrate the epithelium 26. This result could implicate the involvement of some type of cell-to-cell junction(s), since their integrity is generally calcium-dependent. However, other cellular functions that could protect against bacterial traversal are also calcium-dependent, e.g. the roles of SP-D in innate defense 28. Indeed, one of our recent studies showed that P. aeruginosa could partially traverse the tissue paper-blotted corneal epithelium of SP-D knockout mice in vivo 26.
Other data support the possibility that either junctional structures or antimicrobial peptides are involved in epithelial defense against P. aeruginosa traversal. The corneas of mice deficient in MyD88 (Myeloid Differentiation primary response protein 88), a key adaptor protein of innate immunity, are susceptible to P. aeruginosa penetration without the need for tissue paper blotting or EGTA treatment 27. MyD88 is an essential component of TLR (Toll-Like Receptor) signaling and IL-1R signaling, which enables corneal cells to respond to microbial antigens through the activation of cytokines and chemokines, secretion of antimicrobial peptides, and the recruitment of phagocytic cells 29-33.
MyD88 regulation of defenses against bacterial adhesion to, and bacterial penetration of, the corneal epithelium would be consistent with junctional structure involvement in defense, since TLR signaling (dependent on MyD88 for most TLRs), along with other pattern recognition receptors, help regulate the function of tight junctions in other cell types 34. However, MyD88 involvement in defense against bacterial adhesion and traversal may also be due to its importance in regulating the expression of antimicrobial peptides, including human β-defensin-2 (hBD-2) and the cathelicidin LL-37, both of which are expressed by corneal epithelial cells after stimulation with TLR or IL-1R agonists 32, 33, 35, 36. Indeed, we have already shown that hBD-2 is important in protecting the corneal epithelium against P. aeruginosa colonization 37. Our ongoing studies are investigating the relative roles of individual TLRs, and the IL-1R, in defense against P. aeruginosa corneal adhesion and epithelial traversal, and the relative role of epithelial cells versus other resident corneal cell types which also express MyD88-dependent receptors, e.g. macrophages and dendritic cells 29, 31, 38.
Our most recent studies have revealed that corneal epithelial cells express other novel antimicrobial compounds 39, 40. Specifically, we have found that peptide fragments of the intermediate filament protein cytokeratin 6A, KDAMPs (Keratin-Derived Antimicrobial Peptides), isolated from lysates of human corneal epithelial cells, were rapidly bactericidal against multiple clinical isolates of P. aeruginosa, and against other bacterial pathogens, e.g. Streptococcus pyogenes and Staphylococcus aureus. Importantly, knockdown of cytokeratin 6A from which KDAMPs are derived, reduces the antimicrobial activity of human corneal epithelial cell lysates, and in vivo renders the mouse corneal epithelium significantly more susceptible to bacterial adhesion 39. Cytokeratin 6A knockdown did not enable fluorescein staining suggesting that tight junctions remained intact. Whether KDAMP expression or function is MyD88-dependent is to be determined.
The fact that MyD88 regulates the anti-adhesive nature of the corneal epithelium is interesting. Mucins (soluble and membrane-bound) are thought to be important in preventing adhesion of bacteria, such as P. aeruginosa and S. aureus, to corneal epithelial cells 19, 41. The fact that tissue paper blotting enables bacterial adhesion is consistent with that assumption, since it is likely to remove, or at least disrupt, mucins at the corneal surface. Loss of corneal defense against P. aeruginosa adhesion in the MyD88 knockout mouse corneas suggests either that mucin expression is MyD88-dependent, or that the role of mucins is indirect, perhaps via their capacity to sequester MyD88-dependent antimicrobial factors as shown for other tissues 42.
Corneal epithelial cells can internalize bacteria, and can subsequently traffic them to perinuclear vacuoles within the cell where they fail to thrive 8. Our more recent unpublished data indicates that vacuolar acidification reduces the viability of intracellular P. aeruginosa. Whether this is involved in defense against microbial penetration through the healthy corneal epithelium is yet to be determined. Supporting that possibility, however, is our observation that when P. aeruginosa is inoculated onto a healthy rat cornea, most internalized bacteria are found in cells that are readily shed from the eye with rinsing 6. That result supports the notion that internalization/cell shedding is a mechanism for clearing bacteria that manage to adhere to the surface.
If P. aeruginosa does manage to traverse the multilayered corneal epithelium and all of its defenses, the epithelial basement membrane (the basal lamina), composed mostly of extracellular matrix proteins, e.g. laminin and collagen type IV, prevents them from actually entering the corneal stroma. The basal lamina does this in two ways: one physical and the other biochemical. The basal lamina acts as a physical filter because it is a mesh containing pores smaller than the size of most bacteria 43. This filtering role played by the basal lamina explains why making the corneal epithelium susceptible to bacterial adhesion/traversal (using either EGTA or MyD88 knockout mice) does not necessarily result in microbial keratitis (disease/pathology) 26, 27. The pathology that occurs during microbial keratitis requires bacterial entry into the stroma, which then leads to the activation of inflammatory and immune responses and their subsequent damaging sequelae (e.g. see references 4, 5, 44). In the laboratory, the filtering role of the basal lamina can easily be observed using in vitro or in vivo models. Even within corneas made susceptible to disease by scratch injury, penetrating bacteria distant from the scratch-injured area can be seen aligned on the anterior surface of still intact basal lamina 45. After scarification, the cornea regains its resistance to infection within 12 hours, which corresponds to the time that bacteria are no longer able to take that final step into the stroma 46. Interestingly, reacquisition of resistance to corneal infection occurs before barrier function to fluorescein staining is completely reestablished. These results provide further evidence that fluorescein staining is a poor predictor of susceptibility to infection, and that other defense mechanisms (e.g. the basal lamina) can still protect the cornea against bacterial penetration when superficial tight junctions are compromised.
Our in vitro modeling experiments confirmed that basal lamina extracellular matrix proteins can form a barrier to bacterial passage 45. However, that study also showed another role for the basal lamina in defense, which was to improve the barrier function of the epithelial cells growing on top of them. The mechanism(s) by which these proteins impact the barrier function of cells on the opposite side of the multilayer is yet to be determined, but could involve effects on junctional integrity or antimicrobial peptide/mucin expression. Whatever the case, the intact basal lamina is another factor that is lacking in standard cell culture assays that could relate to the increased susceptibility of corneal epithelial cells to bacteria when grown in vitro.
In summary, corneal epithelial-associated barriers to P. aeruginosa consist of defenses against adhesion and defenses against microbial penetration (traversal). The players involved likely include junctional complexes, secreted and internal antimicrobial peptides, mucins, and the basal lamina foundation that provides a physical barrier while also supporting epithelial homeostasis. During and after P. aeruginosa challenge, corneal epithelial defenses are enhanced and regulated by epithelial-derived cytokines and chemokines that can facilitate communication with cells of the immune system to boost corneal defenses.
The bacterial perspective: P. aeruginosa opportunity and adaptability
P. aeruginosa is often referred to as an opportunistic pathogen, in that it requires some form of compromise to host defenses to cause infection. If the opportunity is offered, P. aeruginosa can be a formidable and versatile pathogen, even more destructive to host tissues than “true” pathogens. In addition to its capacity to invade cells and survive intracellularly, or to rapidly kill cells, using ExoS or ExoU respectively, it has other type three secreted toxins that also contribute to pathogenesis in the cornea and other tissues (e.g. refs 47-49). Other virulence factors can also contribute, including proteases, exotoxin A 13, 45, 50, 51, pili through their effects on corneal adhesion and twitching motility 52, 53 and lipopolysaccharide 54, 55. P. aeruginosa also has the ability to form biofilms, which are surface microcolonies surrounded by a polysaccharide-protein matrix, and it can even accomplish this on or in host tissues 56. Biofilms provide a unique and protective microenvironment that favors survival against antimicrobial agents and host immune defenses (see review 57). Biofilms are thought to allow P. aeruginosa, and other microbes, the opportunity to adapt to prevailing environmental conditions through alterations in their gene expression, and sometimes even allow the acquisition of new genes, e.g. encoding antibiotic resistance.
Using a rat model, we found that P. aeruginosa can form biofilms on the posterior surface of contact lenses worn in vivo, and that this was associated with the development of severe microbial keratitis without the necessity for prior scarification injury 56. These results confirmed what clinicians have long suspected; that significant “overt” injury to the cornea is not required for the pathogenesis of contact lens-related infection. When lenses from infected eyes harboring the in vivo grown biofilms were transferred to naïve animals, they were found to cause infections much faster than freshly inoculated lenses (median time reduced from 8 to 2 days).
It is not yet clear whether it is the biofilm itself that shortened infection onset time in the rat model, or if the role of the biofilm was simply to enable the bacteria to survive for long enough to adapt to the in vivo environment. Available data support the latter possibility; i.e. that P. aeruginosa can adapt to in vivo factors to become more virulent. For example, we have found that after P. aeruginosa has already traversed multilayered human corneal epithelium grown in vitro, it acquires an enhanced capacity to traverse naïve cells (~1000 fold) (unpublished data). Comparison of gene expression in the bacteria before and after they had traversed the corneal epithelium revealed numerous changes. Affected bacterial genes included 16 two-component sensor-response regulators which each control the expression of numerous genes. Hundreds of affected bacterial genes were also described as encoding hypothetical proteins indicating a currently unknown function. Much more work will be required to characterize the factors impacted, and to determine which conferred the enhanced capacity to traverse epithelial cells. The genes/gene products/pathways involved are likely to be excellent targets for preventing infection.
Why does contact lens wear predispose to infection?
Contact lens wear is a leading risk factor for P. aeruginosa keratitis 1, 58. The development of silicone hydrogel contact lenses with vastly greater oxygen transmissibility has not reduced the incidence of microbial keratitis 58, suggesting that hypoxia is not critical to pathogenesis. Based on our current knowledge, and because extended wear is a risk factor, we believe that bacterial adaptation coincident with changes to the biochemistry of tear fluid under the contact lens, are the most important contributors.
Live cell imaging of P. aeruginosa reveals that bacteria do not particularly like the apical surface of corneal epithelial cells. While they swim within range of apical cell surfaces as if curious, they remain a significant distance above the cell surface. Only occasionally do bacteria “home in” on the cells, and usually only if the cell is dead or dying. In contrast, they readily bind to areas of exposed glass or plastic between cells, and from that vantage point sometimes gain access to the underside of adjacent cells which are most vulnerable to their virulence strategies 7. Why the exposed apical cell surface repels bacteria is not completely clear, but it could be related to the surface-expressed mucins and/or release of antimicrobial peptides mentioned previously.
Avoidance of the apical cell surface is not unique to the interaction between P. aeruginosa and corneal cells. The gut harbors enormous numbers of bacteria, yet there is a clear zone about 50 microns wide above the epithelial cell surface that contains no microbes, thought maintained by mucins (e.g. Muc2) and associated antimicrobial factors released from the cell surface 42, 59. If the same is true for the ocular surface (where the presence of microbes is probably even less welcome), we expect the corneal surface and tear film above it to be devoid of microbes since it is only about 7 microns thick. At the ocular surface, the very effective sweeping action (shear force) of the eyelids combined with tear flow would make it even more difficult for microbes to get a foothold. Thus, it is not surprising that the eye is so efficient at clearing even very large inocula of bacteria, even those as adaptable and inherently resistant as P. aeruginosa 13.
When a contact lens is placed on the eye this scenario is likely to differ because it provides a surface for bacteria to stick to (which would help them resist physical removal), and it could also enable them to maintain a safe(r) distance from the hostile epithelial surface (which might help them resist being killed). Our experiments with rats confirm that this can indeed happen, since massive mature bacterial biofilms were found to have grown on the back surface of all inoculated worn lenses 56. Once a biofilm forms on this surface, which faces the cornea and tear fluid trapped against it, bacteria within it are likely to be exposed to sub-lethal doses of host-derived antimicrobials and other defense factors. P. aeruginosa has very few nutrient requirements, needing only a few key elements in low concentrations for growth, and is equipped with an unusually large number of genes devoted to adaptation and survival 60. It is already known that these include systems that can respond to host defense factors, resulting in up-regulated resistance to antimicrobial peptides, production of proteases with capacity to break down defense proteins and physical barriers, up-regulation of polysaccharides that help resist recognition and/or phagocytosis by host cells, up-regulation of secreted toxins that damage cells, etc. Thus, P. aeruginosa is in an excellent position to take advantage of the contact lens on the eye, likely explaining why it is the leading cause of contact lens-related infections.
There is a second set of events, however, that is also likely to contribute to the pathogenesis of P. aeruginosa keratitis. Soft contact lens wear allows for very little tear exchange, a factor that would be expected to take a toll on ocular defenses. For example, factors and cells shed from the ocular surface would be less readily removed, and the various tear components, which come from different locations around the ocular surface, could become separated from each other, potentially upsetting the delicate balance between ingredients such as proteases and their respective inhibitors. Molecules important for maintaining homeostasis at the ocular surface could be degraded, either through time or by bacterial proteases, lipases, phospholipases etc. Indeed, we have shown that the protective effects of tears are lost when incubated with P. aeruginosa for several hours 14. Thus, it would not be surprising if the ability of tear fluid to modulate epithelial immunity (as discussed in previous sections of this perspective paper), is compromised in contact lens wear, at least for the critical post-lens tear film that is in contact with the cornea.
Epithelial cells could also suffer directly when wearing a contact lens. We have shown that innate defense responses of cells are blunted after cells wear a contact lens, even in vitro. Human corneal epithelial cells exposed to soft contact lenses in vitro for ~3 days, failed to up-regulate the antimicrobial peptide hBD-2 in response to challenge with P. aeruginosa antigens 61. Further experiments revealed that contact lens-exposed cells failed to activate the transcription factor AP-1 (modulates a range of protective factors) in response to P. aeruginosa antigens, but could still activate NFκB (associated with pro-inflammatory events). Those data suggest that contact lens wear could hinder antimicrobial defenses of the cornea while still allowing potentially damaging pro-inflammatory mediators to compromise epithelial barrier function. Other contact lens-mediated effects on epithelial cell biology in vivo shown by others, including reduced epithelial cell proliferation and differentiation, could also influence corneal innate defense against P. aeruginosa and other pathogens 62.
In some instances, contact lens care solutions could find a way onto the ocular surface. Some of these care solutions have the potential to impact epithelial homeostasis and barrier function 63, 64. However, it is not yet known if lens care solution effects on the cornea are a risk factor for microbial keratitis in humans.
For an infection to occur, the basal lamina would also need to be compromised. How could this happen during lens wear? It is not yet known whether inflammation is an early step in the pathogenesis of contact lens infections. If it is, immune cells infiltrating into the cornea could potentially damage barriers that normally protect against bacterial penetration, including the basal lamina. While the cornea does not normally respond immunologically to microbes outside the cornea, lens wear could potentially compromise the mechanisms that suppress inflammatory or immune responses (perhaps due to changes in tear biochemistry alluded to above), in which case bacteria growing on the back of the contact lens could mediate a host response. Alternatively, the basal lamina might be structurally or biochemically abnormal in those who succumb to contact lens related infections. This could be because the corneal epithelial cells that help make the basal lamina are impacted by lens wear through mechanisms discussed above, or there could be genetic reasons. The latter is feasible considering that the incidence of infection has been surprisingly stable over the years despite the introduction of many new lens and solution types. Indeed, associations between susceptibility to microbial keratitis and single nucleotide polymorphisms in cytokine genes have been reported 1.
A common theme for the above-proposed mechanisms, is the requirement for an extended exposure time. Contact lens biofilm formation and keratitis in vivo, suppression of antimicrobial peptide expression, changes to tear fluid biochemistry, and activation of immune responses, all require time to manifest. The need to wait for these events to unfold is likely to explain the elevated risk of P. aeruginosa keratitis during extended wear of contact lenses 58, and it provides us with avenues for reducing risk.
Conclusions and Future Goals
We are learning that the ocular surface possesses multiple integrated defenses (Fig. 1 and Table 1) that almost universally protect the cornea against P. aeruginosa (the focus of our studies). However, it is clear that these defenses, and the intrinsic resistance of the healthy cornea, have evolved (and are conserved across species) to protect the eye against a broad-range of microbial pathogens, e.g. other bacteria, fungi, viruses and protozoa. This is clearly evidenced in numerous animal models of bacterial, fungal, viral, or protozoan corneal infections. In most of those models, infection occurs only when the corneal epithelium is deeply damaged by injury through to the anterior stroma, or if it is completely by-passed by intrastromal injection 4, 24, 65. Stromal entry of microbial cells then invokes a powerful inflammatory and immune response 4, 5. Even with a contact lens-wearing animal model, i.e. without prior induction of epithelial injury, initial P. aeruginosa infection and disease only occurs after a prolonged exposure 56. Otherwise, the cornea “brushes off” even large numbers of contaminating microbes, even those capable of causing massive cell and tissue destruction, with barely a trace of evidence. Incredibly, it resists P. aeruginosa infection even when bacteria have penetrated the epithelium to the basal lamina, which only occurs when there is significant compromise to tear/epithelial defenses. We believe that “null infection” models (in which the maintenance of health, rather than disease, is the outcome, including those described above) can be a useful complement to traditional infection models (in which disease is the usual outcome). Null infection models will be particularly helpful for understanding the mechanisms that protect us against opportunistic bacteria such as P. aeruginosa. These models would provide a foundation for studies aimed at understanding how and why compromise allows susceptibility, and for developing novel therapies that augment our own defenses. High-resolution imaging of unprocessed corneal tissue can be a useful complement to such efforts, because it allows the relationships between individual bacteria and cells to be studied over time in vivo, which previously required in vitro experimentation with its associated limitations.
While a significant amount of work remains to be done to fully understand contact lens-related corneal infection involving P. aeruginosa, or other causative microbes, we now have clear paths to follow. Our aim is to eliminate this problem, and use the information gained from doing this research to develop new ways to prevent infections in general. A greater availability of lens-wearing animal models, in particular contact lenses that fit mouse eyes, would be extremely valuable for moving forward with this effort.
Our long-term goal is the development of new therapeutic and/or preventive interventions in contact lens-related P. aeruginosa keratitis. Current therapeutic interventions involve anti-Pseudomonal antibiotics, e.g. aminoglycosides or fluoroquinolones, and prevention relies on reducing P. aeruginosa contamination of contact lenses and lens cases using contact lens care (disinfection) solutions. Extending beyond, and improving, these clinical approaches requires two major advances. Firstly, a detailed knowledge of the critical P. aeruginosa factors (virulence genes and proteins) required for survival and adaptation on a contact lens in the lens case and more importantly on the ocular surface, which allow this versatile pathogen the means to breach the corneal epithelial barrier. Secondly, detailed knowledge of the intrinsic host defense mechanisms of the cornea that allow resistance to P. aeruginosa, and how a contact lens induces sufficient compromise to allow bacterial adaptation and virulence to cause infection.
Acknowledgements
A. This work was funded by the National Eye Institute (EY011221) and the National Institute of Allergy and Infectious Diseases (AI079192). Other research support was also provided by the Bill and Melinda Gates Foundation, Alcon, Allergan, CooperVision, and the American Optometric Foundation (Vistakon Research Grants).
D. We thank all of our undergraduate and graduate students, optometry students, post-doctoral fellows and research scientists, and numerous exceptional collaborators and colleagues for their invaluable contributions to this research effort.
Biography
Dr. David Evans received a BSc and PhD (Pharmacy) from the University of Manchester, UK. He completed a post-doctoral fellowship at Harvard Medical School, and is currently a Professor of Biological and Pharmaceutical Sciences, College of Pharmacy, Touro University CA, and Associate Research Scientist, School of Optometry, University of California, Berkeley. Dr. Evans research interests are; pathogenesis of corneal infections, innate immunity, and antimicrobial therapeutics. He has collaborated with Dr. Fleiszig for nearly two decades.

B. Dr. Fleiszig is a paid consultant for Allergan. Drs. Fleiszig and Evans are listed as co-inventors on several US patents (or pending applications) belonging to the University of California, Berkeley, which involve use, or up-regulation, of ocular antimicrobial factors to prevent or treat ocular infections. These patents or applications relate to the use or up-regulation of collectins, defensins, and keratin-derived antimicrobial peptides.

Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stapleton F, Carnt N. Contact lens-related microbial keratitis: how have epidemiology and genetics helped us with pathogenesis and prophylaxis. Eye (Lond) 2012;26(2):185–193. doi: 10.1038/eye.2011.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lichtinger A, Yeung SN, Kim P, et al. Shifting trends in bacterial keratitis in toronto: an 11-year review. Ophthalmology. 2012;119(9):1785–1790. doi: 10.1016/j.ophtha.2012.03.031. [DOI] [PubMed] [Google Scholar]
- 3.Green M, Apel A, Stapleton F. Risk factors and causative organisms in microbial keratitis. Cornea. 2008;27(1):22–27. doi: 10.1097/ICO.0b013e318156caf2. [DOI] [PubMed] [Google Scholar]
- 4.Hazlett LD, Hendricks RL. Reviews for immune privilege in the year 2010: immune privilege and infection. Ocul Immunol Inflamm. 2010;18(4):237–243. doi: 10.3109/09273948.2010.501946. [DOI] [PubMed] [Google Scholar]
- 5.Hazlett LD. Bacterial infections of the cornea (Pseudomonas aeruginosa) Chem Immunol Allergy. 2007;92:185–194. doi: 10.1159/000099269. [DOI] [PubMed] [Google Scholar]
- 6.Fleiszig SM, Zaidi TS, Pier GB. Pseudomonas aeruginosa invasion of and multiplication within corneal epithelial cells in vitro. Infect Immun. 1995;63(10):4072–4077. doi: 10.1128/iai.63.10.4072-4077.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fleiszig SM, Evans DJ, Do N, et al. Epithelial cell polarity affects susceptibility to Pseudomonas aeruginosa invasion and cytotoxicity. Infect Immun. 1997;65(7):2861–2867. doi: 10.1128/iai.65.7.2861-2867.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Angus AA, Lee AA, Augustin DK, et al. Pseudomonas aeruginosa induces membrane blebs in epithelial cells, which are utilized as a niche for intracellular replication and motility. Infect Immun. 2008;76(5):1992–2001. doi: 10.1128/IAI.01221-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Angus AA, Evans DJ, Barbieri JT, Fleiszig SM. The ADP-ribosylation domain of Pseudomonas aeruginosa ExoS is required for membrane bleb niche formation and bacterial survival within epithelial cells. Infect Immun. 2010;78(11):4500–4510. doi: 10.1128/IAI.00417-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fleiszig SM, Zaidi TS, Preston MJ, et al. Relationship between cytotoxicity and corneal epithelial cell invasion by clinical isolates of Pseudomonas aeruginosa. Infect Immun. 1996;64(6):2288–2294. doi: 10.1128/iai.64.6.2288-2294.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finck-Barbancon V, Goranson J, Zhu L, et al. ExoU expression by Pseudomonas aeruginosa correlates with acute cytotoxicity and epithelial injury. Mol Microbiol. 1997;25(3):547–557. doi: 10.1046/j.1365-2958.1997.4891851.x. [DOI] [PubMed] [Google Scholar]
- 12.Fleiszig SM, Wiener-Kronish JP, Miyazaki H, et al. Pseudomonas aeruginosa-mediated cytotoxicity and invasion correlate with distinct genotypes at the loci encoding exoenzyme S. Infect Immun. 1997;65(2):579–586. doi: 10.1128/iai.65.2.579-586.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mun JJ, Tam C, Kowbel D, et al. Clearance of Pseudomonas aeruginosa from a healthy ocular surface involves surfactant protein D and is compromised by bacterial elastase in a murine null-infection model. Infect Immun. 2009;77(6):2392–2398. doi: 10.1128/IAI.00173-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleiszig SM, Kwong MS, Evans DJ. Modification of Pseudomonas aeruginosa interactions with corneal epithelial cells by human tear fluid. Infect Immun. 2003;71(7):3866–3874. doi: 10.1128/IAI.71.7.3866-3874.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwong MS, Evans DJ, Ni M, Cowell BA, Fleiszig SM. Human tear fluid protects against Pseudomonas aeruginosa keratitis in a murine experimental model. Infect Immun. 2007;75(5):2325–2332. doi: 10.1128/IAI.01404-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selinger DS, Selinger RC, Reed WP. Resistance to infection of the external eye: the role of tears. Surv Ophthalmol. 1979;24(1):33–38. doi: 10.1016/0039-6257(79)90145-0. [DOI] [PubMed] [Google Scholar]
- 17.Flanagan JL, Willcox MD. Role of lactoferrin in the tear film. Biochimie. 2009;91(1):35–43. doi: 10.1016/j.biochi.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 18.Mun JJ, Tam C, Evans DJ, Fleiszig SM. Modulation of epithelial immunity by mucosal fluid. Sci Rep. 2011;1:8. doi: 10.1038/srep00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fleiszig SM, Zaidi TS, Ramphal R, Pier GB. Modulation of Pseudomonas aeruginosa adherence to the corneal surface by mucus. Infect Immun. 1994;62(5):1799–1804. doi: 10.1128/iai.62.5.1799-1804.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ni M, Evans DJ, Hawgood S, et al. Surfactant protein D is present in human tear fluid and the cornea and inhibits epithelial cell invasion by Pseudomonas aeruginosa. Infect Immun. 2005;73(4):2147–2156. doi: 10.1128/IAI.73.4.2147-2156.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masinick SA, Montgomery CP, Montgomery PC, Hazlett LD. Secretory IgA inhibits Pseudomonas aeruginosa binding to cornea and protects against keratitis. Invest Ophthalmol Vis Sci. 1997;38(5):910–918. [PubMed] [Google Scholar]
- 22.Dartt DA. Tear lipocalin: structure and function. Ocul Surf. 2011;9(3):126–138. doi: 10.1016/s1542-0124(11)70022-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Callegan MC, Hobden JA, Hill JM, Insler MS, O’Callaghan RJ. Topical antibiotic therapy for the treatment of experimental Staphylococcus aureus keratitis. Invest Ophthalmol Vis Sci. 1992;33(11):3017–3023. [PubMed] [Google Scholar]
- 24.Marquart ME. Animal models of bacterial keratitis. J Biomed Biotechnol. 2011:680642. doi: 10.1155/2011/680642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hazlett LD. Corneal response to Pseudomonas aeruginosa infection. Prog Retin Eye Res. 2004;23(1):1–30. doi: 10.1016/j.preteyeres.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Alarcon I, Tam C, Mun JJ, et al. Factors impacting corneal epithelial barrier function against Pseudomonas aeruginosa traversal. Invest Ophthalmol Vis Sci. 2011;52(3):1368–1377. doi: 10.1167/iovs.10-6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tam C, LeDue J, Mun JJ, et al. 3D quantitative imaging of unprocessed live tissue reveals epithelial defense against bacterial adhesion and subsequent traversal requires MyD88. PLoS One. 2011;6(8):e24008. doi: 10.1371/journal.pone.0024008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta G, Surolia A. Collectins: sentinels of innate immunity. Bioessays. 2007;29(5):452–464. doi: 10.1002/bies.20573. [DOI] [PubMed] [Google Scholar]
- 29.Pearlman E, Johnson A, Adhikary G, et al. Toll-like receptors at the ocular surface. Ocul Surf. 2008;6(3):108–116. doi: 10.1016/s1542-0124(12)70279-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McDermott AM. The role of antimicrobial peptides at the ocular surface. Ophthalmic Res. 2009;41(2):60–75. doi: 10.1159/000187622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar A, Yu FS. Toll-like receptors and corneal innate immunity. Curr Mol Med. 2006;6(3):327–337. doi: 10.2174/156652406776894572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar A, Yin J, Zhang J, Yu FS. Modulation of corneal epithelial innate immune response to pseudomonas infection by flagellin pretreatment. Invest Ophthalmol Vis Sci. 2007;48(10):4664–4670. doi: 10.1167/iovs.07-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McDermott AM, Redfern RL, Zhang B, et al. Defensin expression by the cornea: multiple signalling pathways mediate IL-1beta stimulation of hBD-2 expression by human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2003;44(5):1859–1865. doi: 10.1167/iovs.02-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hiemstra IH, Bouma G, Geerts D, Kraal G, den Haan JM. Nod2 improves barrier function of intestinal epithelial cells via enhancement of TLR responses. Mol Immunol. 2012;52(3-4):264–272. doi: 10.1016/j.molimm.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 35.Redfern RL, Reins RY, McDermott AM. Toll-like receptor activation modulates antimicrobial peptide expression by ocular surface cells. Exp Eye Res. 2011;92(3):209–220. doi: 10.1016/j.exer.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McNamara NA, Van R, Tuchin OS, Fleiszig SM. Ocular surface epithelia express mRNA for human beta defensin-2. Exp Eye Res. 1999;69(5):483–490. doi: 10.1006/exer.1999.0722. [DOI] [PubMed] [Google Scholar]
- 37.Augustin DK, Heimer SR, Tam C, et al. Role of defensins in corneal epithelial barrier function against Pseudomonas aeruginosa traversal. Infect Immun. 2011;79(2):595–605. doi: 10.1128/IAI.00854-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamrah P, Dana MR. Corneal antigen-presenting cells. Chem Immunol Allergy. 2007;92:58–70. doi: 10.1159/000099254. [DOI] [PubMed] [Google Scholar]
- 39.Tam C, Mun JJ, Evans DJ, Fleiszig SM. Cytokeratins mediate epithelial innate defense through their antimicrobial properties. J Clin Invest. 2012;122(10):3665–3677. doi: 10.1172/JCI64416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zasloff M. Defending the cornea with antibacterial fragments of keratin. J Clin Invest. 2012;122(10):3471–3473. doi: 10.1172/JCI65380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blalock TD, Spurr-Michaud SJ, Tisdale AS, et al. Functions of MUC16 in corneal epithelial cells. Invest Ophthalmol Vis Sci. 2007;48(10):4509–4518. doi: 10.1167/iovs.07-0430. [DOI] [PubMed] [Google Scholar]
- 42.Meyer-Hoffert U, Hornef MW, Henriques-Normark B, et al. Secreted enteric antimicrobial activity localises to the mucus surface layer. Gut. 2008;57(6):764–771. doi: 10.1136/gut.2007.141481. [DOI] [PubMed] [Google Scholar]
- 43.Abrams GA, Goodman SL, Nealey PF, Franco M, Murphy CJ. Nanoscale topography of the basement membrane underlying the corneal epithelium of the rhesus macaque. Cell Tissue Res. 2000;299(1):39–46. doi: 10.1007/s004419900074. [DOI] [PubMed] [Google Scholar]
- 44.Willcox MD. Pseudomonas aeruginosa infection and inflammation during contact lens wear: a review. Optom Vis Sci. 2007;84(4):273–278. doi: 10.1097/OPX.0b013e3180439c3e. [DOI] [PubMed] [Google Scholar]
- 45.Alarcon I, Kwan L, Yu C, Evans DJ, Fleiszig SM. Role of the corneal epithelial basement membrane in ocular defense against Pseudomonas aeruginosa. Infect Immun. 2009;77(8):3264–3271. doi: 10.1128/IAI.00111-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee EJ, Evans DJ, Fleiszig SM. Role of Pseudomonas aeruginosa ExsA in penetration through corneal epithelium in a novel in vivo model. Invest Ophthalmol Vis Sci. 2003;44(12):5220–5227. doi: 10.1167/iovs.03-0229. [DOI] [PubMed] [Google Scholar]
- 47.Lee EJ, Cowell BA, Evans DJ, Fleiszig SM. Contribution of ExsA-regulated factors to corneal infection by cytotoxic and invasive Pseudomonas aeruginosa in a murine scarification model. Invest Ophthalmol Vis Sci. 2003;44(9):3892–3898. doi: 10.1167/iovs.02-1302. [DOI] [PubMed] [Google Scholar]
- 48.Zolfaghar I, Evans DJ, Ronaghi R, Fleiszig SM. Type III secretion-dependent modulation of innate immunity as one of multiple factors regulated by Pseudomonas aeruginosa RetS. Infect Immun. 2006;74(7):3880–3889. doi: 10.1128/IAI.01891-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shaver CM, Hauser AR. Relative contributions of Pseudomonas aeruginosa ExoU, ExoS, and ExoT to virulence in the lung. Infect Immun. 2004;72(12):6969–6977. doi: 10.1128/IAI.72.12.6969-6977.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Twining SS, Kirschner SE, Mahnke LA, Frank DW. Effect of Pseudomonas aeruginosa elastase, alkaline protease, and exotoxin A on corneal proteinases and proteins. Invest Ophthalmol Vis Sci. 1993;34(9):2699–2712. [PubMed] [Google Scholar]
- 51.O’Callaghan RJ, Engel LS, Hobden JA, et al. Pseudomonas keratitis. The role of an uncharacterized exoprotein, protease IV, in corneal virulence. Invest Ophthalmol Vis Sci. 1996;37(4):534–543. [PubMed] [Google Scholar]
- 52.Zolfaghar I, Evans DJ, Fleiszig SM. Twitching motility contributes to the role of pili in corneal infection caused by Pseudomonas aeruginosa. Infect Immun. 2003;71(9):5389–5393. doi: 10.1128/IAI.71.9.5389-5393.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alarcon I, Evans DJ, Fleiszig SM. The role of twitching motility in Pseudomonas aeruginosa exit from and translocation of corneal epithelial cells. Invest Ophthalmol Vis Sci. 2009;50(5):2237–2244. doi: 10.1167/iovs.08-2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zaidi TS, Fleiszig SM, Preston MJ, Goldberg JB, Pier GB. Lipopolysaccharide outer core is a ligand for corneal cell binding and ingestion of Pseudomonas aeruginosa. Invest Ophthalmol Vis Sci. 1996;37(6):976–986. [PubMed] [Google Scholar]
- 55.Zaidi TS, Lyczak J, Preston M, Pier GB. Cystic fibrosis transmembrane conductance regulator-mediated corneal epithelial cell ingestion of Pseudomonas aeruginosa is a key component in the pathogenesis of experimental murine keratitis. Infect Immun. 1999;67(3):1481–1492. doi: 10.1128/iai.67.3.1481-1492.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tam C, Mun JJ, Evans DJ, Fleiszig SM. The impact of inoculation parameters on the pathogenesis of contact lens-related infectious keratitis. Invest Ophthalmol Vis Sci. 2010;51(6):3100–3106. doi: 10.1167/iovs.09-4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hoiby N, Ciofu O, Johansen HK, et al. The clinical impact of bacterial biofilms. Int J Oral Sci. 2011;3(2):55–65. doi: 10.4248/IJOS11026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stapleton F, Keay L, Edwards K, et al. The incidence of contact lens-related microbial keratitis in Australia. Ophthalmology. 2008;115(10):1655–1662. doi: 10.1016/j.ophtha.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 59.Johansson ME, Phillipson M, Petersson J, et al. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci U S A. 2008;105(39):15064–15069. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kung VL, Ozer EA, Hauser AR. The accessory genome of Pseudomonas aeruginosa. Microbiol Mol Biol Rev. 2010;74(4):621–641. doi: 10.1128/MMBR.00027-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maltseva IA, Fleiszig SM, Evans DJ, et al. Exposure of human corneal epithelial cells to contact lenses in vitro suppresses the upregulation of human beta-defensin-2 in response to antigens of Pseudomonas aeruginosa. Exp Eye Res. 2007;85(1):142–153. doi: 10.1016/j.exer.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 62.Robertson DM, Cavanagh HD. The Clinical and Cellular Basis of Contact Lens-related Corneal Infections: A Review. Clin Ophthalmol. 2008;2(4):907–917. doi: 10.2147/opth.s3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Robertson DM, Petroll WM, Jester JV, Cavanagh HD. The role of contact lens type, oxygen transmission, and care-related solutions in mediating epithelial homeostasis and pseudomonas binding to corneal cells: an overview. Eye Contact Lens. 2007;33(6 Pt 2):394–398. doi: 10.1097/ICL.0b013e318157e609. discussion 399-400. [DOI] [PubMed] [Google Scholar]
- 64.Gorbet MB, Tanti NC, Crockett B, Mansour L, Jones L. Effect of contact lens material on cytotoxicity potential of multipurpose solutions using human corneal epithelial cells. Mol Vis. 2011;17:3458–3467. [PMC free article] [PubMed] [Google Scholar]
- 65.Leal SM, Jr., Pearlman E. The role of cytokines and pathogen recognition molecules in fungal keratitis - Insights from human disease and animal models. Cytokine. 2012;58(1):107–111. doi: 10.1016/j.cyto.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]