Abstract
Purpose
Urological disorders are the most common cause of pediatric chronic kidney disease. We determined the characteristics of children with urological disorders and assessed the agreement between the newly developed bedside glomerular filtration rate estimating formula with measured glomerular filtration rate in 586 patients in the Chronic Kidney Disease in Children study.
Materials and Methods
The Chronic Kidney Disease in Children study is a prospective, observational cohort of children recruited from 48 sites in the United States and Canada. Eligibility requirements include age 1 to 16 years and estimated glomerular filtration rate by original Schwartz formula 30 to 90 ml/min/1.73 m2. Baseline demographics, clinical variables and glomerular filtration rate were assessed. Bland-Altman analysis was conducted to assess agreement between estimated and measured glomerular filtration rates.
Results
Of the 586 participants with at least 1 glomerular filtration rate measurement 348 (59%) had an underlying urological diagnosis (obstructive uropathy in 118, aplastic/hypoplastic/dysplastic kidneys in 104, reflux in 87 and other condition in 39). Among these patients median age was 9 years, duration of chronic kidney disease was 7 years and age at first visit with a urologist was less than 1 year. Of the patients 67% were male, 67% were white and 21% had a low birth weight. Median height was in the 24th percentile. Median glomerular filtration rate as measured by iohexol plasma disappearance was 44.8 ml/min/1.73 m2. Median glomerular filtration rate as estimated by the Chronic Kidney Disease in Children bedside equation was 44.3 ml/min/1.73 m2 (bias = −0.5, 95% CI −1.7 to 0.7, p = 0.44).
Conclusions
Underlying urological causes of chronic kidney disease were present in 59% of study participants. These children were diagnosed early in life, and many had low birth weight and growth delay. There is good agreement between the newly developed Chronic Kidney Disease in Children estimating equations and measured glomerular filtration rate in this population.
Keywords: congenital, hereditary, and neonatal diseases and abnormalities, glomerular filtration rate, kidney failure, chronic, pediatrics, urology
Urological disorders account for up to 60% of underlying diagnoses in children 0 to 12 years old with chronic kidney disease.1–3 The leading urological causes of chronic kidney disease include obstructive uropathy, reflux nephropathy and kidney aplasia/hypoplasia/dysplasia.1,4,5 Obstructive uropathy and aplastic/hypoplastic/dysplastic kidneys each account for 16% of patients undergoing renal transplantation in the NAPRTCS registry.1 In the ItalKid Project 25.4% of 1,348 pediatric patients had a diagnosis of vesicoureteral reflux, and among children with chronic kidney disease at baseline the risk of ESRD by age 20 was 56%.5 In a recent analysis of the NAPRTCS registry 8.5% of pediatric patients had vesicoureteral reflux as a cause of kidney failure.6 Pediatric urologists have an important role in identifying children at risk for chronic kidney disease in collaboration with the pediatric nephrologist. To optimize the long-term outcome for these children, it has been suggested that renal function be monitored through time.7,8
Although preservation of kidney function is a primary goal of urological treatment, few data exist regarding the best way to monitor kidney function in this patient population. While glomerular filtration rate is considered the best measure of kidney function, serum creatinine alone is a poor indicator of GFR in children with CKD. As an alternative to creatinine, the National Kidney Foundation has released guidelines recommending GFR estimating equations as a preferable measure of kidney function. In the past the original Schwartz formula was recommended, which was initially developed in 1976.9 Recently Schwartz et al demonstrated that the newly derived CKiD equation provides excellent estimation of GFR, better than previously published formulas such as the original Schwartz and Filler equations.10–12
CKiD provides a unique opportunity to study children with urological disease leading to CKD. The objectives of this study were to determine the demographic and clinical characteristics of children in the CKiD cohort with underlying urological disorders, and to present the newly developed CKiD derived estimating equation and updated bedside formula in children with urological disease.
MATERIALS AND METHODS
Chronic Kidney Disease in Children Study
The CKiD study is a prospective, observational cohort of children with moderate CKD.13 There are 2 clinical coordinating centers and 48 recruitment sites in the United States and Canada (http://www.statepi.jhsph.edu/ckid). Institutional review board approval for the study was obtained at each site. The study began in 2003, enrollment commenced in 2005 and longitudinal followup is planned through 2013. Inclusion criteria consisted of age 1 to 16 years and mild to moderate impairment in kidney function as defined by an estimated GFR of 30 to 90 ml/min/1.73 m2 by the original Schwartz formula.9 This level of GFR was consistent with moderate CKD but was not yet severe enough to require dialysis or be considered ESRD. The original Schwartz equation, based on a constant (child height in cm and serum creatinine), is calculated as, [k × Ht(cm)]/SCr, where Ht is height, SCr is serum creatinine, and k = 0.45 for males and females 12 to 18 months, 0.55 for males 19 months to less than 13 years and females 19 months and older, and 0.7 for males 13 to 20 years.9
For this analysis cross-sectional baseline data, including demographics, clinical variables, GFR and physical examination, were assessed at study entry for all participants with an underlying urological diagnosis as ascertained from the eligibility form. Demographics and descriptive epidemiology were determined from the general history and medical history forms.
Measurement and Estimation of Glomerular Filtration Rate
The level of kidney function, iGFR, was measured by the iohexol clearance protocol as described previously.11 Recently a new CKiD derived estimating equation (eGFR) has been developed based on child height, serum creatinine, cystatin C (CysC) and blood urea nitrogen, which is, 39.1[Ht(m)/SCr]0.516 [1.8/CysC]0.294 [30/BUN]0.169 × [1.099]male [Ht(m)/1.4]0.188.10 An abbreviated bedside equation (bedside GFR), updating the original Schwartz equation, has also been developed based on height and serum creatinine, which is, 0.413 × [Ht(cm)/SCr]. We chose to examine the more convenient bedside equation among children with urological disorders in the CKiD cohort.
Statistical Analysis
A cross-sectional analysis of children was performed using baseline data from the CKiD study. A single primary diagnosis was determined by the local practitioner or nephrologist. Diagnoses that were either congenital anomalies of the kidney and urinary tract or acquired structural causes of CKD were categorized as urological and included obstructive uropathy, aplastic/hypoplastic/dysplastic kidneys, reflux nephropathy, prune belly syndrome and other causes.8 Nonparametric statistics (medians and interquartile ranges) were used to describe the demographic and clinical characteristics of the cohort. Kruskal-Wallis analysis, using Wilcoxon rank sum and Fisher’s exact tests, was used to compare characteristics among urological disease subgroups.
Estimated GFR was calculated according to previously published formulas (CKiD eGFR and bedside GFR), and agreement was assessed with comparison of the measured iohexol GFR at the same visit. Agreement was measured by extending methods proposed by Bland and Altman.14 Agreement was specifically described by correlation, bias (relative difference between estimated GFR and iGFR values) and ratio of standard deviations.11 Correlations were calculated with Fisher 95% confidence intervals. In the Bland-Altman plot the bias was calculated as the intercept of the difference between the estimating GFR equation and iGFR centered around the mean of the average between the estimating GFR equation and iGFR. The ratio of standard deviation was calculated as the antilog of the slope of this line as described previously.11 Since a portion of subjects were used to develop the estimated GFR equation, we performed a secondary validation analysis that describes the agreement among subjects who were new to the study since the equation was developed. Enhanced histograms graphically depict the bias, variance and correlation comparing the estimated GFR by the CKiD bedside equation and corresponding iGFR value, showing data for each subject in this subset.
RESULTS
Baseline Characteristics of Cohort
At the baseline visit 586 children had at least 1 measure of GFR available. Of these children 348 (59%) had an underlying urological diagnosis (obstructive uropathy in 118, aplastic/hypoplastic/dysplastic kidneys in 104, reflux nephropathy in 87, prune belly syndrome in 11 and other causes in 28). Other cause diagnoses included hydronephrosis (10 patients); vertebral and vascular anomalies, anal atresia, esophageal atresia and/or tracheoesophageal fistula, and radial and renal anomalies/vertebral, anal, cardiac, tracheal, esophageal, renal and limb abnormalities (6); unknown (4); multiple diagnoses (4); bladder exstrophy (1); cross-fused ectopic kidneys (1); rhabdomyosarcoma (1), and spina bifida (1). Median patient age was 9 years and median duration of CKD was 7 years. Children with underlying urological disorders were predominantly male (67%), white (67%), young at onset of kidney disease (median less than 1 year), and were in the 24th percentile for height and 40th percentile for weight compared to norms at entry into the study. Of the patients 21% had low birth weight and 52% required ICU treatment after delivery. Median serum creatinine was 1.2 mg/dl and median serum cystatin C was 1.8 mg/l. Median GFR as measured by iohexol plasma disappearance for children with urological disorders was 44.8 ml/min/1.73 m2 (normal 90 to 120).15
Among children with CKD due to urological disorders the highest proportion of males was among those with obstructive uropathy (85%). Median age at presentation was less than 1 year for those with obstructive uropathy or aplasia/hypoplasia/dysplasia and 1.7 years for those with a primary diagnosis of reflux. A substantial proportion (21%) of all children with CKD due to urological disease had a history of low birth weight, and 20% had uncontrolled hypertension (systolic or diastolic blood pressure above 95th percentile based on age, gender and height). More than half of those with obstructive uropathy or aplasia/hypoplasia/dysplasia had been in the ICU after delivery, while 36% of those with reflux had been in the neonatal ICU. Of children with urological disorders 291 had seen a urologist at a median age of less than 1 year, 238 had undergone a urological procedure and 151 had had a kidney infection. Of children with reported kidney infection an average of 1 infection was reported during the first year of life. The most commonly reported methods of diagnosis of underlying cause of kidney disease were ultrasound and voiding cystourethrogram (table 1).
Table 1.
Parent reported urological procedures and infection in children with urological disease
| Pts With Urological Disease | Subgroups of Urological Disease
|
p Value* | ||||
|---|---|---|---|---|---|---|
| Obstructive Uropathy | Aplastic/Hypoplastic/Dysplastic | Reflux Nephropathy | Other | |||
| No. urologist visits (%) | 291 (84) | 117 (99) | 61 (59) | 77 (90) | 36 (95) | <0.001 |
| Median yrs age at presentation (IQR) | Less than 1 (less than 1–2) | Less than 1 (0–1) | Less than 1 (0–2) | 1 (0–5) | Less than 1 (0–1) | 0.005 |
| No. urological procedure (%) | 238 (70) | 103 (91) | 42 (40) | 64 (76) | 29 (74) | <0.001 |
| No. kidney infections, ever (%): | 151 (48) | 60 (57) | 27 (28) | 42 (53) | 22 (61) | <0.001 |
| Median No. first yr of life (IQR) | 1 (0, 3) | 1 (0, 3) | 1 (0, 3) | 1 (0, 3) | 2 (0, 3) | 0.726 |
| Median No. yr before baseline visit (IQR) | 0 (0, 1) | 0 (0, 1) | 1 (0, 1) | 0 (0, 0.5) | 1 (0, 2) | 0.001 |
| No. diagnostic method (%): | ||||||
| Ultrasound | 309 (97) | 108 (98) | 91 (96) | 77 (97) | 33 (100) | 0.666 |
| Voiding cystourethrogram | 217 (73) | 80 (75) | 48 (56) | 70 (91) | 19 (68) | <0.001 |
| Nuclear medicine study | 136 (49) | 47 (48) | 29 (35) | 44 (63) | 16 (55) | 0.006 |
| Magnetic resonance imaging | 56 (21) | 22 (23) | 15 (19) | 11 (17) | 8 (27) | 0.636 |
| Computerized tomography | 57 (21) | 21 (22) | 15 (18) | 15 (21) | 6 (21) | 0.947 |
| Excretory urography | 40 (15) | 11 (12) | 11 (14) | 12 (19) | 6 (21) | 0.510 |
| Genetic testing | 24 (9) | 7 (7) | 13 (15) | 2 (3) | 2 (7) | 0.059 |
| Kidney biopsy | 11 (4) | 4 (4) | 4 (4) | 2 (3) | 1 (3) | 0.970 |
| Other | 57 (22) | 17 (18) | 13 (17) | 18 (27) | 9 (32) | 0.191 |
Kruskal-Wallis analysis using Wilcoxon rank sum scores for continuous variables and Fisher’s exact test for categorical variables.
Estimation of Glomerular Filtration Rate
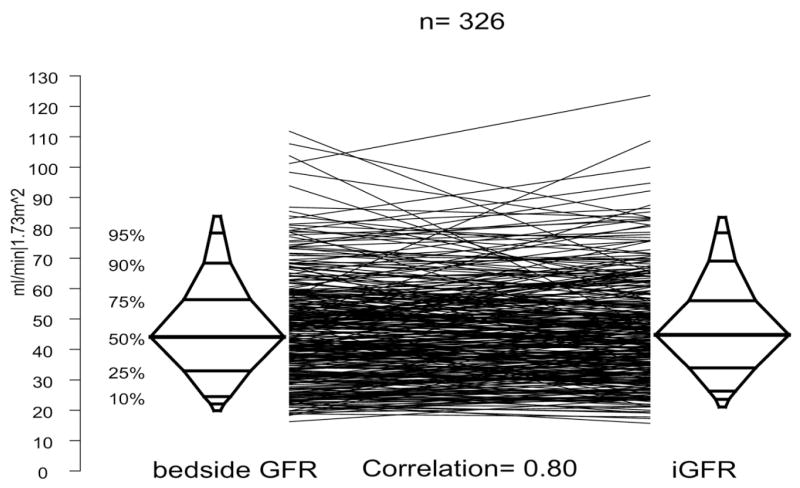
There were 326 subjects who had valid serum creatinine, cystatin C and iGFR measurements, by which we assessed agreement between estimated GFR and iGFR. Median estimated GFR by the full CKiD equation, which includes serum creatinine and cystatin C, was 43.5. The correlation of measured to estimated GFR was excellent for the full (r = 0.85) and abbreviated bedside (r = 0.80) CKiD formulas, although slightly better for the full equation (fig. 1). Median GFR as estimated by the CKiD bedside equation was 44.3. Both equations performed better in this population than the original Schwartz formula, which overestimates significantly as reported previously by the CKiD study.11
Figure 1.
Agreement plots and correlation of CKiD bedside GFR to measured iGFR for children with urological diagnoses in CKiD. Each line represents 1 subject, connecting iGFR measurement with corresponding estimated GFR equation based on serum creatinine. Figure represents subsample of 326 subjects with complete measurement for iGFR and bedside GFR available.
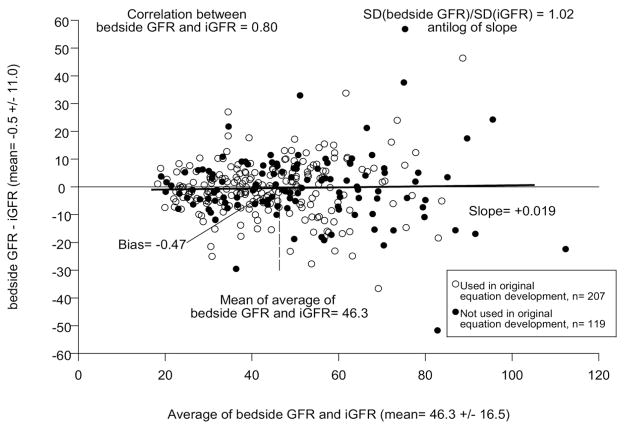
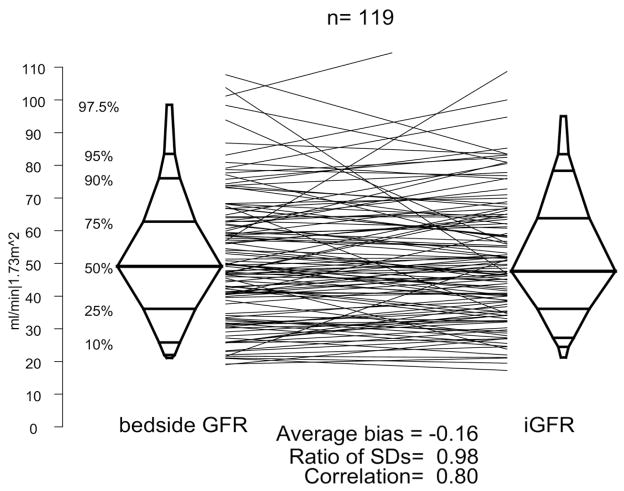
A Bland-Altman plot is presented in figure 2, comparing the CKiD bedside GFR equation with the iGFR measurement. The correlation was good (r = 0.80) with minimal average bias (average difference −0.5 ml/min/1.73 m2) with a minimal change in dispersion (ratio of SDs 1.02). Among the 326 subjects 119 were not used in the equation development and are represented by the solid points in figure 2. We assessed agreement in this subset and present the results in figure 3. Again, the bedside eGFR equation indicated comparable agreement when the subjects were pooled, thus validating the equation in this sub-population (r = 0.81, average bias −0.2 ml/min/1.73 m2 and equal dispersions [ratio of SDs] 0.98).
Figure 2.
Bland-Altman plot for observed iGFR and bedside GFR. Open circles represent participants included in development of estimated GFR equation. Filled circles indicate new participants who were not included in equation development.
Figure 3.
Agreement plot for observed iGFR and bedside GFR in testing data set comprised of participants who were not included in equation development.
An analysis of the agreement of iohexol GFR with the CKiD bedside estimating equation was then performed for each subgroup of children with urological disorders (obstructive uropathy, aplastic/hypoplastic/dysplastic kidneys, reflux nephropathy, other). Table 2 reveals a slight overestimation bias between the bedside and iGFR among the aplastic subgroup. However, no significant bias was observed. The biases were small, all within 2 ml per minute.
Table 2.
Agreement between bedside estimated GFR equation and iGFR
| Condition | No. Pts | Mean Bias (95% CI)* | Ratio of SDs | Correlation |
|---|---|---|---|---|
| Obstructive | 110 | −1.7 (−4.1–0.8) | 0.97 | 0.74 (0.64–0.81) |
| Aplastic | 98 | 1.6 (−0.6–3.8) | 1.14 | 0.80 (0.71–0.86) |
| Reflux | 80 | −1.2 (−3.0–0.6) | 0.97 | 0.88 (0.82–0.92) |
| Other | 38 | −0.9 (−3.9–2.1) | 1.06 | 0.86 (0.74–0.92) |
| Overall | 326 | −0.5 (−1.7–0.7) | 1.02 | 0.80 (0.76–0.83) |
GFR measured as ml/min/1.73 m2 (95% CI).
DISCUSSION
Among children in the CKiD cohort 59% had underlying urological diagnoses. The etiologies causing CKD are similar to those previously reported in large CKD registries, including NAPRTCS and the ItalKid Project.1,5,16 In the CKiD study children with underlying urological disorders were predominantly male, and many had low birth weight and received ICU treatment after birth. Most patients were diagnosed early in life via ultrasound, were seen by a urologist before age 1 year and had undergone a urological procedure. These findings suggest that urologists in partnership with nephrologists have the opportunity to identify children at high risk for kidney disease progression and those comorbid conditions such as high blood pressure, low weight and short stature, which persist into childhood.
In our analysis of the subgroup with urological disorders in the CKiD cohort the newly developed CKiD estimating equations, eGFR and bedside GFR, had good correlation and low bias, indicating good agreement with the measured iohexol GFR, an improvement over prior estimating equations.10,11 The newly developed CKiD bedside GFR estimating equation is a simple, easily used tool that requires only serum creatinine and height, similar to the original Schwartz equation but with an updated coefficient. Thus, this equation can be used by the practicing urologist to assess level of kidney function in this high risk population.
There are also other markers of progression of kidney disease in addition to GFR. In the past nadir serum creatinine was found to be a predictor of future kidney function in children with structural causes of CKD, including posterior urethral valves, vesicoureteral reflux and prune belly syndrome, but was not as reliable a predictor among patients with cloacal malformations.17–20 However, following changing kidney function in children with serum creatinine alone can be misleading. While serum creatinine normally increases as children age, a small increase in serum creatinine (0.1 or 0.2 mg/dl) can portend a significant reduction in kidney function, especially if not accompanied by a corresponding increase in body size and muscle mass. Estimating GFR by calibrating creatinine to height using estimating equations can help mitigate this problem. Serum cystatin C and beta trace protein also hold promise for estimating kidney function in the future.21–23 A prior study of the CKiD cohort demonstrated that proteinuria is also associated with decreased GFR in addition to glomerular causes of CKD and nonwhite race.24
Although this study sample is large, this analysis has a number of limitations. It is cross-sectional in nature and cannot assess causes of decline in kidney function among study participants. Additionally as the formula was developed in the CKiD population (including children with urological and nonurological causes of kidney disease), its excellent agreement with measured GFR in this subgroup analysis is not surprising. However, the levels of agreement were essentially the same when the subset of subjects not used for equation development was analyzed (fig. 3). Additional validation in a larger population of children with urological disease causing CKD may be useful to extend these inferences.
In our analyses of urological diagnosis subgroups another potential limitation was ascertainment of underlying diagnosis causing kidney disease. The attributed cause of kidney disease was physician reported or determined from chart review. However, only 1 primary diagnosis was recorded, and, therefore, it is possible that an individual participant could have multiple urological diagnoses (such as posterior urethral valves and vesicoureteral reflux). In addition, the distribution of causes of urological disease in the CKiD cohort might not be generalizable to the broader population of children with CKD. Selection bias may be present, as these participants were recruited from pediatric nephrology clinics in the United States and Canada. All cases already had compromised kidney function at study entry, and, therefore, likely represent more severe disease than all children with congenital abnormalities of the kidneys and urinary tract. Thus, these findings may have limited extrapolation to a general pediatric urology clinic population.
The new CKiD equations have been developed in a population of children with moderate to severe kidney disease and have not been prospectively validated in a population of children with normal or near normal kidney function and stature. When applied to a population based sample, estimates suggest that the new CKiD equations may underestimate kidney function among children with normal or mildly impaired kidney function.25 However, another study found good agreement of the new CKiD equations in a direct comparison with measured GFR.26
Despite these limitations, this study provides needed information about the characteristics of children with CKD due to urological diagnoses, and more accurate ways to estimate and monitor GFR among these children who are at risk for further decline in kidney function and ultimately ESRD requiring dialysis or transplant. The newly developed CKiD estimating equations may be used to estimate kidney function among children with moderate to severe renal impairment.
CONCLUSIONS
Underlying urological causes of CKD were present in 59% of participants in the CKiD study. These cases were diagnosed early in life, and many were low birth weight and continued to have growth delay. The full and bedside CKiD estimating equations estimated GFR well in this population.
Acknowledgments
Data in this manuscript were created by the Chronic Kidney Disease in Children prospective cohort study (CKiD) with clinical coordinating centers (Principal Investigators) at Children’s Mercy Hospital and the University of Missouri–Kansas City (BAW), Children’s Hospital of Pennsylvania (SLF), Central Biochemistry Laboratory at the University of Rochester Medical Center (GJS), and data coordinating center (Principal Investigator) at the Johns Hopkins Bloomberg School of Public Health (Alvaro Muñoz, Ph.D.). The CKiD study is funded by the National Institute of Diabetes and Digestive and Kidney Diseases, with additional funding from the National Institute of Neurological Disorders and Stroke, the National Institute of Child Health and Human Development, and the National Heart, Lung, and Blood Institute (U01-DK-66143, U01-DK-66174, U01-CK-82194, U01-DK-66116). The project was also supported by grant 1K23DK078671 from the National Institute of Diabetes and Digestive and Kidney Diseases, AUA Foundation, National Cancer Institute and National Kidney Foundation of Maryland Professional Development Award (JLD). The CKiD website is located at http://www.statepi.jhsph.edu/ckid.
Abbreviations and Acronyms
- bedside GFR
abbreviated bedside CKiD GFR estimating equation
- CKD
chronic kidney disease
- CKiD
Chronic Kidney Disease in Children
- eGFR
full CKiD GFR estimating equation
- ESRD
end-stage renal disease
- GFR
glomerular filtration rate
- ICU
intensive care unit
- iGFR
GFR measured by plasma disappearance of iohexol
- NAPRTCS
North American Pediatric Renal Trials and Collaborative Studies
Footnotes
Study received institutional review board approval.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health.
References
- 1.North American Pediatric Renal Trials and Collaborative Studies: NAPRTCS 2007 Annual Report. Washington, DC: EMMES Corp; 2007. [Google Scholar]
- 2.Zilleruelo G, Andia J, Gorman HM, et al. Chronic renal failure in children: analysis of main causes and deterioration rate in 81 children. Int J Pediatr Nephrol. 1980;1:30. [PubMed] [Google Scholar]
- 3.Wong C, Furth S. Epidemiology of renal disease in children. In: Kher K, Makker SP, Schnaper HW, editors. Clinical Pediatric Nephrology. 2. London: Informa Healthcare; 2007. [Google Scholar]
- 4.Roth KS, Koo HP, Spottswood SE, et al. Obstructive uropathy: an important cause of chronic renal failure in children. Clin Pediatr (Phila) 2002;41:309. doi: 10.1177/000992280204100503. [DOI] [PubMed] [Google Scholar]
- 5.Ardissino G, Avolio L, Dacco V, et al. Long-term outcome of vesicoureteral reflux associated chronic renal failure in children. Data from the ItalKid Project. J Urol. 2004;172:305. doi: 10.1097/01.ju.0000129067.30725.16. [DOI] [PubMed] [Google Scholar]
- 6.Novak TE, Mathews R, Martz K, et al. Progression of chronic kidney disease in children with vesicoureteral reflux: the North American Pediatric Renal Trials Collaborative Studies database. J Urol. 2009;182:1678. doi: 10.1016/j.juro.2009.02.085. [DOI] [PubMed] [Google Scholar]
- 7.Chevalier RL. When one kidney is not enough? Kidney Int. 2009;76:475. doi: 10.1038/ki.2009.244. [DOI] [PubMed] [Google Scholar]
- 8.Sanna-Cherchi S, Ravani P, Corbani V, et al. Renal outcomes in patients with congenital anomalies of the kidney and urinary tract. Kidney Int. 2009;76:528. doi: 10.1038/ki.2009.220. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz GJ, Haycock GB, Edelmann CM, Jr, et al. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics. 1976;58:259. [PubMed] [Google Scholar]
- 10.Schwartz GJ, Muñoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartz GJ, Furth S, Cole SR, et al. Glomerular filtration rate via plasma iohexol disappearance: pilot study for chronic kidney disease in children. Kidney Int. 2006;69:2070. doi: 10.1038/sj.ki.5000385. [DOI] [PubMed] [Google Scholar]
- 12.Filler G, Priem F, Vollmer I, et al. Diagnostic sensitivity of serum cystatin for impaired glomerular filtration rate. Pediatr Nephrol. 1999;13:501. doi: 10.1007/s004670050646. [DOI] [PubMed] [Google Scholar]
- 13.Furth SL, Cole SR, Moxey-Mims M, et al. Design and methods of the Chronic Kidney Disease in Children (CKiD) prospective cohort study. Clin J Am Soc Nephrol. 2006;1:1006. doi: 10.2215/CJN.01941205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307. [PubMed] [Google Scholar]
- 15.Schwartz GJ, Work DF. Measurement and estimation of GFR in children and adolescents. Clin J Am Soc Nephrol. 2009;4:1832. doi: 10.2215/CJN.01640309. [DOI] [PubMed] [Google Scholar]
- 16.Warady B, Chadha V. Chronic kidney disease in children, the global perspective. Pediatr Nephrol. 2007;22:1999. doi: 10.1007/s00467-006-0410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeFoor W, Clark C, Jackson E, et al. Risk factors for end stage renal disease in children with posterior urethral valves. J Urol. 2008;180:1705. doi: 10.1016/j.juro.2008.03.090. [DOI] [PubMed] [Google Scholar]
- 18.Caione P, Villa M, Capozza N, et al. Predictive risk factors for chronic renal failure in primary high-grade vesicoureteric reflux. BJU Int. 2004;93:1309. doi: 10.1111/j.1464-410X.04866.x. [DOI] [PubMed] [Google Scholar]
- 19.Noh PH, Cooper CS, Winkler AC, et al. Prognostic factors for long-term renal function in boys with the prune-belly syndrome. J Urol. 1999;162:1399. [PubMed] [Google Scholar]
- 20.Warne SA, Wilcox DT, Ledermann SE, et al. Renal outcome in patients with cloaca. J Urol. 2002;167:2548. [PubMed] [Google Scholar]
- 21.Groesbeck D, Kottgen A, Parekh R, et al. Age, gender, and race effects on cystatin C levels in US adolescents. Clin J Am Soc Nephrol. 2008;3:1777. doi: 10.2215/CJN.00840208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pham-Huy A, Leonard M, Lepage N, et al. Measuring glomerular filtration rate with cystatin c and β-trace protein in children with spina bifida. J Urol. 2003;169:2313. doi: 10.1097/01.ju.0000060205.23406.13. [DOI] [PubMed] [Google Scholar]
- 23.White CA, Akbari A, Doucette S, et al. Estimating GFR using serum beta trace protein: accuracy and validation in kidney transplant and pediatric populations. Kidney Int. 2009;76:784. doi: 10.1038/ki.2009.262. [DOI] [PubMed] [Google Scholar]
- 24.Wong CS, Pierce CB, Cole SR, et al. Association of proteinuria with race, cause of chronic kidney disease, and glomerular filtration rate in the Chronic Kidney Disease in Children study. Clin J Am Soc Nephrol. 2009;4:812. doi: 10.2215/CJN.01780408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fadrowski JJ, Neu AM, Schwartz GJ, et al. Pediatric GFR estimating equations applied to adolescents in the general population. Clin J Am Soc Nephrol. 2011;6:1427. doi: 10.2215/CJN.06460710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Staples A, Leblond R, Watkins S, et al. Validation of the revised Schwartz estimating equation in a predominantly non-CKD population. Pediatr Nephrol. 2010;25:2321. doi: 10.1007/s00467-010-1598-7. [DOI] [PubMed] [Google Scholar]