Abstract
Background
Chronic, high-level lead exposure is a known risk factor for kidney disease. The effect of current low-level environmental lead exposure is less well known, particularly among children, a population generally free from kidney disease risk factors such as hypertension and diabetes mellitus. Therefore, in this study, we investigated the association between lead exposure and kidney function in a representative sample of US adolescents.
Methods
Participants included 769 adolescents aged 12 to 20 years for whom whole blood lead and serum cystatin C were measured in the Third National Health and Nutrition Examination Survey, conducted from 1988–1994. The association between blood lead level and level of kidney function (glomerular filtration rate [GFR]), determined by cystatin C–based and creatinine-based estimating equations, was examined.
Results
Median whole blood lead level was 1.5 μg/dL (to convert to micromoles per liter, multiply by 0.0483), and median cystatin C–estimated GFR was 112.9 mL/min/1.73 m2. Participants with lead levels in the highest quartile (≥3.0 μg/dL) had 6.6 mL/min/1.73 m2–lower estimated GFR (95% confidence interval, −0.7 to −12.6 mL/min/1.73 m2) compared with those in the first quartile (<1 μg/dL). A doubling of blood lead level was associated with a 2.9 mL/min/1.73 m2–lower estimated GFR (95% confidence interval, −0.7 to −5.0 mL/min/1.73 m2). Lead levels were also associated with lower creatinine-based estimated GFR levels, but the association was weaker than with cystatin C–based GFR and not statistically significant.
Conclusions
Higher blood lead levels in a range below the current Centers for Disease Control and Prevention–designated level of concern (10 μg/dL) were associated with lower estimated GFRs in a representative sample of US adolescents. This finding contributes to the increasing epidemiologic evidence indicating an adverse effect of low-level environmental lead exposure.
Lead is a widespread toxicant with well-established detrimental health effects.1 High chronic lead exposure (blood levels >70–80 μg/dL [to convert to micromoles per liter, multiply by 0.0483]) is an established cause of chronic kidney disease (CKD) in adults and children.2–6 At lower levels of exposure (blood levels <10 μg/dL), adverse associations between blood lead and kidney function have been observed in adults.2,7–13 In Taiwanese adults with CKD, higher lead dose at baseline was prospectively associated with more rapid kidney function decline, and chelation to remove lead slowed CKD progression compared with controls.14–16 Most studies of low-level lead exposure and kidney function examined adults at a mean age of 50 years and older with a high prevalence of comorbid conditions and kidney disease risk factors, such as diabetes mellitus, hypertension, and smoking. Few studies have examined the association between low-level lead exposure and kidney function in children and adolescents, who are generally free from these comorbidities.17,18
In 1991, the Centers for Disease Control and Prevention (CDC) lowered the blood lead level of concern in children from 30 to 10 μg/dL in response to studies linking blood lead levels as low as 10 μg/dL with adverse neurodevelopmental effects.19–21 Although growing evidence supports the assertion that chronic lead exposure below 10 μg/dL adversely affects cognitive and cardiovascular function, assessment of the association between low-level lead exposure and kidney function in children and adolescents has been limited by the inability to detect early declines in the glomerular filtration rate (GFR) owing to the high variability and low sensitivity of serum creatinine levels.22–24 Recently, cystatin C level, potentially a more sensitive marker of kidney function than serum creatinine level, was measured in stored serum samples from the Third National Health and Nutrition Examination Survey (NHANES III).25–34 Therefore, we used newly available NHANES III data to investigate the association between blood lead levels and GFRs estimated from serum cystatin C levels in children and adolescents aged 12 to 20 years. We also examined the association between blood lead level and creatinine-based estimated GFRs.
METHODS
STUDY SETTING AND POPULATION
The National Center for Health Statistics, within the CDC, conducted the NHANES III from 1988–1994 using a complex multistage sampling design to obtain a representative sample of the US civilian, noninstitutionalized population aged 2 months and older. The NHANES III study protocols were approved by the institutional review board of the National Center for Health Statistics. Written informed consent was obtained from all participants or, if younger than 18 years, their guardians. Assent was obtained from those aged 12 to 17 years. The participation rate for all NHANES III components (questionnaire, examination, and laboratory) was 77.6%.
In NHANES III, whole blood for lead quantification was collected from all participants 1 year or older, and serum for biochemistry analyses, including serum creatinine level, was collected from those 12 years and older. In 2006, stored surplus serum was used to assay cystatin C in a random sample of 25% of participants aged 12 to 59 years and in all participants with a serum creatinine level greater than 1.2 mg/dL (to convert to micro-moles per liter, multiply by 88.4) in males and greater than 1.0 mg/dL in females.35 Serum cystatin C was measured in 801 NHANES III participants aged 12 to 20 years, including 12 males and 3 females with high serum creatinine levels as defined previously. We excluded participants who were missing data on body mass index (BMI) (n=11), income (n=18), and educational level (n=3), resulting in a final sample size of 769 adolescents.
WHOLE BLOOD LEAD MEASURES
Blood lead was measured at the Environmental Health Sciences Laboratory within the CDC’s National Center for Environmental Health following standardized protocols, including confirmation that collection and storage materials were not contaminated with background lead. Lead was measured by atomic absorption spectrometry using either a PerkinElmer model 5000 or 5100 graphite furnace atomic absorption spectrophotometer (PerkinElmer, Waltham, Massachusetts).36 The limit of detection was 1 μg/dL. For samples with lead levels below this limit (31.0% of study participants), a level equal to the limit of detection divided by the square root of 2 was entered (0.7 μg/dL).37,38 Quality control pools reflecting blood lead levels of 5 μg/dL or less and 20 μg/dL or higher were used for internal quality control. The between-assay coefficients of variation for each of the 7 pools analyzed (mean lead level range, 4.86–53.5 μg/dL) were less than 10%.39
KIDNEY FUNCTION MEASURES
Serum cystatin C level was measured at the Cleveland Clinic Research Laboratory using a particle-enhanced immunonephelometric assay (Dade Behring N Latex cystatin C run on a Dade Behring Nephelometer II; Siemens Healthcare Diagnostics, Deerfield, Illinois).40 The assay range was 0.23 to 7.25 mg/L (to convert to nanomoles per liter, multiply by 74.9). Interassay coefficients of variation for the assay were 5.05% and 4.87% at mean concentrations of 0.97 and 1.90 mg/L, respectively. Serum creatinine level was measured by the modified kinetic Jaffé reaction using a Hitachi 737 analyzer (Roche Diagnostics, Indianapolis, Indiana).
Estimated GFR (measured in milliliters per minute per 1.73 m2) was calculated using a pediatric-specific cystatin C–based equation developed by Filler and Lepage27 (estimated GFR, 91.62[1/cystatin C in milligrams per liter]1.123). Secondary analyses estimated GFR via the creatinine-based formula of Schwartz et al41,42: k(height in centimeters)/(serum creatinine in milligrams per deciliter), where k is 0.7 in boys and 0.55 in girls. Although NHANES III recommends correcting serum creatinine levels to standardize the values to current assays based on an enzymatic laboratory method, this was not done because the original Schwartz equation was derived using creatinine levels determined via the Jaffé reaction.41 We used the original Schwartz equation rather than the more recent GFR estimating equations in the Chronic Kidney Disease in Children cohort study43 because the original Schwartz formula was developed in a population that included children with normal GFRs, whereas the more recent equations were developed in children with CKD and have not yet been validated in healthy children.44
OTHER VARIABLES
Questionnaire information provided the participants’ sex, age, race and ethnicity, urban vs rural residence, household income, and the family reference person’s highest grade of education completed. The family reference person is the person who owned or rented the home in which the youth lived. Residence classification within NHANES III was based on United States Department of Agriculture rural-urban codes.45 The BMI was calculated as weight in kilograms divided by height in meters squared. For participants aged 12 to 18 years, BMI percentiles were calculated based on the CDC’s BMI-for-age sex-specific growth charts, and participants were categorized as obese if their BMI was at the 95th percentile or higher. Participants aged 19 and 20 years were categorized as obese if their BMI was 30 or higher.46 Tobacco smoke exposure was determined based on serum cotinine levels measured by an isotope-dilution high-performance liquid chromatography and atmospheric pressure chemical ionization tandem mass spectrometric method. Tobacco smoke exposure was categorized as undetectable (below the limit of detection, <0.05 ng/mL [to convert to nanomoles per liter, multiply by 5.675]), involuntary (0.05–10.0 ng/mL), and active (>10.0 ng/mL).47,48
STATISTICAL ANALYSIS
All statistical analyses were performed using Stata statistical software, version 9.0 (StataCorp, College Station, Texas) and R statistical software (R Foundation for Statistical Computing, Vienna, Austria). Survey commands were used to account for the NHANES complex sampling design with special sample weights for cystatin C.49,50 The statistical significance level was set at α = .05. All statistical analyses were 2-sided.
Median and interquartile ranges (25th and 75th percentiles) for blood lead levels and estimated GFR were calculated for the entire study population. Linear regression was used to assess associations between blood lead levels and estimated GFR. Lead exposure, the explanatory variable in the linear regression models, was categorized in 3 different ways: (1) as quartiles; (2) as a continuous log-transformed variable; and (3) as restricted quadratic splines to evaluate nonlinear relationships. The restricted quadratic splines were limited to participants with blood lead levels of 10 μg/dL or lower.
Linear regression models were fitted with increasing degrees of adjustment. First, we adjusted for age (continuous), sex, and race/ethnicity (black, Mexican, white, or other). Second, models were further adjusted for urban vs rural residence and tobacco smoke exposure (unexposed, involuntary, or active). Finally, models were further adjusted for obesity, annual household income (<$20 000 vs ≥$20 000), and the educational level of the family reference person (<high school, high school equivalent, or >high school). Secondary analyses, conducted within the same study population, used estimated GFR based on the creatinine-based Schwartz equation as the outcome variable. Models were examined stratified by age, sex, and race/ethnicity. Secondary analyses also evaluated blood pressure, lipid levels, and glucose levels.
RESULTS
Among the study participants, the median whole blood lead level was 1.5 μg/dL, and the median cystatin C–estimated GFR was 112.9 mL/min/1.73 m2 (Table 1). Blood lead levels were higher among males, nonwhites, and participants with higher exposure to tobacco smoke, lower household income, and a family reference person completing less than the 12th grade. Cystatin C–estimated GFR was higher among females, older adolescents, non-whites, participants without active tobacco smoke exposure, and participants for whom the family reference person had a lower educational level.
Table 1.
Blood Lead Levels and Estimated GFR by Participant Characteristicsa
| Characteristic | No. (%) of Participantsb | Blood Lead Level, μg/dL | P Valuec | Cystatin C–Estimated GFR, mL/min/1.73 m2d | P Valuec | Creatinine-Estimated GFR, mL/min/1.73 m2e | P Valuec | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | 769 (100) | 1.5 (0.7–2.9) | … | 112.9 (99.4–126.6) | … | 108.8 (99.1–118.3) | … | |||
| Sex | ||||||||||
| Male | 363 (50.4) | 2.2 (1.1–3.4) |
|
<.001 | 105.8 (93.7–114.5) |
|
<.001 | 114.2 (107.3–127.8) |
|
<.001 |
| Female | 406 (49.6) | 1.1 (0.7–2.2) | 119.4 (108.5–134.6) | 102.4 (93.1–110.7) | ||||||
| Age, y | ||||||||||
| 12–15 | 354 (46.0) | 1.7 (0.7–3.0) |
|
.71 | 108.5 (98.2–119.4) |
|
.001 | 112.3 (102.3–123.3) |
|
.03 |
| 16–20 | 415 (54.0) | 1.4 (0.7–2.0) | 117.7 (104.4–132.5) | 106.4 (96.5–114.0) | ||||||
| Race/ethnicity | ||||||||||
| Black | 262 (14.7) | 2.2 (1.3–3.2) |
|
.003 | 121.1 (105.8–136.8) |
|
<.001 | 103.3 (93.8–111.6) |
|
<.001 |
| Mexican | 269 (8.6) | 2.2 (1.2–3.6) | 117.7 (105.8–130.5) | 112.8 (103.4–122.9) | ||||||
| White | 199 (69.5) | 1.2 (0.7–2.8) | 110.0 (98.2–121.1) | 110.3 (100.8–119.6) | ||||||
| Other | 39 (7.2) | 2.2 (1.1–3.0) | 119.4 (108.5–126.6) | 102.7 (86.8–117.5) | ||||||
| Residence | ||||||||||
| Urban | 341 (44.8) | 2.0 (0.7–3.1) |
|
.19 | 111.4 (98.2–126.6) |
|
.51 | 110.7 (99.6–122.0) |
|
.90 |
| Rural | 428 (55.2) | 1.3 (0.7–2.7) | 112.9 (101.9–128.5) | 108.2 (98.8–115.8) | ||||||
| Tobacco smoke exposure | ||||||||||
| Undetectable | 77 (11.5) | 1.1 (0.7–2.5) |
|
.01 | 112.9 (99.4–124.7) |
|
.002 | 107.3 (99.1–117.0) |
|
.33 |
| Involuntary | 591 (74.3) | 1.4 (0.7–2.9) | 112.9 (100.6–128.5) | 109.4 (99.3–119.6) | ||||||
| Active | 101 (14.2) | 2.2 (1.2–3.8) | 107.1 (91.6–119.4) | 106.8 (96.8–119.6) | ||||||
| Obesef | ||||||||||
| No | 665 (90.6) | 1.5 (0.7–3.0) |
|
.72 | 112.9 (99.4–126.6) |
|
.07 | 110.7 (101.1–121.7) |
|
.72 |
| Yes | 104 (9.4) | 1.6 (0.7–2.7) | 107.1 (97.1–121.1) | 111.4 (97.9–122.0) | ||||||
| Annual household income, $ | ||||||||||
| <20 000 | 395 (37.7) | 2.3 (1.1–4.1) |
|
<.001 | 114.5 (100.6–128.5) |
|
.08 | 109.7 (100.4–120.4) |
|
.08 |
| ≥20 000 | 374 (62.3) | 1.2 (0.7–2.3) | 111.4 (99.4–124.7) | 108.4 (98.7–117.5) | ||||||
| Educational level of family reference person | ||||||||||
| < 12th Grade | 323 (24.7) | 2.2 (1.1–4.0) |
|
.008 | 114.5 (101.9–130.5) |
|
.04 | 109.7 (102.0–123.0) |
|
.15 |
| 12th Grade | 235 (32.6) | 1.4 (0.7–2.5) | 112.9 (99.4–128.5) | 110.0 (99.2–119.8) | ||||||
| > 12th Grade | 211 (42.6) | 1.5 (0.7–2.7) | 111.4 (98.2–121.1) | 107.3 (98.5–115.1) |
Abbreviations: ellipses, not applicable; GFR, glomerular filtration rate.
SI conversion factor: To convert blood lead level to micromoles per liter, multiply by 0.0483.
Data are given as the median (interquartile range) unless otherwise indicated.
Weighted percentage.
P values are adjusted for age and sex.
Cystatin C–estimated GFR was calculated by the equation developed by Filler and Lepage.27
Obesity was defined as a body mass index (calculated as weight in kilograms divided by height in meters squared) at or above the 95th percentile (ages 12–18 years) or 30 or higher (ages 19–20 years).
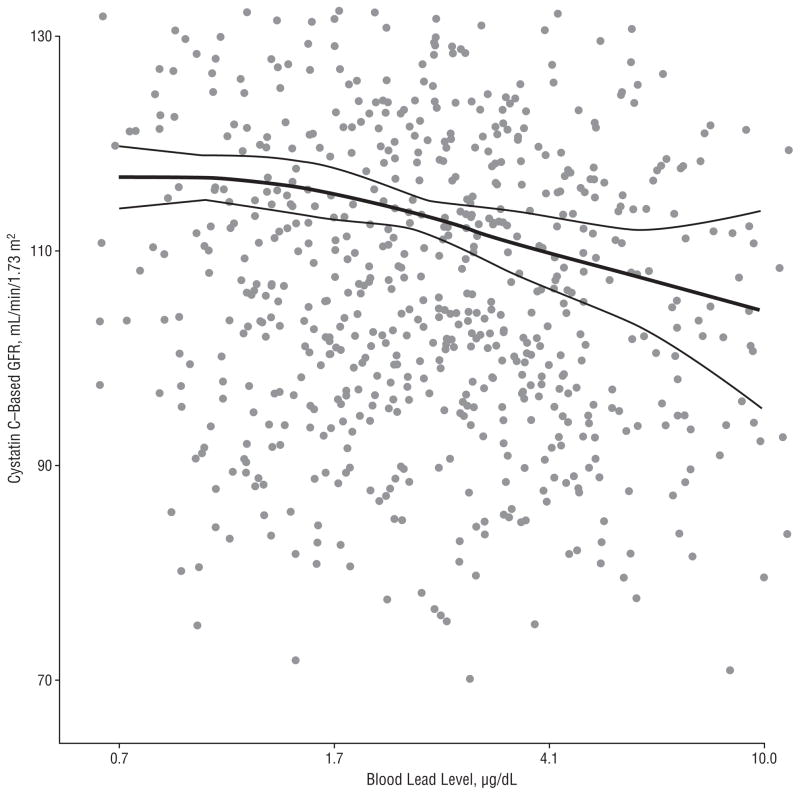
In linear regression analyses, higher blood lead levels were consistently associated with a lower cystatin C–estimated GFR (Table 2). Participants with lead levels in the highest quartile (≥3.0 μg/dL) had a 6.6 mL/min/1.73 m2–lower estimated GFR (95% confidence interval [CI], −0.7 to −12.6 mL/min/1.73 m2) compared with those in the first quartile (lead level <1 μg/dL), with a highly significant test for trend (P=.009). Doubling of blood lead levels was associated with a 2.9 mL/min/1.73 m2–lower estimated GFR in the fully adjusted model (95% CI, −0.7 to −5.0 mL/min/1.73 m2). Restricted quadratic spline analysis showed progressively lower estimated GFRs associated with higher blood lead levels, with no departures from linearity and no threshold points (comparing the fully adjusted spline model with the log-linear model using the Wald F test, P= .29) (Figure). Analyses using the creatinine-based equation to estimate GFR also showed an inverse association between blood lead level and GFR, although the associations were weaker compared with those using the cystatin C–based equation and statistically nonsignificant (Table 2).
Table 2.
Mean Difference in Estimated GFR Associated With Blood Lead Levelsa
| Characteristic | Unadjusted | Model 1b | Model 2c | Model 3d |
|---|---|---|---|---|
| Cystatin C Basede | ||||
| Lead level quartile, μg/dL | ||||
| 1 (<1.0) | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| 2 (1.0–1.5) | −0.5 (−6.8 to 5.8) | −1.1 (−7.4 to 5.1) | −1.1 (−7.1 to 4.8) | −1.4 (−7.4 to 4.5) |
| 3 (1.6–2.9) | −5.8 (−11.6 to −0.1) | −4.1 (−9.5 to 1.2) | −2.7 (−7.7 to 2.4) | −2.6 (−7.3 to 2.2) |
| 4 (>2.9) | −8.8 (−15.7 to −2.0) | −6.6 (−12.7 to −0.5) | −5.2 (−11.3 to 0.9) | −6.6 (−12.6 to −0.7) |
| P value for trend | .003 | .01 | .03 | .009 |
| Per doubling of blood lead level, μg/dLf | −3.8 (−6.3 to −1.3) | −3.0 (−5.2 to −0.7) | −2.4 (−4.6 to −0.2) | −2.9 (−5.0 to −0.7) |
|
| ||||
| Creatinine Basede | ||||
| Lead level quartile, μg/dL | ||||
| 1 (<1.0) | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| 2 (1.0–1.5) | −0.1 (−6.7 to 6.4) | −0.3 (−5.7 to 5.0) | −0.6 (−6.3 to 5.0) | −0.5 (−6.1 to 5.1) |
| 3 (1.6–2.9) | 2.4 (−2.2 to 7.1) | −1.4 (−6.7 to 3.9) | −1.7 (−6.5 to 3.1) | −1.7 (−6.9 to 3.5) |
| 4 (>2.9) | 3.8 (−2.4 to 9.9) | −0.4 (−6.0 to 5.2) | −0.8 (−6.2 to 4.7) | −1.9 (−7.4 to 3.5) |
| P value for trend | .16 | .70 | .59 | .31 |
| Per doubling of blood lead level, μg/dLf | 1.4 (−0.6 to 3.3) | −0.4 (−2.3 to 1.6) | −0.5 (−2.4 to 1.4) | −1.0 (−2.8 to 0.9) |
Abbreviations: CI, confidence interval; GFR, glomerular filtration rate.
SI conversion factor: To convert blood lead level to micromoles per liter, multiply by 0.0483.
N = 769 for all models. Data are given as the difference in estimated GFR (in milliliters per minute per 1.73 m2) compared with the reference category (95% CI) unless otherwise indicated.
Model 1 is adjusted for age, sex, and race/ethnicity.
Model 2 is adjusted for age, sex, race/ethnicity, urban vs rural residence, and tobacco smoke exposure.
Model 3 is adjusted for age, sex, race/ethnicity, urban vs rural residence, tobacco smoke exposure, obesity, annual household income, and educational level of the family reference person.
Cystatin C–estimated GFR was calculated using the Filler and Lepage equation27 and creatinine-estimated GFR using the Schwartz et al equation.41,42
Mean difference in estimated GFR associated with a doubling of the blood lead level (ie, 1–2 or 2–4 μg/dL).
Figure.
Cystatin C–estimated glomerular filtration rate (GFR) by blood lead levels. The thick line represents estimated GFR based on restricted quadratic splines transformation for log-transformed blood lead levels (restricted to ≤10 μg/dL [to convert to micromoles per liter, multiply by 0.0483]) with knots at the 10th, 50th, and 90th percentiles. Thin lines represent corresponding 95% confidence intervals. Data points for the scatterplot represent adjusted blood lead and estimated GFR values and were calculated as the residuals from the weighted linear regression models of log-lead and estimated GFR on the covariates used in model 3 in Table 2. Mean log-lead and estimated GFR levels were added to the residuals to facilitate interpretation of the scatterplot. The horizontal axis is in log scale.
Analyses stratified by age and race/ethnicity were limited by the relatively small sample size. When stratified by sex, the associations were similar to those found in the entire sample. In the fully adjusted model, a 2-fold increase in blood lead levels was associated with 2.3 (95% CI, −4.8 to 0.1 mL/min/1.73 m2) and 3.3 mL/min/1.73 m2–lower cystatin C–estimated GFR (95% CI, −6.2 to −0.3 mL/min/1.73 m2) among males and females, respectively. In sensitivity analyses, we evaluated the effect of adjusting for other kidney disease risk factors. Less than 5% of the cohort was hypertensive (systolic/diastolic blood pressure ≥95th percentile for participants aged 12–17 years or ≥140/90 for participants aged 18–20 years). Inclusion of blood pressure status in the multivariate models did not affect the results. Assessment of hyperlipidemia and hyperglycemia was limited by a lack of fasting in almost 50% of the cohort. Among those fasting, no difference in lead or estimated GFR levels was observed by lipid or glucose status.
COMMENT
In a representative sample of US adolescents, higher blood lead levels were associated with lower estimated GFR after adjustment for factors known to affect blood lead levels and/or GFR. The association was strong and graded throughout the range of blood lead levels, with no obvious threshold. More than 99% of study participants had blood lead levels below 10 μg/dL, the current CDC level of concern for blood lead concentrations in children and adolescents. Previous epidemiologic studies conducted in vulnerable adult populations, such as those with CKD or hypertension, have shown that low-level environmental lead exposure was inversely associated with kidney function in cross-sectional and prospective analyses. Our study extends these findings to a general population sample of US adolescents, indicating that lead exposure at levels common in developed countries is associated with lower kidney function, even in the absence of other co-morbidities.2,7–16 Our findings also suggest that previous studies using creatinine-based estimates of kidney function in healthy populations may have substantially underestimated the adverse association of higher blood lead levels on kidney function.
Exposure to lead has decreased substantially in the United States, primarily owing to public health measures. Federal regulations required phaseout of residential lead-based paint in 1978. Leaded gasoline was phased out in the 1980s and ultimately banned in 1996.1 Despite the elimination of many common industrial uses of lead, most of the US general population still has detectable blood levels. Indeed, the mean blood lead levels in US adolescents aged 12 to 19 years were 1.5 μg/dL and 1.1 μg/dL from 1991 to 1994 and from 1999 to 2000, respectively.1,51,52 Current exposure sources include industry, lead paint, folk remedies, glazed pottery, candy, and drinking water in some urban areas, and certain populations continue to experience high lead exposure, in particular, inner-city children and adults living in areas of low socioeconomic status.1,53–55
Acute high-level exposure to lead causes damage to the proximal tubule in the kidney, and chronic lead exposure is an established cause of interstitial fibrosis in the kidney, possibly through lead-induced oxidative stress.1 Lead exposure has also been associated with bio-markers of kidney injury in population studies. Children living near lead smelters and adolescents working in auto shops in developing nations have consistently shown significant increases in urinary biomarkers of kidney tubular dysfunction such as N-acetyl-β-D-glucosaminidase, retinol binding protein, and α-1-microglobulin in cross-sectional studies.56–60
Although high-dose lead exposure is an established cause of kidney damage, an increasing number of studies implicate low-level environmental lead exposure with decreased kidney function.2 In a representative sample of US adults, blood lead levels even below 5 μg/dL were associated with an increased prevalence of CKD.7,12 In a prospective study of 121 adults with CKD (mean baseline blood lead level, 4.2 μg/dL), Yu et al14 observed a 4-year decline of 4 mL/min/1.73 m2 per 1 μg/dL increment in baseline blood lead level. Also, a randomized clinical trial of 64 adult patients with CKD showed an increase in GFR of 2.1 mL/min/1.73 m2 in the group receiving chelation therapy, designed to reduce the amount of lead in the body, compared with a 6.0 mL/min/1.73 m2 GFR decline among controls during a 27-month follow-up period (P < .001).15
Few studies have evaluated the association between low-level lead and GFR among children and adolescents. Low-level lead exposure was associated with higher serum cystatin C levels among 200 seventeen-year-old Belgian children recruited from 2 industrialized suburbs (mean blood lead levels, 1.8 and 2.7 μg/dL) and a rural area (mean blood lead level, 1.5 μg/dL).18 In contrast, a cross-sectional study of 804 children aged 8 to 12 years living around nonferrous smelters in France, the Czech Republic, and Poland found an inverse association between blood lead levels (means ranging from 3.6–6.5 μg/dL among exposed) and serum creatinine and cystatin C levels.17 The authors suggested that this association could represent glomerular hyperfiltration, a process that occurs very early in kidney injury, before GFR decline. Differences between our findings and this study include the age ranges, differences in laboratory methods for cystatin C determination (automated latex immunoassay vs the particle-enhanced immunonephelometric assay used in our analysis), and differences in multivariate modeling methods and adjustment in the European study.28 Inverse associations between blood lead level and kidney function have also been observed in populations with occupational lead exposure and in one study of adults who had lead poisoning during childhood.61–65
Important strengths of our study include the use of a representative sample of the general US adolescent population, the rigorous quality control of study procedures in NHANES III, and the availability of cystatin C level data measured by a highly precise assay across the clinical range of values.28 Cystatin C is thought to overcome some of the age and muscle mass limitations of serum creatinine as a marker of kidney function, and it has been proposed as a more sensitive and specific marker of kidney function in children and adults.25–34 To estimate GFR, we used the cystatin C–based equation of Filler and Lepage,27 derived from a study of 536 children with a wide range of GFRs (mean [SD], 103 [41] mL/min/1.73 m2) inclusive of those with normal kidney function. Furthermore, Filler and Lepage used the same cystatin C assay in the derivation of the formula as NHANES III. As a secondary analysis, we evaluated the association between blood lead level and the creatinine-based estimated GFR equation of Schwartz et al,41,42 also developed in a pediatric population with a range of GFRs inclusive of healthy children. The point estimates and trends obtained using the Schwartz equation were markedly attenuated compared with the cystatin C–based analyses and were no longer statistically significant, but adjusted analyses showed a similar graded dose-response relationship. The attenuation could be related to measurement error in the Schwartz formula in estimating GFR.66 Serum creatinine level is more affected by age, sex, and race than is cystatin C level among children and adolescents, limiting the precision of creatinine-based GFR estimates.25,41,42,67,68 In addition, creatinine-based estimates are known to perform less well at higher levels of GFR, and it has been repeatedly suggested that cystatin C may be a superior marker of early kidney dysfunction.28
Some limitations of our data need to be considered in the interpretation of these findings. Because this is a cross-sectional study, we cannot rule out the possibility that blood lead levels increase as a consequence of kidney function decline rather than causing the decline. However, in a general population sample of healthy US adolescents with kidney function in the normal range, decreased excretion of lead by the kidney leading to higher levels seems unlikely. Furthermore, a number of studies have found evidence against reverse causation as an explanation for observed associations between lead level and kidney function, even in patients with CKD.2 Prospective studies have shown that baseline lead levels are associated with subsequent decline in kidney function, adding evidence to the cause-effect relationship between lead exposure and subsequent kidney function decline.14,15
Although we adjusted for a variety of biological and environmental factors that might influence blood lead levels and/or kidney function, it is possible that adjustment for such factors was incomplete or unmeasured factors associated both with lead exposure and lower estimated GFR may explain our results. However, other than age, sex, and race, few factors that influence cystatin C levels independent of GFR have been described in adolescents.25 Filtered cystatin C is known to be catabolized primarily by proximal tubular cells in the kidney, and, therefore, it does not return to circulation. Lead is a proximal tubule toxicant, and it is not clear whether this affects cystatin C handling by the kidney.69,70 Dexa-methasone, tumor growth factor β, and exposure of alveolar macrophages to arsenic oxide can cause increased production of cystatin C.28 The effect of lead on cystatin C production and elimination in the body requires further mechanistic studies as well as prospective studies in large population samples.
Cystatin C was measured in only a subset of NHANES III participants, so our study was somewhat limited by sample size. This is, however, one of the largest studies in children examining the association between lead and kidney function, and, despite the relatively small sample size, we saw a consistent association between blood lead level and estimated GFR. The pediatric-based GFR estimating equations used may be limited for participants older than 18. However, the Schwartz equation was derived from a population that included 19- and 20-year-olds, and adult-based estimating equations have generally been derived from populations with much older mean ages. Exclusion of 19- and 20-year-olds from our analysis did not change the observed associations, but the CIs were slightly wider. Finally, because whole blood lead level represents recent and chronic exposure, a single blood measure may be an imperfect marker of chronic lead exposure. Similarly, one measurement of cystatin C or creatinine may be an imperfect marker of GFR. However, the NHANES III data represent one of the few large-scale epidemiologic studies in children in which lead exposure was measured in conjunction with markers of kidney function.
Given the remarkable burden of CKD worldwide, much attention should be given to modifiable factors that cause CKD and are associated with its progression. Although this cross-sectional study does not prove causation, the graded lowering of estimated GFR seen with relatively small increments (approximately 1 μg/dL) in blood lead levels in ranges below the CDC level of concern is striking, particularly given the young and healthy study population. If lead exposure is associated with a lower GFR in the young with no evidence of CKD, the long-term consequences as the population ages and develops traditional CKD risk factors, such as hypertension and diabetes, may be substantial. Worldwide, certain racial/ethnic and lower socioeconomic groups have a greater burden of CKD.71–77 In the United States, African Americans have the highest reported incidence and prevalence of treated end-stage renal disease.78 Environmental factors have been hypothesized to contribute to this burden. Further study should assess whether greater lead exposure contributes to the disproportionate burden of CKD in certain racial/ethnic groups. Efforts to reassess the CDC’s current lead level of concern should incorporate the results of this and other recent studies of environmental lead exposure.
CONCLUSIONS
In this representative sample of healthy US adolescents, higher whole blood lead levels were associated with lower levels of cystatin C–estimated GFR. Although banning lead in gasoline and paint has resulted in a decrease in lead exposure during the past few decades, low-level environmental lead exposure continues, with a disproportionate burden among certain groups, such as minority populations and those of lower socioeconomic status.52 Given the prevalence of CKD in the United States, with an estimated 26 million Americans affected, confirmation of the role of lead in decreased kidney function and subsequent assessment of acceptable lead levels is necessary.79,80
Acknowledgments
Funding/Support: This work was supported by a Young Investigator Award from the National Kidney Foundation (Dr Fadrowski), grant K23ES016514 from the National Institute of Environmental Health Science (Dr Fadrowski), grant K24DK078737 from the National Institute of Diabetes, Digestive, and Kidney Disorders (Dr Furth), and grant U01DK066174 to the National Institute of Diabetes and Digestive and Kidney Diseases/National Heart, Lung and Blood Institute/The Eunice Kennedy Shriver National Institute of Child Health and Human Development–supported Chronic Kidney Disease in Children prospective cohort study (Dr Furth).
Role of the Sponsor: The National Kidney Foundation and National Institutes of Health played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Financial Disclosure: None reported.
Author Contributions: Dr Fadrowski had full access to all the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis. Study concept and design: Fadrowski, Navas-Acien, Guallar, Weaver, and Furth. Acquisition of data: Fadrowski. Analysis and interpretation of data: Fadrowski, Navas-Acien, Tellez-Plaza, Guallar, Weaver, and Furth. Drafting of the manuscript: Fadrowski and Navas-Acien. Critical revision of the manuscript for important intellectual content: Navas-Acien, Tellez-Plaza, Guallar, Weaver, and Furth. Statistical analysis: Fadrowski, Navas-Acien, and Tellez-Plaza. Administrative, technical, or material support: Fadrowski, Navas-Acien, and Tellez-Plaza.
References
- 1.Agency for Toxic Substances and Disease Registry. Toxicological Profile for Lead. Atlanta, GA: US Department of Health and Human Services, Public Health Service; 2007. [Google Scholar]
- 2.Ekong EB, Jaar BG, Weaver VM. Lead-related nephrotoxicity: a review of the epidemiologic evidence. Kidney Int. 2006;70(12):2074–2084. doi: 10.1038/sj.ki.5001809. [DOI] [PubMed] [Google Scholar]
- 3.Khalil-Manesh F, Gonick HC, Cohen AH, et al. Experimental model of lead nephropathy, I: continuous high-dose lead administration. Kidney Int. 1992;41 (5):1192–1203. doi: 10.1038/ki.1992.181. [DOI] [PubMed] [Google Scholar]
- 4.Steenland K, Selevan S, Landrigan P. The mortality of lead smelter workers: an update. Am J Public Health. 1992;82(12):1641–1644. doi: 10.2105/ajph.82.12.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inglis JA, Henderson DA, Emmerson BT. The pathology and pathogenesis of chronic lead nephropathy occurring in Queensland. J Pathol. 1978;124(2):65–76. doi: 10.1002/path.1711240202. [DOI] [PubMed] [Google Scholar]
- 6.Wedeen RP, Malik DK, Batuman V. Detection and treatment of occupational lead nephropathy. Arch Intern Med. 1979;139(1):53–57. [PubMed] [Google Scholar]
- 7.Muntner P, Menke A, DeSalvo KB, Rabito FA, Batuman V. Continued decline in blood lead levels among adults in the United States: the National Health and Nutrition Examination Surveys. Arch Intern Med. 2005;165(18):2155–2161. doi: 10.1001/archinte.165.18.2155. [DOI] [PubMed] [Google Scholar]
- 8.Staessen JA, Lauwerys RR, Buchet JP, et al. The Cadmibel Study Group. Impairment of renal function with increasing blood lead concentrations in the general population. N Engl J Med. 1992;327(3):151–156. doi: 10.1056/NEJM199207163270303. [DOI] [PubMed] [Google Scholar]
- 9.Payton M, Hu H, Sparrow D, Weiss ST. Low-level lead exposure and renal function in the Normative Aging Study. Am J Epidemiol. 1994;140(9):821–829. doi: 10.1093/oxfordjournals.aje.a117330. [DOI] [PubMed] [Google Scholar]
- 10.Kim R, Rotnitsky A, Sparrow D, Weiss S, Wager C, Hu H. A longitudinal study of low-level lead exposure and impairment of renal function: the Normative Aging Study. JAMA. 1996;275(15):1177–1181. [PubMed] [Google Scholar]
- 11.Tsaih SW, Korrick S, Schwartz J, et al. Lead, diabetes, hypertension, and renal function: the Normative Aging Study. Environ Health Perspect. 2004;112(11):1178–1182. doi: 10.1289/ehp.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muntner P, He J, Vupputuri S, Coresh J, Batuman V. Blood lead and chronic kidney disease in the general United States population: results from NHANES III. Kidney Int. 2003;63(3):1044–1050. doi: 10.1046/j.1523-1755.2003.00812.x. [DOI] [PubMed] [Google Scholar]
- 13.Akesson A, Lundh T, Vahter M, et al. Tubular and glomerular kidney effects in Swedish women with low environmental cadmium exposure. Environ Health Perspect. 2005;113(11):1627–1631. doi: 10.1289/ehp.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu CC, Lin JL, Lin-Tan DT. Environmental exposure to lead and progression of chronic renal diseases: a four-year prospective longitudinal study. J Am Soc Nephrol. 2004;15(4):1016–1022. doi: 10.1097/01.asn.0000118529.01681.4f. [DOI] [PubMed] [Google Scholar]
- 15.Lin JL, Lin-Tan DT, Hsu KH, Yu CC. Environmental lead exposure and progression of chronic renal diseases in patients without diabetes. N Engl J Med. 2003;348(4):277–286. doi: 10.1056/NEJMoa021672. [DOI] [PubMed] [Google Scholar]
- 16.Lin JL, Lin-Tan DT, Li YJ, Chen KH, Huang YL. Low-level environmental exposure to lead and progressive chronic kidney diseases. Am J Med. 2006;119(8):707.e1–707.e9. doi: 10.1016/j.amjmed.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 17.de Burbure C, Buchet JP, Leroyer A, et al. Renal and neurologic effects of cadmium, lead, mercury, and arsenic in children: evidence of early effects and multiple interactions at environmental exposure levels. Environ Health Perspect. 2006;114(4):584–590. doi: 10.1289/ehp.8202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Staessen JA, Nawrot T, Hond ED, et al. Renal function, cytogenetic measurements, and sexual development in adolescents in relation to environmental pollutants: a feasibility study of biomarkers. Lancet. 2001;357(9269):1660–1669. doi: 10.1016/s0140-6736(00)04822-4. [DOI] [PubMed] [Google Scholar]
- 19.Meyer PA, Pivetz T, Dignam TA, Homa DM, Schoonover J, Brody D Centers for Disease Control and Prevention. Surveillance for elevated blood lead levels among children—United States, 1997–2001. MMWR Surveill Summ. 2003;52(10):1–21. [PubMed] [Google Scholar]
- 20.Preventing Lead Poisoning in Young Children: a Statement by the Centers for Disease Control. Atlanta, GA: Centers for Disease Control; 1991. [Google Scholar]
- 21.Increased Lead Absorption in Young Children: a Statement by the Centers for Disease Control. Atlanta, GA: Centers for Disease Control; 1975. [Google Scholar]
- 22.Canfield RL, Henderson CR, Jr, Cory-Slechta DA, Cox C, Jusko TA, Lanphear BP. Intellectual impairment in children with blood lead concentrations below 10 microg per deciliter. N Engl J Med. 2003;348(16):1517–1526. doi: 10.1056/NEJMoa022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Navas-Acien A, Guallar E, Silbergeld EK, Rothenberg SJ. Lead exposure and cardiovascular disease–a systematic review. Environ Health Perspect. 2007;115 (3):472–482. doi: 10.1289/ehp.9785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lanphear BP, Hornung R, Khoury J, et al. Low-level environmental lead exposure and children’s intellectual function: an international pooled analysis. Environ Health Perspect. 2005;113(7):894–899. doi: 10.1289/ehp.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Groesbeck D, Kottgen A, Parekh R, et al. Age, gender, and race effects on cystatin C levels in US adolescents. Clin J Am Soc Nephrol. 2008;3(6):1777–1785. doi: 10.2215/CJN.00840208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andersen TB, Eskild-Jensen A, Frokiaer J, Brochner-Mortensen J. Measuring glomerular filtration rate in children: can cystatin C replace established methods? a review. Pediatr Nephrol. 2009;24(5):929–941. doi: 10.1007/s00467-008-0991-y. [DOI] [PubMed] [Google Scholar]
- 27.Filler G, Lepage N. Should the Schwartz formula for estimation of GFR be replaced by cystatin C formula? Pediatr Nephrol. 2003;18(10):981–985. doi: 10.1007/s00467-003-1271-5. [DOI] [PubMed] [Google Scholar]
- 28.Newman DJ. Cystatin C. Ann Clin Biochem. 2002;39(pt 2):89–104. doi: 10.1258/0004563021901847. [DOI] [PubMed] [Google Scholar]
- 29.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function: measured and estimated glomerular filtration rate. N Engl J Med. 2006;354(23):2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 30.Perrone RD, Madias NE, Levey AS. Serum creatinine as an index of renal function: new insights into old concepts. Clin Chem. 1992;38(10):1933–1953. [PubMed] [Google Scholar]
- 31.Madero M, Sarnak MJ, Stevens LA. Serum cystatin C as a marker of glomerular filtration rate. Curr Opin Nephrol Hypertens. 2006;15(6):610–616. doi: 10.1097/01.mnh.0000247505.71915.05. [DOI] [PubMed] [Google Scholar]
- 32.Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis. 2002;40(2):221–226. doi: 10.1053/ajkd.2002.34487. [DOI] [PubMed] [Google Scholar]
- 33.Roos JF, Doust J, Tett SE, Kirkpatrick CM. Diagnostic accuracy of cystatin C compared to serum creatinine for the estimation of renal dysfunction in adults and children: a meta-analysis. Clin Biochem. 2007;40(5–6):383–391. doi: 10.1016/j.clinbiochem.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 34.Ylinen EA, Ala-Houhala M, Harmoinen AP, Knip M. Cystatin C as a marker for glomerular filtration rate in pediatric patients. Pediatr Nephrol. 1999;13(6):506–509. doi: 10.1007/s004670050647. [DOI] [PubMed] [Google Scholar]
- 35.Köttgen A, Selvin E, Stevens LA, Levey AS, Van Lente F, Coresh J. Serum cystatin C in the United States: the Third National Health and Nutrition Examination Survey (NHANES III) Am J Kidney Dis. 2008;51(3):385–394. doi: 10.1053/j.ajkd.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 36.Miller DT, Paschal DC, Gunter EW, Stroud PE, D’Angelo J. Determination of lead in blood using electrothermal atomisation atomic absorption spectrometry with a L’vov platform and matrix modifier. Analyst. 1987;112(12):1701–1704. doi: 10.1039/an9871201701. [DOI] [PubMed] [Google Scholar]
- 37.US Department of Health and Human Services. National Center for Health Statistics. [Accessed June 8, 2009];Third National Health and Nutrition Examination Survey, 1988–1994, NHANES III second laboratory data file. ftp://ftp.cdc.gov/pub/Health_Statistics/NCHS/Datasets/NHANES/NHANESIII/2A/lab2-acc.pdf.
- 38.Hornung RW, Reed LD. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg. 1990;(5):546–551. [Google Scholar]
- 39.Gunter EW, Lewis BG, Koncikowski SM US Department of Health and Human Services. [Accessed April 1, 2009];Laboratory procedures used for the Third National Health and Nutrition Examination Survey (NHANES III), 1988–1994. http://www.cdc.gov/nchs/data/nhanes/nhanes3/cdrom/nchs/manuals/labman.pdf.
- 40.Finney H, Newman DJ, Gruber W, Merle P, Price CP. Initial evaluation of cystatin C measurement by particle-enhanced immunonephelometry on the Behring nephelometer systems (BNA, BN II) Clin Chem. 1997;43(6 pt 1):1016–1022. [PubMed] [Google Scholar]
- 41.Schwartz GJ, Haycock GB, Spitzer A. Plasma creatinine and urea concentration in children: normal values for age and sex. J Pediatr. 1976;88(5):828–830. doi: 10.1016/s0022-3476(76)81125-0. [DOI] [PubMed] [Google Scholar]
- 42.Schwartz GJ, Brion LP, Spitzer A. The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am. 1987;34(3):571–590. doi: 10.1016/s0031-3955(16)36251-4. [DOI] [PubMed] [Google Scholar]
- 43.Furth SL, Cole SR, Moxey-Mims M, et al. Design and methods of Chronic Kidney Disease in Children (CKiD) prospective cohort study. Clin J Am Soc Nephrol. 2006;1(5):1006–1015. doi: 10.2215/CJN.01941205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwartz GJ, Munoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20(3):629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.United States Department of Agriculture. Economic Research Service. [Accessed February 3, 2009];Rural-urban continuum codes. http://www.ers.usda.gov/Data/RuralUrbanContinuumCodes/
- 46.Centers for Disease Control and Prevention. [Accessed February 3, 2009];Healthy weight, assessing your weight, body mass index. http://www.cdc.gov/healthyweight/assessing/bmi/index.html.
- 47.Benowitz NL. Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol Rev. 1996;18(2):188–204. doi: 10.1093/oxfordjournals.epirev.a017925. [DOI] [PubMed] [Google Scholar]
- 48.Pirkle JL, Bernert JT, Caudill SP, Sosnoff CS, Pechacek TF. Trends in the exposure of nonsmokers in the U.S. population to secondhand smoke: 1988–2002. Environ Health Perspect. 2006;114(6):853–858. doi: 10.1289/ehp.8850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lumley T. Survey: analysis of complex survey samples. [Accessed July 14, 2009];R statistical software package, version 2.9.1 (2009-06-26) http://cran.r-project.org/web/packages/survey.
- 50.National Center for Health Statistics. Centers for Disease Control and Prevention Web site. Documentation, codebook, and frequencies. Surplus sera laboratory component: cystatin C (Surplus Sera) [Accessed February 3, 2009];Survey years 1988–1994. ftp://ftp.cdc.gov/pub/Health_Statistics/NCHS/Datasets/NHANES/NHANESIII/27a/SSCYSTAT.pdf.
- 51.Pirkle JL, Kaufmann RB, Brody DJ, Hickman T, Gunter EW, Paschal DC. Exposure of the U.S. population to lead, 1991–1994. Environ Health Perspect. 1998;106(11):745–750. doi: 10.1289/ehp.98106745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.National Center for Environmental Health. Third National Report on Human Exposure to Environmental Chemicals. Atlanta, GA: Centers for Disease Control and Prevention; 2005. pp. 38–44. NCEH Pub. No. 05-0570. [Google Scholar]
- 53.Levin R, Brown MJ, Kashtock ME, et al. Lead exposures in U.S. children, 2008: implications for prevention. Environ Health Perspect. 2008;116(10):1285–1293. doi: 10.1289/ehp.11241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin C, Kim R, Tsaih SW, Sparrow D, Hu H. Determinants of bone and blood lead levels among minorities living in the Boston area. Environ Health Perspect. 2004;112(11):1147–1151. doi: 10.1289/ehp.6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lanphear BP, Burgoon DA, Rust SW, Eberly S, Galke W. Environmental exposures to lead and urban children’s blood lead levels. Environ Res. 1998;76 (2):120–130. doi: 10.1006/enrs.1997.3801. [DOI] [PubMed] [Google Scholar]
- 56.Oktem F, Arslan MK, Dundar B, Delibas N, Gultepe M, Ergurhan Ilhan I. Renal effects and erythrocyte oxidative stress in long-term low-level lead-exposed adolescent workers in auto repair workshops. Arch Toxicol. 2004;78(12):681–687. doi: 10.1007/s00204-004-0597-5. [DOI] [PubMed] [Google Scholar]
- 57.Sönmez F, Dönmez O, Sönmez HM, Keskinog lu A, Kabasakal C, Mir S. Lead exposure and urinary N-acetyl β D glucosaminidase activity in adolescent workers in auto repair workshops. J Adolesc Health. 2002;30(3):213–216. doi: 10.1016/s1054-139x(01)00307-x. [DOI] [PubMed] [Google Scholar]
- 58.Fels LM, Wunsch M, Baranowski J, et al. Adverse effects of chronic low level lead exposure on kidney function: a risk group study in children. Nephrol Dial Transplant. 1998;13(9):2248–2256. doi: 10.1093/ndt/13.9.2248. [DOI] [PubMed] [Google Scholar]
- 59.Verberk MM, Willems TE, Verplanke AJ, De Wolff FA. Environmental lead and renal effects in children. Arch Environ Health. 1996;51(1):83–87. doi: 10.1080/00039896.1996.9935998. [DOI] [PubMed] [Google Scholar]
- 60.Bernard AM, Vyskocil A, Roels H, Kriz J, Kodl M, Lauwerys R. Renal effects in children living in the vicinity of a lead smelter. Environ Res. 1995;68(2):91–95. doi: 10.1006/enrs.1995.1012. [DOI] [PubMed] [Google Scholar]
- 61.Weaver VM, Lee BK, Ahn KD, et al. Associations of lead biomarkers with renal function in Korean lead workers. Occup Environ Med. 2003;60(8):551–562. doi: 10.1136/oem.60.8.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weaver VM, Schwartz BS, Ahn KD, et al. Associations of renal function with polymorphisms in the delta-aminolevulinic acid dehydratase, vitamin D receptor, and nitric oxide synthase genes in Korean lead workers. Environ Health Perspect. 2003;111(13):1613–1619. doi: 10.1289/ehp.6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roels H, Lauwerys R, Konings J, et al. Renal function and hyperfiltration capacity in lead smelter workers with high bone lead. Occup Environ Med. 1994;51(8):505–512. doi: 10.1136/oem.51.8.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hsiao CY, Wu HD, Lai JS, Kuo HW. A longitudinal study of the effects of long-term exposure to lead among lead battery factory workers in Taiwan (1989–1999) Sci Total Environ. 2001;279(1–3):151–158. doi: 10.1016/s0048-9697(01)00762-8. [DOI] [PubMed] [Google Scholar]
- 65.Hu H. A 50-year follow-up of childhood plumbism: hypertension, renal function, and hemoglobin levels among survivors. Am J Dis Child. 1991;145(6):681–687. doi: 10.1001/archpedi.1991.02160060099029. [DOI] [PubMed] [Google Scholar]
- 66.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 suppl 1):S1–S266. [PubMed] [Google Scholar]
- 67.Schwartz GJ, Feld LG, Langford DJ. A simple estimate of glomerular filtration rate in full-term infants during the first year of life. J Pediatr. 1984;104(6):849–854. doi: 10.1016/s0022-3476(84)80479-5. [DOI] [PubMed] [Google Scholar]
- 68.Léger F, Bouissou F, Coulais Y, Tafani M, Chatelut E. Estimation of glomerular filtration rate in children. Pediatr Nephrol. 2002;17(11):903–907. doi: 10.1007/s00467-002-0964-5. [DOI] [PubMed] [Google Scholar]
- 69.Ferguson MA, Vaidya VS, Bonventre JV. Biomarkers of nephrotoxic acute kidney injury. Toxicology. 2008;245(3):182–193. doi: 10.1016/j.tox.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tenstad O, Roald AB, Grubb A, Aukland K. Renal handling of radiolabelled human cystatin C in the rat. Scand J Clin Lab Invest. 1996;56(5):409–414. doi: 10.3109/00365519609088795. [DOI] [PubMed] [Google Scholar]
- 71.Norris KC, Agodoa LY. Unraveling the racial disparities associated with kidney disease. Kidney Int. 2005;68(3):914–924. doi: 10.1111/j.1523-1755.2005.00485.x. [DOI] [PubMed] [Google Scholar]
- 72.Rostand SG, Kirk KA, Rutsky EA, Pate BA. Racial differences in the incidence of treatment for end-stage renal disease. N Engl J Med. 1982;306(21):1276–1279. doi: 10.1056/NEJM198205273062106. [DOI] [PubMed] [Google Scholar]
- 73.McDonald SP, Russ GR, Kerr PG, Collins JF. Australia and New Zealand Dialysis and Transplant Registry. ESRD in Australia and New Zealand at the end of the millennium: a report from the ANZDATA registry. Am J Kidney Dis. 2002;40 (6):1122–1131. doi: 10.1053/ajkd.2002.36943. [DOI] [PubMed] [Google Scholar]
- 74.Lambie M, Richards N, Smith S. Ethnicity, age and incidence rates for renal replacement therapy (RRT) in Birmingham, UK: 1990–2004. Nephrol Dial Transplant. 2008;23(12):3983–3987. doi: 10.1093/ndt/gfn366. [DOI] [PubMed] [Google Scholar]
- 75.Perkovic V, Cass A, Patel AA, et al. InterASIA Collaborative Group. High prevalence of chronic kidney disease in Thailand. Kidney Int. 2008;73(4):473–479. doi: 10.1038/sj.ki.5002701. [DOI] [PubMed] [Google Scholar]
- 76.Merkin SS. Exploring the pathways between socioeconomic status and ESRD. Am J Kidney Dis. 2008;51(4):539–541. doi: 10.1053/j.ajkd.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 77.Ward MM. Socioeconomic status and the incidence of ESRD. Am J Kidney Dis. 2008;51(4):563–572. doi: 10.1053/j.ajkd.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 78.Collins AJ, Foley RN, Herzog C, et al. United States Renal Data System 2008 Annual Data Report Abstract. Am J Kidney Dis. 2009;53(suppl 1):vi–vii. S8–S374. doi: 10.1053/j.ajkd.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 79.Levey AS, Andreoli SP, DuBose T, Provenzano R, Collins AJ. CKD: common, harmful, and treatable; World Kidney Day 2007. Am J Kidney Dis. 2007;49(2):175–179. doi: 10.1053/j.ajkd.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 80.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298(17):2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]