Abstract
The goal was to determine recurrent or second primary status for late stomal malignancies, 16 and 17 years post-total laryngectomy in two laryngeal squamous cell carcinoma (LSCC) patients, based on DNA methylation signatures and HPV typing. Adopting a literature review based definition of late stomal recurrences as new primaries at the site of the stoma or neopharynx occurring more than 5 years after total laryngectomy, we employed a multi-gene candidate approach to examine promoter methylation in 24 tumor suppressor genes and PCR-based assays for HPV status offered additional insights into whether the late stomal tumors post total laryngectomy were related or not. The primary tumor for Patient 1 was negative for HPV but had aberrant hypermethylation of APC, MLH1, and BRCA1. The stomal biopsy 17-years later showed presence of HPV-16 without any methylated genes. In Patient 2, HPV-11 and promoter methylation of APC identified in the primary tumor was also observed in the stomal malignancy 16 years post total laryngectomy. Additional information provided by molecular typing for HPV and methylation markers underscored Patient 1’s and 2’s late stomal presentation as most likely a second primary and recurrence, respectively. DNA methylation markers are particularly advantageous because DNA methylation is an early event in tumorigenesis, and the epigenetic modification, 5-methylcytosine, is a stable marker. Molecular marks to discern genetic heterogeneity or relatedness of stomal malignancies several years post-total laryngectomy can provide clues to their status as either second primaries or likely recurrences. Our results support the hypothesis that a subset of stomal recurrences after total laryngectomy represents second primary tumors.
Keywords: DNA methylation, HPV status, second primary, recurrences
Introduction
Laryngeal cancer represents the largest subgroup of head and neck cancers (1). Roughly 12,250 new cases of laryngeal cancer are diagnosed each year in the U.S (2). Given the fundamental role the larynx plays in human speech and communication, determining the optimal management of laryngeal cancers is critical. Treatment options comprise radiotherapy, surgery, chemotherapy or a combination of modalities (3). Despite refinement of multimodal therapies over the last 20 years, five-year survival rates of 40% have remained static since the mid-1980s (4).
Laryngeal cancer usually presents as dysphonia or hoarseness of the voice. Squamous cell carcinoma of the larynx (LSCC) is the dominant histological type. Risk factors include tobacco and alcohol use, gastric acid reflux and HPV (particularly type 16). The most common site for LSCC is the glottis. The standard treatment for LSCC is a laryngectomy wherein the larynx is surgically removed.
In 1873, Billroth performed the first total laryngectomy as a treatment modality for laryngeal cancer. Since then, surgeons have adopted the total laryngectomy as mainstay treatment for extensive and invasive cases. However, this has not been without consequence as stomal recurrence after total laryngectomy has become one of the most serious complications in managing laryngeal carcinoma (5).
Primarily, stomal recurrence is defined as “a diffuse infiltrate of neoplastic tissue at the junction of amputated trachea and skin usually documented within a 5-year disease-free interval (DFI)” (5). The overall incidence for squamous cell carcinoma stomal recurrence is 6% and varies anywhere from 2.5 to 15% (6, 7). Additionally, it is of note that the majority of stomal recurrences usually present within one year post-total laryngectomy with greater than 90% presenting within 2 years (8). Current and past literature examining late recurrence of laryngeal cancer is scant with the greatest disease-free interval documented at 30 years post-conservative treatment (9). However, recurrence post-total laryngectomy has been explicitly noted after a disease-free interval of only 6.5 years (10).
Additionally, a caveat to this definition of recurrence is that this term is reserved for the return of disease of the same histological type usually within a 3 to 5-year disease-free interval. Any return of disease beyond this interval is generally termed a new or second primary. The criteria for a second primary were initially outlined in 1932 by Warren and Gates as a tumor that is definitively malignant, distinct from the original primary, with the possibility of metastasis excluded (11). Moreover, the time interval between two malignancies can be further classified into simultaneous, synchonronous (within 6 months) or metachronous (greater than 6 months) categories to provide further distinction (12).
The risk factors for stomal recurrence have been widely studied and include subglottic extension of the primary tumor, size of primary tumor and pre-operative trachostomies (7, 13). The main theories regarding the pathogenesis of stomal recurrence suggest that it occurs because of tumor cell implantation via perioperative implantation during tracheostomy or due to lymphatic spread mainly by subglottic lymphatic drainage (13).
With the advent of tumor marker identification, the lines between recurrence, metastasis and second primaries can be delineated further by examining the molecular characteristics of the tumors. Recurrent genomic aberrations are good indicators of genes that are causally associated with transformation, cancer development or progression. Microsatellite analysis of stomal recurrences after total laryngectomy support the hypothesis that a subset of late stomal recurrences are likely second primary tumors, genetically unrelated to the primary laryngeal lesion (14).
Epigenetics is the regulation of changes in gene expression by mechanisms that do not involve changes in DNA sequence. Perhaps the best known epigenetic process, in part because it has been easiest to study with existing technology, is DNA methylation. This is the addition (hypermethylation) or removal (hypomethylation) of a methyl group (CH3). Hypermethylation is a well described DNA modification that has been implicated in normal mammalian development (15), imprinting (16), and X chromosome inactivation (17). However, recent studies have identified hypermethylation as a probable cause in the development of various cancers (18). In squamous head and neck cancer (HNSCC), recent comprehensive high-throughput methods have underscored the contribution of both genetic (19, 20) and epigenetic events (21, 22), often working together (23), in the development and progression of HNSCC. Previous studies from our group (24, 25) have demonstrated that epigenetic events of DNA hypermethylation underlie the pathogenesis of benign sinonasal and recurrent laryngeal papillomas, establishing a monoclonal origin for recurrent respiratory papillomatosis (RRP) as well support a monoclonal progression for malignant transformation to LSCC in some RRP cases
In addition to tobacco and alcohol, epidemiological and laboratory evidence now warrant the conclusion that, the human papilloma virus (HPV) is a causative agent for some HNSCC (26, 27) and an independent risk factor for oropharyngeal HNSCC (28–30). The prevalence of HPV infection was significantly higher among patients with oropharyngeal SCC (35.6%) than among those with oral (23.5%) or laryngeal (24.0%) SCC (31).
The goal of our study was to use epigenetic events of promoter methylation and HPV infection status to determine recurrent or second primary status for two late stomal malignancies 16 and 17 years post-total laryngectomy. We adopted a literature review based definition of late stomal recurrences as new primaries at the site of the stoma or neopharynx occurring more than 5 years after total laryngectomy. We employed a multi-gene candidate approach to examine for promoter methylation in 24 tumor suppressor genes and PCR-based assays for HPV-16 and HPV-11 status to molecularly asses the clonal relationship between the primary and late stomal tumor lesions.
Materials and methods
Cohort
Clinical history for Patient 1
Case #1
W.W. was a 63 year-old male who was diagnosed with T2N0MO squamous cell carcinoma of the left true vocal cord in February of 1991. He completed radiation therapy consisting of 7000 rads in April of 1991. In June of 1991, he underwent salvage total laryngectomy for persistent disease. He remained disease-free until July of 2008, when he presented with dysphagia. An esophagoscopy was performed and a mass was noted in the neopharynx. Biopsy of this region revealed squamous cell carcinoma, T4N1M0. The patient underwent cervical esophagectomy with stomal resection, bilateral selective neck dissections, free jejunal flap reconstruction of total laryngopharyngectomy defect as well as pectoralis major flap reconstruction of stoma in August 2008. Two months post-operatively, he was noted to have recurrence of disease bilaterally in the posterior triangles. He underwent palliative chemotherapy and passed away in March 2009.
Case #2
W.E. was a 67 year-old male with a history of a T3N1MX of the right pyriform sinus status post total laryngectomy and right neck dissection with Internal Jugular sacrifice and post operative chemoradiation therapy in 1993. In 1995, the patient has a biopsy taken of his stoma site for bleeding that was deemed negative for histopathologic disease. He presented again in November 2009 with increased difficulty with speech through his tracheoesophageal puncture (TEP) and episodes of bleeding from his stoma site. At this time, stomal biopsy was positive for stomal/esophageal squamous cell carcinoma. The patient was not deemed to be an otolaryngological surgical candidate at that time and was to undergo chemoradiative treatment. The patient presented in March 2010 with symptomatic spinal cord compression and underwent a T2 Corpectomy with C6-T4 fusion by neurosurgery. W.E. presented to the ER in April 2010 symptoms of severe sepsis and multi-organ failure and after a prolonged hospital course, died in June 2010.
Patient tissue material for this study was obtained according to the Henry Ford Health System institutional review board protocols.
Molecular Studies
The two primary LSCC patients presenting with late stomal malignancy 16 and 17 years post total laryngectomy was examined for aberrant DNA methylation in 24 tumor suppressor genes and HPV status. Archival tissue DNA, extracted from microdissected LSCC lesions, was interrogated for methylation status using the multi-gene methylation-specific multiplex ligation-dependent probe amplification (MS-MLPA) assay.
The Methylation-Specific Multiplex Ligation Dependent Probe Amplification (MS-MLPA) Assay
MS-MLPA, a modification of the conventional MLPA assay allows for the simultaneous detection of changes in methylation status as well as copy number changes of approximately 41 different DNA sequences in a single reaction requiring only 20 ng of human DNA (23, 32, 33).
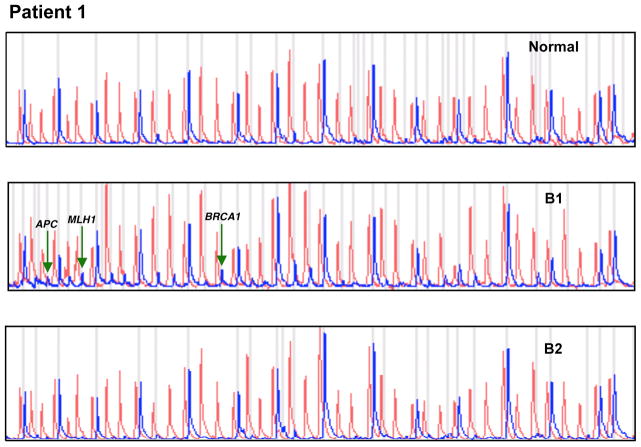
Briefly, the MS-MLPA panel in the presence of HhaI detects aberrant promoter hypermethylation by taking advantage of a HhaI site in the gene probes of interest. The control gene probes, without a HhaI site, serve as undigested controls. A normal control DNA sample will generate individual peaks for all probes in the absence of HhaI and 15 separate peaks in the presence of HhaI (Figure 1).
Figure 1.
MS-MLPA assay demonstrating methylation of APC, MLH1 and BRCA1 in biopsy 1 (B1, primary tumor). No methylation in biopsy 2 (B2, 17 years post total laryngectomy).
Gene probe panels
The 41 gene probe panel (Table 1) interrogates 38 unique genes implicated in cancer including LSCC for losses and gains and methylation status in two separate reactions (one in the absence of the methyl-sensitive enzyme HhaI and one in the presence of the HhaI enzyme). Because there are two probes each for MLH1, RASSF1 and BRCA2, a normal control DNA sample will generate 41 individual peaks in the absence of HhaI and 15 individual peaks in the presence of HhaI.
Table 1.
Methylation-specific MLPA Probe Panel (ME001B)
| No. | Chromosomal Locus | Gene probe |
|---|---|---|
| 1 | 01p36 | TP73 |
| 2 | 02q22.3 | CASP8 |
| 3 | 03p25.3 | VHL |
| 4 | 03p24 | RARB |
| 5 | 03p21.1 | MLH1 |
| 6 | 03p21.1 | MLH1 |
| 03p22 | CTNNB1 | |
| 7 | 03p21.3 | RASSF1 |
| 8 | 03p21.3 | RASSF1 |
| 9 | 03p14.2 | FHIT |
| 03q21 | CASR | |
| 10 | 05q21 | APC |
| 11 | 06q25.1 | ESR1 |
| 06q26 | PARK2 | |
| 07q21.3 | CDK6 | |
| 12 | 09p21 | CDKN2A |
| 13 | 09p21 | CDKN2B |
| 14 | 09q34.1 | DAPK1 |
| 10p14 | AI651963 | |
| 10p12.1 | CREM | |
| 15 | 10q23.3 | PTEN |
| 16 | 11p12 | CD44 |
| 17 | 11q13 | GSTP1 |
| 18 | 11q23 | ATM |
| 19 | 11q23 | IGSF4 |
| 12p13 | TNFRSF1A | |
| 12p13 | TNFRSF7 | |
| 20 | 12q13.1 | CDKN1B |
| 12q23 | PAH | |
| 21 | 12q24.33 | CHFR |
| 22 | 13q12.3 | BRCA2 |
| 13q12.3 | BRCA2 | |
| 14q24.3 | MLH3 | |
| 16p13.3 | TSC2 | |
| 16q22.1 | CDH1 | |
| 23 | 16q24.2 | CDH13 |
| 24 | 17p13.3 | HIC1 |
| 25 | 17q21 | BRCA1 |
| 18q21.3 | BCL2 | |
| 19q13 | KLK3 | |
| 26 | 22q12.3 | TIMP3 |
Multiple probes for genes are shown in bold.
HPV Detection
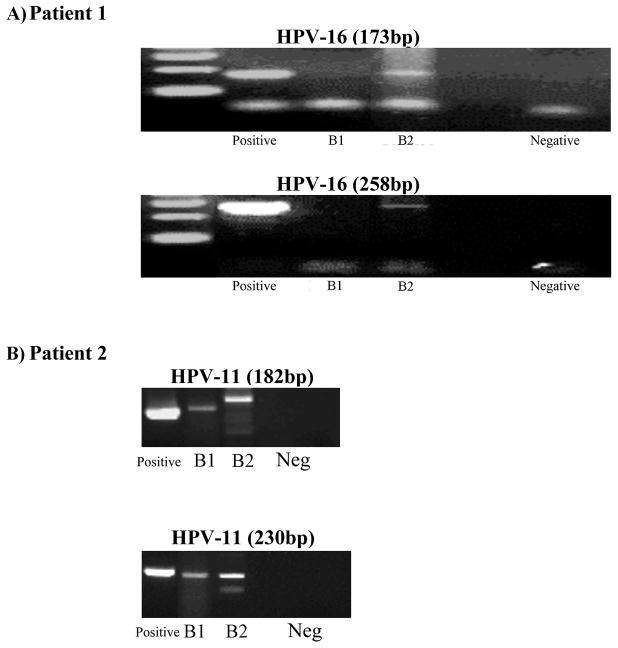
HPV status for the primary and late stomal tumor biopsies was identified and confirmed by PCR for HPV-11 and HPV-16 sequences using multiple HPV type specific primers especially designed to amplify less than 300 base pair DNA fragment lengths (34) (Figure 2).
Figure 2.
Figure 2A. Patient 1 is HPV negative in biopsy 1 (B1, primary tumor) but is positive for HPV-16 in biopsy 2 (B2, 17 years post total laryngectomy) by two sets of primers for HPV-16. (Positive: HPV positive control; Negative: HPV negative control)
Figure 2B. Patient 2 is positive for HPV-11 in biopsies 1 and 2 (B1 and B2, primary tumor and 16 years post total laryngectomy, respectively) by two sets of primers for HPV-11. (Positive: HPV positive control; Neg: HPV negative control)
Results
DNA methylation and HPV typing results for the two cases are summarized in Table 2
Table 2.
Summary of methylation and HPV status
| Patient # | Date of Diagnosis | Biopsy type | Date of TL | HPV | Methylation |
|---|---|---|---|---|---|
| Patient 1 | 2/5/1991 | primary | 7/24/1991 TL | negative | APC, MLH1, BRCA1 |
| 8/1/2008 | stomal | HPV-16 | No methylation | ||
| Patient 2 | 4/13/1993 | primary | 4/13/1993 TL | HPV-11 | APC, MLH1 |
| 11/3/2009 | stomal | HPV-11 | APC |
TL = Total Laryngectomy
Patient 1
DNA from the primary tumor biopsy (Biopsy 1) was HPV-negative (Figure 2A). MS-MLPA indicated aberrant methylation of APC, MLH1, and BRCA1 (Figure 1).
DNA from the post-total laryngectomy 17 years later (Biopsy 2) was HPV-16 positive and was absent for methylated genes in the MS-MLPA panel.
Patient 2
DNA from the primary tumor biopsy (Biopsy 1) and the stomal biopsy (Biopsy 2) 16 years post-total laryngectomy was HPV-11 positive (Figure 2B). MS-MLPA indicated aberrant methylation of APC in both, primary and late stomal biopsies (Figure 3).
Figure 3.
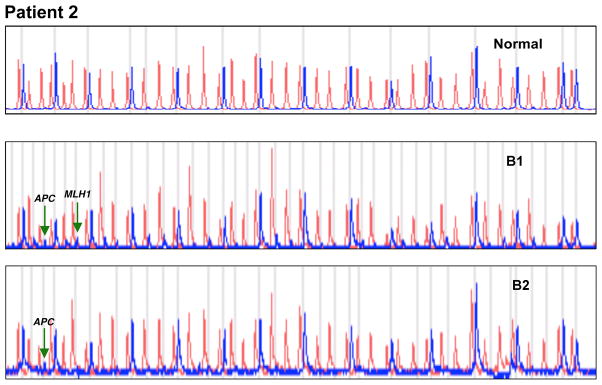
MS-MLPA assay demonstrating methylation of APC in biopsies 1 and 2 (B1 and B2, primary tumor and 16 years post total laryngectomy, respectively). Additional methylation of MLH1 detected in biopsy 1.
Discussion
The extent of heterogeneity or lack of clonality of tumors is an important question that underscores the biologic propensity of a cancer cell to persist, progress and metastasize. A basic mechanism of tumor resistance to cancer treatment is that tumors are composed of a multitude of variant cells and cell sub populations that differ with respect to phenotypic and genotypic characteristics (tumor heterogeneity) (35, 36). Cellular and molecular heterogeneity pose the greatest challenges to effective treatment modalities and disease management in HNSCC. Another challenge to effective treatment is the phenomenon of “tumor repopulation”, which is the potential for tumor cells to proliferate rapidly after any treatment. It is worthy of mention that between the initial biopsy and the next tissue sample, both patients in this report had undergone not only surgical treatment with histopathologic negative margins, but also chemoradiative therapy, the latter likely serving as a contributing factor in their subsequent stomal malignancies.
Among prevailing theories regarding the pathogenesis of stomal recurrences, submucosal extension and lymph node metastases are probably more important mechanisms than cancer cell implantation (13). While clinically, the delineation between a recurrence and a new primary hinges upon the length of the disease free interval (DFI), it was our objective to provide a more definitive classification for tumors that present beyond the 5-year DFI. Stomal recurrence after total laryngectomy has become a serious complication and due to the dismal prognosis of stomal recurrence the focus is on prevention. Knowledge of whether tumor stomal cells post total laryngectomy are related clones (monoclonal) or distinct clonal populations from a primary LSCC with DFI can offer perspectives for patient management and prognostication.
The debate whether second primary cancers are new tumors or recurrences/metastases began in 1953 by Slaughter and colleagues (37) who introduced the concept of “field cancerization” to help explain the high incidence of second primary neoplasms in squamous epithelium. Existing literature largely clusters around parameters established by Slaughter stating that most recurrences occur within 2cm of the primary lesion and within 36 months from primary treatment. Molecular genetics support for field cancerization in synchronous primary cancer of the oral cavity was evident from the finding of different p53 mutations in the right tonsillar pillar-soft palate tumor, and a left retromolar trigone tumor (38). In studies of X chromosome inactivation in second primary cancers arising in women, Bedi and colleagues (35) demonstrated inactivation of the same allele in both tumors. Evidence employing fluorescence in situ approaches suggests a monoclonal origin of second primary tumors (39).
The hypothesis that a subset of stomal recurrences after total laryngectomy may be second primary tumors, i.e., genetically unrelated to the primary laryngeal lesion (14) is supported by microsatellite analysis of stomal recurrences after total laryngectomy. Of the 5 informative patients, 2 demonstrated discordant alterations in the recurrent tumor indicative of clonal heterogeneity, 2 demonstrated identical alterations, and 1 showed an additional alteration in the recurrent tumor(14).
Epigenetic events of promoter hypermethylation are emerging as one of the most promising molecular strategies for cancer detection and represent important tumor-specific markers occurring early in tumor progression. Our group has demonstrated using high-throughput methods, the contribution of both genetic (19, 20) and epigenetic events (22), often working together (23), in the development and progression of HNSCC. Promoter hypermethylation is emerging as one of the most promising cancer detection strategies for several reasons. DNA methylation markers are particularly advantageous because DNA methylation is an early event in tumorigenesis, and the epigenetic modification, 5-methylcytosine, is a stable marker amenable to PCR-based methylation assays using whole genomic DNA from fresh/frozen tissue, cell lines, as well as formalin fixed paraffin tumor tissue DNA (23–25, 40). Additionally, aberrant promoter hypermethylation always occurs in virtually the same location within an affected gene, allowing a single PCR primer to be applicable to all patients for examination of the methylation status of a specific gene. This sharply contrasts with genomic biomarkers such as DNA mutations in genes such as p53 or mitochondrial genes (41), which often involve myriad different base changes at many locations within the gene even in cancers of the same histologic types. Thus, classification based on promoter methylation profiling may well be a more promising approach than expression profiling since these DNA-based techniques are not subject to the problems of tissue preservation and the potential pitfalls of tissue heterogeneity.
In this study, DNA methylation patterns pointed to lack of genetic relatedness for primary and late stomal biopsies in Patient 1. Aberrant methylation of APC, MLH1, and BRCA1 detected in the primary biopsy was not observed in the late stomal biopsy. None of the 24 tumor suppressor genes in the MS-MLPA panel showed methylation in the stomal biopsy 17 years post total laryngectomy. In Patient 2, the primary tumor and the late stomal malignancy 16 years later post- total laryngectomy had aberrant methylation of APC suggesting retention of a monoclonal population for over a decade before the patient became symptomatic. APC (adenomatosis polyposis coli) is a tumor suppressor gene originally implicated in colon cancer. Genetic and epigenetic alterations in this gene have since been recognized in other malignancies including oral squamous cell carcinoma (OSCC), gastric cancers and esophageal adenocarcinomas. Uesugi et al. (42) previously reported mutations and/or deletions of APC in primary OSCC and suggested that loss of APC function contributes to carcinogenesis in the oral region. APC inactivation as a result of promoter hypermethylation occurred in 25% of OSCC cell (42). In a primary LSCC cohort of 79 patients, aberrant methylation of APC occurred in 16% of the tumors (43). In our study, aberrant methylation of APC was a common event in both primary tumors supporting methylation of APC as an early event in LSCC tumorigenesis.
Both cases had methylation of MLH1 in the primary biopsies. MLH1 is a DNA mismatch repair gene that is frequently mutated in hereditary nonpolyposis colon cancer (HNPCC) (44). In sporadic colorectal cancers (CRC), this gene is found to be inactivated by promoter methylation. In addition to CRC, methylation of MLH1 has been reported in breast cancer (45), uterine cancer (46) and acute myeloid leukemia (47). Sasiadek et al. (48) found a correlation of both LOH and hypermethylation with the loss of expression for MLH1 in laryngeal cancer.
HPV infection status offered additional insights into whether the late stomal tumors post total laryngectomy were related or not. For cervical cancer, a persistent viral status represents a necessary although not sufficient basis for HPV-related lesions (49, 50), where persistent infection with a specific high risk HPV type must be maintained for at least 2 years (51). In Patient 1, HPV-16 was detected only in the stomal biopsy suggesting a later onset viral infection and strengthening the likelihood of the late stomal malignancy in Patient 1 as a second primary.
The association between HPV and head and neck cancer was established as early as the 1980’s (52) and more recently, HPV-16 positive oropharyngeal cancers have been shown to have improved outcomes with chemoradiative treatment (53, 54). A study in 2008 by The American Cancer Society found that the burden of HPV-oropharyngeal cancers is second only to cervical cancer and thus the potential benefit of prophylactic vaccination is significant (55). Given that W.W.’s initial tumor specimen was HPV negative, the question arises as to whether this molecular knowledge may have been employed to advocate the administration of the HPV vaccine thereby possibly preventing the development of a second primary.
In Patient 2, HPV-11 was detected in the primary tumor and the stomal tumor 16 years post total laryngectomy. HPV types 6 and 11 are etiologic agents for laryngeal papillomas (56–58). Studies on HPV typing in benign laryngeal papillomas have demonstrated an association of HPV type 11 with a more aggressive course of the disease (59, 60). According to Lele et al (61), HPV-11 infection may be an early event in progression of recurrent respiratory papillomatosis to carcinoma. Patient 2’s medical history did not indicate any history of respiratory papillomatosis.
In conclusion, the use of DNA methylation patterns as well as HPV status can serve as an added monitor for following patients who have undergone extensive surgical resection and chemoradiative therapy of primary tumors. Our documentation of two cases of stomal malignancies presenting 16 and 17 years post-total laryngectomy using molecular typing for HPV and methylation signatures as a second (62) primary (Patient 1) and a recurrence (Patient 2), demonstrates the utility of molecular approaches in differentiating multiple tumors, providing a snapshot of tumorigenic evolution over an extended period of time.
Acknowledgments
Supported by NIH R01 DE 15990
References
- 1.Clayman GLLS, Laramore GF, et al. Neoplasms of the head and neck. Vol. 2000. B.C. Becker Inc; New York: 2000. section 27. [Google Scholar]
- 2.Horner MJRL, Krapcho M, Neyman N, Aminou R, Howlader N, Altekruse SF, Feuer EJ, Huang L, Mariotto A, Miller BA, Lewis DR, Eisner MP, Stinchcomb DG, Edwards BK. SEER Cancer Statistics Review, 1975–2006. National Cancer Institute; Bethesda, MD: 2009. [Google Scholar]
- 3.Wilson J. Effective Head and Neck Cancer Management. 3. British Association of Otorhinolaryngologists Head and Neck Surgeons; London: 2000. [Google Scholar]
- 4.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153–156. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 5.Imauchi Y, Ito K, Takasago E, Nibu K, Sugasawa M, Ichimura K. Stomal recurrence after total laryngectomy for squamous cell carcinoma of the larynx. Otolaryngol Head Neck Surg. 2002;126:63–66. doi: 10.1067/mhn.2002.121515. [DOI] [PubMed] [Google Scholar]
- 6.Keim WF, Shapiro MJ, Rosin HD. Study of Postlaryngectomy Stomal Recurrence. Arch Otolaryngol. 1965;81:183–186. doi: 10.1001/archotol.1965.00750050190014. [DOI] [PubMed] [Google Scholar]
- 7.Zbaren P, Greiner R, Kengelbacher M. Stoma recurrence after laryngectomy: an analysis of risk factors. Otolaryngol Head Neck Surg. 1996;114:569–575. doi: 10.1016/S0194-59989670248-6. [DOI] [PubMed] [Google Scholar]
- 8.Esteban F, Moreno JA, Delgado-Rodriguez M, Mochon A. Risk factors involved in stomal recurrence following laryngectomy. J Laryngol Otol. 1993;107:527–531. doi: 10.1017/s0022215100123618. [DOI] [PubMed] [Google Scholar]
- 9.Rubin J, Johnson JT, Myers EN. Stomal recurrence after laryngectomy: interrelated risk factor study. Otolaryngol Head Neck Surg. 1990;103:805–812. doi: 10.1177/019459989010300523. [DOI] [PubMed] [Google Scholar]
- 10.Thomas R. Late Recurrence of Laryngeal Cancer. J Laryngol Otol. 1964;78:1123–1124. doi: 10.1017/s0022215100063271. [DOI] [PubMed] [Google Scholar]
- 11.Modlin B, Ogura JH. Post-laryngectomy tracheal stomal recurrences. Laryngoscope. 1969;79:239–250. doi: 10.1288/00005537-196902000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Warren SGO. Multiple primary malignant tumors: A survey of the literature and a statistical study. American Journal of Cancer. 1932;16:1358–1414. [Google Scholar]
- 13.Yotakis J, Davris S, Kontozoglou T, Adamopoulos G. Evaluation of risk factors for stomal recurrence after total laryngectomy. Clin Otolaryngol Allied Sci. 1996;21:135–138. doi: 10.1111/j.1365-2273.1996.tb01317.x. [DOI] [PubMed] [Google Scholar]
- 14.Santoro R, Franchi A, Tempesti C, Sardi I, Polli G. Stomal recurrence following total laryngectomy: clinical and molecular analysis of a series. Ann Otol Rhinol Laryngol. 2003;112:594–599. doi: 10.1177/000348940311200704. [DOI] [PubMed] [Google Scholar]
- 15.Costello JF, Plass C. Methylation matters. J Med Genet. 2001;38:285–303. doi: 10.1136/jmg.38.5.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li E, Beard C, Jaenisch R. Role for DNA methylation in genomic imprinting. Nature. 1993;366:362–365. doi: 10.1038/366362a0. [DOI] [PubMed] [Google Scholar]
- 17.Pfeifer GP, Tanguay RL, Steigerwald SD, Riggs AD. In vivo footprint and methylation analysis by PCR-aided genomic sequencing: comparison of active and inactive X chromosomal DNA at the CpG island and promoter of human PGK-1. Genes Dev. 1990;4:1277–1287. doi: 10.1101/gad.4.8.1277. [DOI] [PubMed] [Google Scholar]
- 18.Costello JF, Fruhwald MC, Smiraglia DJ, et al. Aberrant CpG-island methylation has non-random and tumour-type-specific patterns. Nat Genet. 2000;24:132–138. doi: 10.1038/72785. [DOI] [PubMed] [Google Scholar]
- 19.Worsham MJ, Pals G, Schouten JP, et al. Delineating genetic pathways of disease progression in head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2003;129:702–708. doi: 10.1001/archotol.129.7.702. [DOI] [PubMed] [Google Scholar]
- 20.Worsham MJ, Chen KM, Tiwari N, et al. Fine-mapping loss of gene architecture at the CDKN2B (p15INK4b), CDKN2A (p14ARF, p16INK4a), and MTAP genes in head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2006;132:409–415. doi: 10.1001/archotol.132.4.409. [DOI] [PubMed] [Google Scholar]
- 21.Sanchez-Cespedes M, Okami K, Cairns P, Sidransky D. Molecular analysis of the candidate tumor suppressor gene ING1 in human head and neck tumors with 13q deletions. Genes Chromosomes Cancer. 2000;27:319–322. doi: 10.1002/(sici)1098-2264(200003)27:3<319::aid-gcc13>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 22.Maruya S, Issa JP, Weber RS, et al. Differential methylation status of tumor-associated genes in head and neck squamous carcinoma: incidence and potential implications. Clin Cancer Res. 2004;10:3825–3830. doi: 10.1158/1078-0432.CCR-03-0370. [DOI] [PubMed] [Google Scholar]
- 23.Worsham MJ, Chen KM, Meduri V, et al. Epigenetic events of disease progression in head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2006;132:668–677. doi: 10.1001/archotol.132.6.668. [DOI] [PubMed] [Google Scholar]
- 24.Stephen JK, Vaught LE, Chen KM, et al. An epigenetically derived monoclonal origin for recurrent respiratory papillomatosis. Arch Otolaryngol Head Neck Surg. 2007;133:684–692. doi: 10.1001/archotol.133.7.684. [DOI] [PubMed] [Google Scholar]
- 25.Stephen JK, Vaught LE, Chen KM, et al. Epigenetic events underlie the pathogenesis of sinonasal papillomas. Mod Pathol. 2007;20:1019–1027. doi: 10.1038/modpathol.3800944. [DOI] [PubMed] [Google Scholar]
- 26.Gillison ML, Lowy DR. A causal role for human papillomavirus in head and neck cancer. Lancet. 2004;363:1488–1489. doi: 10.1016/S0140-6736(04)16194-1. [DOI] [PubMed] [Google Scholar]
- 27.Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 28.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.D’Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 30.Chen AA, Marsit CJ, Christensen BC, et al. Genetic variation in the vitamin C transporter, SLC23A2, modifies the risk of HPV16-associated head and neck cancer. Carcinogenesis. 2009;30:977–981. doi: 10.1093/carcin/bgp076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14:467–475. doi: 10.1158/1055-9965.EPI-04-0551. [DOI] [PubMed] [Google Scholar]
- 32.Nygren AO, Ameziane N, Duarte HM, et al. Methylation-specific MLPA (MS-MLPA): simultaneous detection of CpG methylation and copy number changes of up to 40 sequences. Nucleic Acids Res. 2005;33:e128. doi: 10.1093/nar/gni127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen K, Sawhney R, Khan M, et al. Methylation of multiple genes as diagnostic and therapeutic markers in primary Head and Neck Squamous Cell Carcinoma. Arch Otolaryngol Head Neck Surg. 2007;133:1131–1138. doi: 10.1001/archotol.133.11.1131. [DOI] [PubMed] [Google Scholar]
- 34.Stephen JK, Vaught LE, Chen KM, Shah V, Schweitzer VG, Gardner G, Benninger MS, Worsham MJ. Consistent aberrant DNA methylation patterns in laryngeal papillomas. International Journal of Head and Neck Surgery. 2010;1:69–77. doi: 10.5005/jp-journals-10001-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bedi GC, Westra WH, Gabrielson E, Koch W, Sidransky D. Multiple head and neck tumors: evidence for a common clonal origin. Cancer Res. 1996;56:2484–2487. [PubMed] [Google Scholar]
- 36.Heppner GH, Miller BE. Therapeutic implications of tumor heterogeneity. Semin Oncol. 1989;16:91–105. [PubMed] [Google Scholar]
- 37.Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6:963–968. doi: 10.1002/1097-0142(195309)6:5<963::aid-cncr2820060515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 38.Koch WM, Boyle JO, Mao L, Hakim J, Hruban RH, Sidransky D. p53 gene mutations as markers of tumor spread in synchronous oral cancers. Arch Otolaryngol Head Neck Surg. 1994;120:943–947. doi: 10.1001/archotol.1994.01880330029006. [DOI] [PubMed] [Google Scholar]
- 39.Worsham MJ, Wolman SR, Carey TE, Zarbo RJ, Benninger MS, Van Dyke DL. Common clonal origin of synchronous primary head and neck squamous cell carcinomas: analysis by tumor karyotypes and fluorescence in situ hybridization. Hum Pathol. 1995;26:251–261. doi: 10.1016/0046-8177(95)90054-3. [DOI] [PubMed] [Google Scholar]
- 40.Stephen JK, Vaught LE, Chen KM, et al. Epigenetic events underlie the pathogenesis of sinonasal papillomas. Mod Pathol. 2007 doi: 10.1038/modpathol.3800944. [DOI] [PubMed] [Google Scholar]
- 41.Fliss MS, Usadel H, Caballero OL, et al. Facile detection of mitochondrial DNA mutations in tumors and bodily fluids. Science. 2000;287:2017–2019. doi: 10.1126/science.287.5460.2017. [DOI] [PubMed] [Google Scholar]
- 42.Uesugi H, Uzawa K, Kawasaki K, et al. Status of reduced expression and hypermethylation of the APC tumor suppressor gene in human oral squamous cell carcinoma. Int J Mol Med. 2005;15:597–602. [PubMed] [Google Scholar]
- 43.Stephen JK, Chen K, Shah V, et al. DNA hypermethylation markers of poor outcome in laryngeal cancer. Clinical Epigenetics. 2010 doi: 10.1007/s13148-010-0005-3. ePub ahead of print June 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu B, Nicolaides NC, Markowitz S, et al. Mismatch repair gene defects in sporadic colorectal cancers with microsatellite instability. Nat Genet. 1995;9:48–55. doi: 10.1038/ng0195-48. [DOI] [PubMed] [Google Scholar]
- 45.Huang KT, Dobrovic A, Yan M, et al. DNA methylation profiling of phyllodes and fibroadenoma tumours of the breast. Breast Cancer Res Treat. doi: 10.1007/s10549-010-0970-4. [DOI] [PubMed] [Google Scholar]
- 46.Seeber LM, Zweemer RP, Marchionni L, et al. Methylation profiles of endometrioid and serous endometrial cancers. Endocr Relat Cancer. 17:663–673. doi: 10.1677/ERC-10-0014. [DOI] [PubMed] [Google Scholar]
- 47.Griffiths EA, Gore SD, Hooker CM, et al. Epigenetic differences in cytogenetically normal versus abnormal acute myeloid leukemia. Epigenetics. :5. doi: 10.4161/epi.5.7.12558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sasiadek MM, Stembalska-Kozlowska A, Smigiel R, Ramsey D, Kayademir T, Blin N. Impairment of MLH1 and CDKN2A in oncogenesis of laryngeal cancer. Br J Cancer. 2004;90:1594–1599. doi: 10.1038/sj.bjc.6601679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Munoz N, Bosch FX, de Sanjose S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 50.Herrero R, Hildesheim A, Bratti C, et al. Population-based study of human papillomavirus infection and cervical neoplasia in rural Costa Rica. J Natl Cancer Inst. 2000;92:464–474. doi: 10.1093/jnci/92.6.464. [DOI] [PubMed] [Google Scholar]
- 51.Campisi G, Giovannelli L. Controversies surrounding human papilloma virus infection, head & neck vs oral cancer, implications for prophylaxis and treatment. Head Neck Oncol. 2009;1:8. doi: 10.1186/1758-3284-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Syrjanen K, Syrjanen S, Pyrhonen S. Human papilloma virus (HPV) antigens in lesions of laryngeal squamous cell carcinomas. ORL J Otorhinolaryngol Relat Spec. 1982;44:323–334. doi: 10.1159/000275612. [DOI] [PubMed] [Google Scholar]
- 53.Nichols AC, Faquin WC, Westra WH, et al. HPV-16 infection predicts treatment outcome in oropharyngeal squamous cell carcinoma. Otolaryngol Head Neck Surg. 2009;140:228–234. doi: 10.1016/j.otohns.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 54.Chen R, Aaltonen LM, Vaheri A. Human papillomavirus type 16 in head and neck carcinogenesis. Rev Med Virol. 2005;15:351–363. doi: 10.1002/rmv.471. [DOI] [PubMed] [Google Scholar]
- 55.Gillison ML, Chaturvedi AK, Lowy DR. HPV prophylactic vaccines and the potential prevention of noncervical cancers in both men and women. Cancer. 2008;113:3036–3046. doi: 10.1002/cncr.23764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mounts P, Shah KV, Kashima H. Viral etiology of juvenile- and adult-onset squamous papilloma of the larynx. Proc Natl Acad Sci U S A. 1982;79:5425–5429. doi: 10.1073/pnas.79.17.5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gabbott M, Cossart YE, Kan A, Konopka M, Chan R, Rose BR. Human papillomavirus and host variables as predictors of clinical course in patients with juvenile-onset recurrent respiratory papillomatosis. J Clin Microbiol. 1997;35:3098–3103. doi: 10.1128/jcm.35.12.3098-3103.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gissmann L, Wolnik L, Ikenberg H, Koldovsky U, Schnurch HG, zur Hausen H. Human papillomavirus types 6 and 11 DNA sequences in genital and laryngeal papillomas and in some cervical cancers. Proc Natl Acad Sci U S A. 1983;80:560–563. doi: 10.1073/pnas.80.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hartley C, Hamilton J, Birzgalis AR, Farrington WT. Recurrent respiratory papillomatosis--the Manchester experience, 1974–1992. J Laryngol Otol. 1994;108:226–229. [PubMed] [Google Scholar]
- 60.Lie ES, Karlsen F, Holm R. Presence of human papillomavirus in squamous cell laryngeal carcinomas. A study of thirty-nine cases using polymerase chain reaction and in situ hybridization. Acta Otolaryngol. 1996;116:900–905. doi: 10.3109/00016489609137949. [DOI] [PubMed] [Google Scholar]
- 61.Lele SM, Pou AM, Ventura K, Gatalica Z, Payne D. Molecular events in the progression of recurrent respiratory papillomatosis to carcinoma. Arch Pathol Lab Med. 2002;126:1184–1188. doi: 10.5858/2002-126-1184-MEITPO. [DOI] [PubMed] [Google Scholar]
- 62.Baker AH, Zaltsman AB, George SJ, Newby AC. Divergent effects of tissue inhibitor of metalloproteinase-1, -2, or -3 overexpression on rat vascular smooth muscle cell invasion, proliferation, and death in vitro. TIMP-3 promotes apoptosis. J Clin Invest. 1998;101:1478–1487. doi: 10.1172/JCI1584. [DOI] [PMC free article] [PubMed] [Google Scholar]