Summary
Background
Elevated urine 11-dehydro TXB2, an indicator of persistent thromboxane generation in aspirin-treated patients, correlates with adverse cardiovascular outcome and has recently been identified as an independent risk factor for vein graft thrombosis after cardiac bypass surgery in the Reduction in Graft Occlusion Rates (RIGOR) study. The polyclonal antibody-based ELISA used to measure 11-dehydro TXB2 in these previous studies is no longer clinically available and has been supplanted by a Food and Drug Administration (FDA)-cleared second-generation monoclonal antibody-based ELISA.
Objectives
To compare the laboratory and clinical performance of the first- and second-generation assays in a well-defined study population.
Methods
11-dehydro TXB2 was quantified in 451 urine samples from 229 Reduction in Graft Occlusion Rates (RIGOR) subjects using both ELISA. Ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) and spiking studies were used to investigate discordant assay results. The association of 11-dehydro TXB2 to clinical outcome was assessed for each assay using multivariate modeling.
Results
Median 11-dehydro TXB2 levels were higher by monoclonal antibody- compared with polyclonal antibody-based ELISA (856 vs. 399 pg mg−1 creatinine, P < 0.000001), with the latter providing values similar to UPLC-MS/MS. This discrepancy was predominantly as a result of cross-reactivity of the monoclonal antibody with 11-dehydro-2,3-dinor TXB2, a thromboxane metabolite present in a similar concentration but with a poor direct correlation with 11-dehydro TXB2. In contrast to the first-generation ELISA, 11-dehydro TXB2 measured by the monoclonal antibody-based ELISA failed to associate with the risk of vein graft occlusion.
Conclusion
Quantification of urine 11-dehydro TXB2 by monoclonal antibody-based ELISA was confounded by interference from 11-dehydro-2,3-dinor TXB2 which reduced the accuracy and clinical utility of this second-generation assay.
Keywords: 11-dehydro thromboxane B2, aspirin, ELISA, thrombosis, vein graft
Introduction
Thromboxane A2 (TXA2) is a short-lived eicosanoid derived from cyclooxygenase-1 (COX-1)-dependent metabolism of arachidonic acid [1,2]. In healthy individuals, the vast majority of TXA2 is generated by platelets in response to an array of physiologic agonists. TXA2 not only mediates activation of the platelet in which it is formed, but is also released where it can activate adjacent quiescent platelets and stimulate arterial vasoconstriction through binding to specific cellular receptors.
Aspirin (ASA) effectively inhibits platelet TXA2 formation and suppresses agonist-induced platelet activation by irreversibly acetylating the serine −530 residue of the COX-1 enzyme [3]. As a result of this antiplatelet effect, ASA significantly reduces the risk of a myocardial infarction and stroke in patients with atherosclerosis [4]. However, growing evidence indicates that some patients continue to have high levels of agonist-induced platelet activation in spite of ASA therapy and therefore remain at an increased risk for cardiovascular events [5,6]. Assays which quantify TXA2 production as a means of gauging the effectiveness of ASA therapy and cardiovascular risk are therefore desirable.
The direct measurement of thromboxane A2 is not clinically feasible because its strained bicyclic oxetane acetal moiety rapidly undergoes aqueous nucleophilic attack in vivo to yield thromboxane B2 (TXB2). TXB2 itself is extensively metabolized via two major pathways, β-oxidation and dehydrogenation, to dozens of different metabolites [7]. One of these metabolites, 11-dehydro TXB2, is a thermodynamically stable and biologically inert prostanoid that is secreted into the urine and forms the basis for several clinical assays that measure systemic TXA2 production as a means of assessing ASA responsiveness [8,9]. The first such commercially available assay for clinical use (marketed as ASPIRINcheck® by Esoterix Inc., Austin, TX, USA) was a competitive ELISA utilizing a rabbit polyclonal anti-11-dehydro TXB2 primary antibody that reported values normalized to urine creatinine. Several clinical studies employing this assay found a significant relationship between elevated urine 11-dehydro TXB2 and the risk of stroke, myocardial infarction and death in ASA-treated patients with established cardiovascular disease or multiple cardiovascular risk factors [10,11]. More recently, we found elevated urine 11-dehydro TXB2 to be a novel risk factor for early thrombotic occlusion of vein grafts in ASA-treated patients after coronary artery bypass graft (CABG) surgery [12]. Unfortunately, this assay is no longer commercially available and has been supplanted by a Food and Drug Administration (FDA)-cleared second-generation assay (marketed as AspirinWorks Test® by Corgenix Medical Corp., Broomfield, CO, USA) which is based on an ELISA utilizing a mouse monoclonal anti-11-dehydro TXB2 primary antibody [13]. To date, there have been little data published comparing the two assays or the ability of this second-generation assay to predict clinical outcome.
The primary goal of this study was to compare the relative performance of the first-and second generation ELISA-based assays at measuring urine 11-dehydro TXB2 in a cohort of CABG surgery patients receiving ASA therapy. Using ultra-performance liquid chromatography/tandem mass spectrometry (UPLC-MS/MS) as an independent method to measure the concentration of 11-dehydro TXB2, we observed significant differences in the accuracy of the two assays. Further investigation with analyte spiking studies revealed that this was predominantly because of differences in primary antibody specificity. Finally, we determined the relative strength of the association of urine 11-dehydro TXB2 measured by both assays with the risk of early vein graft thrombotic occlusion.
Methods
Patient population
The Reduction in Graft Occlusion Rates (RIGOR) study is a multicenter, observational study investigating risk factors for early vein graft thrombosis in 368 subjects undergoing first-time CABG surgery. Detailed descriptions of the study and its principal findings have previously been reported [12,14,15]. Human subject research review board approval was obtained at all participating sites and signed written consent was obtained from all participants. Subjects were administered ASA (300–325 mg) within 24 h of surgery and were given a supply of 325 mg enteric-coated ASA at discharge with instructions to take one pill daily for at least 6 months, at which time vein graft patency was assessed by multidetector computed tomography coronary angiography. Graft patency was adjudicated by at least two blinded reviewers as previously described [14]. As part of the study protocol, blood was collected 3 days and 6 months after surgery to assess platelet function using a wide array of assays as previously described [12,16]. In particular, shear-depend platelet activation was quantified by the closure time (CT) measured by the Platelet Function Analyzer-100 (PFA-100; Siemens Healthcare Diagnostics, Newark, DE, USA) using a collagen/adenosine diphosphate (CADP) agonist cartridge. Urine was simultaneously collected at these same time points, immediately centrifuged and stored at −70 °C prior to batch analysis for 11-dehydroTXB2.
ELISA assays
Measurement of urine 11-dehydro TXB2 was performed in duplicate first using the first-generation polyclonal antibody-based ELISA (#519501, Cayman Chemical, Ann Arbor, MI, USA) by Esoterix Laboratory Services, Inc. according to the manufacturer’s instructions (coefficient of variance = 10% and lower limit of detection of 25 pg mL−1) and then in the same samples after a second freeze/thaw cycle using the second-generation monoclonal antibody-based ELISA (AspirinWorks Test Kit; Corgenix) performed in duplicate by Corgenix according to the manufacturer’s instructions (coefficient of variance = 10% and lower limit of detection of 222 pg mL−1). To compare the specificities of the primary antibodies, pooled urine from subjects with low levels of 11-dehydro TXB2 by the polyclonal antibody-based ELISA (pooled mean 22 ± 8 pg mL−1) were spiked with increasing concentrations (0.01–10 ng mL−1) of purified thromboxane B2, 11-dehydro TXB2, 2,3-dinor TXB2 and 11-dehydro-2,3,-dinor TXB2 purchased from Sigma-Aldrich, St. Louis, MO, USA. Samples were simultaneously assayed in duplicate using the polyclonal-and monoclonal-based ELISA assays according to the manufacturer’s instructions.
Mass spectrometry
Quantitation of urine 11-dehydro TXB2, 2,3-dinor TXB2 and 11-dehydro-2,3,-dinor TXB2 in urine was achieved by UPLC-MS/MS, using reverse phase chromatography, negative ion electrospray introduction and selected reaction monitoring. 11-dehydro TXB2, tetradeuterated (d4) 11-dehydro TXB2 and 11-dehydro-2,3-dinor TXB2 were purchased from Cayman Chemical Co. 2,3-dinor TXB2 was converted to 18O2-2,3-dinor TXB2 by labeling with H218O according to the method of Pickett and Murphy [17]. H218O was purchased from Cambridge Isotope Laboratories, Andover, MA, USA. To each 1.0 mL of urine, 5.0 ng d4-11-dehydro TXB2 was added as an internal standard. After equilibrating for 15 min, 20 μL of formic acid was added and the sample applied to a StrataX solid phase extraction cartridge (Phenomenex, Torrence, CA, USA). For samples in which 11-dehydro TXB2 and 2,3,-dinor TXB2 were to be quantified, 5.0 ng each d4-11-dehydro TXB2 and 18O2-2,3-dinor TXB2 were added. After equilibrating for 15 min, 0.5 mL of methoxyamine HCl (1 g mL−1 H2O) was added and the sample was allowed to stand for 15 min at room temperature to form the methoxime (MO) derivative. The sample was then applied to the StrataX SPE cartridge. The cartridge was sequentially washed with 1 mL each water and 5% acetonitrile in water then dried by application of a house vacuum for 15 min. Compounds of interest were eluted with 1 mL of 5% acetonitrile in ethyl acetate. After drying under a nitrogen stream, samples were dissolved in 200 μL 20% acetonitrile in Millipore water (EMD Millipore, Billerica, MA, USA) for analysis.
Samples were introduced to a 200 mm × 2.1 mm × 1.9 μm Hypersil GOLD C18 UPLC column in an Accela UPLC system which was online with a Quantum Ultra AM (ThermoFisher, Waltham, MA, USA). The mobile phase was generated from (i) Millipore water and (ii) 5% methanol/95% acetonitrile, each containing 0.005% acetic acid adjusted to pH 5.7 with ammonium hydroxide. The flow rate was 350 μL min−1. For 11-dehydroTXB2/11-dehydro-2,3-dinor TXB2 samples, a gradient starting at 20% B with a linear ramp to 38% B over 18 min was used. Under these conditions, the retention time for 11-dehydro TXB2 and d4-11-dehydro TXB2 was approximately 15.4 min, and the retention time for 11-dehydro-2,3-dinor TXB2 was approximately 8.5 min. The relative response was determined by injection of a standard curve consisting of varying amounts of 11-dehydro-2,3-dinor TXB2 and a constant amount of d4-11-dehydro TXB2. For 11-dehydro TXB2/2,3-dinor TXB2 samples, a gradient starting at 15% B with a linear ramp to 40% B over 20 min was used. The resulting retention time for 11-dehydro TXB2 and d4-11-dehydro TXB2 was approximately 18.7 min, and the retention time for 2,3-dinor TXB2 MO was approximately 12.7 min. The collision gas was argon at 1.5 mTorr. The collision energy was 16 eV for 11-dehydro TXB2, 11 eV for 11-dehydro-2,3-dinor TXB2 and 15 eV for 2,3-dinor TXB2 MO. The skimmer offset was 6 eV. The transitions monitored were m/z 371 → 309 for d4-11-dehydro TXB2, m/z 367 → 305 for endogenous 11-dehydro TXB2, m/z 374 → 155 for 18O2-2,3-dinor TXB2 MO and m/z 370 → 155 for 2,3-dinor TXB2 MO. The MS/MS characteristics of 11-dehydro-2,3-dinor TXB2 were determined by the infusion of authentic material. As 11-dehydro-2,3-dinor TXB2 eluted significantly earlier than the internal standard, three transitions were monitored to ensure positive identification of the correct UPLC peak. These were m/z 339 → 277, m/z 339 → 235, and m/z 339 → 197. The m/z 339 → 277 transition was used for quantification, but all three peaks were required to be present at the correct retention time in the correct ratios.
Statistics
Inter-assay differences between monoclonal and polyclonal antibody-based ELISA were examined preliminarily with Wilcoxon’s paired rank test and linear regression with Pearson’s correlation coefficient followed by the Bland–Altman analysis [18]. The relationships between UPLC-MS/MS and the first- and second generation assays were investigated with linear regression. The inter-assay analyses were performed using R (http://cran.r-project.org).
For correlation with clinical outcome, univariate analyses were performed on a per graft basis for the odds of occlusion vs. patency using both the polyclonal- and monoclonal-based assay results as either continuous variables tested by Pearson’s correlation coefficient or a categorical variable (e.g. above or below the assay-appropriate quartile) tested by Fisher’s exact test. The results from a more extended univariate analysis were entered into multivariate analysis by removing the highly correlated covariates (e.g. rho = 0.7) and choosing variables with a statistical significance of P = 0.15 or those supported by the literature. These were entered into a multilevel random effects model. For multivariate analyses, a four-level model was explored initially: analyzing SVG outcome with random intercepts for patient, surgeon, hospital and multi-segmented SVGs considered as single grafts. Because random intercepts on surgeon, hospital and multi-segmented SVGs were not significant, the analysis thereafter included clustering on the patient alone. Stepwise backwards elimination of variables was performed to generate several potential models. Akaike Information Criterion was used for model comparison. Analyses were performed using Stata/MP version 10.0 for Windows software (Stata, Inc., College Station, TX, USA). Differences were considered significant when P < 0.05.
Results
Inter-assay correlation and agreement with mass spectrometry
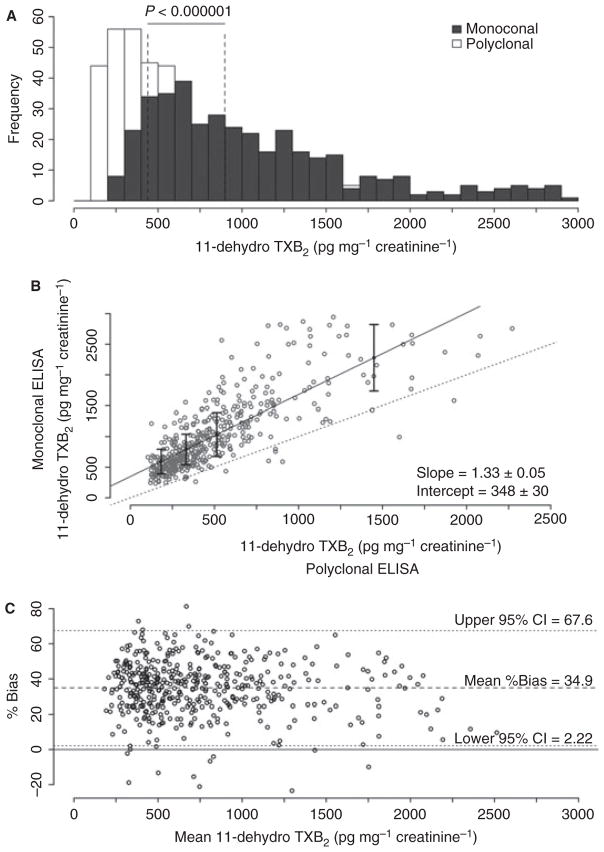
The concentration of 11-dehydro TXB2 normalized to creatinine was measured by each ELISA in 451 urine samples obtained 3 days and 6 months after CABG surgery from 229 RIGOR subjects on ASA monotherapy. The distribution of values for the assays differed markedly (Fig. 1A), with median 11-dehydro TXB2 values obtained by the second-generation monoclonal antibody-based assay being significantly higher than those obtained by the first-generation polyclonal antibody-based assay (856 vs. 399 pg mg−1 creatinine, P < 0.000001). While regression analysis demonstrated a linear relationship between respective assay values, the degree of constant and proportional bias (348 ± 30 ng mg−1 creatinine and 1.33 ± 0.05, respectively; Fig. 1B) was more than expected for assays intended to measure the same analyte. There was also poor inter-assay agreement by Bland–Altman analysis with a mean bias of 35% and a wide limit of agreement (2–68%; Fig. 1C), confirming that the two assays cannot be considered interchangeable, even with use of a correction factor.
Fig. 1.
Inter-assay correlation of 11-dehydro TXB2 results in 451 Reduction in Graft Occlusion Rates (RIGOR) urine samples measured by polyclonal and monoclonal antibody-based ELISAs. (A) Distribution of result values. Dashed lines denote respective median values. (B) Scatter plot of the result values. Solid line denotes the regression trend line and the dashed line has a slope = 1. (C) Bland–Altman plot of percent bias vs. mean.
To determine the relative performance of each ELISA to a non-immunologic detection method, 11-dehydro TXB2 was quantified by UPLC-MS/MS in urine samples from 29 RIGOR subjects used for the above analyses. Results using the first-generation polyclonal antibody-based ELISA did not differ significantly from those obtained by mass spectrometry using the paired t-test (P = 0.21) and by linear regression analysis (Fig. 2) had a slope that was close to unity (1.08 ± 0.08) with a small constant bias (0.072 ± 0.03 ng mL−1). In contrast, results from the second-generation monoclonal antibody-based ELISA were statistically different from those obtained by mass spectrometry (P = 0.00004) and by linear regression had a slope greater than unity (2.20 ± 0.22) with a large constant bias (0.21 ± 0.09 ng mL−1).
Fig. 2.
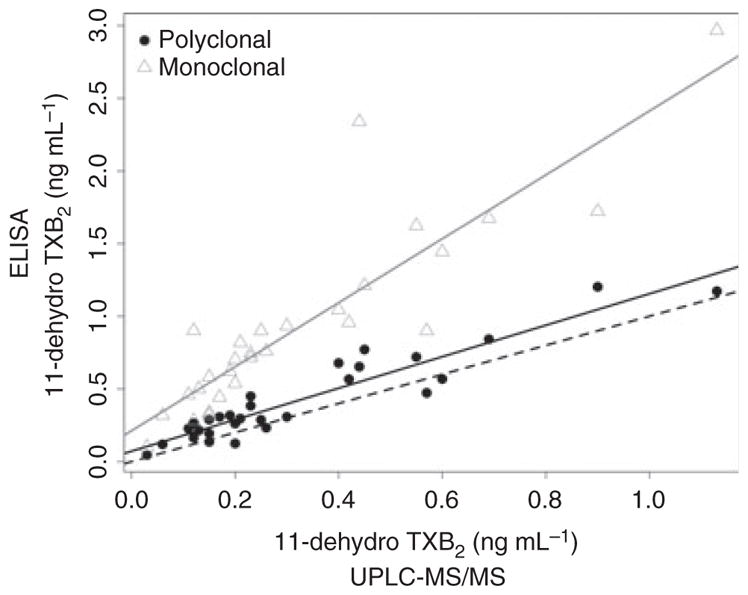
Correlation of ELISA results with mass spectrometry. Gray and black solid lines denote regression lines for 11-dehydro TXB2 results measured by the monoclonal and polyclonal antibody-based ELISAs, respectively. Dashed line has a slop of one.
Relative specificity of primary antibodies
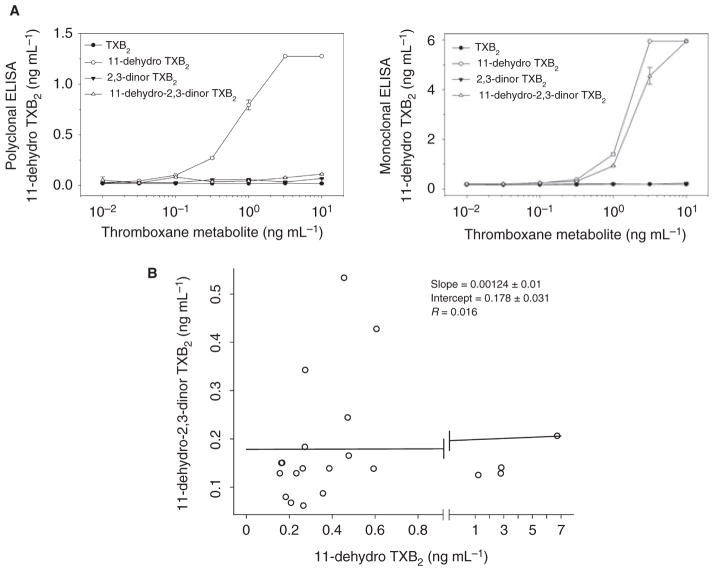
Both ELISAs utilize primary antibodies that predominantly recognize 11-dehydro-TXB2 but exhibit variable cross-reactivity with other related TXB2 species [19,20]. To determine if the discrepancy in assay results could be explained by differences in primary antibody specificity, pooled urine samples from several RIGOR subjects with very low concentrations of 11-dehydro-TXB2 were spiked with increasing concentrations of purified TXB2, 11-dehydro-TXB2, 2,3-dinor TXB2 and 11-dehydro 2,3-dinor TXB2 then analyzed for 11-dehydro TXB2 by each ELISA. Whereas there was no significant interference from non-11-dehydro TXB2 metabolites with the polyclonal anti-body-based ELISA, the monoclonal antibody-based ELISA exhibited marked interference from 11-dehydro-2,3-dinor TXB2 (Fig. 3A). To confirm that this interference is clinically relevant, the concentration of 11-dehydro-2,3-dinor TXB2 was quantified by UPLC-MS/MS in 21 of the 29 remaining urine samples. While 11-dehydro-2,3-dinor TXB2 and 11-dehydro TXB2 concentrations were frequently of a similar magnitude, there was no significant correlation between the two by linear regression analysis (Fig. 3B).
Fig. 3.
Differences in primary antibody specificity between assays. (A) Pooled urine sample with low concentration of 11-dehydro TXB2 were spiked with an increasing concentration of the indicated thromboxane metabolites and apparent 11-dehydro TXB2 levels determined by polyclonal (left panel) and monoclonal antibody-based ELISA (right panel). (B) Correlation between urine 11-dehydro 2,3-dinor TXB2 and 11-dehydro TXB2 levels determined by performance liquid chromatography/tandem mass spectrometry (UPLC-MS/MS).
Correlation with clinical outcome
We previously found that urine 11-dehydro TXB2, measured 6 months after CABG surgery using the polyclonal antibody-based ELISA, was an independent risk factor for early vein graft thrombotic occlusion [12]. Given the differences between the assays, we sought to determine if 11-dehydro TXB2 measured in these same samples using the second-generation monoclonal antibody-based ELISA would equally correlate with outcome. Figure 4 shows the prevalence of vein graft occlusion in RIGOR subjects stratified by quartile of 11-dehydro TXB2 as determined by each ELISA. Vein graft occlusion was most prevalent in subjects in the highest quartile of 11-dehydro TXB2 (= 450 pg mg−1 creatinine) measured with the first-generation polyclonal antibody-based ELISA whereas it was most prevalent in subjects above the median 11-dehydro TXB2 value (= 681 pg mg−1 creatinine) measured with the second-generation monoclonal antibody-based ELISA.
Fig. 4.
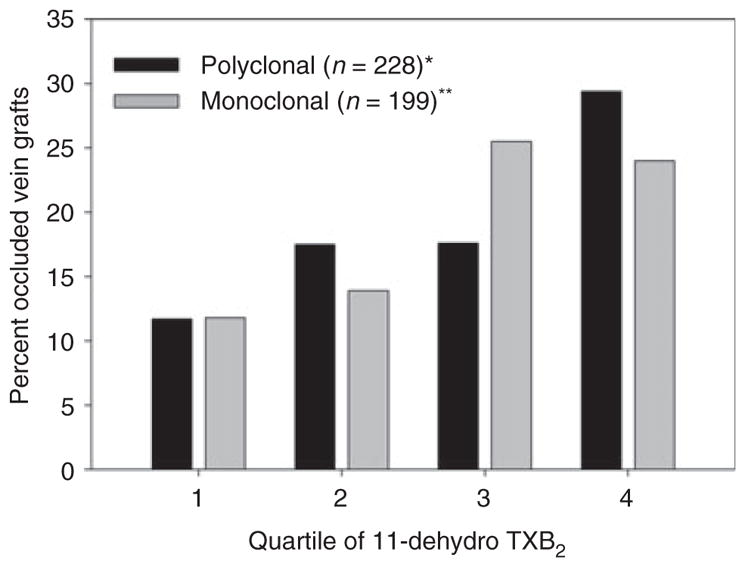
Percentage of occluded vein grafts in Reduction in Graft Occlusion Rates (RIGOR) subjects stratified by quartile of urine 11-dehydro TXB2. *P = 0.004; **P = 0.01.
By univariate analyses, vein graft occlusion did correlate with 11-dehydro TXB2 measured by both assays when expressed as a continuous variable and correlated best with the upper quartile and median values when expressed as a binary variable for the polyclonal and monoclonal antibody-based ELISAs, respectively (Table 1). In multivariate analyses, taking into account a wide array of known and suspected risk factors, 11-dehydro TXB2 measured by the polyclonal antibody-based ELISA remained significantly associated with the risk of vein graft occlusion when expressed as both continuous and binary variables [12]. Other variables significantly associated with vein graft occlusion included shear-dependent platelet activation as measured by PFA-100 closure time using the CADP agonist cartridge and small target vessel size, historically considered one of the strongest predictors of early graft failure [12,21,22]. In contrast, analogous multivariate modeling revealed that 11-dehydro TXB2 measured with the monoclonal antibody-based ELISA and analyzed either as continuous (model 1) or binary (model 2) variables failed to significantly associate with the risk of vein graft occlusion (Table 2).
Table 1.
Univariate analyses of the correlation between urine 11-dehydro TXB2 and the risk of early vein graft occlusion
| Variable | Polyclona
|
Monoclonal
|
||
|---|---|---|---|---|
| Odds ratio | P-value | Odds ratio | P-value | |
| 11-dehydro TXB2 (ln pg mg−1 creatinine)* | 2.13 | 0.004 | 2.06 | 0.022 |
| 11-dehydro TXB2 (= vs. < median)† | 2.12 | 0.025 | 2.68 | 0.008 |
| 11-dehydro TXB2 (= vs. < upper quartile)‡ | 3.13 | 0.001 | 1.45 | 0.379 |
Odds of vein graft occlusion for every variable unit increase.
Polyclonal ELISA = 331 pg mg−1 creatinine; monoclonal ELI-SA = 681 pg mg−1 creatinine.
Polyclonal ELISA = 450 pg mg−1 creatinine; monoclonal ELI-SA = 992 pg mg−1 creatinine.
11-dehydro TXB2 = 11-dehydro thromboxane B2.
Table 2.
Multivariate analyses of the correlation between risk factors and risk of early vein graft occlusion
| Variable | Polyclonal
|
Monoclonal
|
||||
|---|---|---|---|---|---|---|
| Odds ratio | P-value | 95% CI | Odds ratio | P-value | 95% CI | |
| Model 1 | ||||||
| PFA-100 CADP CT (s)* | 0.98 | 0.002 | 0.97–0.99 | 0.97 | 0.002 | 0.96–0.99 |
| Target vessel diameter = 1.5 mm | 2.36 | 0.009 | 1.23–4.53 | 1.98 | 0.066 | 0.96–4.12 |
| 11-dehydroTXB2 (ln pg mg −1 creatinine)* | 1.73 | 0.047 | 1.10–2.99 | 1.23 | 0.500 | 0.67–2.28 |
| Female gender | 2.31 | 0.052 | 0.99–6.38 | 3.22 | 0.012 | 1.30–8.00 |
| Non-white race | 2.48 | 0.069 | 0.97–6.38 | 2.99 | 0.025 | 1.15–7.79 |
| Model 2 | ||||||
| PFA-100 CADP (CT = 88 vs. > 88 s) | 2.85 | 0.006 | 1.36–6.00 | 3.97 | 0.001 | 1.76–8.97 |
| Target vessel diameter = 1.5 mm | 2.38 | 0.011 | 1.22–4.61 | 2.16 | 0.041 | 1.03–4.54 |
| 11-dehydro TXB2 (= 450 vs. < 450 pg mg−1 creatinine) | 2.59 | 0.015 | 1.20–5.56 | – | – | – |
| 11-dehydro TXB2 (= 681vs. < 681 pg mg−1 creatinine) | – | – | – | 1.63 | 0.199 | 0.77–3.43 |
| Female gender | 2.13 | 0.089 | 0.89–5.12 | 2.73 | 0.032 | 1.09–6.85 |
| Non-white race | 1.90 | 0.200 | 0.71–5.04 | 2.77 | 0.031 | 1.10–6.98 |
PFA-100, platelet function assay 100; CT, closure time (seconds); 11-dehydro TXB2, 11-dehydro thromboxane B2.
Odds of vein graft occlusion for every variable unit increase.
Discussion
The major findings of this study were: (i) urine 11-dehydro TXB2 was more accurately measured by the first-generation ELISA employing a rabbit polyclonal primary antibody than the second-generation ELISA employing a mouse monoclonal primary antibody; (ii) the mouse monoclonal primary antibody exhibited significant cross-reactivity with 11-dehydro 2,3-dinor TXB2, which is present in substantial but variable concentrations in the urine of patients with established cardiovascular disease; and (iii) 11-dehydro TXB2 measured with the first-generation polyclonal antibody-based ELISA more strongly correlated with vein graft occlusion 6 months after CABG surgery than does 11-dehydro TXB2 measured with the second-generation monoclonal antibody-based ELISA.
Our comparative analyses found significant differences in both the analytical and clinical performance of the two ELISA platforms that have been used to measure urine 11-dehydro TXB2 clinically. These differences were predominantly as a result of the presence of a coexisting TXB2 metabolite, 11-dehydro-2,3-dinor TXB2, which is detected by the monoclonal primary antibody used in the second-generation ELISA but not the polyclonal primary antibody used in the first-generation ELISA. The reported affinity of the monoclonal antibody is 3.3-fold greater for 11-dehydro-2,3-dinor TXB2 than it is for 11-dehydro TXB2 [20]. In our analysis, to account fully for the difference between the assay results, the monoclonal primary antibody would need to have an affinity 5.4-fold greater for 11-dehydro-2,3-dinor TXB2 than for 11-dehydro TXB2. Thus, while interference by 11-dehydro-2,3-dinor TXB2 accounts for most of the observed difference between assay results, we cannot exclude the presence of other unmeasured TXB2 metabolites that may also interfere with the second-generation ELISA assay.
Our data further reveal poor intra-individual correlation between 11-dehydro TXB2 and 11-dehydro-2,3-dinor TXB2 in subjects with cardiovascular disease on aspirin therapy. This is consistent with data observed in healthy aspirin-naïve subjects where the proportion of both metabolites in the urine varied substantially [23]. Because of this inconsistent relationship in TXB2 metabolite concentrations, results from these two assays cannot be correlated with the use of a simple conversion factor. Selective removal of 11-dehydro-2,3-dinor TXB2 to eliminate interference is also unlikely to be technically or commercially feasible. This non-interchangeability of assays has significant clinical implications. Two large studies, the Heart Outcomes Prevention Evaluation (HOPE) study and the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management and Avoidance (CHARISMA) study, demonstrated the predictive value of measuring urine 11-dehydro TXB2 using the polyclonal antibody-based ELISA to define cardiovascular risk [10,11]. As the second-generation monoclonal antibody-based ELISA has not been clinically validated to this degree, the clinical significance of the numerical difference in 11-dehydro TXB2 results between the two assays was largely unknown. By re-measuring 11-dehydro TXB2 in stored urine samples from the well-defined RIGOR study population and analyzing data using the same statistical modeling, we found the monoclonal antibody-based assay to be inferior to the polyclonal antibody-based assay at detecting an association between 11-dehydro TXB2 and the risk of early vein graft thrombosis after CABG surgery. This suggests that any utility of the second-generation assay in identifying cardiovascular risk in other patient populations will need to be specifically investigated and cannot be assumed by extrapolation of results from previous studies that measured 11-dehydro TXB2 by the first-generation assay.
Investigations into the metabolic fate of TXB2 have described two main degradation pathways: (i) dehydrogenation to 11-dehydro TXB2,; and (ii) β-oxidation to 2,3-dinor TXB2 [24,25]. Both appear to occur mainly in the liver, kidney and lung [26]. Under physiologic conditions, enzyme-mediated dehydrogenation accounts for the majority of thromboxane metabolism and results in a more stable product [27–29]. Dehydrogenation and β-oxidation of TXB2 can both occur, resulting in the formation of 11-dehydro-2,3-dinor TXB2, a metabolite first identified in human urine by exhaustive mass spectral scanning for thromboxane metabolites after a high-dose injection of TXB2 [7]. Although relatively little is known about this specific thromboxane metabolite, it has been reported to be present in comparable but variable abundance to that of 11-dehydro TXB2 in the urine of healthy normal individuals and is suppressible by aspirin therapy [23]. Our study is the first to describe urine levels of 11-dehydro-2,3 dinor TXB2 (median 112, range: 28–434 pg mL−1 creatinine) and their variable relationship to levels of 11-dehydro TXB2 in patients with known cardiovascular disease on ASA therapy. It is currently not known what factors determine the relative production of 11-dehydro-2,3 dinor TXB2 and whether measuring this particular analyte has any clinical prognostic utility.
We acknowledge there are several potential limitations to the above results. First, all the analyses were initially performed by the polyclonal ELISA and were re-frozen prior to thawing and analysis with the monoclonal ELISA. While the spiking studies demonstrate unequivocally that the monoclonal ELISA suffers from interference by 11-dehydro-2,3 dinor TXB2, there is the possibility that some bias could have resulted from the additional freeze-thaw cycle. This probably is a minor problem as there does not appear to be a stability problem with TXB2 metabolites during multiple freeze-thaw cycles and the same bias can be demonstrated when comparing monoclonal antibody-based ELISA and LC-MS/MS results, which were performed on samples frozen and thawed a comparable number of times. Second, we did not have a control group for assessing 11-dehydro-2,3-dinor in the general population as the clinical study being addressed in RIGOR did not require a group of healthy volunteers. The characterization of this molecule in other patient populations will be necessary to extrapolate its significance beyond its capability to interfere with the monoclonal ELISA in the context presented here.
In conclusion, the first and second-generation ELISA used for clinical determination of thromboxane generation and aspirin responsiveness yield significantly different measurements of 11-dehydro TXB2 and are not interchangeable. This is as a result of cross-reactivity of the monoclonal antibody with 11-dehydro-2,3-dinor TXB2, a molecule present in a relatively high concentration but with poorly correlation to 11-dehydro TXB2 in our patient population. Results obtained with the second-generation assay therefore cannot be assumed to have the same predictive value for clinical outcome that has been demonstrated in clinical studies measuring 11-dehydro TXB2 using the first-generation ELISA assay.
Acknowledgments
This study was supported by grants from the Flight Attendant Medical Research Foundation (to J.J.R.) and from the National Institutes of Health (PO1HL062250 to GAF) as well as material support from the Corgenix Medical Company, Broomfield, CO and the Johns Hopkins Institute for Clinical and Translational Research (funded by UL1 RR025005 from the National Center for Research Resources, National Institutes of Health). The parent Reduction in Grafts Occlusion Rates study was supported by grants from The Medicines Company, AstraZeneca Pharmaceuticals, Sanofi-BMS, and received material support from Siemens Healthcare Diagnostics, Inc. and GlaxoSmithKline.
Footnotes
Addendum
J.J. Rade had full access to all of the data in this study and takes responsibility for the integrity of the data and accuracy of the analyses. Study design: M.T. Olson, T.S. Kickler and J.J. Rade. Data Acquisition: M.T. Olson, J.A. Lawson, J. Jani, G.A. FitzGerald and J.J. Rade. Data Interpretation and Analysis: M.T. Olson, J.A. Lawson and J.J. Rade. Statistical Analysis: R.C. McLean. Manuscript drafting: M.T. Olson, J.J. Rade. Manuscript editing and final approval: All authors.
Disclosure of Conflict of Interest
J.J. Rade and T.S. Kickler received royalty payments as part of a licensing agreement between Siemens Healthcare Diagnostics and Johns Hopkins University; J.J. Rade has received honoraria from Corgenix Medical Corporation for speaking at sponsored CME-approved symposia. All other authors report no conflicts.
References
- 1.Hamberg M, Svensson J, Samuelsson B. Thromboxanes: a new group of biologically active compounds derived from prostaglandin endoperoxides. Proc Natl Acad Sci U S A. 1975;72:2994–8. doi: 10.1073/pnas.72.8.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhagwat SS, Hamann PR, Still WC, Bunting S, Fitzpatrick FA. Synthesis and structure of the platelet aggregation factor thromboxane A2. Nature. 1985;315:511–3. doi: 10.1038/315511a0. [DOI] [PubMed] [Google Scholar]
- 3.Awtry EH, Loscalzo J. Aspirin. Circulation. 2000;101:1206–18. doi: 10.1161/01.cir.101.10.1206. [DOI] [PubMed] [Google Scholar]
- 4.Antiplatelet Trialists’ Collaboration. Collaborative overview of randomised trials of antiplatelet therapy–I: prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. BMJ. 2002;324:71–86. [PMC free article] [PubMed] [Google Scholar]
- 5.Krasopoulos G, Brister SJ, Beattie WS, Buchanan MR. Aspirin “resistance” and risk of cardiovascular morbidity: systematic review and meta-analysis. BMJ. 2008;336:195–8. doi: 10.1136/bmj.39430.529549.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Snoep JD, Hovens MM, Eikenboom JC, van der Bom JG, Huisman MV. Association of laboratory-defined aspirin resistance with a higher risk of recurrent cardiovascular events: a systematic review and meta-analysis. Arch Intern Med. 2007;167:1593–9. doi: 10.1001/archinte.167.15.1593. [DOI] [PubMed] [Google Scholar]
- 7.Roberts LJ, Sweetman BJ, Oates JA. Metabolism of thromboxane B2 in man. Identification of twenty urinary metabolites. J Biol Chem. 1981;256:8384–93. [PubMed] [Google Scholar]
- 8.Ciabattoni G, Maclouf J, Catella F, FitzGerald GA, Patrono C. Radioimmunoassay of 11-dehydrothromboxane B2 in human plasma and urine. Biochim Biophys Acta. 1987;918:293–7. doi: 10.1016/0005-2760(87)90233-5. [DOI] [PubMed] [Google Scholar]
- 9.Karon BS, Wockenfus A, Scott R, Hartman SJ, McConnell JP, Santrach PJ, Ja3e AS. Aspirin responsiveness in healthy volunteers measured with multiple assay platforms. Clin Chem. 2008;54:1060–5. doi: 10.1373/clinchem.2007.101014. [DOI] [PubMed] [Google Scholar]
- 10.Eikelboom JW, Hirsh J, Weitz JI, Johnston M, Yi Q, Yusuf S. Aspirin-resistant thromboxane biosynthesis and the risk of myocardial infarction, stroke, or cardiovascular death in patients at high risk for cardiovascular events. Circulation. 2002;105:1650–5. doi: 10.1161/01.cir.0000013777.21160.07. [DOI] [PubMed] [Google Scholar]
- 11.Eikelboom JW, Hankey GJ, Thom J, Bhatt DL, Steg PG, Montalescot G, Johnston SC, Steinhubl SR, Mak KH, Easton JD, Hamm C, Hu T, Fox KAA, Topol EJ. Incomplete inhibition of thromboxane biosynthesis by acetylsalicylic acid. determinants and effect on cardiovascular risk. Circulation. 2008;118:1690. doi: 10.1161/CIRCULATIONAHA.108.768283. [DOI] [PubMed] [Google Scholar]
- 12.Gluckman TJ, McLean RC, Schulman SP, Kickler TS, Shapiro EP, Conte JV, McNicholas KW, Segal JB, Rade JJ. Effects of aspirin responsiveness and platelet reactivity on early vein graft thrombosis after coronary artery bypass graft surgery. J Am Coll Cardiol. 2011;57:1069–77. doi: 10.1016/j.jacc.2010.08.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geske FJ, Guyer KE, Ens G. AspirinWorks: a new immunologic diagnostic test for monitoring aspirin effect. Mol Diagn Ther. 2008;12:51–4. doi: 10.1007/BF03256268. [DOI] [PubMed] [Google Scholar]
- 14.Gluckman TJ, Segal JB, Schulman SP, Shapiro EP, Kickler TS, Prechel MM, Conte JV, Walenga JM, Shafique I, Rade JJ. Effect of anti-platelet factor-4/heparin antibody induction on early saphenous vein graft occlusion after coronary artery bypass surgery. J Thromb Haemost. 2009;7:1457–64. doi: 10.1111/j.1538-7836.2009.03526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McLean RC, Nazarian SM, Gluckman TJ, Schulman SP, Thiemann DR, Shapiro EP, Conte JV, Thompson JB, Shafique I, McNicholas KW, Villines TC, Laws KM, Rade JJ. Relative importance of patient, procedural and anatomic risk factors for early vein graft thrombosis after coronary artery bypass graft surgery. J Cardiovasc Surg (Torino) 2011;52:877–85. [PMC free article] [PubMed] [Google Scholar]
- 16.Nazarian SM, Thompson JB, Gluckman TJ, Laws K, Jani JT, Kickler TS, Rade JJ. Clinical and laboratory factors associated with shear-dependent platelet hyper-reactivity in patients on chronic aspirin therapy. Thromb Res. 2009;126:379–83. doi: 10.1016/j.thromres.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pickett WC, Murphy RC. Enzymatic preparation of carboxyl oxygen-18 labeled prostaglandin F2 alpha and utility for quantitative mass spectrometry. Anal Biochem. 1981;111:115–21. doi: 10.1016/0003-2697(81)90237-2. [DOI] [PubMed] [Google Scholar]
- 18.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10. [PubMed] [Google Scholar]
- 19.Cayman Chemical. Product Information:11-dehydro Thromboxane B2 EIA Antiserum. Oct 15, 2010. [419502] [Google Scholar]
- 20.Caymen Chemical. Product Information:11-dehydro Thromboxane B2 Monoclonal Antibody. Feb 7, 2012. [419512] [Google Scholar]
- 21.Paz MA, Lupon J, Bosch X, Pomar JL, Sanz G. Predictors of early saphenous vein aortocoronary bypass graft occlusion. The GESIC Study Group. Ann Thorac Surg. 1993;56:1101–6. doi: 10.1016/0003-4975(95)90024-1. [DOI] [PubMed] [Google Scholar]
- 22.Shah PJ, Gordon I, Fuller J, Seevanayagam S, Rosalion A, Tatoulis J, Raman JS, Buxton BF. Factors affecting saphenous vein graft patency: clinical and angiographic study in 1402 symptomatic patients operated on between 1977 and 1999. J Thorac Cardiovasc Surg. 2003;126:1972–7. doi: 10.1016/s0022-5223(03)01276-5. [DOI] [PubMed] [Google Scholar]
- 23.Chiabrando C, Rivoltella L, Alberti E, Bonollo M, Djurup R, Fanelli R. Urinary excretion and origin of 11-dehydro-2,3-dinor-thromboxane B2 in man. Prostaglandins. 1993;45:401–11. doi: 10.1016/0090-6980(93)90117-p. [DOI] [PubMed] [Google Scholar]
- 24.Diczfalusy U. Beta-oxidation of eicosanoids. Prog Lipid Res. 1994;33:403–28. doi: 10.1016/0163-7827(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 25.Benigni A, Chiabrando C, Perico N, Fanelli R, Patrono C, FitzGerald GA, Remuzzi G. Renal metabolism and urinary excretion of thromboxane B2 in the rat. Am J Physiol. 1989;257:F77–85. doi: 10.1152/ajprenal.1989.257.1.F77. [DOI] [PubMed] [Google Scholar]
- 26.Remuzzi G, FitzGerald GA, Patrono C. Thromboxane synthesis and action within the kidney. Kidney Int. 1992;41:1483–93. doi: 10.1038/ki.1992.217. [DOI] [PubMed] [Google Scholar]
- 27.Catella F, FitzGerald GA. Paired analysis of urinary thromboxane B2 metabolites in humans. Thromb Res. 1987;47:647–56. doi: 10.1016/0049-3848(87)90103-4. [DOI] [PubMed] [Google Scholar]
- 28.Lawson JA, Patrono C, Ciabattoni G, FitzGerald GA. Long-lived enzymatic metabolites of thromboxane B2 in the human circulation. Anal Biochem. 1986;155:198–205. doi: 10.1016/0003-2697(86)90247-2. [DOI] [PubMed] [Google Scholar]
- 29.Catella F, Healy D, Lawson JA, FitzGerald GA. 11-Dehydrothrom-boxane B2: a quantitative index of thromboxane A2 formation in the human circulation. Proc Natl Acad Sci U S A. 1986;83:5861–5. doi: 10.1073/pnas.83.16.5861. [DOI] [PMC free article] [PubMed] [Google Scholar]