Summary
The partitioning of secretory and membrane protein-encoding mRNAs to the endoplasmic reticulum (ER), and their translation on ER-associated ribosomes, governs access to the secretory/exocytic pathways of the cell. As mRNAs encoding secretory and membrane proteins comprise approximately 30% of the transcriptome, the localization of mRNAs to the ER represents an extraordinarily prominent, ubiquitous, and yet poorly understood RNA localization phenomenon.
The partitioning of mRNAs to the ER is generally thought to be achieved by the signal recognition particle (SRP) pathway. In this pathway, mRNA localization to the ER is determined by the translation product – translation yields an N-terminal signal sequence or topogenic signal that is recognized by the SRP and the resulting mRNA-ribosome-SRP complex is then recruited to the ER membrane. Recent studies have demonstrated that mRNAs can be localized to the ER via a signal sequence and/or translation-independent pathway(s) and that discrete sets of cytosolic protein-encoding mRNAs are enriched on the ER membrane, though they lack an encoded signal sequence. These key findings reopen investigations into the mechanism(s) that govern mRNA localization to the ER.
In this contribution, we describe two independent methods that can be utilized to study this important and poorly understood aspect of eukaryotic cell biology. These methods comprise two independent means of fractionating tissue culture cells to yield free/cytosolic polyribosomes and ER membrane-bound polyribosomes. Detailed methods for the fractionation and characterization of the two polyribosome pools are provided.
Keywords: mRNA localization, endoplasmic reticulum, cytosol, polyribosome, rRNA, mRNA
1. Introduction
The endoplasmic reticulum (ER) is the site of synthesis of secretory and membrane proteins, which comprise ca. 30% of the cell’s proteome1. A fundamental question in cell biology concerns the cellular mechanisms that compartmentalize the synthesis of secretory and membrane proteins to the ER. Pioneering in vitro studies in the 1980s by Blobel and colleagues established the signal recognition particle (SRP) pathway as (a) the mechanism of mRNA partitioning to the ER2-5. In this model, all newly exported mRNAs initiate translation on cytosolic ribosomes. In the case of mRNAs encoding secretory or membrane proteins, translation yields the synthesis of an N-terminal signal sequence or a transmembrane domain which is recognized by the SRP, resulting in a suppression of protein synthesis. The ribosome-nascent polypeptide-SRP complex is then recruited to the ER via binding interactions with the ER-resident SRP-receptor. Upon binding of the ribosome-nascent polypeptide-SRP complex to the ER, SRP is released, translation resumes, and the growing peptide is co-translationally translocated into the ER for further processing.
While this mechanism has been widely accepted to be the mechanism by which mRNA are partitioned between the ER and the cytosol, several experimental observations indicate that SRP-independent mechanisms contribute to mRNA partitioning in the cell. For example, several groups have observed significant overlap in the composition of cytoplasmic and membrane-associated mRNAs6-8. Though the Signal Hypothesis predicts that signal sequence-encoding mRNAs would be present, perhaps at low enrichment, in cytosolic polysomes, this model does not provide a mechanism for how mRNAs lacking encoded signal sequences would be partitioned to the ER. In addition, genetic ablation of components of the SRP pathway in yeast does not affect the viability of the organism9. Instead, disabling of the SRP pathway function is compensated by an expansion of ER, indicating that the cells are able to adapt to the absence of the SRP pathway by activating or expanding compensatory pathways9. Consistent with these findings, depletion of SRP54 (the signal peptide binding SRP subunit) by RNA interference in trypanosomes is lethal, loss of SRP54 does not affect the processing of signal peptide containing proteins10. In HeLa cells, RNAi knockdown of SRP54 does not have any effect on the growth or viability of the cells. Further, loss of SRP54 affects the expression of the membrane receptor DR4 but not DR5, suggesting that cells have multiple pathways to bring about mRNA partitioning between the cytosol and ER11. In support of the existence of an alternative, SRP-independent mRNA partitioning mechanism, our group recently reported that deletion of the encoded signal sequence of Grp94, an ER-localized mRNA, or mutational loss of its translation function did not disrupt mRNA localization to the ER12. In fact, specific subsets of mRNAs encoding cytoplasmic and nucleoplasmic proteins have been consistently observed to be enriched on the ER, even thought they do not encode a signal peptide6-8, 13. A very recent report from the Walter lab suggests a mechanism by which such non-canonical mRNA partitioning can occur; in yeast, the localization of HAC1 mRNA to the ER is mediated by a conserved element in the 3′ UTR14. However, it is not known whether such direct mRNA localization is an exception to the common rule of SRP-dependent partitioning or if it represents a broader, primary mechanism by which all mRNAs are sorted between the cytosol and the ER. In order to study this phenomenon, it is essential to systematically analyze individual mRNAs and assess their partitioning between the cytosol and the ER.
This paper describes two methods for analyzing mRNA partitioning between the ER and the cytosol. The first method, sequential detergent extraction, takes advantage of the difference in the lipid composition of the plasma membrane and the ER membrane. Digitonin, a ß-sterol binding detergent that selectively solubilizes the cholesterol-rich plasma membrane and leaves the ER and nuclear membrane intact, is used to release cytosolic polysomes. Then, the permeabilized cells are washed and the ER-bound polyribosomes solubilized by any of a variety of detergents, including dodecylmaltoside, NP40, or an admixture of Nonidet P-40 (NP40) and sodium deoxycholate (DOC). The second method, mechanical homegenization followed by differential centrifugation, is a variation of the classical differential centrifugation method for cellular fractionation that was developed by Claude in the 1940s and later perfected by Palade and others15, 16.
Over the past decades, the field of mRNA localization has been niched to studies in Drosophila embryos and budding yeast model17, 18. There is a critical need for more studies on the mechanisms of subcellular mRNA localization in higher eukaryotic cells to gain a broader understanding of this important biological phenomenon. The methods outlined in this paper provide tools needed to study mRNA partitioning between the cytosol and the ER, a ubiquitous mRNA sorting process that, conservatively, directs the subcellular localization of >30% of the transcriptome.
2. Materials
As compared to DNA, RNA is very susceptible to degradation due both to nonspecific cleavage in the presence of divalent cations, and more importantly, the near ubiquitous presence of RNAse activity. Thus, great care should be taken to avoid RNAse contamination at every step of the following experiments. The most common sources of RNAse are human skin and microbial growth in stock solutions. Hence, gloves should always be worn while handling RNA and stock solutions should be stored in small aliquots and discarded at frequent intervals.
To reduce/eliminate RNAse contamination, buffers and solutions can be treated with 0.1% (v/v) diethyl pyrocarbonate (DEPC) (see Note 1) overnight at 37°C and then autoclaved for 15 minutes to remove unreacted DEPC. Buffers containing free amine groups (TRIS, HEPES, etc) and solutions/buffers that cannot be autoclaved (sucrose, MOPS, etc) cannot be DEPC-treated. Such solutions/buffers should be made up using molecular biology grade, RNAse-free powdered reagents and DEPC-treated water. All glassware should be baked at 400°C for 4 hours to inactivate RNAses. Sterile disposable tips and tubes are generally RNAse-free. Non-disposable plastic ware (such as ultracentrifuge tubes) can be treated twice with RNAZap (Ambion) or 0.2% SDS, and rinsed thoroughly in DEPC-treated water.
2.1 Cell Culture
HEK 293T cell line (ATCC).
Cell-culture medium: Dulbecco’s modified Eagle’s medium (DMEM; Mediatech) supplemented with 10% fetal bovine serum (FBS; Invitrogen).
Trypsin-EDTA (Invitrogen).
Phosphate buffered saline (PBS) (Mediatech).
2.2 Fractionation by sequential detergent extraction
1% (w/v) digitonin (Calbiochem) in DMSO (freeze in 100 μl aliquots; see Note 2).
RNaseOut™ Recombinant Ribonuclease Inhibitor: 40 U/μl stock. Store at −20°C (Invitrogen; Cat. no. 17-0969-01).
Complete™ Protease Inhibitor Cocktail: Complete™ EDTA-free (Roche Molecular Biochemicals; Cat. no. 1-873-580). Make a 100× stock in DMSO and store at −20°C. Use at 1×.
Diethyl pyrocarbonate (DEPC)-treated water. Prepare as a 0.1% (v/v) solution and incubate at 37°C overnight. Autoclave for 15 minutes to destroy unreacted DEPC.
Stock solutions: 4M potassium acetate (KOAc); 1M potassium 4-(2-hydroxyethyl)-1-piperazineethanesulfonate (K-HEPES); 1M magnesium acetate (Mg(OAc)2); 0.2M ethyleneglycol bis (2-aminoethylether)-N, N, N’, N’–tetraacetic acid (EGTA) pH 8.0; 10% (v/v) Nonidet P-40 (NP-40); 10% (w/v) sodium deoxycholate (DOC).
Permeabilization buffer: 110mM KOAc, 25mM K-HEPES pH 7. 2, 2.5mM Mg(OAc)2, 1mM EGTA, 0.015% digitonin, 1mM DTT, 50μg/ml CHX, 1× Complete Protease Inhibitor Cocktail, 40U/mL RNaseOUT™. Digitonin, DTT, CHX, Complete Protease Inhibitor Cocktail and RNaseOUT™ must be added fresh.
Wash buffer: 110mM KOAc, 25mM K-HEPES pH 7.2, 2.5mM Mg(OAc)2, 1mM EGTA, 0.004% digitonin, 1mM DTT, 50μg/ml CHX. Digitonin, DTT and CHX must be added fresh.
Lysis buffer: 400mM KOAc, 25mM K-HEPES pH 7.2, 15mM Mg(OAc)2, 1% (v/v) NP-40, 0.5% (w/v) DOC, 1mM DTT, 50μg/ml CHX, 1× Complete Protease Inhibitor Cocktail, 40U/mL RNase Out. DTT, CHX, Complete Protease Inhibitor Cocktail and RNaseOUT™ must be added fresh.
Sucrose cushion: 0.5M sucrose in lysis buffer.
2.3 Fractionation by differential centrifugation
Cell scraper (Fisher Scientific).
Cell homogenizer (Isobiotech, Heidelberg, Germany)
3ml Luer-Lok™ syringes (BD Pharmingen)
Stock solutions: 200mM KCl, 150mM MgCl2, 1M Tris-Cl pH 7.4, 2M Sucrose, 100mM dithiothreitol (DTT; freeze in 100 μl aliquots), 10mg/ml cycloheximide (CHX) (freeze in 100 μl aliquots).
Hypotonic lysis buffer (HLB): 10mM KCl, 7.5mM MgCl2, 50mM Tris-HCl, pH 7.4, 1mM DTT, 50μg/ml CHX, 1× Complete Protease Inhibitor Cocktail, 40U/ml RNAseOUT™. Add cycloheximide, DTT, Complete Protease Inhibitor Cocktail and RNAseOUT™ just prior to using the solution.
Polycarbonate ultracentrifuge tubes, 11 × 34 mm (Beckman; Cat. No. 343778).
2.4 Immunoflourescence microscopy
Coverslips.
Fixing solution: 4% formaldehyde in PBS.
Acetone.
Blocking solution: 1% (w/v) BSA and 0.2% (v/v) Triton X-100 in PBS.
Primary antibody diluted in 1% (w/v) BSA and 0.05% (v/v) Triton X-100 in PBS.
Fluor-conjugated secondary antibody in 1% (w/v) BSA and 0.05% (v/v) Triton X-100 in PBS.
Anti-fade mounting medium.
Nail polish, to seal the coverslip.
2.5 Polysome analysis
15% sucrose in lysis buffer.
40% sucrose in lysis buffer.
Polyallomer centrifuge tubes, 14 × 89 mm (Beckman; cat # 331372).
Teledyne/Isco gradient fractionator with a continuous UV flow cell.
2.6 RNA and protein extraction
TRIzol® Reagent (Invitrogen; see Note 3).
Chloroform.
Isopropanol.
0.3M guanidine chloride in 95% isopropanol.
Ethanol.
75% (v/v) ethanol.
Nuclease-free water (to resuspend RNA).
1% SDS (to resuspend protein).
2.7 Denaturing formaldehyde agarose gel electrophoresis and Northern blotting
10× MOPS: 0.2M 3-(N-morpholino)propanesulfonic acid (MOPS), 80mM sodium acetate (NaOAc), 10mM EDTA pH 7.4. This buffer is light sensitive and should be stored in an amber bottle. The color of this solution slowly changes to orange with time. This does not affect its activity.
Agarose (electrophoresis grade).
37.5% (w/v) formaldehyde.
Formamide (deionized).
RNA tracking dye (Ambion).
SyBR safe RNA dye (Invitrogen) (optional).
DNA/RNA gel electrophoresis apparatus.
20× SSC: 3M NaCl, 0.3M NaOAc pH 7.0.
10N sodium hydroxide (NaOH).
Northern transfer buffer: 5× SSC, 10mM NaOH.
Methylene blue stain: 0.02% (w/v) methylene blue in 0.2M NaOAc pH 5.2.
Hybond XL membrane (Amersham Pharmacia Biotech).
Whatman 3MM filter paper or equivalent.
Paper towels.
Stratalinker UV Crosslinker (Stratagene).
T4 Polynucleotide kinase (PNK) (New England Biolabs).
100μM oligonucleotide directed against RNA sequence to be detected.
γ-[32P]-dATP at 6000Ci/mmol; end-labeling grade (MP Biomedicals).
Sephadex G-25 quick spin column or equivalent (Roche).
Scintillation fluid, counting tubes, and beta-counter.
ExpressHyb hybridization solution (Clontech).
Hybridization oven and glass tubes.
Low-stringency wash buffer: 0.5× SSC, 0.1% (w/v) SDS in deionized water.
High-stringency wash buffer: 0.1× SSC, 0.1% (w/v) SDS in deionized water.
Phosphorimager plates, cassettes and scanner (Typhoon 9400; GE Healthcare).
2.8 SDS polyacrylamide gel electrophoresis and Western blotting
12.5% denaturing polyacrylamide gel; 0.75mm thick.
Gel electrophoresis system (BioRad).
5× gel loading dye: 0.2M Tris-HCl pH 6.8, 10% (v/v) glycerol, 10% (w/v) SDS, 0.05% (w/v) bromophenol blue, 10mM ß-mercaptoethanol (BME). Add ß-BME to the sample buffer just prior to use.
5× SDS running buffer: 250mM Tris-HCl, 2M glycine, 1% (w/v) SDS.
CAPS transfer buffer: 50mM 3-[cyclohexylamino]-1-propane sulfonic acid (Sigma C2632) pH 11, 0.075% SDS, 20% (v/v) methanol.
Nitrocellulose membrane (Biorad).
Ponceau stain: 0.1%(w/v) Ponceau S, 5% (v/v) acetic acid.
PBS-T: 1× PBS, 0.2% Tween-20.
5% milk in PBS-T.
Primary and secondary antibodies.
Enhanced chemiluminescence (ECL) reagents (Denville Scientific).
X-ray film and cassette (Denville Scientific).
Fixer/developer system.
3. Methods
3.1 Methods for cellular fractionation
Cellular fractionation has been used for several decades to analyze the molecular composition of the various cellular components. In order to study mRNA partitioning between the cytosol and the ER, we aim to separate the fractions that are enriched for cytosolic polysomes from those enriched in membrane-bound polysomes. This chapter describes two independent methods of cellular fractionation namely, 1) sequential detergent extraction, and 2) mechanical fractionation followed by differential centrifugation.
3.1.1 Fractionation by sequential detergent extraction
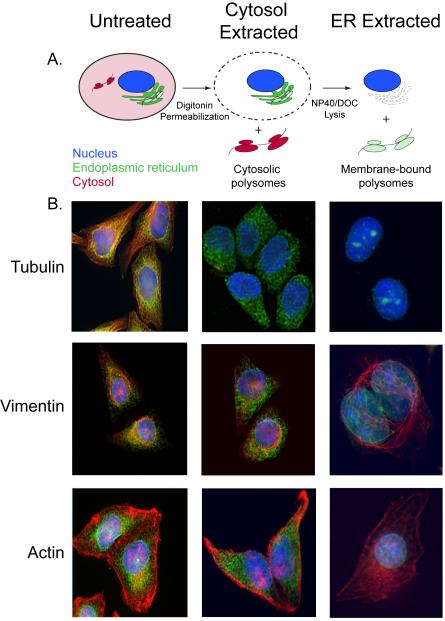
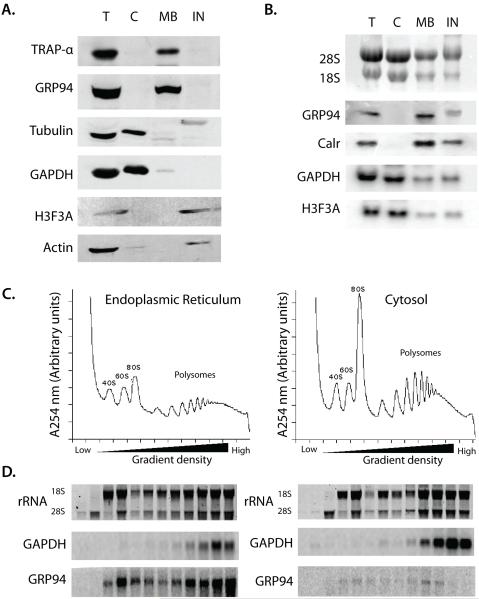
This method takes advantage of the relatively high cholesterol content of the plasma membrane, as compared to other cellular membranes. Digitonin is a ß-sterol binding detergent that selectively solubilizes the plasma membrane, leaving the ER- and nuclear membranes intact. Hence, sequential treatment with digitonin followed by a more lytic detergent, such as an NP-40/DOC cocktail, yields cytosolic- and membrane-bound polysome fractions, respectively (schematically illustrated in Fig. 1A). The various steps of the sequential detergent extraction procedure have been validated by immunofluorescene microscopy, where it can be seen that disruption of the plasma membrane with digitonin results in the release of (depolymerized) tubulin, without effect on the ER, the actin cytoskeleton, or the intermediate filament network (Fig. 1 B). Following addition of the ER lysis buffer, the ER fraction is recovered in a soluble fraction and the nuclei, actin cytoskeleton, and intermediate filament network remain (Fig. 1B). Companion immunoblot analyses of marker protein distributions show that the cytosolic proteins GAPDH and tubulin are present in the cytosol fraction, as expected, and the ER-membrane proteins, TRAPα and ER-lumenal protein, GRP94 are present in the ER fraction (Fig. 2 A). The NP-40 insoluble material consists primarily of nuclear and cytoskeletal elements, as evidenced by the marker proteins histone H3 and actin, respectively (Fig. 2 A). Similarly, Northern blot analysis of the mRNA composition of the cytosol and membrane fractions show that the cytosol fraction is enriched for mRNAs encoding histone (H3F3A) and GAPDH, whereas the membrane fraction is enriched in mRNAs encoding ER resident proteins, such as GRP94 and calreticulin (Fig. 2 B).
Figure 1. Characterization of the detergent fractionation method through immunofluorescence microscopy.
A) Schematic representation of the effect of the permeabilization and lysis on cells. Upon digitonin treatment, the plasma membrane is solubilized, allowing the recovery of cytosolic polysomes (shown in red). During the subsequent lysis with NP40/DOC mixture, the ER is solubilized and membrane-bound polysomes (shown in green) recovered, leaving the nucleus intact (shown in blue). B) Cells were immunostained during the three stages of detergent extraction (untreated, permeabilized and lysed) using antibodies against TRAPα (green), tubulin (red), vimentin (red) and actin (red). Nucleus is stained with DAPI (blue).
Figure 2. Validation of the detergent fractionation method via Western and Northern blot analysis.
A) Protein from the T, C, M and IN fractions were extracted using the TRIzol® Reagent and equivalent amounts of protein were resolved on a 10% SDS polyacrylamide gel. The proteins were transferred to a nitrocellulose membrane and the analyzed for the presence of ER resident (TRAPα and GRP94), cytosolic (tubulin and GAPDH) and nuclear (Histone 3) proteins by western blotting. Primary antibodies were used at a dilution of 1:3000 and HRP-conjugated secondary antibodies were used at 1:5000. B) RNA from the total (T), cytosol (C), membrane-bound (M) and insoluble (IN) fractions derived from the sequential detergent fractionation procedure were resolved on a denaturing agarose gel and transferred to a nylon membrane. The ribosomal RNA were stained using methylene blue and the profile was documented. The membrane was then probed for membrane-bound mRNAs (GRP94 and calreticulin) and cytosolic mRNAs (GAPDH and Histone H3F3A) using γ-[32P] labeled antisense oligonucleotides. Following overnight exposure on phosphorimager plates, images were collected using Typhoon 9400 and image size/contrast using Adobe Photoshop 7. 0.
The method described below is for cells grown in monolayer. However, the protocol can be easily adapted for non-adherent cells by performing permeabilization, wash and lysis in suspension and pelleting cells at 3000 × g for 5 minutes between the different steps. The volumes of reagents mentioned in the following protocol are scaled to extract polysomes from 10 million cells.
Seed HEK293T cells in a T75 flask to be 80-90% confluent on the day of the experiment.
Aspirate the media and wash the cells once with 10 ml of PBS (room temperature).
Treat the cells with 10ml ice-cold PBS containing 50μg/ml CHX for 10 minutes, on ice (see Notes 4, 5). Perform all remaining steps on ice using ice-cold reagents.
Add 1 ml of permeabilization buffer to the cells, taking care not to dislodge the cells (see Note 6, 7) and incubate for 5 minutes. Tilt the flask to drain the soluble material (cytosol fraction) and collect the cytosol in a pre-cooled microcentrifuge tube.
Wash cells gently with 5ml of wash buffer.
Treat the cells with 1ml of lysis buffer for 5 minutes. Drain and collect the NP-40 soluble material (membrane fraction).
Clarify both the cytosolic and membrane fractions at 7500 × g for 10 minutes to remove cell debris. Transfer the supernatants to clean, pre-chilled microcentrifuge tubes.
The various steps of this process can be visualized by immunofluorescence microscopy by staining for TRAPα (ER), tubulin (cytosol), vimentin (intermediate filaments) and actin (cytoskeleton) (see Section 3.2) (Fig. 1 B)
The polysome profiles of the cytosolic and membrane-bound fractions can be analyzed by layering 1ml of the cytosolic and membrane fractions on 15-40% linear sucrose gradients and subjecting them to velocity sedimentation (see Section 3.3) (Fig. 2C).
If the polyribosomes in the cytosol and membrane fractions need to be recovered for downstream applications, layer the clarified lysate over 1/3 volume of 0.5M sucrose in the same buffer as that of the sample. Centrifuge at 60K rpm for 40 minutes in a Beckman TLA 100.2 rotor at 4°C. Ribosome pellets will appear clear and glassy.
To analyze the RNA/protein content in the cytosol and membrane fractions (Fig. 2B), directly extract using 1 ml TRIzol® Reagent per 0.25 ml of sample (see Section 3.4). Samples in TRIzol® can be frozen at −70°C for storage prior to processing.
3.1.2 Fractionation by differential centrifugation
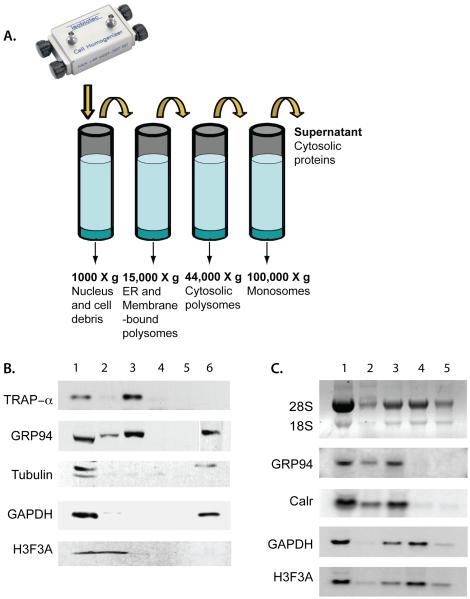
A cell cracker, or more technically, a ball-bearing homogenizer, is a precision device that efficiently and reproducibly disrupts cell structure while maintaining organelle integrity. The following protocol allows for efficient recovery of membrane-bound and cytosolic polysomes. By immunoblot analysis of marker protein distribution, we identified conditions that allowed separation of the nucleus from the ER (as evidenced by the relative absence of the nuclear marker histone H3 in the ER fraction; Fig. 3 A) and the ER-bound polysomes from free-polysomes (Fig. 3 B). Using this method, ER membranes (indicated by the ER membrane marker, TRAPα) were recovered in a 15,000 × g spin (Fig. 3 A) as were, appropriately, ER-bound polysomes (Fig. 3 B). The 44,000 × g spin, which should theoretically sediment free polysomes (based on K-factor calculations) yields a fraction enriched for cytosolic polysomes (Fig. 3 B). The supernatant from the 100,000 × g spin (which theoretically sediments monosomes and individual ribosomes) contains the cytosolic proteins such as histone H3 and GAPDH, as expected, and also GRP94 (ER lumen protein) which is partially released during cell homogenization.
Figure 3. Validation of the mechanical fractionation method via Western and Northern blot analysis.
A) A schematic representation of the mechanical fractionation method of cellular fractionation. B) Protein from the fractions 1 – 6 were analyzed for ER, cytosol and nuclear proteins by Western blot as described in Fig. 1 A. C) Total RNA (1), RNA extracted from the pellets from centrifugation at 500 × g (2), 15,000 × g (3), 44,000 × g (4), 100,000 × g (5) and that from the supernatant from the 100,000 × g spin were analyzed by Northern blot as described in Fig. 1 B.
Aspirate media from cells and wash the cells once with 10ml PBS at room temperature.
Add 6 ml of ice-cold PBS to each well and scrape cells with a cell scraper. All subsequent steps were performed on ice, using ice-cold solutions.
Pellet cells at 1000 × g (3.3k) for 4 minutes at 4°C. (Extract cell pellets from two wells of the 6-well plate with 1ml of the TRIzol® Reagent; see Note 8).
Resuspend pellets from the remaining 4 wells in 4 ml of ice-cold hypotonic lysis buffer (HLB).
Let cells swell on ice for 10 minutes.
Rinse the cell cracker (pre-cooled and kept on ice) with cold HLB using 5ml Luer-Lok™ syringes (see Note 9). Fill a pre-cooled 5ml Luer-Lok™ syringe with the cell suspension, avoiding air bubbles. Pass the cell suspension through pre-cooled Cell Cracker with 12 μm clearance 12 times (6 pushes on each syringe). Pool the lysates and adjust the volume to 4ml if needed.
Adjust homogenate to 250mM sucrose and 4mM MgCl2.
Centrifuge at 1000 × g (2.3k) for 5 minutes at 4°C to pellet unbroken cells and nucleii (Solubilize the pellets in TRIzol® Reagent; see Note 8).
Centrifuge the supernatant from step 8 at 15,000 × g (12.7k) for 15 minutes at 4°C (solubilize the pellets in TRIzol® Reagent).
Centrifuge the supernatant from step 9 at 44,000 × g for 15 minutes at 4°C (solubilize the pellets in TRIzol® Reagent).
Centrifuge the supernatant from step 10 at 100,000 × g for 1 hour at 4°C (solubilize the pellets in TRIzol® Reagent).
Add 1ml TRIzol® Reagent to 250 μl of the supernatant from 100,000x g spin (see Note 10).
Proceed to TRIzol® extraction (Section 3.5) or freeze the samples at −80°C until RNA/Protein extraction.
3.2 Immunoflourescence microscopy
Plate cells onto glass coverslips 12-24 hours prior to analysis.
Rinse the cells in PBS.
Fix the cells in 4% paraformaldehyde in PBS for 15 minutes at room temperature.
Rinse thrice with PBS for 5 minutes each.
Permeabilize the fixed cells in either ice-cold PBS, 1% (v/v) Triton X-100 for 10 minutes on ice, or ice-cold acetone, with incubation at −20°C for 10 minutes.
Rinse thrice with PBS for 5 minutes each.
Block using the blocking solution for 1 hour at room temperature, or overnight at 4°C.
Add primary antibody solution (just enough to cover the cell layer) and incubate at room temperature for 1 hour. Alternatively, invert the coverslip onto a 50μL drop of the primary antibody solution, on Parafilm, and incubate for 1 hr at RT.
Rinse thrice with PBS for 5 minutes each.
Repeat step 8 with secondary antibody solution and incubate for 1 hr at RT, in the dark.
Rinse thrice with PBS for 5 minutes each.
Mount the cover slip on a slide using anti-fade mounting medium.
Seal slides with nail polish.
3.3 Polysome gradient analysis
Pour 15-40% linear sucrose gradient as follows:
Add 5 ml of 15% sucrose in a Beckman centrifuge tube (SW40)
Underlay 5ml of 40% sucrose solution slowly so that the interface is not disturbed. (see Note 11).
Cover the tube with parafilm and slowly tip the tube so that it is laying on its side. Support the tube on both sides with microcentrifuge tube racks to prevent rolling.
Let the gradient form over 2 hours.
Carefully tip the tube back up and place it on ice for at least 30 minutes. If needed, the gradient can be stably stored on ice overnight.
Overlay the sample on the sucrose gradient and centrifuge at 45000 × g for 3 hours.
Fractionate the gradient, either via an automated gradient fractionator (i.e., Teledyne/ISCO) or manually, In the absence of a gradient fractionator the centrifuge tubes can be manually punctured, fractions collected, and the UV absorbance (254 nm) measured for each fraction to generate the polyribosome trace.
3.4 RNA and protein extraction using the
TRIzol® Reagent TRIzol® Reagent can be used to sequentially extract RNA and protein from the same biological sample.
Incubate the TRIzo®-treated samples at room temperature for 10 minutes to allow dissociation of nucleoprotein complexes (see Note 8).
Spin the TRIzol®-treated samples at 10,000× g for 10 minutes to remove insoluble material (optional).
Add 200 μl of chloroform to each sample and vortex for 15 seconds.
Incubate at room temperature for 3 minutes to allow phase separation.
Spin at maximum speed for 15 minutes on a table-top centrifuge at 4°C.
RNA isolation: Carefully transfer the aqueous phase (containing the RNA) to a clean tube (see Note 12) and add 0.5 ml of isopropanol. Mix well and incubate at room temperature for 10 minutes to precipitate RNA (see Note 13). Spin at 13000× g for 10 minutes to pellet the RNA. Wash the RNA pellet with 1ml of 75% ethanol. Air dry the pellet for 2-3 minutes (do not allow the pellet to over dry) and re-suspend the RNA in appropriate volume of DEPC-treated water (see Note 14).
Protein isolation: Remove any remaining aqueous phase and as much of the interphase as possible without removing the organic phase which contains the protein. Add 0.5 ml of ethanol to the organic phase to precipitate any DNA. Incubate for 5 minutes at room temperature and spin at 3000× g for 5 minutes to remove the DNA. The DNA pellet will be barely visible and very soft. Take care to not disturb it while removing the supernatant. To precipitate the protein, add 0.5ml of isopropanol per 0.75ml of the organic phase-ethanol mixture and incubate for 10 minutes at room temperature to precipitate the protein. Spin at 13000× g for 10 minutes to pellet the protein. Wash the pellet for 20 minutes with 1ml of 0.4M guanidine hydrochloride in 95% isopropanol at room temperature. Repeat this step for a total of three washes. Wash using 1ml of ethanol to remove the salt and air-dry the pellet for 2-3 minutes. Resuspend the protein pellet in 1% SDS. If the pellet does not go into solution upon incubation at room temperature for 15-20 minutes, heating the sample at 65°C for 15 minutes with occasional vortexing will assist solubilization (see Note 15).
Measure the RNA and protein concentrations spectrophotometrically. To obtain a more accurate sense of the protein concentration, standard protein quantification assays such as the BCA assay (Pierce) should be used.
3.5 Denaturing formaldehyde agarose gel electrophoresis and Northern blotting
Rinse the gel tray, comb and the electrophoresis apparatus with 0.2% SDS and DEPC-treated water.
Formaldehyde agarose gel: Boil 1g of agarose in 82ml DEPC-treated water. Add 10ml of 10× MOPS buffer to the melted agarose. When the solution cools to about 60°C, add 8ml of formaldehyde in a fume hood (see Note 16, 17).
Formamide/formaldehyde sample buffer: Mix 200μl of formamide, 70μl of formaldehyde, 30 μl of 10× MOPS and 27μl tracking dye (Ambion). Add 24 μl of sample buffer to 8 μl of RNA sample and heat the samples for 10 minutes at 65°C. Cool the samples to room temperature and load.
Run the gel in 1× MOPS buffer at 120V for 2 hours (see Note 18).
Set up the Northern transfer as follows: Cut one piece of Hybond™ membrane and six pieces of Whatman 3MM filter paper to the size of the gel. Soak the gel and the membrane in Northern transfer buffer for 5-10 minutes. Assemble the Northern transfer by sandwiching the gel and the membrane between 6 filter paper squares (three on each side) and place this assembly on a stack of paper towels. Gently roll a glass pipette over the assembled transfer stack to eliminate any trapped air. Wet two long pieces of the filter paper precut to the width of the gel and place one end on top of the transfer stack and dip the other end in transfer buffer. This will serve as a wick to facilitate downward capillary transfer (see Note 19). Place the gel tray on top of the transfer stack to limit buffer evaporation. Transfer for 2-12 hours.
Crosslink RNA to the membrane using the auto crosslink option setting on a Strategene Stratalinker. After this point, the reagents do not need to be RNAse-free.
Stain the membrane with methylene blue stain and record the rRNA banding pattern.
Destain the membrane in water and prehybridize the membrane using ExpressHyb™ hybridization solution for 30 minutes. The hybridization should be carried out at 42°C when using oligo probes, and at 50°C when using random probes (see Note 20).
Radiolabeled probes: oligonucleotide probes can be end-labeled with γ-[32P] ATP using T4 polynucleotide kinase (New England Biolabs). Alternately, probes can be made by random priming of a fragment of the target gene using α-[32P] CTP, with a first strand cDNA synthesis kit (Ambion). Unincorporated α-[32P] ATP is removed using a G-25 Sephadex quick spin column.
Measure the radiolabeled probe on a scintillation counter and add 1 × 107 cpm [32P]-labeled DNA probe to the prehybridized membrane. It is important to denature the random probes by boiling at 95°C and rapidly cooling on ice before adding to the blot.
Hybridize the probe for 8 hours to overnight.
Rinse the membrane in low-stringency wash buffer for 30 minutes, followed by high-stringency wash buffer for 30 minutes.
Place the blot on pre-wet filter paper, cover with Saran Wrap and expose to a phosphorimager screen for 3 hours or longer and scan (see Note 21).
Radiolabeled probes can be stripped off a membrane by washing in boiling 0.5% SDS solution. (see Note 22).
The membrane can be reprobed using a different probe by repeating steps 8 through 14.
3.6 SDS polyacrylamide gel electrophoresis and Western blotting
Dilute the protein samples 1:4 in 4× sample buffer. Boil the samples at 95°C for 5 minutes. Cool and load 20μg of protein per lane on a 10% SDS polyacrylamide gel.
Run the gel at a constant voltage of 120V until the dye front reaches the end of the gel, in 1× SDS running buffer.
Soak the gel in CAPS transfer buffer.
Cut nitrocellulose membrane to the dimensions of the gel and soak in CAPS buffer.
Semi-dry Western transfer: assemble the transfer by sandwiching the membrane and the gel between four pieces of pre-wet Whatman 3MM filter paper (cut to the dimensions of the gel), two pieces per side.
Perform Western transfer at a constant current of 100mA for 30 minutes.
Ponceau stain the membrane to check for efficient transfer.
Destain the membrane in water and block in 5% milk in PBS-T for 1 hour at room temperature. (see Note 23).
Wash the membrane in 1× PBS-T once for 15 minutes and twice for 5 minutes.
Add the primary antibody diluted to the appropriate final concentration in 2% milk in PBS-T to the blot and incubate for 1 hour at room temperature. (see Note 24).
Repeat step 10.
Add the HRP-conjugated secondary antibody in 2% milk in PBS-T and incubate for 1 hour at room temperature. (see Notes 23, 24).
Repeat step 10.
Develop the blot using ECL reagents.
Acknowledgements
The authors thank Angela Jockheck-Clark for critical reading of the manuscript. We also wish to thank Mayya Shveygert and the Gromeier lab for providing GAPDH and H3 antibodies. This work was supported by NIH grant GM-077382 to CVN.
Footnotes
DEPC is a suspected carcinogen. Avoid inhalation and skin contact and always handle in a fume hood.
Digitonin stock solutions are not stable for long periods of time even when frozen. We recommend making up small volumes of stock solution and not using them for longer than a week.
TRIzol® Reagent contains phenol and should be used with caution. Standard safety procedures should be followed to dispose phenol-containing solutions.
HEK293T cells lift very easily. Care should be taken to avoid dislodging them e.g. pipetting reagents gently on to the sides of the tissue culture vessel. Coating the surface of glass culture vessels with poly-D-lysine (Millipore) may enhance attachment of the cells.
The 10-minute incubation of cells on ice at this point is important to enable microtubule depolymerization. If cells are not incubated on ice for a sufficiently long period, tubilin will be primarily present in the insoluble fraction, rather than the cytosol.
This procedure can be easily scaled up or down by correspondingly changing the volume of reagents used. The main factor to consider is that the amount of the reagents used should be sufficient to cover the entire surface of the cell monolayer.
For adherent cells such as HEK293T, it is important to perform the permeabilization on the monolayer. Adherent cells when lifted do not allow efficient extraction of polysomes potentially due to rounding up and contraction of the cells during lifting.
It may be necessary to pass the TRIzol®-ized sample through 27 1/2 gauge needle to completely shear the DNA. If shearing the DNA by this method, extreme care should be taken to avoid the TRIzol® Reagent from splattering on to skin/eyes.
Luer-Lok™ syringes are necessary to avoid sample loss while using the cell cracker; normal syringes tend to detach due to the high pressure generated when cells pass through.
Alternately, precipitate the protein from the supernatant fraction using tricholoroacetic acid method and RNA using lithium chloride method. Please refer to Maniatis et. al.19 for detailed protocols.
Keep the tip of the needle only slightly under the interface so that when the needle is retracted, it does not leave a track of 40% sucrose through the 15% layer.
Be careful to not touch the interphase with the micropippetor tip as this reduces the quality of the RNA preparation. It would be best to try not to recover the last 5% of the aqueous phase.
If working with small quantities of RNA, precipitate at −20°C and/or add 10ug of yeast tRNA as a carrier to aid precipitation.
Even trace amounts of DEPC can inactivate most polymerases and other enzymes. Hence, use commercial nuclease-free water if RNA will be used for molecular biology applications downstream.
While there is a notion that the protein pellet from TRIzol® extraction is hard to resuspend, in our hands, it has been quite easy to get the protein pellet to solubilize even without the heating step. We have confirmed by PAGE that the profile of proteins from HEK293T extracted by TRIzol®, or directly extracted in sample buffer, are identical. However, it is possible that protein derived from some cell lines and/or tissues may prove harder to solubilize.
Formaldehyde is highly toxic. All waste generated (including the gel) should be disposed following standard safety procedures.
If required, 2 μl of SyBr safe dye can be added to the gel solution to enable visualization of the RNA after electrophoresis.
The 1X MOPS buffer can be reused several times unless RNAse contamination is suspected.
Check and make sure there is no buffer short circuit between the wick and the paper towels. If required, place pieces of parafilm around the edge of the gel to prevent the wick from touching the paper towels. This is to ensure that the buffer transfer occurs only through the gel.
This is only a general rule of thumb and works in our hands for most of the probes we use in the lab. The stringency of the hybridization depends on the temperature of hybridization. Hence it may be required to identify an optimal hybridization temperature for a given probe depending on its length, Tm, etc. to ensure specificity and sensitivity.
Drying of the membrane hybridized with the probe fixes the probe to the membrane and make it very difficult to strip. Hence care should be taken to avoid membrane drying if it is to be reprobed. In the event of the membrane drying, it could be placed in −20°C for an extended priod, to allow the radiolabel to decay before reprobing for another RNA.
Efficient stripping may require multiple washes in boiling 0.5% SDS. Stripping of the probes may need to be checked by exposing the blot overnight to a phosphoimager screen.
This step can be done at 4°C overnight.
If you reuse antibody solutions, omit sodium azide in the HRP-conjugated secondary antibody stock solution. Sodium azide inhibits the activity of the HRP enzyme.
Contributor List:
Sujatha Jagannathan, Department of Cell Biology, Duke University Medical Center, Durham, NC 27710
Christine Nwosu, Department of Cell Biology, Duke University Medical Center, Durham, NC 27710
Christopher V. Nicchitta, PhD., Department of Cell Biology, Duke University Medical Center, Durham, NC 27710
References
- 1.Stevens TJ, Arkin IT. Do more complex organisms have a greater proportion of membrane proteins in their genomes? Proteins. 2000;39:417–20. doi: 10.1002/(sici)1097-0134(20000601)39:4<417::aid-prot140>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 2.Walter P, Ibrahimi I, Blobel G. Translocation of proteins across the endoplasmic reticulum. I. Signal recognition protein (SRP) binds to in-vitro-assembled polysomes synthesizing secretory protein. J Cell Biol. 1981;91:545–50. doi: 10.1083/jcb.91.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walter P, Blobel G. Translocation of proteins across the endoplasmic reticulum. II. Signal recognition protein (SRP) mediates the selective binding to microsomal membranes of in-vitro-assembled polysomes synthesizing secretory protein. J Cell Biol. 1981;91:551–6. doi: 10.1083/jcb.91.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walter P, Blobel G. Translocation of proteins across the endoplasmic reticulum III. Signal recognition protein (SRP) causes signal sequence-dependent and site-specific arrest of chain elongation that is released by microsomal membranes. J Cell Biol. 1981;91:557–61. doi: 10.1083/jcb.91.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lingappa VR, Blobel G. Early events in the biosynthesis of secretory and membrane proteins: the signal hypothesis. Recent Prog Horm Res. 1980;36:451–75. doi: 10.1016/b978-0-12-571136-4.50018-8. [DOI] [PubMed] [Google Scholar]
- 6.Lerner RS, Seiser RM, Zheng T, et al. Partitioning and translation of mRNAs encoding soluble proteins on membrane-bound ribosomes. RNA. 2003;9:1123–37. doi: 10.1261/rna.5610403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diehn M, Eisen MB, Botstein D, Brown PO. Large-scale identification of secreted and membrane-associated gene products using DNA microarrays. Nat Genet. 2000;25:58–62. doi: 10.1038/75603. [DOI] [PubMed] [Google Scholar]
- 8.Mueckler MM, Pitot HC. Structure and function of rat liver polysome populations. I. Complexity, frequency distribution, and degree of uniqueness of free and membrane-bound polysomal polyadenylate-containing RNA populations. J Cell Biol. 1981;90:495–506. doi: 10.1083/jcb.90.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mutka SC, Walter P. Multifaceted physiological response allows yeast to adapt to the loss of the signal recognition particle-dependent protein-targeting pathway. Mol Biol Cell. 2001;12:577–88. doi: 10.1091/mbc.12.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu L, Liang XH, Uliel S, Unger R, Ullu E, Michaeli S. RNA interference of signal peptide-binding protein SRP54 elicits deleterious effects and protein sorting defects in trypanosomes. J Biol Chem. 2002;277:47348–57. doi: 10.1074/jbc.M207736200. [DOI] [PubMed] [Google Scholar]
- 11.Ren YG, Wagner KW, Knee DA, Aza-Blanc P, Nasoff M, Deveraux QL. Differential regulation of the TRAIL death receptors DR4 and DR5 by the signal recognition particle. Mol Biol Cell. 2004;15:5064–74. doi: 10.1091/mbc.E04-03-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pyhtila B, Zheng T, Lager PJ, Keene JD, Reedy MC, Nicchitta CV. Signal sequence- and translation-independent mRNA localization to the endoplasmic reticulum. RNA. 2008;14:445–53. doi: 10.1261/rna.721108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diehn M, Bhattacharya R, Botstein D, Brown PO. Genome-scale identification of membrane-associated human mRNAs. PLoS Genet. 2006;2:e11. doi: 10.1371/journal.pgen.0020011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aragon T, van Anken E, Pincus D, et al. Messenger RNA targeting to endoplasmic reticulum stress signalling sites. Nature. 2008 doi: 10.1038/nature07641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Claude A. Fractionation of mammalian liver cells by differential centrifugation: II. Experimental procedures and results. Journal of Experimental Medicine. 1946;84:61–89. [PubMed] [Google Scholar]
- 16.Duve C. Exploring cells with a centrifuge. Science. 1975;189:186–94. doi: 10.1126/science.1138375. [DOI] [PubMed] [Google Scholar]
- 17.Palacios IM, Johnston D. Getting the message across: the intracellular localization of mRNAs in higher eukaryotes. Annu Rev Cell Dev Biol. 2001;17:569–614. doi: 10.1146/annurev.cellbio.17.1.569. [DOI] [PubMed] [Google Scholar]
- 18.Paquin N, Chartrand P. Local regulation of mRNA translation: new insights from the bud. Trends Cell Biol. 2008;18:105–11. doi: 10.1016/j.tcb.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A laboratory manual. Cold Spring Harbor Press; Cold Spring Harbor, New York, USA: 2001. [Google Scholar]