Abstract
Objectives
To characterize the use of mechanical ventilation in the emergency department (ED), with respect to ventilator settings, monitoring, and titration; and to determine the incidence of progression to acute lung injury (ALI) after admission, examining the influence of factors present in the ED on ALI progression.
Methods
This was a retrospective, observational cohort study of mechanically ventilated patients with severe sepsis and septic shock (June 2005 to May 2010), presenting to an academic ED with an annual census of >95,000 patients. All patients in the study (n = 251) were analyzed for characterization of mechanical ventilation use in the ED. The primary outcome variable of interest was the incidence of ALI progression after ICU admission from the ED and risk factors present in the ED associated with this outcome. Secondary analyses included ALI present in the ED and clinical outcomes comparing all patients progressing to ALI versus no ALI. To assess predictors of progression to ALI, statistically significant variables in univariable analyses at a p ≤ 0.10 level were candidates for inclusion in a bidirectional, stepwise, multivariable logistic regression analysis.
Results
Lung-protective ventilation was used in 68 patients (27.1%), and did not differ based on ALI status. Delivered tidal volume was highly variable, with a median tidal volume delivered of 8.8 mL/kg ideal body weight (IBW) (IQR 7.8 to 10.0), and a range of 5.2 to 14.6 mL/kg IBW. Sixty-nine patients (27.5%) in the entire cohort progressed to ALI after admission to the hospital, with a mean onset of 2.1 days (SD ± 1 day). Multivariable logistic regression analysis demonstrated that a higher body mass index, higher Sequential Organ Failure Assessment score, and ED vasopressor use were associated with progression to ALI. There was no association between ED ventilator settings and progression to ALI. Compared to patients who did not progress to ALI, patients progressing to ALI after admission from the ED had an increase in mechanical ventilator duration, vasopressor dependence, and hospital length of stay.
Conclusions
Lung-protective ventilation is uncommon in the ED, regardless of ALI status. Given the frequency of ALI in the ED, the progression shortly after ICU admission, and the clinical consequences of this syndrome, the effect of ED-based interventions aimed at reducing the sequelae of ALI should be investigated further.
INTRODUCTION
Mechanical ventilation in emergency department (ED) patients has not been rigorously studied, despite the frequency of endotracheal intubation in critically ill ED patients.1 Mechanical ventilation is an age-dependent intervention, and patients over age 65 years are expected to constitute more than 20% of the population by the year 2025.2,3 With the increasing use of the ED as the safety net for the health care system, longer lengths of stay (LOS) while awaiting intensive care unit (ICU) beds, and the extensive amount of critical care being provided in the ED, emergency physicians (EPs) are being expected to manage a greater number of mechanically ventilated patients for increasing periods of time.4-8 Despite these facts, there is a lack of descriptive data to characterize how mechanical ventilation is used in the ED, as previous data have been restricted to the ICU.9,10
Acute lung injury (ALI) is common, deadly, and expensive.11,12 Despite robust epidemiological data addressing the incidence and outcomes of the syndrome, little attention has been focused on patients in the ED.11,12 After onset, it remains difficult to treat, and tidal volume reduction remains the only consistently accepted therapy in ALI.13 While lower tidal volume has become the standard for patients with established ALI, adherence to lung-protective ventilation remains low in the ICU, and there are little data on its use in patients with ALI in the ED.14-17
The most appropriate mechanical ventilation strategy in patients at risk for ALI is controversial.18-21 Patients developing ALI have worse clinical outcomes compared to those who do not develop the syndrome.22-27 These facts, combined with the limited treatment options that exist for ALI, have led to increased interest in the prevention of ALI.28 Unfortunately, while the ED is the portal of entry for some of the highest risk patients for ALI, previous studies on factors associated with ALI progression have been restricted to the ICU, the operating room, or general ward.22-26,29-34 The effect that the ED may have on the progression to ALI is unknown.
This study was performed with several objectives: 1) to characterize the use of mechanical ventilation in the ED, with respect to ventilator settings, monitoring, and titration; 2) to determine the incidence of progression to ALI after admission, and risk factors present in the ED associated with this outcome; 3) to describe the incidence of ALI in the ED in mechanically ventilated patients and to assess compliance with lung-protective ventilation in these patients; and 4) to assess outcome differences between patients progressing to ALI versus those without ALI. We hypothesized that lung-protective ventilation would be uncommon in the ED, and would not differ based on ALI status; we also hypothesized that ALI in the ED and progression to ALI after ED admission would be common, and that factors present during the ED stay would influence this outcome.
METHODS
Study Design
This was a retrospective observational cohort study. This observational study is reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies.35 Financial support for this project was provided in part from the National Center for Research Resources (NCRR), and the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through Grant Number UL1 TR000448. The funding organization played no role in the study concept, design, data analysis, or writing of the manuscript. This study was approved by the Human Research Protection Office at the principal investigator’s institution with waiver of informed consent.
Study Setting and Population
This study was conducted at a university-affiliated, urban teaching hospital (1,250 beds), with an annual ED census of > 95,000 patients. Over a 5-year period [June 2005 (registry inception)-May 2010 (study conception)], all mechanically ventilated patients enrolled in a severe sepsis registry were eligible for inclusion. Criteria for inclusion in the registry included suspected infection with a lactate level ≥ 4 mmol/L, or systolic blood pressure (sBP) ≤ 90 mmHg after initial fluid bolus.
Study Protocol
Patients with severe sepsis or septic shock were identified as receiving mechanical ventilation in the ED by registry query and verified by review of the medical record. Baseline patient characteristics, LOS, treatment variables, and outcome variables were collected from the electronic medical record. All ventilator parameters were abstracted from the medical record by data collectors trained in data collection and project details, prior to data collection. To ensure uniform data collection and accuracy, all variables were defined prior to data extraction and placed in a standardized format during the data collection process. Regular meetings and monitoring of data collection were performed. Upon completion of data collection, two other (separate) data abstractors verified all records for accuracy, and cross-checked all data with electronic medical records.
Acute lung injury was defined according to the American European Consensus Conference (AECC) definition.36 These criteria include: 1) bilateral alveolar infiltrates on chest x-ray, 2) hypoxemia with a partial pressure of oxygen to fraction of inspired oxygen ratio (PaO2:FiO2) ≤ 300, and 3) no clinical evidence of left atrial hypertension. Acute respiratory distress syndrome (ARDS) refers to a PaO2:FiO2 ratio of ≤ 200. For the purposes of this article, “ALI” encompasses both ALI and ARDS. Given the interobserver variation in chest x-ray interpretation, to verify the presence of bilateral infiltrates the official chest x-ray report was searched for keywords indicative of bilateral infiltrates (e.g. “lung infiltrates, pulmonary edema”) as previously described.37-40 Patients were assumed to have clinical evidence of left atrial hypertension if they had a past medical history of congestive heart failure or dialysis-dependent end-stage renal disease, or had depressed left ventricular function on an echocardiogram obtained within 24 hours of the development of pulmonary edema. Patients were also assumed to have clinical evidence of left atrial hypertension if they had a widened vascular pedicle width on chest radiography, as previously described.41,42 In patients without arterial blood gas measurements, the oxygenation criteria was determined by using the pulse oximeter:fraction of inspired oxygen (SpO2:FiO2) ratio as previously described.43 The presence of two consecutive radiographic and oxygenation criteria was also required for ALI diagnosis. When more than one value was present, the worst value was selected. Lung-protective ventilation was defined as the use of tidal volume <8 mL/kg ideal body weight (IBW), as this was the upper limit of tidal volume allowed by previous investigation of low tidal volume ventilation in ALI.13 Severe sepsis and septic shock were defined as previously described.44,45
The baseline patient characteristics included: age, height, sex, weight, IBW, body mass index (BMI), ED LOS, patient comorbidities, vital signs, hemodynamics, and laboratory values. Modified Acute Physiology and Chronic Health Evaluation (APACHE) II and Sequential Organ Failure Assessment (SOFA) scores were determined. These scores omit the neurologic function component (because of previously reported potential challenges with inter-rater agreement on determination of Glasgow Coma Scale score).46-48 IBW was derived from the height and sex according to the formula: males, 50 +0.91 × (height in centimeters − 152.4); females, 45.5 + 0.91 × (height in centimeters − 152.4).
Ventilator variables included ventilator mode, tidal volume, positive end-expiratory pressure (PEEP), FiO2, peak airway pressure, mean airway pressure, and inspiratory plateau airway pressure.
Process of care variables included antibiotic administration, time to initiation of intravenous antibiotics, intravenous fluid administered over the first six hours, vasopressor use, blood product administration, and use of central venous pressure (CVP) or central venous oxygen saturation (ScvO2) to monitor resuscitation.
All patients in the study were analyzed for characterization of mechanical ventilation use in the ED. The primary outcome variable of interest was the incidence of ALI progression after ICU admission from the ED and risk factors present in the ED associated with this outcome. The assessment of progression to ALI was restricted to patients alive at least 24 hours after admission, and those without histories of heart failure or dialysis-dependence, and was only assessed over the first five days after admission, as previous data indicate that progression to ALI occurs early during ICU stay.22-25,30,32,49 Secondary analyses included ALI present in the ED and clinical outcomes comparing all patients progressing to ALI versus no ALI (change in SOFA score, dialysis-dependent acute kidney injury, mechanical ventilation duration, duration of vasopressor use, hospital LOS, and hospital mortality).
Data Analysis
Descriptive statistics, including mean (± standard deviation [SD]), median (interquartile range [IQR]), and frequency distributions were used to assess the characteristics of the patient cohort. To assess predictors of progression to ALI, continuous and categorical variables were compared using an unpaired t-test, Wilcoxon’s test, chi-square test, or Fisher’s exact test, as appropriate. Variables with less than 10% missing data and statistically significant in univariable analyses at a p ≤ 0.10 level were candidates for inclusion in a bidirectional stepwise, multivariable, logistic regression analysis. The stepwise regression method selected variables for inclusion or exclusion from the model in a sequential fashion based on the significance level of 0.10 for entry and 0.10 for removal. Collinearity was assessed, and the model used variables that contributed information that was statistically independent of the other variables in the model. Because of the high correlation between two measures of body composition (BMI and weight), only one of the multiple measures was included in the multivariable logistic regression model. Therefore, the measure with the strongest univariable association was selected (BMI). Adjusted odds ratios (aORs) and corresponding 95% confidence intervals (CIs) are reported for variables in the multivariable model, adjusted for all variables in the model. To assess clinical outcomes based on ALI status, chi-square, analysis of variance (ANOVA) using rank-transformed data, and Tukey’s HSD was used to compare groups. All tests were two-tailed, and a p value < 0.05 was considered statistically significant. A sample size calculation was not performed a priori, as the primary outcome was descriptive and to characterize the use of mechanical ventilation in the ED. Based on previous data examining a similar patient cohort in the ICU (n = 160), our sample size of 251 patients was recognized as likely to be adequate for investigation of ED-based parameters associated with progression to ALI.23
RESULTS
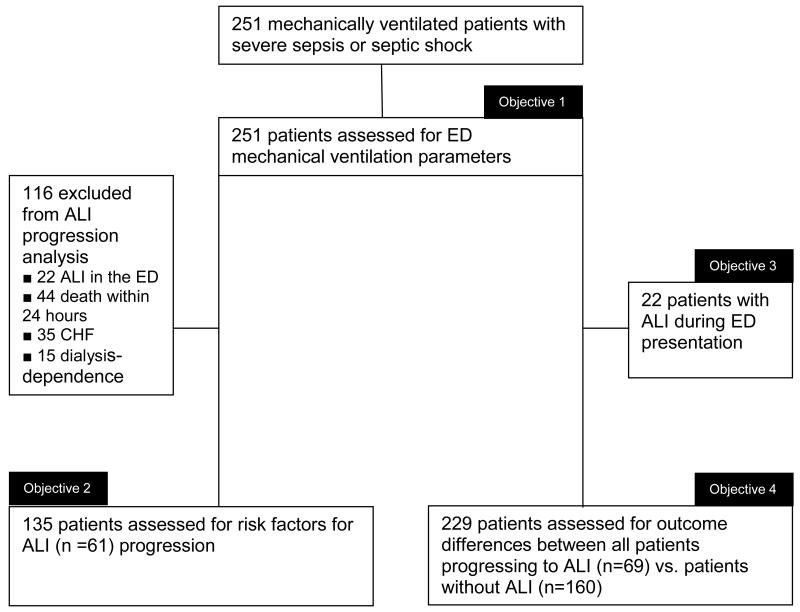
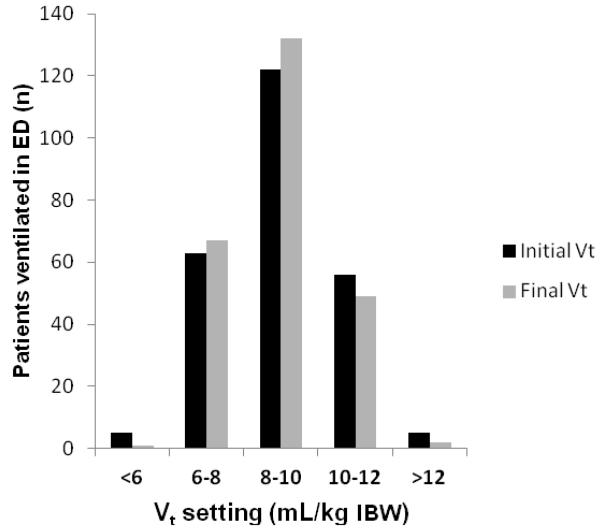
Two hundred fifty-one mechanically ventilated patients were included in the study (Figure 1). Table 1 shows the baseline characteristics of the study population. Ventilator variables are presented in Table 2. In the entire cohort, volume-targeted ventilation was used in 242 patients (96.4%). Median tidal volume delivered was 8.8 mL/kg IBW (IQR 7.8 to 10.0), with a range of 5.2 to 14.6 mL/kg IBW. Figure 2 demonstrates this distribution of tidal volume delivered in the ED, showing the difference in initial and final tidal volume in the patients who did have tidal volume adjusted while in the ED (n = 25). Lung-protective ventilation was used in 68 (27.1%) patients. Inspiratory plateau pressure was recorded in 76 (30.3%) of patients. With respect to titration of settings, in addition to the data in Table 2, 174 (69.3%) patients had no ventilator parameters changed in the ED.
Figure 1.
Flow diagram depicting the patients analyzed to achieve each objective of the study.
ALI = acute lung injury; CHF = congestive heart failure
Table 1. Characteristics of mechanically ventilated ED patients.
| Characteristics, n=251 | |
|---|---|
| Baseline | |
| Age (years) | 62.9 (51.2-77.9) |
| Male, n (%) | 129 (51.4) |
| Comorbidities, n (%) | |
| Diabetes | 81 (32.3) |
| Cirrhosis | 9 (3.6) |
| CHF | 40 (15.9) |
| Dialysis | 25 (10.0) |
| Malignancy | 59 (23.5) |
| COPD | 43 (17.1) |
| Height (in) | 66.9 (64.0-70.0) |
| Weight (kg) | 73.0 (57.9-87.9) |
| IBW (kg) | 63.8 (54.6-73.0) |
| BMI | 25.5 (21.0-30.4) |
| Temperature | 36.7 (36.4-37.8) |
| Heart rate | 109.5 (88.0-130.0) |
| RR | 20.0 (18.0-26.0) |
| sBP | 102.5 (80.0-133.5) |
| dBP | 61.0 (48.5-80.0) |
| SpO2 | 93.0 (88.0-99.0) |
| Lactate | 3.7 (2.0-7.3) |
| APACHE II* | 24.0 (19.0-29.0) |
| SOFA* | 8.0 (6.0-11.0) |
| CVP (n=135) | 11.0 (8.0-17.0) |
| ScvO2 (n=102) | 77.0 (66.0-84.0) |
| ED LOS (hours) | 5.5 (4.2-7.5) |
| Process of Care Variables | |
| Time to antibiotic administration (hours) | 2.0 (1.3-3.0) |
| Fluids over first 6 hours (liters) | 3.0 (2.0-4.0) |
| Vasopressor use, n (%) | 191 (76.1) |
| Blood product administration, n (%) | 28 (11.2) |
| ScvO2 ≥ 70% achieved, n (%)† | 80 (78.4) |
CHF = congestive heart failure; COPD = chronic obstructive pulmonary disease; IBW = ideal body weight; BMI = body mass index; RR = respiratory rate; sBP = systolic blood pressure; dBP = diastolic blood pressure; SpO2 = pulse oximetry; APACHE = Acute Physiology and Chronic Health Evaluation; SOFA = Sequential Organ Failure Assessment Score; CVP = central venous pressure; ScvO2 = central venous oxygen saturation; LOS = length of stay Continuous variables are reported as median (interquartile range).
Refers to the 102 patients with ScvO2 monitored while in the ED.
Table 2. Ventilator Variables and Care in the ED.
| Ventilator Settings | Entire Cohort (n=251) |
No ALI* (n=229) | ALI in ED* (n=22) | Developed ALI after admission (n=69) |
p * |
|---|---|---|---|---|---|
| Ventilator Mode | |||||
| VC-AC, n (%) | 242 (96.4) | 222 (96.9) | 20 (90.9) | 64 (92.8) | 0.75 |
| Other | 9 (3.6) | 7 (3.1) | 2 (9.1) | 5 (7.2) | |
| Tidal volume, mL | 547 (480.5-642.5) | 535 (467.0-630.5) | 560.1 (495.0-654.2) | 565.1 (501.1-656.2) | 0.37 |
| Tidal volume, mL/kg IBW |
8.8 (7.8-10.0) | 8.7 (7.8-9.9) | 9.0 (8.0-10.1) | 9.0 (7.9-9.9) | 0.40 |
| Lung protective ventilation, n (%) |
68 (27.1) | 64 (27.9) | 4 (18.2) | 19 (27.5) | 0.82 |
| PEEP | 5.0 (5.0-5.0) | 5.0 (5.0-5.0) | 5.0 (5.0-5.0) | 5.0 (5.0-5.0) | 0.87 |
| FiO2 | 95.1 (100-100) | 94.0 (100-100) | 97.0 (100-100) | 96.5 (100-100) | 0.69 |
|
| |||||
| Monitored Variables | |||||
| Peak pressure, cm H2O (n=225) |
29.0 (23.0-35.0) | 28.5 (22.4-34.2) | 34.2 (27.8-40.1) | 30.0 (23.0-37.0) | 0.55 |
| Mean pressure, cmH2O (n=218) |
10.0 (8.0-12.0) | 9.9 (8.0-11.8) | 12.2 (10.1-14.1) | 10.0 (9.0-12.0) | 0.42 |
| Plateau pressure, cmH2O (n=76) |
20.0 (15.0-25.0) | 21.8 (14.5-24.8) | 22.0 (15.5-26.5) | 23.0 (16.0-27.0) | 0.47 |
|
| |||||
| Titration Variables | |||||
| 1st tidal volume setting in ED = highest tidal volume, n (%) |
236 (94) | 215 (93.9) | 21 (95.5) | 68 (98.6) | 0.85 |
| Non-protective ventilation time (minutes) |
230.0 (0.0-354.0) | 225.7 (0.0-352.1) | 250.9 (0.0-450.5) | 248.3 (0.0-420.9) | 0.62 |
| Exposure to FiO2 100%, (minutes) |
251.0 (148.0-373.0) | 241.0 (139.2-365.6) | 290.5 (187.8-429.9) | 262.5 (159.4-385.0) | 0.38 |
| 1st ICU setting same as last ED setting, n (%) |
207 (86.3) | 187 (81.7) | 20 (90.1) | 60 (87.0) | 0.78 |
| Exposure to same tidal volume for 24 hour, n (%) |
60 (24.8) | 57 (24.9) | 3 (13.7) | 26 (37.7) | 0.22 |
p value comparison of ED ALI patients vs. no ALI
ALI = acute lung injury; VC = volume controlled; AC = assist control; IBW = ideal body weight; PEEP = positive end expiratory pressure; FiO2 = fraction of inspired oxygen
Continuous variables are reported as median (interquartile range).
Figure 2.
Delivered tidal volume in the ED
Of the 251 patients mechanically ventilated in the ED, 68 (27.1%) received lung-protective ventilation (<8 mL/kg IBW). Twenty-five patients (10.0%) had tidal volume adjusted while in the ED (n=15, increase in tidal volume; n=10, decrease in tidal volume). Vt = tidal volume; IBW = ideal body weight
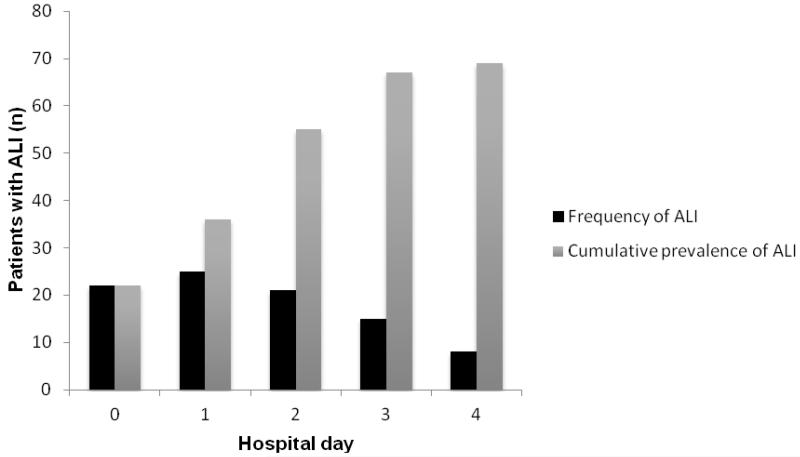
Sixty-nine patients (27.5%) in the entire cohort progressed to ALI after admission to the hospital, with a mean onset of 2.1 days (SD ± 1 day) (Figure 3). There was no difference in ventilator variables in these patients (Table 2). After exclusion of 116 patients with either ALI at the time of ED presentation, those with congestive heart failure (CHF) or dialysis, and those who died within 24 hours of admission, 135 patients were assessed for risk factors for ALI progression (Figure 1). Results of the univariable analysis are presented in Table 3. Multivariable logistic regression analysis demonstrated that a higher BMI, higher SOFA score, and ED vasopressor use were associated with progression to ALI (Table 4).
Figure 3.
Frequency and cumulative prevalence of ALI according to hospital day
Hospital day 0 refers to the ED. Frequency of ALI represents the development of new cases of ALI on an individual hospital day (e.g. 25 new cases of ALI development on hospital day 1). Cumulative prevalence of ALI represents the total number of ALI cases present on an individual hospital day, excluding those cases experiencing death.
ALI = acute lung injury
Table 3. Risk factors for progression to ALI.
| Factor | Progression to ALI (n=61) |
No progression to ALI (n=74) |
P |
|---|---|---|---|
| Baseline characteristics | |||
| Age (yrs) | 62.4 (±15.6) | 63.1 (±17.4) | 0.82 |
| Male, n (%) | 30 (49.2) | 45 (60.8) | 0.18 |
| Comorbidities, n (%) | |||
| Diabetes | 21 (34.4) | 22 (29.7) | 0.55 |
| Cirrhosis | 3 (4.9) | 2 (2.7) | 0.66 |
| Malignancy | 15 (24.6) | 16 (21.6) | 0.68 |
| COPD | 11 (18.0) | 10 (13.5) | 0.47 |
| Height (in) | 66.8 (±4.0) | 66.8 (±3.3) | 0.96 |
| Weight (kg) | 80.6 (60.0-93.2) | 69.7 (57.2-81.8) | 0.02 |
| IBW (kg) | 63.4 (±11.1) | 63.9 (±9.1) | 0.79 |
| BMI | 27.5 (22.7-32.3) | 23.9 (20.4-28.7) | 0.006 |
| Lactate | 3.8 (2.3-6.4) | 3.1 (1.8-5.1) | 0.44 |
| APACHE II | 24.0 (±5.9) | 21.8 (±5.9) | 0.03 |
| SOFA | 9.0 (±3.3) | 7.3 (±3.8) | 0.01 |
| CVP | 12.3 (±5.1) | 10.4 (±4.5) | 0.10 |
| ScvO2 | 77.0 (68.0-84.0) | 78.0 (69.0-84.0) | 0.88 |
| ED LOS | 5.5 (4.3-7.0) | 4.9 (3.9-7.1) | 0.46 |
|
| |||
| Process of Care Variables | |||
|
| |||
| Time to antibiotic administration (hours) |
1.7 (1.2-2.8) | 2.0 (1.3-2.8) | 0.64 |
| Fluids over first 6 hours (liters) | 3.3 (2.0-4.0) | 4.0 (2.0-5.0) | 0.34 |
| Vasopressor use, n (%) | 50 (82.0) | 46 (62.2) | 0.01 |
| Blood product administration, n (%) |
3 (4.9) | 9 (12.2) | 0.14 |
| ScvO2 ≥ monitored, n (%) | 31 (50.8) | 49 (66.2) | 0.37 |
| ScvO2 ≥ 70% achieved, n (%) | 22 (36.1) | 25 (33.8) | 0.97 |
|
| |||
| Ventilator Variables | |||
|
| |||
| Ventilator mode VC-AC, n (%) |
56 (91.8) | 72 (97.3) | 0.12 |
| PEEP | 5.0 (5.0-5.0) | 5.0 (5.0-5.0) | 0.85 |
| FiO2 | 100.0 (100.0-100.0) | 100.0 (100.0-100.0) | 0.18 |
| Tidal volume, mL/kg IBW | 8.6 (7.7-9.7) | 8.8 (7.7-9.6) | 0.83 |
| Lung-protective ventilation, n (%) |
18 (29.5) | 24 (33.3) | 0.63 |
| Peak pressure, cmH2O | 29.0 (23.0-36.0) | 28.0 (25.0-33.0) | 0.74 |
| Mean pressure, cmH2O | 10.0 (9.0-12.0) | 9.0 (8.0-11.0) | 0.11 |
| Plateau pressure, cmH2O | 23.0 (16.0-27.0) | 17.5 (15.0-24.0) | 0.24 |
CHF = congestive heart failure; COPD = chronic obstructive pulmonary disease; IBW = ideal body weight; BMI = body mass index; APACHE = Acute Physiology and Chronic Health Evaluation; SOFA = Sequential Organ Failure Assessment Score; CVP = central venous pressure; ScvO2 = central venous oxygen saturation; LOS = length of stay; VC = volume controlled; AC = assist control; PEEP = positive end expiratory pressure; FiO2 = fraction of inspired oxygen
Continuous variables are reported as mean (± standard deviation) and median (interquartile range) unless otherwise noted.
Table 4. Multivariate analysis for factors associated with development of acute lung injury.
| Variable | aOR | 95% CI | P |
|---|---|---|---|
| BMI | 1.09 | 1.03-1.14 | <0.001 |
| SOFA | 1.13 | 1.03-1.25 | 0.03 |
| Vasopressor use | 2.80 | 1.16-7.20 | 0.02 |
BMI = body mass index; SOFA = Sequential Organ Failure Assessment Score; aOR = adjusted odds ratio
Twenty-two patients (8.8%) had ALI at the time of ED presentation (Table 2). These patients were ventilated no differently than patients without ALI at the time of ED presentation. Median tidal volume was 9.0 mL/kg IBW (IQR 8.0 to 10.1) in ALI patients, compared to 8.7 mL/kg IBW (IQR 7.8 to 9.9) in patients without ALI (p = 0.40). There was also no difference in monitoring or titration variables.
Compared to patients who did not progress to ALI, patients progressing to ALI after admission from the ED had an increase in mechanical ventilator duration, vasopressor dependence, and hospital LOS (Table 5).
Table 5. Clinical outcomes comparing all patients that progressed to ALI vs. no ALI progression.
| Outcome | ALI (n=69) | No ALI (n=160) | P |
|---|---|---|---|
| Δ SOFA | 3.0 (−1.0 to 4.0) | 3.0 (−0.5 to 5.0) | 0.17 |
| Dialysis, n (%) | 11 (15.9) | 31 (19.5) | 0.70 |
| Mechanical ventilation duration (hours) |
132.7 (79.6 to 201.9) | 42.0 (13.5 to 112.6) | <0.001 |
| Vasopressor duration (hours) |
57.8 (23.2 to 122.3) | 19.6 (9.4 to 53.7) | <0.001 |
| Hospital LOS, hours | 182.3 (112.3 to 325.1) | 80.6 (16.7 to 186.5) | <0.001 |
| Mortality, n (%) | 35 (50.7) | 82 (51.3) | 0.78 |
ALI = acute lung injury; SOFA = Sequential Organ Failure Assessment Score; LOS = length of stay
Continuous variables are reported as median (interquartile range).
Δ: refers to the change in SOFA score from ED baseline to 24 hours
DISCUSSION
Information regarding the mechanical ventilation practices used in critically ill ED patients is vital before any consideration of therapeutic interventions can occur. The findings from this study provide some new information regarding mechanically ventilated ED patients, built on previous findings in this field by extending it into the ED environment, and offer potential insight into future areas for improvement in clinical care and research. Our current findings have several implications.
We first sought to characterize how mechanical ventilation was used in the ED. We found that tidal volume ranges were highly variable (as high as 14.6 mL/kg IBW) and that lung-protective ventilation was uncommon in the ED setting. Animal data and human studies indicate that ventilator-induced lung injury (VILI) can develop within hours, and progression to ALI occurs early in the course of respiratory failure.22,23,25,27,32,50-52 Our findings suggest that, while no evidence-based guidelines exist for ED mechanical ventilation, prolonged ED LOS may expose patients to iatrogenic injury from excessively high tidal volume. Similar findings from the operating room (OR) suggest that in areas traditionally thought of as providing a relatively time-limited amount of mechanical ventilation, excessive initial tidal volume may indeed influence clinical outcome.53 Furthermore, unlike patients in the OR who are most often extubated after surgery, critically ill ED patients will likely continue to receive mechanical ventilation after admission from the ED. This may be especially important in the setting of another finding of the current study: the ventilator is often not titrated in the ED, and terminal ED settings are commonly continued in the ICU. The influence that initial care in the ED has on clinical care and subsequent therapy has been previously documented.54-58 Our findings suggest that this “therapeutic momentum” may be true for mechanical ventilation as well. Finally, inspiratory plateau pressure is infrequently monitored in mechanically ventilated patients in the ED. Plateau pressure is a surrogate for pulmonary over-distention, and an important and easily measured index of lung compliance and predictor of VILI. It is a strong predictor of mortality early in the course of ALI, and routine monitoring of this important parameter could be important in the limitation of lung injury in mechanically ventilated patients in the ED.59
Acute lung injury is relatively common in mechanically ventilated ED patients (8.8%). The epidemiology of ALI in the ICU has been well described, yet very few data exist from patients in the ED.11,12,17,60 The success of early ED-based interventions in other high-mortality critical care syndromes (e.g. severe sepsis) suggests that early care of ALI may improve outcome.58,61 This premise is further supported by our finding that patients with established lung injury (for which management guidelines exist) are managed in a similar fashion to those without lung injury, and ALI patients are exposed to tidal volumes well above those established as safe.13 A ventilation strategy that limits tidal volume is standard care for patients with ALI, and has shown long- and short-term outcome benefit.13,16 Despite this, compliance with this intervention remains poor.16 Given the influence of ED interventions on subsequent care, low tidal volume ventilation initiated in the ED may not only improve care, but also improve compliance with low tidal volume ventilation subsequently in the ICU.
Progression to ALI is common (27.5%) and occurs early after admission (2.1 days). Previous data have shown similar findings with ALI progression rates of 6.2% to 44%, and onset of 5 hours to 3.7 days in ICU patients.22-25,27,30,32,33,49,62 These findings, combined with our ventilation data, suggest that the ED may be an optimal location for ALI prevention trials to begin.28 The development of ALI represents an intersection between patient risk factors and care delivery risk factors, such as packed cell transfusion, antibiotics, and fluid balance.22,23,32,49
In this current analysis, focusing on factors present in the ED, BMI, vasopressor use, and a higher degree of organ dysfunction were predictive of progression to ALI. The link between obesity and ALI is interesting. Obesity alters immunity and, similar to ALI, represents a pro-inflammatory state, with chronic elevation of pro-inflammatory cytokines.63 Pulmonary mechanics are also altered, with a decrease in chest wall and lung compliance.64 The net effect can be an increase in pleural pressure which, if PEEP is not set effectively, can serve to promote both alveolar collapse at end-expiration, and to increase lung distention and transpulmonary pressure (alveolar minus pleural pressure), putting the patient at risk for VILI. Using this rationale, obese patients may be a subset of patients deriving benefit from PEEP levels higher than typically used.65 Data also show that clinician-set tidal volume is higher in obese patients, despite height (not weight) being the prime determinant of lung volume.66,67 Similar associations have been demonstrated between obesity and ALI development in ICU patients.68,69 Given the increase in the obesity epidemic, our data give further insight into patients most likely to benefit from ED-based ALI prevention strategies.70 In addition, future ALI prevention trials will need to control for each of these confounding variables while assessing patient populations with similar baseline risk of progressing to ALI.68,71
The progression to ALI after ED admission was not associated with an increase in mortality, which is contradictory to previous work.22-27,33 This was driven by the higher than expected mortality in the non-ALI group. Our study did show that ALI progression was associated with an increase in mechanical ventilation, vasopressor requirements, and hospital LOS, which is consistent with previous work.22,25-27 Given the lack of available treatment options after ALI onset, our data further support the need for ALI prevention.
LIMITATIONS
The retrospective design limits ability to draw causation. However, a temporal relationship between factors present in the ED and subsequent development of ALI suggests a cause-effect relationship.72 We also attempted to improve transparency and improve reporting by adhering to guideline recommendations.35 An increasing body of evidence indicates that the guidelines do improve the overall quality of research reporting.73
With respect to the descriptive mechanical ventilation data, we went to extensive lengths to verify accuracy, yet this remains a single-center study. These findings should be reproduced in other EDs and sites to verify that similar room for improvement exists elsewhere.
Our data set was restricted to patients with sepsis. This is a high-risk group for ALI, and patients at lower risk for complications (e.g. intubation strictly for airway protection) may progress to ALI at a much lower rate, and therefore may not benefit from ALI prevention strategies.
We defined lung-protective ventilation as 8mL/kg IBW, as this was the upper level of tidal volume used in previous studies of ALI and ARDS.13 True lung-protective ventilation limits both tidal volume and end-inspiratory stretch (as reflected by plateau pressure). We did not include a pressure limit to define lung-protective, as there were no previous data in the literature to suggest how often this monitoring parameter is even measured in the ED setting. The lack of adherence to lung-protective ventilation in the ED in our study is congruent with that seen in the ICU, therefore giving some face validity to our definition.16
The majority of data suggest that lower tidal volume is associated with a decrease in progression to ALI.74 However, as only 68 patients (27.1%) were ventilated with lung-protective ventilation, our study was likely underpowered to show any relationship between tidal volume and ALI development. It should also be noted that in patients without ALI, the most appropriate tidal volume to deliver is controversial, so “lung-protective” may truly be subjective in this population until further data prove otherwise.18-21
Defining ALI retrospectively can be challenging. Given the interobserver variability in chest x-ray interpretation, we chose to use the official x-ray interpretation for the presence of bilateral infiltrates. The new Berlin definition of ARDS attempts to address this variability by stating that the chest radiograph should include “bilateral opacities consistent with pulmonary edema that are not fully explained by effusions, lobar/lung collapse, or nodules/masses.”75 Going forward we would opt for consensus review among investigators after viewing a set of training chest radiographs indicative of the potential spectrum of images that may represent ARDS.75 We opted for a conservative approach when labeling patients with ALI, and were intentionally very restrictive in our definition of ALI. The differentiation between cardiogenic/hydrostatic pulmonary edema and ALI is difficult and no criterion standard exists.76-78 In patients with heart failure and dialysis-dependent renal failure, hydrostatic pulmonary edema is a common reason for endotracheal intubation in the ED. We therefore assumed left atrial hypertension in these patients, but we also recognize that some of these patients may have had ALI as well. This is supported by data showing that myocardial depression in sepsis is common, and ALI and left atrial hypertension also frequently co-exist.79-81 This may have caused the underestimation of ALI in patients with a history of heart failure or dialysis, as well as sepsis-induced myocardial depression.23,34,82 In the future, a shift away from excluding patients with potential left atrial hypertension to excluding patients with only left atrial hypertension as a cause for respiratory failure is recommended.75 However, with the ED ALI rate and progression rate in our study congruent with previous studies of similar design, we believe our diagnosis of ALI to be accurate.
We also recognize that ALI and ARDS have undergone a recent definitional change.83 We report the definition of ALI according to the AECC criteria, as this was the definitional criteria in place during the conduct of the study.36
CONCLUSIONS
Lung-protective ventilation is uncommon in ED patients with respiratory failure, and the ventilator is infrequently titrated. Acute lung injury is a common complication early in the course of mechanical ventilation, and is associated with increasing body mass index, organ failure, and vasopressor use. Given the frequency of acute lung injury in the ED, the progression shortly after intensive care unit admission, and the negative clinical consequences of syndrome, the effect of ED-based interventions aimed at reducing the sequelae of acute lung injury should be investigated further.
Acknowledgements
The authors would like to acknowledge Karen Steger-May, MA, from the Division of Biostatistics for assistance with the statistical analysis of these data.
Footnotes
Presentation: Society for Academic Emergency Medicine annual meeting, May 2012, Chicago, Illinois.
Disclosures: Financial support for this project was provided to Brian M. Fuller, MD, as part of the Postdoctoral Mentored Training Program in Clinical Investigation. This publication was made possible by the National Center for Research Resources (NCRR) and the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through Grant Number UL1 TR000448. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors have no conflicts of interest or disclosures to declare.
Contributor Information
Brian M. Fuller, Division of Emergency Medicine, Washington University School of Medicine in St. Louis, St. Louis, MO.
Nicholas M. Mohr, Department of Emergency Medicine, Department of Anesthesiology, Division of Critical Care, Roy J. and Lucille A. Carver College of Medicine, University of Iowa, Iowa City, IA.
Matthew Dettmer, Division of Emergency Medicine, Washington University School of Medicine in St. Louis, St. Louis, MO.
Sarah Kennedy, Division of Emergency Medicine, Washington University School of Medicine in St. Louis, St. Louis, MO.
Kevin Cullison, Division of Emergency Medicine, Washington University School of Medicine in St. Louis, St. Louis, MO.
Rebecca Bavolek, Division of Emergency Medicine, Washington University School of Medicine in St. Louis, St. Louis, MO.
Nicholas Rathert, Division of Emergency Medicine, Washington University School of Medicine in St. Louis, St. Louis, MO.
Craig McCammon, Barnes-Jewish Hospital, St. Louis, MO.
REFERENCES
- 1.Sagarin MJ, Barton ED, Chng YM, Walls RM. Airway management by US and Canadian emergency medicine residents: a multicenter analysis of more than 6,000 endotracheal intubation attempts. Ann Emerg Med. 2005;46:328–36. doi: 10.1016/j.annemergmed.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Carson SS, Cox CE, Holmes GM, Howard A, Carey TS. The changing epidemiology of mechanical ventilation: a population-based study. J Inten Care Med. 2006;21:173–82. doi: 10.1177/0885066605282784. [DOI] [PubMed] [Google Scholar]
- 3.Needham DM, Bronskill SE, Calinawan JR, Sibbald WJ, Pronovost PJ, Laupacis A. Projected incidence of mechanical ventilation in Ontario to 2026: preparing for the aging baby boomers. Crit Care Med. 2005;33:574–9. doi: 10.1097/01.ccm.0000155992.21174.31. [DOI] [PubMed] [Google Scholar]
- 4.Fromm RE, Gibbs LR, McCallum WB, et al. Critical care in the emergency department: a time-based study. Crit Care Med. 1993;21:970–6. doi: 10.1097/00003246-199307000-00009. [DOI] [PubMed] [Google Scholar]
- 5.McCaig LF, Nawar EW. National hospital ambulatory medical care survey: 2004 emergency department summary. Adv Data. 2006;18(340):1–34. [PubMed] [Google Scholar]
- 6.Nelson M, Waldrop RD, Jones J, Randall Z. Critical care provided in an urban emergency department. Am J Emerg Med. 1998;16:56–9. doi: 10.1016/s0735-6757(98)90066-3. [DOI] [PubMed] [Google Scholar]
- 7.Svenson J, Besinger B, Stapczynski JS. Critical care of medical and surgical patients in the ED: length of stay and initiation of intensive care procedures. Am J Emerg Med. 1997;15:654–7. doi: 10.1016/s0735-6757(97)90181-9. [DOI] [PubMed] [Google Scholar]
- 8.Varon J, Fromm RE, Levine RL. Emergency department procedures and length of stay for critically ill medical patients. Ann Emerg Med. 1994;23:546–9. doi: 10.1016/s0196-0644(94)70075-3. [DOI] [PubMed] [Google Scholar]
- 9.Esteban A, Anzueto A, Frutos F, et al. Characteristics and outcomes in adult patients receiving mechanical ventilation. JAMA. 2002;287:345–55. doi: 10.1001/jama.287.3.345. [DOI] [PubMed] [Google Scholar]
- 10.Fuller B, Mohr NM, Dettmer M, Cullison K, Bavolek R, Rathert N. Lung protective ventilation is uncommon among ED patients (Abstract) Acad Emerg Med. 2012;19(Suppl 1):s93. [Google Scholar]
- 11.Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–93. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 12.Rubenfeld GD, Herridge MS. Epidemiology and outcomes of acute lung injury. Chest. 2007;131:554–62. doi: 10.1378/chest.06-1976. [DOI] [PubMed] [Google Scholar]
- 13.The Acute Respiratory Distress Syndrome Network Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–8. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 14.Weinert CR, Gross CR, Marinelli WA. Impact of randomized trial results on acute lung injury ventilator therapy in teaching hospitals. Am J Resp Crit Care Med. 2003;167:1304–9. doi: 10.1164/rccm.200205-478OC. [DOI] [PubMed] [Google Scholar]
- 15.Young MP, Manning HL, Wilson DL, et al. Ventilation of patients with acute lung injury and acute respiratory distress syndrome: Has new evidence changed clinical practice? Crit Care Med. 2004;32:1260–5. doi: 10.1097/01.ccm.0000127784.54727.56. [DOI] [PubMed] [Google Scholar]
- 16.Needham DM, Colantuoni E, Mendez-Tellez PA, et al. Lung protective mechanical ventilation and two year survival in patients with acute lung injury: prospective cohort study. Br Med J. 2012;344:e2124. doi: 10.1136/bmj.e2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hou P, Elie-Turenne MC, Mitani A, et al. Towards prevention of acute lung injury: frequency and outcomes of emergency department patients at-risk - a multicenter cohort study. Int J Emerg Med. 2012;5:22. doi: 10.1186/1865-1380-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gattinoni L. Counterpoint: Is low tidal volume mechanical ventilation preferred for all patients on ventilation? No. Chest. 2011;140:11–3. doi: 10.1378/chest.11-0827. [DOI] [PubMed] [Google Scholar]
- 19.Hubmayr R. Point: Is low tidal volume mechanical ventilation preferred for all patients on ventilation? Yes. Chest. 2011;140:9–11. doi: 10.1378/chest.11-0825. [DOI] [PubMed] [Google Scholar]
- 20.Mohr N, Fuller BM. Low tidal volume ventilation should be the routine ventilation strategy of choice for all emergency department patients. Ann Emerg Med. 2012;60:215–6. doi: 10.1016/j.annemergmed.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 21.Wright B, Slesinger TL. Low tidal volume should not routinely be used for emergency department patients requiring mechanical ventilation. Ann Emerg Med. 2012;60:216–7. doi: 10.1016/j.annemergmed.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kahn JM, Caldwell EC, Deem S, Newell DW, Heckbert SR, Rubenfeld GD. Acute lung injury in patients with subarachnoid hemorrhage: incidence, risk factors, and outcome. Crit Care Med. 2006;34:196–202. doi: 10.1097/01.ccm.0000194540.44020.8e. [DOI] [PubMed] [Google Scholar]
- 23.Iscimen R, Yilmaz M, Cartin-Ceba R, et al. Risk factors for the development of acute lung injury in patients with septic shock: an observational cohort study. Crit Care Med. 2008;36(5):1518–22. doi: 10.1097/CCM.0b013e31816fc2c0. [DOI] [PubMed] [Google Scholar]
- 24.Gajic O, Frutos-Vivar F, Esteban A, Hubmayr RD, Anzueto A. Ventilator settings as a risk factor for acute respiratory distress syndrome in mechanically ventilated patients. Inten Care Med. 2005;31:922–6. doi: 10.1007/s00134-005-2625-1. [DOI] [PubMed] [Google Scholar]
- 25.Gajic O, Dara SI, Mendez JL, et al. Ventilator-associated lung injury in patients without acute lung injury at the onset of mechanical ventilation. Crit Care Med. 2004;32:1817–24. doi: 10.1097/01.ccm.0000133019.52531.30. [DOI] [PubMed] [Google Scholar]
- 26.Fernández-Pérez ER, Sprung J, Afessa B, et al. Intraoperative ventilator settings and acute lung injury after elective surgery: a nested case control study. Thorax. 2009;64:121–7. doi: 10.1136/thx.2008.102228. [DOI] [PubMed] [Google Scholar]
- 27.Mascia L, Zavala E, Bosma K, et al. High tidal volume is associated with the development of acute lung injury after severe brain injury: an international observational study. Crit Care Med. 2007;35:1815–20. doi: 10.1097/01.CCM.0000275269.77467.DF. [DOI] [PubMed] [Google Scholar]
- 28.Spragg RG, Bernard GR, Checkley W, et al. Beyond mortality: future clinical research in acute lung injury. Am J Respir Crit Care Med. 2010;181:1121–7. doi: 10.1164/rccm.201001-0024WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blum JM, Stentz MJ, Park PK. Predictors of postoperative acute lung injury in a low incidence surgical population [Abstract] Anesthesiology. 2011:A790. doi: 10.1097/ALN.0b013e3182794975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Determann RM, Royakkers A, Wolthuis EK, et al. Ventilation with lower tidal volumes as compared with conventional tidal volumes for patients without acute lung injury: a preventive randomized controlled trial. Crit Care. 2010;14:R1. doi: 10.1186/cc8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hughes CG, Weavind L, Banerjee A, Mercaldo ND, Schildcrout JS, Pandharipande PP. Intraoperative risk factors for acute respiratory distress syndrome in critically ill patients. Anesth Analg. 2010;111:464–7. doi: 10.1213/ANE.0b013e3181d8a16a. [DOI] [PubMed] [Google Scholar]
- 32.Jia X, Malhotra A, Saeed M, Mark RG, Talmor D. Risk factors for ARDS in patients receiving mechanical ventilation for > 48 h. Chest. 2008;133:853–61. doi: 10.1378/chest.07-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yilmaz M, Keegan MT, Iscimen R, et al. Toward the prevention of acute lung injury: Protocol-guided limitation of large tidal volume ventilation and inappropriate transfusion. Crit Care Med. 2007;35:1660–6. doi: 10.1097/01.CCM.0000269037.66955.F0. [DOI] [PubMed] [Google Scholar]
- 34.Ferguson ND, Frutos-Vivar F, Esteban A, et al. Clinical risk conditions for acute lung injury in the intensive care unit and hospital ward: a prospective observational study. Crit Care. 2007;11:R96. doi: 10.1186/cc6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147:573–7. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 36.Bernard GR, Artigas A, Brigham KL, et al. The American-European consensus conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–24. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 37.Meade MO, Cook RJ, Guyatt GH, et al. Interobserver variation in interpreting chest radiographs for the diagnosis of acute respiratory distress syndrome. Am J Respir Crit Care Med. 2000;161:85–90. doi: 10.1164/ajrccm.161.1.9809003. [DOI] [PubMed] [Google Scholar]
- 38.Solti I, Cooke CR, Xia F, Wurfel MM. Automated classification of radiology reports for acute lung injury: comparison of keyword and machine learning based natural language processing approaches; Bioinformatics and Biomedicine Workshop, 2009 BIBMW 2009 IEEE International Conference; pp. 314–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rubenfeld GD, Caldwell E, Granton J, Hudson LD, Matthay MA. Interobserver variability in applying a radiographic definition for ARDS. Chest. 1999;116:1347–53. doi: 10.1378/chest.116.5.1347. [DOI] [PubMed] [Google Scholar]
- 40.Beards S, Jackson A, Hunt L, et al. Interobserver variation in the chest radiograph component of the lung injury score. Anaesthesia. 1995;50:928–32. doi: 10.1111/j.1365-2044.1995.tb05921.x. [DOI] [PubMed] [Google Scholar]
- 41.Wesley Ely E, Smith AC, Chiles C, et al. Radiologic determination of intravascular volume status using portable, digital chest radiography: a prospective investigation in 100 patients. Criti Care Med. 2001;29:1502–12. doi: 10.1097/00003246-200108000-00002. [DOI] [PubMed] [Google Scholar]
- 42.Ely EW, Haponik EF. Using the chest radiograph to determine intravascular volume status: the role of vascular pedicle width. Chest. 2002;121:942–50. doi: 10.1378/chest.121.3.942. [DOI] [PubMed] [Google Scholar]
- 43.Rice TW, Wheeler AP, Bernard GR, Hayden DL, Schoenfeld DA, Ware LB. Comparison of the Spo2/Fio2 Ratio and the Pao2/Fio2 Ratio in Patients With Acute Lung Injury or ARDS. Chest. 2007;132:410–7. doi: 10.1378/chest.07-0617. [DOI] [PubMed] [Google Scholar]
- 44.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250–6. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 45.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–55. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 46.Vincent JL, de Mendonca A, Cantraine F, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Crit Care Med. 1998;26:1793–800. doi: 10.1097/00003246-199811000-00016. [DOI] [PubMed] [Google Scholar]
- 47.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22:707–10. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 48.Vincent JL, Angus DC, Artigas A, et al. Effects of drotrecogin alfa (activated) on organ dysfunction in the PROWESS trial. Crit Care Med. 2003;31:834–903. doi: 10.1097/01.CCM.0000051515.56179.E1. [DOI] [PubMed] [Google Scholar]
- 49.Plurad D, Martin M, Green D, et al. The decreasing incidence of late posttraumatic acute respiratory distress syndrome: the potential role of lung protective ventilation and conservative transfusion practice. J Trauma. 2007;63:1–7. doi: 10.1097/TA.0b013e318068b1ed. [DOI] [PubMed] [Google Scholar]
- 50.Muscedere J, Mullen J, Gan K, Slutsky A. Tidal ventilation at low airway pressures can augment lung injury. Am J Respir Crit Care Med. 1994;149:1327–34. doi: 10.1164/ajrccm.149.5.8173774. [DOI] [PubMed] [Google Scholar]
- 51.Tremblay L, Valenza F, Ribeiro SP, Li J, Slutsky AS. Injurious ventilatory strategies increase cytokines and c-fos m-RNA expression in an isolated rat lung model. J Clin Invest. 1997;99:944–52. doi: 10.1172/JCI119259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pasero D, Davi A, Guerriero F, et al. High tidal volume as an independent risk factor for acute lung injury after cardiac surgery [Abstract] Intensive Care Med. 2008;34(Supplement 1):398. [Google Scholar]
- 53.Lellouche F, Dionne S, Simard S, Bussières J, Dagenais F. High tidal volumes in mechanically ventilated patients increase organ dysfunction after cardiac surgery. Anesthesiology. 2012;116:1072–82. doi: 10.1097/ALN.0b013e3182522df5. [DOI] [PubMed] [Google Scholar]
- 54.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–7. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 55.Kumar A, Roberts D, Wood K, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589–96. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 56.Wood K. Major pulmonary embolism: review of a pathophysiologic approach to the golden hour of hemodynamically significant pulmonary embolism. Chest. 2002;121:877–905. doi: 10.1378/chest.121.3.877. [DOI] [PubMed] [Google Scholar]
- 57.Fuller B, Mohr NM, Mueller K, Skrupky L, McCammon C. Emergency department vancomycin use: dosing practices and associated outcomes. J Emerg Med. 2012 doi: 10.1016/j.jemermed.2012.09.036. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. New Engl J Med. 2001;345:1368–77. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 59.Checkley W, Brower R, Korpak A, Thompson BT. Effects of a clinical trial on mechanical ventilation practices in patients with acute lung injury. Am J Respir Crit Care Med. 2008;177:1215–22. doi: 10.1164/rccm.200709-1424OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goyal M, Houseman D, Johnson NJ, Christie J, Mikkelsen ME, Gaieski DF. Prevalence of acute lung injury among medical patients in the emergency department. Acad Emerg Med. 2012;19:E1011–8. doi: 10.1111/j.1553-2712.2012.01429.x. [DOI] [PubMed] [Google Scholar]
- 61.Wang HE, Shapiro NI, Angus DC, Yealy DM. National estimates of severe sepsis in United States emergency departments. Crit Care Med. 2007;35:1928–36. doi: 10.1097/01.CCM.0000277043.85378.C1. [DOI] [PubMed] [Google Scholar]
- 62.Determann RM, Royakkers A, Wolthuis EK, et al. Ventilation with lower tidal volumes as compared with conventional tidal volumes for patients without acute lung injury: a preventive randomized controlled trial. Crit Care. 2010;14:R1. doi: 10.1186/cc8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tilg H, Moschen A. Role of adiponectin and PBEF/visfatin as regulators of inflammation: involvement in obesity-associated diseases. Clin Sci. 2008;114:275–88. doi: 10.1042/CS20070196. [DOI] [PubMed] [Google Scholar]
- 64.McCallister JW, Adkins EJ, O’Brien JM., Jr Obesity and acute lung injury. Clin Chest Med. 2009;30:495–508. doi: 10.1016/j.ccm.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Talmor D, Sarge T, Malhotra A, et al. Mechanical ventilation guided by esophageal pressure in acute lung injury. New Engl J Med. 2008;359:2095–104. doi: 10.1056/NEJMoa0708638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.O’Brien JM, Jr, Welsh CH, Fish RH, Ancukiewicz M, Kramer AM. Excess body weight is not independently associated with outcome in mechanically ventilated patients with acute lung injury. Ann Intern Med. 2004;140:338–45. doi: 10.7326/0003-4819-140-5-200403020-00009. [DOI] [PubMed] [Google Scholar]
- 67.Morris AE, Stapleton RD, Rubenfeld GD, Hudson LD, Caldwell E, Steinberg KP. The association between body mass index and clinical outcomes in acute lung injury. Chest. 2007;131:342–8. doi: 10.1378/chest.06-1709. [DOI] [PubMed] [Google Scholar]
- 68.Gajic O, Dabbagh O, Park PK, et al. Early identification of patients at risk of acute lung injury: evaluation of lung injury prediction score in a multicenter cohort study. Am J Respir Crit Care Med. 2011;183:462–70. doi: 10.1164/rccm.201004-0549OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gong MN, Bajwa EK, Thompson BT, Christiani DC. Body mass index is associated with the development of acute respiratory distress syndrome. Thorax. 2010;65:44–50. doi: 10.1136/thx.2009.117572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Adams KF, Schatzkin A, Harris TB, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. New Engl J Med. 2006;355:763–78. doi: 10.1056/NEJMoa055643. [DOI] [PubMed] [Google Scholar]
- 71.Elie-Turenne MC, Hou PC, et al. Towards prevention of acute lung injury: identification of emergency department patients at risk. Int J Emerg Med. 2012;5:33. doi: 10.1186/1865-1380-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Newman T, Browner WS, Hulley SB. Enhancing Causal Inference in Observational Studies. In: Hully SB, Cummings SR, Browner WS, editors. Designing Clinical Research. Lippincott Williams & Wilkins; Philadelphia, PA: 2007. pp. 127–46. [Google Scholar]
- 73.Plint A, Moher D, Morrison A, et al. Does the CONSORT checklist improve the quality of reports of randomised controlled trials? A systematic review. Med J of Austral. 2006;185:263–7. doi: 10.5694/j.1326-5377.2006.tb00557.x. [DOI] [PubMed] [Google Scholar]
- 74.Fuller BM, Mohr NM, Drewry AM, Carpenter CR. Lower tidal volume at initiation of mechanical ventilation may reduce progression to acute respiratory distress syndrome-a systematic review. Crit Care. 2013;17:R11. doi: 10.1186/cc11936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ferguson ND, Fan E, Camporota L, et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Inten Care Med. 2012:1–10. doi: 10.1007/s00134-012-2682-1. [DOI] [PubMed] [Google Scholar]
- 76.Schmickl CN, Shahjehan K, Li G, et al. Decision support tool for early differential diagnosis of acute lung injury and cardiogenic pulmonary edema in medical critically ill patients: acute lung injury vs cardiogenic pulmonary edema. Chest. 2012;141:43–50. doi: 10.1378/chest.11-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ware LB, Matthay MA. Acute pulmonary edema. New Engl J Med. 2005;353:2788–96. doi: 10.1056/NEJMcp052699. [DOI] [PubMed] [Google Scholar]
- 78.Fein A, Goldberg S, Walkenstein M, Dershaw B, Braitman L, Lippmann M. Is pulmonary artery catheterization necessary for the diagnosis of pulmonary edema? Am Rev Respir Dis. 1984;129:1006. doi: 10.1164/arrd.1984.129.6.1006. [DOI] [PubMed] [Google Scholar]
- 79.Parker MM, Shelhamer JH, Bacharach SL, et al. Profound but reversible myocardial depression in patients with septic shock. Ann Intern Med. 1984;100:483–90. doi: 10.7326/0003-4819-100-4-483. [DOI] [PubMed] [Google Scholar]
- 80.Vieillard-Baron A, Caille V, Charron C, Belliard G, Page B, Jardin F. Actual incidence of global left ventricular hypokinesia in adult septic shock. Crit Care Med. 2008;36:1701–6. doi: 10.1097/CCM.0b013e318174db05. [DOI] [PubMed] [Google Scholar]
- 81.Ferguson ND, Meade MO, Hallett DC, Stewart TE. High values of the pulmonary artery wedge pressure in patients with acute lung injury and acute respiratory distress syndrome. Inten Care Med. 2002;28:1073–7. doi: 10.1007/s00134-002-1354-y. [DOI] [PubMed] [Google Scholar]
- 82.Hudson LD, Milberg JA, Anardi D, Maunder RJ. Clinical risks for development of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1995;151:293–301. doi: 10.1164/ajrccm.151.2.7842182. [DOI] [PubMed] [Google Scholar]
- 83.The ARDS Definition Task Force Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–33. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]