Abstract
The discovery of disorders that are associated with antibodies to neuronal cell-surface proteins has led to a paradigm shift in our understanding of CNS autoimmunity. These disorders can occur in patients with or without cancer—often children or young adults who develop psychosis, catatonic or autistic features, memory problems, abnormal movements, or seizures that were previously considered idiopathic. The autoantigens in such cases have crucial roles in synaptic transmission, plasticity and peripheral nerve excitability. Patients can be comatose or encephalopathic for months and yet fully recover with supportive care and immunotherapy. By contrast, disorders in which the antibodies target intracellular antigens, and in which T-cell-mediated irreversible neuronal degeneration occurs, show a considerably poorer response to treatment. In this article, we review the various targets of neuronal antibodies, focusing predominantly on autoantigens located on the cell surface or synapses—namely, N-methyl-d-aspartate receptors, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors, γ-aminobutyric acid receptors, leucine-rich glioma-inactivated protein 1, contactin-associated protein-like 2, and metabotropic glutamate receptors. We also provide an algorithm to identify and assess antibodies that bind to cell-surface and synaptic antigens.
Introduction
Antibodies that target neuronal epitopes were first recognized in patients with paraneoplastic neuronopathy, cerebellar degeneration, or encephalitis. In these disorders, the target antigens are nuclear or cytoplasmic proteins, such as Hu, Yo and Ma2, to which antibodies have limited accessibility. Accordingly, many of these antibodies are not directly pathogenic, but instead indicate a T-cell-mediated immune response against the corresponding neuronal antigens (Figure 1). A second group of antibody-related brain disorders involves antibodies that target intracellular synaptic proteins, such as 65 kDa glutamic acid decarboxylase (GAD65) and amphiphysin. These antigens might be vulnerable to antibody-mediated disruption during synaptic vesicle fusion and reuptake, but whether T-cell-mediated pathogenic mechanisms are more important than antibody-mediated mechanisms remains a topic of debate (Figure 1).
Figure 1.
Autoantigens and mechanisms of neuronal dysfunction. a | Antibodies to intracellular antigens, such as HuD, might not be pathogenic but could instead indicate a T-cell-mediated response against neurons. b | Intracellular synaptic antigens, such as GAD65, may be targeted by both antibodies and T-cell-mediated mechanisms. Which of these mechanisms is more important remains controversial. c | Cell-surface receptors are functionally disrupted by antibodies. Antibodies against NMDAR and AMPAR have been shown to cause receptor cross-linking and internalization. Abbreviations: AMPAR, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; GAD65, 65 kDa glutamic acid decarboxylase; NMDAR, N-methyl-d-aspartate receptor.
More recently, a third group of brain disorders was identified in which the antibodies target cell-surface or synaptic proteins and are associated with encephalitis (Figure 1). The antigens include the N-methyl-d-aspartate receptor (NMDAR);1 the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR);2 the γ-aminobutyric acid receptor-B (GABAB receptor);3 two protein targets that were previously thought to be voltage-gated potassium channels (VGKCs) but are now known to be leucine-rich glioma-inactivated protein 1 (LGI1) and contactin-associated protein-like 2 (Caspr2);4–6 the glycine receptor (GlyR);7 and the metabotropic glutamate receptor mGluR5.8
The encephalitides associated with antibodies against cell-surface antigens differ from those related to intracellular antigens in several important respects. First, the cell-surface target antigens are disrupted by the antibodies. Second, the association with malignancy is much less consistent. Third, symptoms can more commonly be reversed with treatment. Last, the symptoms relate to the disruption of the target antigen. Exceptions to these rules are the disorders associated with cerebellar degeneration and antibodies against cell-surface antigens, including mGluR1 and voltage-gated calcium channels (VGCCs). The cerebellar degeneration that results from immune responses to these antigens is poorly responsive to immunotherapy, and the presence of VGCC antibodies almost always indicates an underlying small-cell lung cancer (SCLC). Antibodies to VGCC also occur in Lambert–Eaton myasthenic syndrome, which is responsive to immunotherapy and may occur with or without cancer.
This Review describes the targets of neuronal autoantibodies, focusing particularly on cell-surface and synaptic autoantigens, and provides an algorithm for interpretation of results of antibody tests.
Nuclear and cytoplasmic antigens
The strong association of some neuron-specific antibodies with cancer, and the expression of the target antigens by neurons and cancer cells, led to these antibodies being collectively termed `paraneoplastic' or `onconeuronal' antibodies. The main neurological syndromes, the associated cancers, and the functions of the target antigens of these antibodies are shown in Table 1.
Table 1.
Neuronal nonsynaptic autoantibody targets and associated syndromes
| Antigen | Antigen function | Tumour association | Syndromes | Mechanisms | Prognosis |
|---|---|---|---|---|---|
| Hu proteins (primarily HuD, but also HuC, Hel-N1 and Hel-N2)112 | HuD is important for neuronal RNA handling, cell-cycle regulation and cell development113,114 | Small-cell lung cancer115 | Neuropathy (often purely sensory), cerebellitis, limbic encephalitis, autonomic dysfunction and/or brainstem encephalitis | Antibodies are not directly pathogenic; possibly T-cell-mediated | 20% survival at 3 years (encephalitis is slightly more likely to cause death than is cancer) |
| Collapsin response mediator protein 5 | Regulation of neurite outgrowth, and neurogenesis116 | Small-cell lung cancer and thymoma117 | Neuropathy, uveoretinal symptoms, ataxia or limbic encephalitis117 | Possibly T-cell-mediated22 | Longer survival than with anti-Hu syndromes (48 versus 11 months)117 |
| Ma1118 | Promotion of apoptosis | Diverse (lung, skin, gastrointestinal and renal) | Limbic encephalitis, cerebellitis, brainstem encephalitis or polyneuropathy | Probably T-cell-mediated rather than antibody-mediated21 | In a series of 13 patients, nine deteriorated, three stabilized and one improved119 |
| Ma2 (also known as Ta)118 | Not known | Germ cell tumours (especially in young men) | Limbic encephalitis, brainstem encephalitis, polyneuropathy or cerebellitis119 | Not known | In a case series 33% improved, 21% stabilized and 46% deteriorated119 |
| Yo proteins (also known CDR1 and CDR2) | CDR1 is strongly expressed in Purkinje cells; function unknown120 CDR2 may be involved in cell cycle regulation, mitosis, and transcriptional regulation121 | Specific to women Almost all are eventually diagnosed with breast or gynaecological cancer122 | Paraneoplastic cerebellar degeneration | Conflicting data in patients regarding a role for T cells27,28 Antibodies trigger neuronal cell death in slice culture29 | Tumours may respond, but neurological symptoms are often unresponsive122 |
| Ri proteins (also known as Nova-1 and Nova-2) | Nova-1 is an RNA-binding protein expressed by subcortical neurons Function of Nova-2 is not known | Breast cancer | Nova-1: cerebellar degeneration, encephalitis, myelitis, opsoclonus myoclonus123,124 Nova-2: paraneoplastic opsoclonus myoclonus ataxia,125 myoclonus, encephalitis, cerebellar degeneration and myelitis | Antibodies may prevent binding of Nova-1 to RNA126 Unclear whether antibodies are pathogenic; comorbid antibodies are common and can occur in asymptomatic cancer patients127 | Three of six patients improved; median survival >69 months in one series123 |
| Tr | Found in Purkinje neurons;128 function not known | Hodgkin lymphoma | Paraneoplastic cerebellar degeneration129 | Not known | Relatively good: median survival >113 months123 |
| Zinc finger protein ZIC 4 | Important for brain development | Small-cell lung cancer | Paraneoplastic cerebellar degeneration130 | Antibodies may not be pathogenic; 80% of patients have other antibodies as well | Not known |
| Gephyrin and GABARAP | Associated with GABAergic transmission | Gephyrin: mediastinal carcinoma GABARAP: not known | Stiff-person syndrome131,132 | Not known | Not known |
Abbreviations: GABA, γ-aminobutyric acid; GABARAP, GABA receptor-associated protein.
In these disorders, the antibodies are found in the patient's CNS, but are not thought to be directly pathogenic. For example, studies of patients with Hu antibodies showed IgG bound to the nuclei of neurons, and the antibodies were enriched in the cerebrospinal fluid (CSF) owing to intrathecal antibody synthesis.9 Injection of Hu antibodies into experimental animals, however, did not cause symptoms.10 Furthermore, immunization of mice with Hu resulted in the presence of Hu antibodies in serum and antitumour activity, but no neurological consequences.11,12 Interestingly, although 20% of patients with SCLC have serum Hu antibodies, less than 0.01% of patients with SCLC develop paraneoplastic neurological syndromes.13
T-cell-mediated mechanisms might be responsible for disorders associated with antibodies to nuclear or cytoplasmic antigens. Indeed, T-cell responses to HuD have been demonstrated in patients with paraneoplastic encephalomyelitis.14 Brain or peripheral nerve tissues from these patients show more infiltration by T lymphocytes than by B lymphocytes,9,15 and T cells are found in close contact with neurons that express MHC class I molecules.16 Immunization of mice with peptides derived from HuD also gives rise to cytotoxic T lymphocytes that target Hu, although these animals do not develop neurological symptoms.17
Expression of Hu in SCLC is thought to trigger the Hu-directed autoimmune response—a hypothesis that is supported by the presence of Hu antibodies in transgenic mice that are predisposed to develop SCLC.18 Normal mice, but not HuD-null mice, have profound tolerance for Hu, and this type of tolerance probably protects most patients with SCLC from autoimmune symptoms.19 A substantial proportion of HuD-reactive CD8+ lymphocytes from patients are type 2, which are less cytotoxic than type 1 CD8+ lymphocytes. This finding might account for the lack of cytotoxic neuronal activity in animal models of, and in most patients with, SCLC.20 The mechanism through which this tolerance has been overcome in patients who present with autoimmune symptoms is unknown.
T-cell-mediated mechanisms that target other intracellular antigens have also been proposed.21 A postmortem study of patients with antibodies against collapsin response mediator protein 5 found evidence of T-cell-mediated pathology,22 and postmortem studies of patients with antibodies against Yo (also known as CDR2) showed T-cell infiltration of the cerebellum with extensive loss of Purkinje cells, but not deposits of IgG or complement, or B-cell infiltrates.23–25 Two reports described cytotoxic T-cell responses to Yo in patients with Yo antibodies,26,27 although these findings could not be replicated in a subsequent study.28 In one study, Yo antibodies were taken up by cerebellar neurons in slice cultures and triggered cell death,29 whereas in other studies, Yo antibodies injected intraventricularly30 or intraperitoneally in combination with blood–brain barrier disruption31 were taken up by Purkinje cells but did not cause neuronal death.
In summary, antibodies to intracellular cytoplasmic and nuclear antigens do not seem to be directly pathogenic but, rather, are markers of T-cell responses that target neurons. Response to therapy tends to be poor in neurological disorders associated with these autoantibodies, perhaps owing to irreversible neuronal death.
Intracellular synaptic antigens
GAD65 and amphiphysin are intracellular synaptic antigens (Table 2). GAD65 is the form of glutamatic acid decarboxylase that is concentrated in presynaptic terminals,32 and amphiphysins are proteins of the BAR superfamily—molecules that are crucial for clathrin-mediated endocytosis, which is required for recycling of synaptic vesicles.33 Unlike the antigens discussed in the previous section, the epitopes of GAD65 and amphiphysin can be exposed to antibodies during synaptic vesicle fusion and reuptake (Figure 1).
Table 2.
Synaptic autoantibody targets and associated syndromes
| Antigen | Antigen function | Tumour association | Syndromes | Mechanisms | Prognosis |
|---|---|---|---|---|---|
| Intracellular antigens | |||||
| 65 kDa glutamic acid decarboxylase | Crucial for synthesis of GABA | Usually none; four of 61 had neuroendocrine tumours in one series35 | Most commonly stiff-person syndrome or cerebellitis Observed in many other syndromes | Evidence for both T-cell-mediated and antibody-mediated mechanisms Pathogenic role in other syndromes is not clear | Not known |
| Amphiphysin | Important for recycling of synaptic vesicles | Breast cancer (90%) | Stiff-person syndrome | Antibodies are directly pathogenic | Median time to death 33 months in one series38 |
| Extracellular antigens | |||||
| NMDAR | Crucial for learning and memory | Ovarian teratoma (frequency varies according to patient's age; rare in children) | Characteristic clinical syndrome | Antibodies disrupt NMDAR function by cross-linking and internalization of receptors | 75% of patients have good outcomes |
| Leucine-rich glioma-inactivated protein 1 | Secreted protein that regulates presynaptic Kv1 channels and postsynaptic AMPARs | Usually none | Limbic encephalitis, often with seizures, hyponatraemia, and/or myoclonus | Unknown | Approximately 80% of patients have good outcomes |
| Contactin-associated protein-like 2 | Organizes Kv1 channels on myelinated axons | Thymoma | Encephalitis and/or peripheral nerve hyperexcitability | Unknown | Approximately 80% of patients have good outcomes |
| GABAB receptor | Mediates inhibitory synaptic transmission | Small-cell lung cancer | Encephalitis with prominent and severe seizures | Unknown | Nine of 10 treated patients showed some improvement |
| AMPAR | Crucial for learning and memory | Lung, breast and thymic cancers | Limbic encephalitis | Antibodies cause cross-linking and internalization of AMPARs | Nine of 10 treated patients showed some improvement |
| P/Q-type voltage-gated calcium channels | Crucial for calcium influx into presynaptic terminals | Small-cell lung cancer | Lambert-Eaton myasthenic syndrome and/or cerebellitis | Antibodies block channels at presynaptic terminal of neuromuscular junction; possibly also pathogenic in CNS | Not known for CNS syndrome |
| Metabotropic glutamate receptor 1 | Crucial for cerebellar function | Hodgkin lymphoma | Cerebellitis | Unknown | Mixed response to treatment |
| Metabotropic glutamate receptor 5 | Crucial for hippocampal function | Hodgkin lymphoma | Ophelia syndrome | Unknown | Patients usually respond to treatment |
Abbreviations: AMPAR, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; GABA, γ-aminobutyric acid; NMDAR, N-methyl-d-aspartate receptor.
GAD65 antibodies are associated with stiff-person syndrome (SPS) and cerebellar ataxia, but have also been reported in other syndromes such as limbic encephalitis and epilepsy.34–36 Part of this diversity could be due to the common association of GAD65-targeted antibodies with other autoantibodies.2–4 Amphiphysin antibodies are also found in association with SPS, particularly in patients with breast cancer.37,38
Evidence exists that both cell-mediated and antibody-mediated mechanisms underlie the pathogenic effects of GAD65 antibodies. GAD65 antibodies from most patients with SPS decrease the synthesis of GABA in tissue extracts, but whether this response occurs in vivo is unclear.39 GAD65 antibodies from some patients with SPS have also been reported to increase the excitability of spinal cord neurons and cause abnormal spontaneous motor neuron discharges—findings that are of potential relevance to SPS.40 A notable study in mice, however, suggests that T cells are the primary pathogenic drivers in disorders associated with GAD65 antibodies. For this study, the researchers generated mice with a T-cell response to GAD65, and then isolated GAD65-specific T cells and transferred these T-cell clones to GAD65-naive mice.41 The immunized mice developed encephalomyelitis, and transfer of clones of GAD65-specific T cells to naive mice caused similar neurological symptoms.42 Interestingly, transfer of the T cells to mice lacking B cells produced similar symptoms, the only difference being the development of GAD65 antibodies in mice with B cells; these antibodies did not alter the course of the disease.
Amphiphysin antibodies might be pathogenic. Passive transfer to mice of either IgG from two patients with these antibodies or affinity-purified amphiphysin antibodies resulted in stiffness and muscle spasms in the animals.43,44 The antibodies alter GABAergic neurotransmission, possibly by reducing the surface expression of sodium–potassium–chloride cotransporter 1.45 The antibodies are internalized in presynaptic terminals of spinal inhibitory neurons,44 and disrupt inhibitory synaptic transmission in the spinal cord and the recycling of inhibitory synaptic vesicles. Conversely, postmortem study of a patient with a syndrome associated with amphiphysin antibodies showed a CD8-predominant T-cell infiltrate in the brain, spinal cord and dorsal root ganglia.38
Overall, therefore, intracellular synaptic antigens are important for the function of inhibitory synapses. Although disruption of these proteins by antibodies might be responsible for some of the symptoms seen in patients, T-cell-mediated mechanisms also seem to be involved.
Cell-surface and synaptic antigens
Antibodies against cell-surface and synaptic antigens are being identified with increasing frequency (Table 2). The associated syndromes often mimic genetic or pharmacological disruption of the target antigens. Studying the functions of these important proteins will, therefore, prove helpful in improving our understanding of patients' symptoms and the functions of these proteins in humans.
NMDA receptors
Since its discovery in 2007,1 the disorder associated with NMDAR antibodies has been reported in over 500 patients, including children,46 women with or without teratoma, and men.47 In the California Encephalitis Project, which focuses on diagnosis of encephalitis of unclear aetiology, the incidence of encephalitis associated with NMDAR antibodies currently surpasses that of encephalitis with viral aetiology identified in young patients.48
NMDAR antibodies are associated with a characteristic syndrome that frequently includes prodromal symptoms resembling a viral illness, followed in a few days or weeks by prominent psychiatric symptoms, catatonia, agitation, seizures, decreased level of consciousness, abnormal movements, and autonomic instability.49 This acute stage is followed by a prolonged phase of recovery, during which executive functions are altered and psychiatric symptoms often resurface. About 75% of patients ultimately recover, usually slowly, over a period of months.47 These symptoms resemble those associated with NMDAR antagonists, such as phencyclidine.47
The only tumour that is strongly associated with anti-NMDAR encephalitis is ovarian teratoma.50 Prompt removal of this tumour, together with immunotherapy, is associated with more-rapid improvement of the encephalitis.47
The NMDAR is crucial for synaptic plasticity, learning, and memory. NMDAR antibodies produce effects similar to genetic disruption of the NMDAR. Mice with partial genetic disruption of the NR1 subunit of the NMDAR—the subunit that is the primary target of the antibodies—show impaired learning and stereotyped behavioural abnormalities that are suggestive of schizophrenia, and mice with profound disruption of NR1 die early of respiratory failure.51
Several lines of evidence support a pathogenic role for NMDAR antibodies in patients. First, these antibodies decrease the levels of synaptic NMDAR and disrupt NMDAR-dependent synaptic currents in cultured neurons.49,52 These effects are reversible and depend on antibody-mediated capping and internalization of the receptor, as Fab fragments do not have these effects.52 Second, patients with NMDAR antibodies have a considerably better prognosis than those with, for example, Hu antibodies, and most patients recover.47 Patients with NMDAR antibodies may even recover after many months of coma,53 consistent with functional disruption of synaptic transmission rather than irreversible T-cell-mediated neuronal damage. Third, pathology studies have shown antibody deposition in patients' brains, but without complement or substantial neuronal death.50 Last, infusion of antibodies into rodent brains results in decreased levels of synaptic NMDARs, increased glutamate release, impaired glutamate regulation, and increased corticomotor excitability.54,55
Proteins related to the VGKC Shaker family
Antibodies that bind the VGKC complex have been detected in patients with peripheral nerve hyperexcitability, encephalitis, Morvan syndrome, or various other disorders.56,57 Initial reports suggested that patients' antibodies bind to the VGKCs Kv1.1 and Kv1.2.56,58 Recent studies, however, have shown that LGI1 and Caspr2 are the main autoantigens.4–6 A third autoantigen that has been implicated in a minority of patients is contactin-2,6 a cell adhesion protein that is expressed on neuronal axons and myelinating cells. However, antibodies against contactin-2 usually occur in association with those targeting LGI1 or Caspr2, and have been identified in other disorders,59 raising doubts about the importance of these antibodies.
LGI1 is a secreted synaptic protein that binds to presynaptic ADAM23 (disintegrin and metalloproteinase domain-containing protein 23) and postsynaptic ADAM22, and associates with and regulates Kv1.1 and Kv1.2, as well as AMPARs.60 Human LGI1 mutations result in autosomal dominant lateral temporal lobe epilepsy.61 Caspr2 is a transmembrane axonal protein of the neurexin IV superfamily that is localized to the juxtaparanode of myelinated axons. Its extracellular domain interacts with contactin-2,62 and it connects with the cytoskeleton via protein 4.1B (Figure 2). Caspr2, contactin-2 and protein 4.1B are all necessary to concentrate Kv1.1 and Kv1.2 channels in the juxtaparanode.63 This localization is important for the proper electrical functioning of axons,64 although the mechanism by which Caspr2 concentrates Kv1.1 and Kv1.2 channels is unknown.
Figure 2.
Caspr2 interaction with juxtaparanodal proteins. Caspr2 is an axonal protein that binds contactin-2 in cis and trans orientations to organize the juxtaparanodal region. Caspr2 links to PDZ-binding proteins, and to the cytoskeleton via protein 4.1B. Caspr2 organizes Kv1 potassium channels in the juxtaparanodal region, although the underlying mechanism remains to be determined. Abbreviation: Caspr2, contactin-associated protein-like 2.
The discovery that proteins associated with Kv1.1 and Kv1.2, rather than the VGKCs themselves, are the autoantibody targets explains some of the diversity of symptoms among patients with these antibodies. For example, LGI1 is primarily a CNS protein, and LGI1 antibodies are associated with a CNS disorder.
LGI1
LGI1 antibodies are associated with limbic encephalitis, several types of seizures, and frequent hyponatraemia.4,6 The disorder is rarely paraneoplastic, and the response to treatment is often good. Some patients develop myoclonic movements which, in the setting of rapidly progressive encephalitis, can resemble Creutzfeldt–Jakob disease.4,65 The identification of LGI1 as a major target of so-called VGKC antibodies has clarified several aspects of the associated disorder. For example, the myoclonic movements, which were previously under-recognized, have recently been characterized as faciobrachial dystonic seizures66 or, as we believe is more accurate, tonic seizures,67 owing to the fact that these movements can also affect the lower extremities and are preceded by electrodecremental EEG events. Interestingly, Lgi1-null mice show several types of seizures, including tonic seizures, that result in a lethal phenotype.68
LGI1 antibodies probably cause reversible CNS synaptic dysfunction by several mechanisms. The antibodies may prevent binding of LGI1 to the receptors that it regulates, or they might act on the LGI1–ADAM protein complex. Alternatively, LGI1 antibodies could disrupt currents mediated by Kv1.1 and Kv1.2, and/or impair AMPAR function, either indirectly by blocking LGI1-mediated regulation of these proteins or directly by disrupting the entire protein complex. A study involving application of serum from a patient with LGI1 antibodies to a hippocampal slice preparation showed effects similar to application of a Kv1.1 and Kv1.2 antagonist, a finding that requires replication.69
Caspr2
Caspr2 antibodies are associated with autoimmune encephalitis, peripheral nerve hyperexcitability, and Morvan syndrome.4–6,70 PNS manifestations can either precede or follow those in the CNS, in some cases by several years. Some patients with these antibodies have thymoma, but most do not have tumours. Response to immunotherapy is relatively good in most patients.5 In some cases, symptoms overlapping those of myasthenia gravis develop, which can result in a complex syndrome with a superficial resemblance to motor neuron disease.5
Mutations in the human gene encoding Caspr2 (CNTNAP2) are associated with autism, epilepsy, Tourette syndrome, cortical dysplasia, obsessive–compulsive disorder, Pitt–Hopkins syndrome, and other mental disabilities.71–73 Mice with Caspr2 deletion show analogous behavioural defects and symptoms.74 Interestingly, common variants of the CNTNAP2 gene in healthy individuals are associated with abnormal language processing and are a risk factor for autism.75
Caspr2 antibodies probably act by disrupting axonal potassium currents, although why the occurrence of PNS and CNS symptoms varies so widely among patients is unknown. Factors such as differences in time to establishment of intrathecal antibody synthesis, or in the structure of tight, septate-like junctions of myelinating cells around the axons—structures that could limit the ability of antibodies to reach Caspr2—may explain this variability.
AMPA receptors
AMPAR antibodies are associated with limbic encephalitis and psychiatric symptoms, particularly in patients with tumours of the lung, breast or thymus.2,76,77 AMPARs are ionotropic glutamate receptors that are important for excitatory neurotransmission. Structurally, they are heterotetramers comprising various combinations of four subunits (GluR1–4).78 Channels containing GluR1 and GluR2 are strongly regulated by activity, and are crucial for various forms of long-term synaptic plasticity.78
Antibodies target the extracellular domains of GluR1 and GluR2 subunits of the receptor, and inhibit receptor function through mechanisms similar to those of NMDAR antibodies, causing a reversible decrease in synaptic AMPARs and associated synaptic currents.2 In a case series, most (nine of 10) patients responded to treatment, but relapses were frequent.2
GABAB receptors
Patients with GABAB receptor antibodies present with limbic encephalitis that is characterized by a tendency towards severe seizures or status epilepticus.3 Approximately half of the patients identified to date have SCLC cells (which express GABAB receptors), and antibodies targeting GABAB receptors could be the most common autoantibody in patients with lung cancer and autoimmune encephalitis.79 The GABAB receptor is a G protein-coupled receptor that is expressed both presynaptically and postsynaptically. Each receptor comprises a B1 subunit, which is important for agonist binding, and a B2 subunit, which is required for effecting intracellular signalling.80 The antibodies bind to the extracellular domain of the B1 subunit. Genetic or pharmacological disruption of the GABAB receptor results in seizures and cognitive impairment.80
GABAB receptor antibodies probably act by disrupting receptor signalling, although they do not seem to mediate antibody-mediated capping and internalization, as has been demonstrated for antibodies against NMDARs and AMPARs (E. Lancaster et al., unpublished work). These differences may relate to the presynaptic location of many GABAB receptors.
Voltage-gated calcium channels
VGCC antibodies are strongly associated with Lambert–Eaton myasthenic syndrome, a neuromuscular disorder that typically causes proximal weakness and autonomic symptoms. These symptoms are attributed to autoimmune disruption of neuromuscular transmission by antibodies to P/Q-type VGCCs.81 Patients with VGCC antibodies may develop a cerebellar syndrome with or without the neuromuscular junction disorder.82 VGCC antibodies can also occur in patients with other paraneoplastic neurological disorders or in patients with cancer who do not have a neurological syndrome.83 A recent study demonstrated VGCC antibodies in eight of 67 patients with sporadic, previously unexplained cerebellar degeneration.84 VGCC antibodies are pathogenic at the neuromuscular junction and might also affect cerebellar neurotransmission.85–87 Autopsy studies of patients with VGCC antibody-associated cerebellar degeneration showed depletion of cerebellar P/Q-type VGCCs and antibody binding to the remaining channels, supporting a pathogenic role for VGCC antibodies in the CNS.88
Glycine receptors
Antibodies against the α1 subunit of GlyR have been reported in a few patients with the syndrome progressive encephalomyelitis with rigidity and myoclonus,7 and in patients with symptoms of rigidity, exaggerated startle responses, diplopia, ataxia and myoclonus.89,90 Some of these patients responded to immunotherapy. No tumours have been associated with these antibodies. We have observed antibodies to GlyR subunits, including but not limited to α1, in patients with SPS (E. Lancaster et al., unpublished work).
GlyR is an ionotropic receptor expressed in the brainstem and spinal cord. Activation of the receptor generates a chloride current that is inhibitory in most adult neurons, which have a low concentration of intracellular chloride.91 GlyRs are composed of five subunits (α1–4 and β). Genetic disruption of GlyR subunits results in hereditary hyperekplexia (startle disease) in humans,92 and similar disorders in mice, cattle and dogs.93 Pharmacological inhibition of GlyR with strychnine results in rigidity, painful and disabling muscle spasms, and exaggerated startle responses.94 The response of patients to immunotherapy and the resemblance of their symptoms to the genetic and pharmacological syndromes argue in favour of a functional effect of the autoantibodies.
Metabotropic glutamate receptors
Antibodies targeting mGluR1 were first reported in two patients with cerebellar ataxia and a history of Hodgkin lymphoma.95 Two patients with cerebellar ataxia but without Hodgkin lymphoma have subsequently been reported.8,96 Antibodies against mGluR5 were recently reported in two patients with Ophelia syndrome—a rare disorder involving psychiatric symptoms and cognitive and memory impairments that occurs in the setting of Hodgkin lymphoma.97 These patients improved rapidly with antitumour therapy.8
mGluR1 and mGlur5 constitute the group 1 mGluRs and show a high level of sequence homology with one another.98 Despite this close homology, the receptors serve distinct functions: mGluR5 is important for long-term depression of synapses in the hippocampus and for learning,99 whereas mGluR1 is crucial for rapid synaptic transmission in the cerebellum.100 Interestingly, patients' antibodies to mGluR1 or mGluR5 are always specific for one receptor type.8,95
Injection of mGluR1 antibodies near the cerebellum in rodents causes ataxia within 30 min. This ataxia reaches a maximum after 2–4 h and then resolves over 1 day. Moreover, application of mGluR1 antibodies to cerebellar slices disrupts synaptic long-term depression, further supporting the notion that the cerebellum is the target site of these antibodies.101 However, a postmortem study of a patient with mGluR1 antibodies showed a substantial reduction in the number of Purkinje cells, with the surviving neurons having truncated dendritic arbours.101 Purkinje cells express mGluR1,102 but no T-cell infiltrates were observed, so the mechanism by which these Purkinje neurons were lost is unclear.
Antibody tests
Caveats to interpretation
Identification of novel autoantigens has resulted in the development of new diagnostic assays. Tests for intracellular antigens frequently involve immunohistochemistry per formed on tissue arrays and immunoblotting of recombinant proteins. Immunohistochemistry can have limitations when several autoantibodies coexist in a single patient, but this technique has the advantage of detecting novel antibodies. By contrast, immunoblotting of recombinant proteins can be more specific, but does not identify novel antibodies.
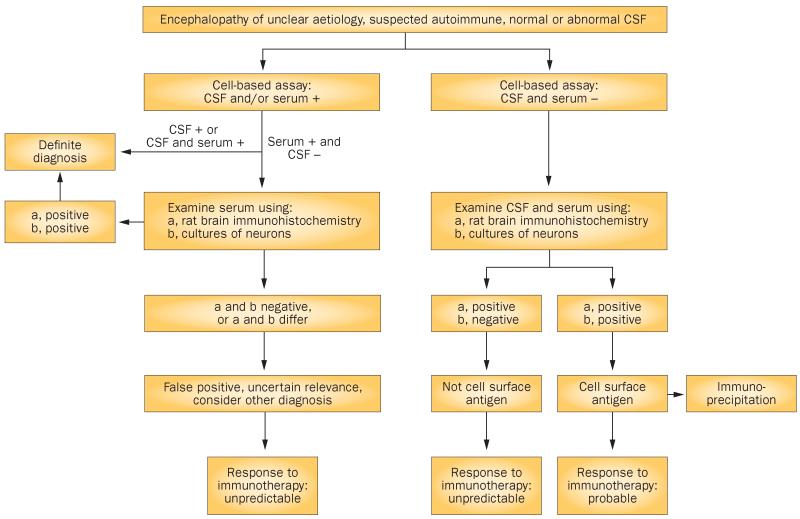
The presence of antibodies against neuronal cell-surface proteins was initially defined according to reactivity of patient serum and CSF in three antigen-binding assays49,52—brain tissue optimized for presentation of cell-surface antigens, cultured dissociated neurons, and recombinant cells expressing the antigen of interest (cell-based assay). These criteria have since been simplified to the cell-based assay, sometimes using only the patient's serum and not the CSF. Because the readout is not quantitative, interpretation of the results or score depends on the experience of the researcher. Moreover, serum is often screened at low dilutions (<1:100), which can increase background artefacts and produce misleading, false-positive results despite rigorous controls (Figure 3). Serum that is weakly positive in the cell-based assay can test negative according to the other two criteria. In such situations, the CSF is often negative and the associated symptoms are atypical (for example, related to mitochondrial disease, schizophrenia or prion disease), which all suggest a false-positive result and call into question the clinical significance of low serum antibody levels (J. Dalmau, unpublished work). Therefore, as the diagnostic criteria have been simplified, the diagnostic accuracy has decreased. Although the initial set of criteria may be time-consuming to assess in clinical high-throughput laboratories, our studies indicate that the inclusion of CSF analysis in cell-based assays is sufficient to raise the sensitivity and specificity of testing close to those of the three `gold standard' criteria.
Figure 3.
Algorithm for identification and assessment of antibodies to neuronal cell-surface antigens. Comprehensive assessment of known and novel antibodies to cell-surface antigens depends on examination of the reactivity of serum and CSF. Some patients may have normal results on routine CSF studies (for example, cell counts, protein levels and oligoclonal bands) but test positive for specific antibodies to neuronal cell-surface antigens (for example, leucine-rich glioma-inactivated protein 1 or the N-methyl-d-aspartate receptor) in the CSF. In patients with symptomatic encephalitis, we always test for antibodies in CSF, as antibodies can be present in or absent from serum. Cases with only low levels of antibodies in the CSF have been reported, although the relevance and identity of these antibodies are unclear. In such cases, confirmation that antibodies react with the cell surface of neurons (as tested in cell culture) and with the brain neuropil (as tested in rat brain slice preparations) is strongly recommended. Detection of a novel cell-surface reactivity should be followed by characterization of the antigen by immunoprecipitation and mass spectrometry. Abbreviation: CSF, cerebrospinal fluid.
These limitations and variation of clinical criteria to define a disease could explain different results among investigators. For example, a study involving a cell-based assay showed that three of 46 (6.5%) patients with schizophrenia had serum NMDAR antibodies (NR subunit not specified), whereas another study using the three criteria outlined above found that none of 100 patients with schizophrenia had IgG NR1 antibodies (which are typically associated with anti-NMDAR encephalitis).103
Guidelines for antibody identification
A recently published review104 included response to immunotherapy as a component of criteria to define disorders related to cell-surface antigens. However, patients with syndromes of unknown aetiology only have restricted access to empirical immunotherapy, making those criteria impractical. Furthermore, patients with rapidly progressive encephalopathy and inflammatory CSF changes—as occurs, for example, in CNS angiitis—may respond to empirical immunotherapy105 and, conversely, patients with encephalitis and antibodies against cell-surface antigens do not always respond to immunotherapy.4,49 Together, these factors could result in misclassification of disorders associated with antibodies to neuronal cell-surface proteins.
In our opinion, a disorder qualifies as being related to neuronal cell-surface antigens only when reactivity to such antigens is demonstrated (Figure 3).106 CSF analysis was crucial in our identification of novel antigens, including NMDAR,1 AMPAR,2 GABAB receptor,3 mGluR5,8 neurexin,107 and LGI1 and Caspr2.4 Indeed, the first clue that eventually led to immunoprecipitation of these antigens arose from careful selection of patients with similar syndromes to one another and CSF reactivity to the neuropil of rat brain. The patterns of serum immunostaining in the same patients were more variable than those of CSF, owing to nonspecific background staining, the presence of other antibodies, or alteration of reactivity by intravenous immunoglobulin or plasma exchange, which are sometimes used empirically to treat suspected autoimmune disorders.
Selection of patients with the same phenotype is crucial to avoid confusing results and allow antigen characterization. For example, Caspr2 was recently reported to be an autoantigen in idiopathic cerebellar ataxia.108 However, the index case had prominent limbic encephalitis, and in subsequent studies peripheral nerve hyperexcitability was identified (L. Bataller, personal communication). Among other patients with anti-Caspr2 antibodies, only two had isolated cerebellar dysfunction without other auto-immune symptoms and neither of these patients' serum reacted with tissue from the cerebellum, where Caspr2 is strongly expressed.5
Conclusions and future directions
Several categories of neuronal autoantibody targets exist, including cytoplasmic or nuclear antigens, intracellular synaptic proteins, and the more recently described cell-surface or synaptic proteins. Cell-surface and synaptic proteins are particularly interesting because the associated antibodies seem to be pathogenic, and they produce symptoms that are more responsive to immunotherapy than those related to intracellular antigens. Furthermore, such antibodies are associated with syndromes that resemble genetic or pharmacological models in which the corresponding antigen is disrupted. The discovery of these disorders has led to the development of diagnostic tests which, although highly specific, have some limitations when CSF is excluded from analysis. The use of serum and CSF is important not only for the diagnosis of known disorders, but also for the characterization of new autoantigens, as shown in our algorithm for antibody and antigen interpretation (Figure 3).
In addition to the disorders described here, other syndromes mediated by neuronal antibodies are likely to exist, and not all of these syndromes may show the classical symptoms of rapidly progressive encephalitis with CSF pleocytosis. Indeed, serum IgA antibodies against NMDARs were recently identified in a group of patients who presented with slowly progressive cognitive decline, and CSF that was normal or showed mild abnormalities in routine clinical tests.109
Many questions about these disorders remain unanswered. For example, the trigger of the autoimmune response in many patients is unknown. As discussed above, only a fraction of patients with Hu antibodies develop symptoms, and most patients with ovarian teratomas do not develop NMDAR-targeted autoimmunity. Why do the usual mechanisms of self-tolerance fail in other patients? And how does the antibody response become established in the CNS?110 Robust intrathecal antibody synthesis usually accompanies some of these disorders, and migration of B cells into the CNS might be necessary for symptoms to occur. Could this event be targeted to prevent symptoms? The answers to these questions may result in new therapies.
Animal models of syndromes related to neuronal antibodies would allow pathogenesis and treatments to be further explored, but recreation of some immunological findings or symptoms in animals does not necessarily equate to a disease model, particularly when the target antigen is behind the blood–brain barrier.17,21 Infusion of antibodies directly into the CSF of animals does not entirely overcome this problem, as such antibodies might not access the brain parenchyma. Additional strategies include development of a B-cell and/or T-cell response to various antigens in knockout animals for the same antigens and then transferring the B cells or T cells to wild-type animals.19
Prompt immunotherapy is often associated with improved outcomes in encephalitis associated with antibodies to cell-surface antigens,4,47 but which therapies (plasma exchange, intravenous immunoglobulin, steroids, cyclophosphamide or rituximab) provide the greatest benefit is unknown. A preliminary report has found that 44% of patients with anti-NMDAR encephalitis fail first-line therapies, but second-line therapies (rituximab and cyclophosphamide) are usually effective.111 As increasing numbers of patients with neuronal antibodies are identified, clinical trials should be conducted to determine the optimal treatments for these disorders. Towards this goal, a trial of prolonged immunotherapy with rituximab in patients with anti-NMDAR encephalitis has recently been approved by the NIH (Principal Investigators: A. Nath and J. Dalmau). With continued improvements in diagnosis and treatment, increasing numbers of patients with these disorders will live long and productive lives.
Key points
-
■
Antibodies that target neuronal antigens are becoming increasingly recognized
-
■
Antibodies to intracellular neuronal antigens may mark a T-cell response that targets neurons
-
■
Antibodies to cell-surface and synaptic antigens are associated with seizures and psychosis, as well as disorders of memory, behaviour, cognition and movement; such antibodies may be directly pathogenic
-
■
Many patients with antibodies to cell-surface antigens respond to treatment
-
■
Assessment of clinical phenotype and analysis of serum and cerebrospinal fluid are crucial for identification of known and novel autoantibodies
Acknowledgements
This work is supported in part by grants to J. Dalmau from the NIH (RO1NS077851 and RO1MH094741), the National Cancer Institute (RO1CA089054), Fundació la Marató TV3 and Fondo de Investigaciones Sanitarias (FIS, PI11/01780), and Euroimmun. This work is also supported in part by grants to E. Lancaster from the National Organization for Rare Disorders and the Dana Foundation.
Footnotes
Author contributions Both authors contributed to researching data for the article, discussion of the content, writing the article, and review and/or editing of the manuscript before submission.
Competing interests E. Lancaster declares associations with the following companies: Lundbeck, Talecris. J. Dalmau declares associations with the following companies/organizations: Euroimmun, University of Pennsylvania. See the article online for full details of the relationships.
References
- 1.Dalmau J, et al. Paraneoplastic anti-N-methyl-d-aspartate receptor encephalitis associated with ovarian teratoma. Ann. Neurol. 2007;61:25–36. doi: 10.1002/ana.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lai M, et al. AMPA receptor antibodies in limbic encephalitis alter synaptic receptor location. Ann. Neurol. 2009;65:424–434. doi: 10.1002/ana.21589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lancaster E, et al. Antibodies to the GABAB receptor in limbic encephalitis with seizures: case series and characterisation of the antigen. Lancet Neurol. 2010;9:67–76. doi: 10.1016/S1474-4422(09)70324-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lai M, et al. Investigation of LGI1 as the antigen in limbic encephalitis previously attributed to potassium channels: a case series. Lancet Neurol. 2010;9:776–785. doi: 10.1016/S1474-4422(10)70137-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lancaster E, et al. Investigations of Caspr2, an autoantigen of encephalitis and neuromyotonia. Ann. Neurol. 2011;69:303–311. doi: 10.1002/ana.22297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Irani SR, et al. Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan's syndrome and acquired neuromyotonia. Brain. 2010;133:2734–2748. doi: 10.1093/brain/awq213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hutchinson M, et al. Progressive encephalomyelitis, rigidity, and myoclonus: a novel glycine receptor antibody. Neurology. 2008;71:1291–1292. doi: 10.1212/01.wnl.0000327606.50322.f0. [DOI] [PubMed] [Google Scholar]
- 8.Lancaster E, et al. Antibodies to metabotropic glutamate receptor 5 in the Ophelia syndrome. Neurology. 2011;77:1698–1701. doi: 10.1212/WNL.0b013e3182364a44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dalmau J, Furneaux HM, Rosenblum MK, Graus F, Posner JB. Detection of the anti-Hu antibody in specific regions of the nervous system and tumor from patients with paraneoplastic encephalomyelitis/sensory neuronopathy. Neurology. 1991;41:1757–1764. doi: 10.1212/wnl.41.11.1757. [DOI] [PubMed] [Google Scholar]
- 10.Sillevis Smitt PA, Manley GT, Posner JB. Immunization with the paraneoplastic encephalomyelitis antigen HuD does not cause neurologic disease in mice. Neurology. 1995;45:1873–1878. doi: 10.1212/wnl.45.10.1873. [DOI] [PubMed] [Google Scholar]
- 11.Sillevis Smitt P, Manley G, Dalmau J, Posner J. The HuD paraneoplastic protein shares immunogenic regions between PEM/PSN patients and several strains and species of experimental animals. J. Neuroimmunol. 1996;71:199–206. doi: 10.1016/s0165-5728(96)00153-1. [DOI] [PubMed] [Google Scholar]
- 12.Carpentier AF, et al. DNA vaccination with HuD inhibits growth of a neuroblastoma in mice. Clin. Cancer Res. 1998;4:2819–2824. [PubMed] [Google Scholar]
- 13.Darnell RB, Posner JB. Paraneoplastic syndromes involving the nervous system. N. Engl. J. Med. 2003;349:1543–1554. doi: 10.1056/NEJMra023009. [DOI] [PubMed] [Google Scholar]
- 14.Rousseau A, et al. T cell response to Hu-D peptides in patients with anti-Hu syndrome. J. Neurooncol. 2005;71:231–236. doi: 10.1007/s11060-004-1723-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Voltz R, Dalmau J, Posner JB, Rosenfeld MR. T-cell receptor analysis in anti-Hu associated paraneoplastic encephalomyelitis. Neurology. 1998;51:1146–1150. doi: 10.1212/wnl.51.4.1146. [DOI] [PubMed] [Google Scholar]
- 16.Plonquet A, et al. Oligoclonal T-cells in blood and target tissues of patients with anti-Hu syndrome. J. Neuroimmunol. 2002;122:100–105. doi: 10.1016/s0165-5728(01)00452-0. [DOI] [PubMed] [Google Scholar]
- 17.Plonquet A, et al. Peptides derived from the onconeural HuD protein can elicit cytotoxic responses in HHD mouse and human. J. Neuroimmunol. 2003;142:93–100. doi: 10.1016/s0165-5728(03)00269-8. [DOI] [PubMed] [Google Scholar]
- 18.Kazarian M, et al. Immune response in lung cancer mouse model mimics human anti-Hu reactivity. J. Neuroimmunol. 2009;217:38–45. doi: 10.1016/j.jneuroim.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeLuca I, Blachere NE, Santomasso B, Darnell RB. Tolerance to the neuron-specific paraneoplastic HuD antigen. PLoS ONE. 2009;4:e5739. doi: 10.1371/journal.pone.0005739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts WK, et al. Patients with lung cancer and paraneoplastic Hu syndrome harbor HuD-specific type 2 CD8+ T cells. J. Clin. Invest. 2009;119:2042–2051. doi: 10.1172/JCI36131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pellkofer H, et al. Modelling paraneoplastic CNS disease: T-cells specific for the onconeuronal antigen PNMA1 mediate autoimmune encephalomyelitis in the rat. Brain. 2004;127:1822–1830. doi: 10.1093/brain/awh205. [DOI] [PubMed] [Google Scholar]
- 22.Cross SA, et al. Paraneoplastic autoimmune optic neuritis with retinitis defined by CRMP-5-IgG. Ann. Neurol. 2003;54:38–50. doi: 10.1002/ana.10587. [DOI] [PubMed] [Google Scholar]
- 23.Verschuren MC, van Bergen CJ, van Gastel-Mol EJ, Bogers AJ, van Dongen JJ. A DNA binding protein in human thymocytes recognizes the T cell receptor-δ-deleting element ψJα. J. Immunol. 1996;156:3806–3814. [PubMed] [Google Scholar]
- 24.Giometto B, et al. Sub-acute cerebellar degeneration with anti-Yo autoantibodies: immunohistochemical analysis of the immune reaction in the central nervous system. Neuropathol. Appl. Neurobiol. 1997;23:468–474. doi: 10.1111/j.1365-2990.1997.tb01323.x. [DOI] [PubMed] [Google Scholar]
- 25.Storstein A, Krossnes BK, Vedeler CA. Morphological and immunohistochemical characterization of paraneoplastic cerebellar degeneration associated with Yo antibodies. Acta Neurol. Scand. 2009;120:64–67. doi: 10.1111/j.1600-0404.2008.01138.x. [DOI] [PubMed] [Google Scholar]
- 26.Albert ML, et al. Tumor-specific killer cells in paraneoplastic cerebellar degeneration. Nat. Med. 1998;4:1321–1324. doi: 10.1038/3315. [DOI] [PubMed] [Google Scholar]
- 27.Albert ML, Austin LM, Darnell RB. Detection and treatment of activated T cells in the cerebrospinal fluid of patients with paraneoplastic cerebellar degeneration. Ann. Neurol. 2000;47:9–17. [PubMed] [Google Scholar]
- 28.Sutton IJ, Steele J, Savage CO, Winer JB, Young LS. An interferon-γ ELISPOT and immunohistochemical investigation of cytotoxic T lymphocyte-mediated tumour immunity in patients with paraneoplastic cerebellar degeneration and anti-Yo antibodies. J. Neuroimmunol. 2004;150:98–106. doi: 10.1016/j.jneuroim.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 29.Greenlee JE, et al. Purkinje cell death after uptake of anti-Yo antibodies in cerebellar slice cultures. J. Neuropathol. Exp. Neurol. 2010;69:997–1007. doi: 10.1097/NEN.0b013e3181f0c82b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Graus F, et al. Effect of intraventricular injection of an anti-Purkinje cell antibody (anti-Yo) in a guinea pig model. J. Neurol. Sci. 1991;106:82–87. doi: 10.1016/0022-510x(91)90198-g. [DOI] [PubMed] [Google Scholar]
- 31.Greenlee JE, Burns JB, Rose JW, Jaeckle KA, Clawson S. Uptake of systemically administered human anticerebellar antibody by rat Purkinje cells following blood–brain barrier disruption. Acta Neuropathol. 1995;89:341–345. doi: 10.1007/BF00309627. [DOI] [PubMed] [Google Scholar]
- 32.Soghomonian JJ, Martin DL. Two isoforms of glutamate decarboxylase: why? Trends Pharmacol. Sci. 1998;19:500–505. doi: 10.1016/s0165-6147(98)01270-x. [DOI] [PubMed] [Google Scholar]
- 33.Wu Y, Matsui H, Tomizawa K. Amphiphysin I and regulation of synaptic vesicle endocytosis. Acta Med. Okayama. 2009;63:305–323. doi: 10.18926/AMO/31822. [DOI] [PubMed] [Google Scholar]
- 34.Pittock SJ, et al. Glutamic acid decarboxylase autoimmunity with brainstem, extrapyramidal, and spinal cord dysfunction. Mayo Clin. Proc. 2006;81:1207–1214. doi: 10.4065/81.9.1207. [DOI] [PubMed] [Google Scholar]
- 35.Saiz A, et al. Spectrum of neurological syndromes associated with glutamic acid decarboxylase antibodies: diagnostic clues for this association. Brain. 2008;131:2553–2563. doi: 10.1093/brain/awn183. [DOI] [PubMed] [Google Scholar]
- 36.Malter MP, Helmstaedter C, Urbach H, Vincent A, Bien CG. Antibodies to glutamic acid decarboxylase define a form of limbic encephalitis. Ann. Neurol. 2010;67:470–478. doi: 10.1002/ana.21917. [DOI] [PubMed] [Google Scholar]
- 37.Murinson BB, Guarnaccia JB. Stiff-person syndrome with amphiphysin antibodies: distinctive features of a rare disease. Neurology. 2008;71:1955–1958. doi: 10.1212/01.wnl.0000327342.58936.e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pittock SJ, et al. Amphiphysin autoimmunity: paraneoplastic accompaniments. Ann. Neurol. 2005;58:96–107. doi: 10.1002/ana.20529. [DOI] [PubMed] [Google Scholar]
- 39.Ishida K, Mitoma H, Mizusawa H. Reversibility of cerebellar GABAergic synapse impairment induced by anti-glutamic acid decarboxylase autoantibodies. J. Neurol. Sci. 2008;271:186–190. doi: 10.1016/j.jns.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 40.Manto MU, Hampe CS, Rogemond V, Honnorat J. Respective implications of glutamate decarboxylase antibodies in stiff person syndrome and cerebellar ataxia. Orphanet J. Rare Dis. 2011;6:3. doi: 10.1186/1750-1172-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burton AR, et al. On the pathogenicity of autoantigen-specific T-cell receptors. Diabetes. 2008;57:1321–1330. doi: 10.2337/db07-1129. [DOI] [PubMed] [Google Scholar]
- 42.Burton AR, et al. Central nervous system destruction mediated by glutamic acid decarboxylase-specific CD4+ T cells. J. Immunol. 2010;184:4863–4870. doi: 10.4049/jimmunol.0903728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sommer C, et al. Paraneoplastic stiff-person syndrome: passive transfer to rats by means of IgG antibodies to amphiphysin. Lancet. 2005;365:1406–1411. doi: 10.1016/S0140-6736(05)66376-3. [DOI] [PubMed] [Google Scholar]
- 44.Geis C, et al. Stiff person syndrome-associated autoantibodies to amphiphysin mediate reduced GABAergic inhibition. Brain. 2010;133:3166–3180. doi: 10.1093/brain/awq253. [DOI] [PubMed] [Google Scholar]
- 45.Geis C, et al. Stiff person syndrome associated anti-amphiphysin antibodies reduce GABA associated [Ca2+]i rise in embryonic motoneurons. Neurobiol. Dis. 2009;36:191–199. doi: 10.1016/j.nbd.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 46.Florance NR, et al. Anti-N-methyl-d-aspartate receptor (NMDAR) encephalitis in children and adolescents. Ann. Neurol. 2009;66:11–18. doi: 10.1002/ana.21756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 2011;10:63–74. doi: 10.1016/S1474-4422(10)70253-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gable MS, Sheriff H, Dalmau J, Tilley DH, Glaser CA. The frequency of autoimmune N-methyl-d-aspartate receptor encephalitis surpasses that of individual viral etiologies in young individuals enrolled in the california encephalitis project. Clin. Infect. Dis. 2012;54:899–904. doi: 10.1093/cid/cir1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dalmau J, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1091–1098. doi: 10.1016/S1474-4422(08)70224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tuzun E, et al. Evidence for antibody-mediated pathogenesis in anti-NMDAR encephalitis associated with ovarian teratoma. Acta Neuropathol. 2009;118:737–743. doi: 10.1007/s00401-009-0582-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Belforte JE, et al. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat. Neurosci. 2010;13:76–83. doi: 10.1038/nn.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hughes EG, et al. Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. J. Neurosci. 2010;30:5866–5875. doi: 10.1523/JNEUROSCI.0167-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iizuka T, et al. Anti-NMDA receptor encephalitis in Japan: long-term outcome without tumor removal. Neurology. 2008;70:504–511. doi: 10.1212/01.wnl.0000278388.90370.c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Manto M, Dalmau J, Didelot A, Rogemond V, Honnorat J. In vivo effects of antibodies from patients with anti-NMDA receptor encephalitis: further evidence of synaptic glutamatergic dysfunction. Orphanet J. Rare Dis. 2010;5:31. doi: 10.1186/1750-1172-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Manto M, Dalmau J, Didelot A, Rogemond V, Honnorat J. Afferent facilitation of corticomotor responses is increased by IgGs of patients with NMDA-receptor antibodies. J. Neurol. 2011;258:27–33. doi: 10.1007/s00415-010-5674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hart IK, et al. Autoantibodies detected to expressed K+ channels are implicated in neuromyotonia. Ann. Neurol. 1997;41:238–246. doi: 10.1002/ana.410410215. [DOI] [PubMed] [Google Scholar]
- 57.Tan KM, Lennon VA, Klein CJ, Boeve BF, Pittock SJ. Clinical spectrum of voltage-gated potassium channel autoimmunity. Neurology. 2008;70:1883–1890. doi: 10.1212/01.wnl.0000312275.04260.a0. [DOI] [PubMed] [Google Scholar]
- 58.Kleopa KA, Elman LB, Lang B, Vincent A, Scherer SS. Neuromyotonia and limbic encephalitis sera target mature Shaker-type K+ channels: subunit specificity correlates with clinical manifestations. Brain. 2006;129:1570–1584. doi: 10.1093/brain/awl084. [DOI] [PubMed] [Google Scholar]
- 59.Boronat A, et al. Analysis of antibodies to surface epitopes of contactin-2 in multiple sclerosis. J. Neuroimmunol. 2012;244:103–106. doi: 10.1016/j.jneuroim.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fukata Y, et al. Disruption of LGI1-linked synaptic complex causes abnormal synaptic transmission and epilepsy. Proc. Natl Acad. Sci. USA. 2010;107:3799–3804. doi: 10.1073/pnas.0914537107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morante-Redolat JM, et al. Mutations in the LGI1/Epitempin gene on 10q24 cause autosomal dominant lateral temporal epilepsy. Hum. Mol. Genet. 2002;11:1119–1128. doi: 10.1093/hmg/11.9.1119. [DOI] [PubMed] [Google Scholar]
- 62.Chatzopoulou E, et al. Structural requirement of TAG-1 for retinal ganglion cell axons and myelin in the mouse optic nerve. J. Neurosci. 2008;28:7624–7636. doi: 10.1523/JNEUROSCI.1103-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gu C, Gu Y. Clustering and activity tuning of Kv1 channels in myelinated hippocampal axons. J. Biol. Chem. 2011;286:25835–25847. doi: 10.1074/jbc.M111.219113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou L, Messing A, Chiu SY. Determinants of excitability at transition zones in Kv1.1-deficient myelinated nerves. J. Neurosci. 1999;19:5768–5781. doi: 10.1523/JNEUROSCI.19-14-05768.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Geschwind MD, et al. Voltage-gated potassium channel autoimmunity mimicking Creutzfeldt–Jakob disease. Arch. Neurol. 2008;65:1341–1346. doi: 10.1001/archneur.65.10.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Irani SR, et al. Faciobrachial dystonic seizures precede Lgi1 antibody limbic encephalitis. Ann. Neurol. 2011;69:892–900. doi: 10.1002/ana.22307. [DOI] [PubMed] [Google Scholar]
- 67.Andrade DM, Tai P, Dalmau J, Wennberg R. Tonic seizures: a diagnostic clue of anti-LGI1 encephalitis? Neurology. 2011;76:1355–1357. doi: 10.1212/WNL.0b013e3182152808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chabrol E, et al. Electroclinical characterization of epileptic seizures in leucine-rich, glioma-inactivated 1-deficient mice. Brain. 2010;133:2749–2762. doi: 10.1093/brain/awq171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lalic T, Pettingill P, Vincent A, Capogna M. Human limbic encephalitis serum enhances hippocampal mossy fiber-CA3 pyramidal cell synaptic transmission. Epilepsia. 2011;52:121–131. doi: 10.1111/j.1528-1167.2010.02756.x. [DOI] [PubMed] [Google Scholar]
- 70.Irani SR, et al. Morvan's syndrome: clinical and serological observations in 29 cases. Ann. Neurol. doi: 10.1002/ana.23577. http://dx.doi.org/10.1002/ana.23577. [DOI] [PubMed]
- 71.Verkerk AJ, et al. CNTNAP2 is disrupted in a family with Gilles de la Tourette syndrome and obsessive compulsive disorder. Genomics. 2003;82:1–9. doi: 10.1016/s0888-7543(03)00097-1. [DOI] [PubMed] [Google Scholar]
- 72.Strauss KA, et al. Recessive symptomatic focal epilepsy and mutant contactin-associated protein-like 2. N. Engl. J. Med. 2006;354:1370–1377. doi: 10.1056/NEJMoa052773. [DOI] [PubMed] [Google Scholar]
- 73.Gregor A, et al. Expanding the clinical spectrum associated with defects in CNTNAP2 and NRXN1. BMC Med. Genet. 2011;12:106. doi: 10.1186/1471-2350-12-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Penagarikano O, et al. Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits. Cell. 2011;147:235–246. doi: 10.1016/j.cell.2011.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Whalley HC, et al. Genetic variation in CNTNAP2 alters brain function during linguistic processing in healthy individuals. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2011;156B:941–948. doi: 10.1002/ajmg.b.31241. [DOI] [PubMed] [Google Scholar]
- 76.Bataller L, et al. Reversible paraneoplastic limbic encephalitis associated with antibodies to the AMPA receptor. Neurology. 2010;74:265–267. doi: 10.1212/WNL.0b013e3181cb3e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Graus F, et al. The expanding clinical profile of anti-AMPA receptor encephalitis. Neurology. 2010;74:857–859. doi: 10.1212/WNL.0b013e3181d3e404. [DOI] [PubMed] [Google Scholar]
- 78.Granger AJ, Gray JA, Lu W, Nicoll RA. Genetic analysis of neuronal ionotropic glutamate receptor subunits. J. Physiol. 2011;589:4095–4101. doi: 10.1113/jphysiol.2011.213033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Boronat A, Sabater L, Saiz A, Dalmau J, Graus F. GABAB receptor antibodies in limbic encephalitis and anti-GAD-associated neurologic disorders. Neurology. 2011;76:795–800. doi: 10.1212/WNL.0b013e31820e7b8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bettler B, Kaupmann K, Mosbacher J, Gassmann M. Molecular structure and physiological functions of GABAB receptors. Physiol. Rev. 2004;84:835–867. doi: 10.1152/physrev.00036.2003. [DOI] [PubMed] [Google Scholar]
- 81.Titulaer MJ, Lang B, Verschuuren JJ. Lambert–Eaton myasthenic syndrome: from clinical characteristics to therapeutic strategies. Lancet Neurol. 2011;10:1098–1107. doi: 10.1016/S1474-4422(11)70245-9. [DOI] [PubMed] [Google Scholar]
- 82.Clouston PD, et al. Paraneoplastic cerebellar degeneration. III. Cerebellar degeneration, cancer, and the Lambert–Eaton myasthenic syndrome. Neurology. 1992;42:1944–1950. doi: 10.1212/wnl.42.10.1944. [DOI] [PubMed] [Google Scholar]
- 83.Lennon VA, et al. Calcium-channel antibodies in the Lambert–Eaton syndrome and other paraneoplastic syndromes. N. Engl. J. Med. 1995;332:1467–1474. doi: 10.1056/NEJM199506013322203. [DOI] [PubMed] [Google Scholar]
- 84.Burk K, Wick M, Roth G, Decker P, Voltz R. Antineuronal antibodies in sporadic late-onset cerebellar ataxia. J. Neurol. 2010;257:59–62. doi: 10.1007/s00415-009-5262-8. [DOI] [PubMed] [Google Scholar]
- 85.Pinto A, Iwasa K, Newland C, Newsom-Davis J, Lang B. The action of Lambert–Eaton myasthenic syndrome immunoglobulin G on cloned human voltage-gated calcium channels. Muscle Nerve. 2002;25:715–724. doi: 10.1002/mus.10087. [DOI] [PubMed] [Google Scholar]
- 86.Lang B, Pinto A, Giovannini F, Newsom-Davis J, Vincent A. Pathogenic autoantibodies in the Lambert–Eaton myasthenic syndrome. Ann. NY Acad. Sci. 2003;998:187–195. doi: 10.1196/annals.1254.019. [DOI] [PubMed] [Google Scholar]
- 87.Liao YJ, et al. Anti-Ca2+ channel antibody attenuates Ca2+ currents and mimics cerebellar ataxia in vivo. Proc. Natl Acad. Sci. USA. 2008;105:2705–2710. doi: 10.1073/pnas.0710771105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fukuda T, et al. Reduction of P/Q-type calcium channels in the postmortem cerebellum of paraneoplastic cerebellar degeneration with Lambert–Eaton myasthenic syndrome. Ann. Neurol. 2003;53:21–28. doi: 10.1002/ana.10392. [DOI] [PubMed] [Google Scholar]
- 89.Piotrowicz A, Thumen A, Leite MI, Vincent A, Moser A. A case of glycine-receptor antibody-associated encephalomyelitis with rigidity and myoclonus (PERM): clinical course, treatment and CSF findings. J. Neurol. 2011;258:2268–2270. doi: 10.1007/s00415-011-6078-x. [DOI] [PubMed] [Google Scholar]
- 90.Mas N, et al. Antiglycine-receptor encephalomyelitis with rigidity. J. Neurol. Neurosurg. Psychiatry. 2011;82:1399–1401. doi: 10.1136/jnnp.2010.229104. [DOI] [PubMed] [Google Scholar]
- 91.Hernandes MS, Troncone LR. Glycine as a neurotransmitter in the forebrain: a short review. J. Neural Transm. 2009;116:1551–1560. doi: 10.1007/s00702-009-0326-6. [DOI] [PubMed] [Google Scholar]
- 92.Shiang R, et al. Mutations in the α1 subunit of the inhibitory glycine receptor cause the dominant neurologic disorder, hyperekplexia. Nat. Genet. 1993;5:351–358. doi: 10.1038/ng1293-351. [DOI] [PubMed] [Google Scholar]
- 93.Harvey RJ, Topf M, Harvey K, Rees MI. The genetics of hyperekplexia: more than startle! Trends Genet. 2008;24:439–447. doi: 10.1016/j.tig.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 94.Makarovsky I, et al. Strychnine—a killer from the past. Isr. Med. Assoc. J. 2008;10:142–145. [PubMed] [Google Scholar]
- 95.Sillevis Smitt P, et al. Paraneoplastic cerebellar ataxia due to autoantibodies against a glutamate receptor. N. Engl. J. Med. 2000;342:21–27. doi: 10.1056/NEJM200001063420104. [DOI] [PubMed] [Google Scholar]
- 96.Marignier R, et al. Metabotropic glutamate receptor type 1 autoantibody-associated cerebellitis: a primary autoimmune disease? Arch. Neurol. 2010;67:627–630. doi: 10.1001/archneurol.2010.51. [DOI] [PubMed] [Google Scholar]
- 97.Carr I. The Ophelia syndrome: memory loss in Hodgkin's disease. Lancet. 1982;1:844–845. doi: 10.1016/s0140-6736(82)91887-6. [DOI] [PubMed] [Google Scholar]
- 98.Nicoletti F, et al. Metabotropic glutamate receptors: from the workbench to the bedside. Neuropharmacology. 2011;60:1017–1041. doi: 10.1016/j.neuropharm.2010.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Simonyi A, Schachtman TR, Christoffersen GR. Metabotropic glutamate receptor subtype 5 antagonism in learning and memory. Eur. J. Pharmacol. 2010;639:17–25. doi: 10.1016/j.ejphar.2009.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hildebrand ME, et al. Functional coupling between mGluR1 and Cav3.1 T-type calcium channels contributes to parallel fiber-induced fast calcium signaling within Purkinje cell dendritic spines. J. Neurosci. 2009;29:9668–9682. doi: 10.1523/JNEUROSCI.0362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Coesmans M, et al. Mechanisms underlying cerebellar motor deficits due to mGluR1-autoantibodies. Ann. Neurol. 2003;53:325–336. doi: 10.1002/ana.10451. [DOI] [PubMed] [Google Scholar]
- 102.Ichise T, et al. mGluR1 in cerebellar Purkinje cells essential for long-term depression, synapse elimination, and motor coordination. Science. 2000;288:1832–1835. doi: 10.1126/science.288.5472.1832. [DOI] [PubMed] [Google Scholar]
- 103.Masdeu JC, et al. Serum IgG antibodies against the NMDA receptor not detected in schizophrenia. Biol. Psychiatry. 2012;71:53S. doi: 10.1176/appi.ajp.2012.12050646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zuliani L, Graus F, Giometto B, Bien C, Vincent A. Central nervous system neuronal surface antibody associated syndromes: review and guidelines for recognition. J. Neurol. Neurosurg. Psychiatry. 2012;83:638–645. doi: 10.1136/jnnp-2011-301237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hajj-Ali RA, Singhal AB, Benseler S, Molloy E, Calabrese LH. Primary angiitis of the CNS. Lancet Neurol. 2011;10:561–572. doi: 10.1016/S1474-4422(11)70081-3. [DOI] [PubMed] [Google Scholar]
- 106.Ances BM, et al. Treatment-responsive limbic encephalitis identified by neuropil antibodies: MRI and PET correlates. Brain. 2005;128:1764–1777. doi: 10.1093/brain/awh526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lancaster E, Martinez-Hernandez E, Dalmau J. Encephalitis and antibodies to synaptic and neuronal cell surface proteins. Neurology. 2011;77:179–189. doi: 10.1212/WNL.0b013e318224afde. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Becker EB, et al. Contactin-associated protein-2 antibodies in non-paraneoplastic cerebellar ataxia. J. Neurol. Neurosurg. Psychiatry. 2012;83:437–440. doi: 10.1136/jnnp-2011-301506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pruss H, et al. IgA NMDA receptor antibodies are markers of synaptic immunity in slow cognitive impairment. Neurology. doi: 10.1212/WNL.0b013e318258300d. http://dx.doi.org/10.1212/WNL.0b013e318258300d. [DOI] [PMC free article] [PubMed]
- 110.Martinez-Hernandez E, et al. Analysis of complement and plasma cells in the brain of patients with anti-NMDAR encephalitis. Neurology. 2011;77:589–593. doi: 10.1212/WNL.0b013e318228c136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Titulaer M, et al. Clinical features, treatment, and outcome of 500 patients with anti-NMDA receptor encephalitis [abstract] Neurology. 2012;78(Meeting abstracts 1):PL01.001. [Google Scholar]
- 112.Dalmau J, Furneaux HM, Gralla RJ, Kris MG, Posner JB. Detection of the anti-Hu antibody in the serum of patients with small cell lung cancer—a quantitative western blot analysis. Ann. Neurol. 1990;27:544–552. doi: 10.1002/ana.410270515. [DOI] [PubMed] [Google Scholar]
- 113.Hubers L, et al. HuD interacts with survival motor neuron protein and can rescue spinal muscular atrophy-like neuronal defects. Hum. Mol. Genet. 2011;20:553–579. doi: 10.1093/hmg/ddq500. [DOI] [PubMed] [Google Scholar]
- 114.Okano HJ, Darnell RB. A hierarchy of Hu RNA binding proteins in developing and adult neurons. J. Neurosci. 1997;17:3024–3037. doi: 10.1523/JNEUROSCI.17-09-03024.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dalmau J, Graus F, Rosenblum MK, Posner JB. Anti-Hu-associated paraneoplastic encephalomyelitis/sensory neuronopathy. A clinical study of 71 patients. Medicine (Baltimore) 1992;71:59–72. doi: 10.1097/00005792-199203000-00001. [DOI] [PubMed] [Google Scholar]
- 116.Veyrac A, et al. CRMP5 regulates generation and survival of newborn neurons in olfactory and hippocampal neurogenic areas of the adult mouse brain. PLoS ONE. 2011;6:e23721. doi: 10.1371/journal.pone.0023721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Honnorat J, et al. Onco-neural antibodies and tumour type determine survival and neurological symptoms in paraneoplastic neurological syndromes with Hu or CV2/CRMP5 antibodies. J. Neurol. Neurosurg. Psychiatry. 2009;80:412–416. doi: 10.1136/jnnp.2007.138016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Graus F, et al. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J. Neurol. Neurosurg. Psychiatry. 2004;75:1135–1140. doi: 10.1136/jnnp.2003.034447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dalmau J, et al. Clinical analysis of anti-Ma2-associated encephalitis. Brain. 2004;127:1831–1844. doi: 10.1093/brain/awh203. [DOI] [PubMed] [Google Scholar]
- 120.Furneaux HM, et al. Characterization of a cDNA encoding a 34-kDa Purkinje neuron protein recognized by sera from patients with paraneoplastic cerebellar degeneration. Proc. Natl Acad. Sci. USA. 1989;86:2873–2877. doi: 10.1073/pnas.86.8.2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.O'Donovan KJ, Diedler J, Couture GC, Fak JJ, Darnell RB. The onconeural antigen cdr2 is a novel APC/C target that acts in mitosis to regulate c-myc target genes in mammalian tumor cells. PLoS ONE. 2010;5:e10045. doi: 10.1371/journal.pone.0010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rojas I, et al. Long-term clinical outcome of paraneoplastic cerebellar degeneration and anti-Yo antibodies. Neurology. 2000;55:713–715. doi: 10.1212/wnl.55.5.713. [DOI] [PubMed] [Google Scholar]
- 123.Shams'ili S, et al. Paraneoplastic cerebellar degeneration associated with antineuronal antibodies: analysis of 50 patients. Brain. 2003;126:1409–1418. doi: 10.1093/brain/awg133. [DOI] [PubMed] [Google Scholar]
- 124.Pittock SJ, Lucchinetti CF, Lennon VA. Anti-neuronal nuclear autoantibody type 2: paraneoplastic accompaniments. Ann. Neurol. 2003;53:580–587. doi: 10.1002/ana.10518. [DOI] [PubMed] [Google Scholar]
- 125.Yang YY, Yin GL, Darnell RB. The neuronal RNA-binding protein Nova-2 is implicated as the autoantigen targeted in POMA patients with dementia. Proc. Natl Acad. Sci. USA. 1998;95:13254–13259. doi: 10.1073/pnas.95.22.13254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Buckanovich RJ, Darnell RB. The neuronal RNA binding protein Nova-1 recognizes specific RNA targets in vitro and in vivo. Mol. Cell Biol. 1997;17:3194–3201. doi: 10.1128/mcb.17.6.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Drlicek M, et al. Antibodies of the anti-Yo and anti-Ri type in the absence of paraneoplastic neurological syndromes: a long-term survey of ovarian cancer patients. J. Neurol. 1997;244:85–89. doi: 10.1007/s004150050054. [DOI] [PubMed] [Google Scholar]
- 128.Graus F, et al. Immunological characterization of a neuronal antibody (anti-Tr) associated with paraneoplastic cerebellar degeneration and Hodgkin's disease. J. Neuroimmunol. 1997;74:55–61. doi: 10.1016/s0165-5728(96)00205-6. [DOI] [PubMed] [Google Scholar]
- 129.Bernal F, et al. Anti-Tr antibodies as markers of paraneoplastic cerebellar degeneration and Hodgkin's disease. Neurology. 2003;60:230–234. doi: 10.1212/01.wnl.0000041495.87539.98. [DOI] [PubMed] [Google Scholar]
- 130.Bataller L, Wade DF, Fuller GN, Rosenfeld MR, Dalmau J. Cerebellar degeneration and autoimmunity to zinc-finger proteins of the cerebellum. Neurology. 2002;59:1985–1987. doi: 10.1212/01.wnl.0000038352.01415.ce. [DOI] [PubMed] [Google Scholar]
- 131.Dalakas MC. Advances in the pathogenesis and treatment of patients with stiff person syndrome. Curr. Neurol. Neurosci. Rep. 2008;8:48–55. doi: 10.1007/s11910-008-0009-y. [DOI] [PubMed] [Google Scholar]
- 132.Espay AJ, Chen R. Rigidity and spasms from autoimmune encephalomyelopathies: stiff-person syndrome. Muscle Nerve. 2006;34:677–690. doi: 10.1002/mus.20653. [DOI] [PubMed] [Google Scholar]